Summary
Bariatric surgery is currently the most effective treatment for obesity. Not only do these types of surgeries produce significant weight loss but also they improve insulin sensitivity and whole body metabolic function. The aim of this review is to explore how altered physiology of adipose tissue may contribute to the potent metabolic effects of some of these procedures. This includes specific effects on various fat depots, the function of individual adipocytes and the interaction between adipose tissue and other key metabolic tissues. Besides a dramatic loss of fat mass, bariatric surgery shifts the distribution of fat from visceral to the subcutaneous compartment favoring metabolic improvement. The sensitivity towards lipolysis controlled by insulin and catecholamines is improved, adipokine secretion is altered and local adipose inflammation as well as systemic inflammatory markers decreases. Some of these changes have been shown to be weight loss independent, and novel hypothesis for these effects includes include changes in bile acid metabolism, gut microbiota and central regulation of metabolism. In conclusion bariatric surgery is capable of improving aspects of adipose tissue function and do so in some cases in ways that are not entirely explained by the potent effect of surgery.
Keywords: Adipose, bariatric surgery, obesity
Introduction
Bariatric surgery is widely acknowledged as the most effective treatment for obesity, and intensive efforts over the past few years have not only added to our understanding of the mechanisms by which surgery improves metabolism and resolves type 2 diabetes in some patients but have also shifted our understanding of how metabolism is regulated. Mechanical explanations for the success of surgery such as restriction of stomach volume and intestinal malabsorption have given way to physiological explanations that emphasize alterations in gut signals to other organs (1,2). A key question is the degree to which these signals have direct or indirect impacts on adipose tissue metabolic function. A growing body of evidence links adipose tissue dysfunction to key aspects of the metabolic dysregulation that accompanies excess body weight. For this reason, our aim with the current review is to provide an overview of how adipose tissue responds to bariatric surgery and whether there are weight loss independent mechanisms involved in these responses.
Bariatric surgery procedures
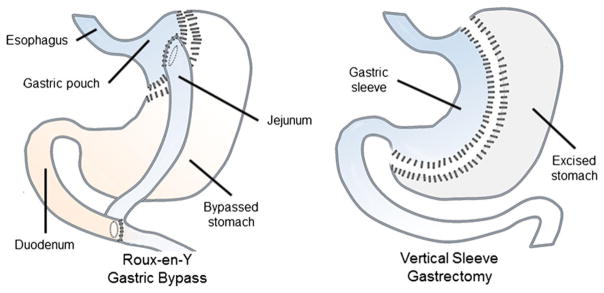
The two dominant bariatric operations used in the clinic are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (3) (Fig. 1). The RYGB leaves the patient with a small stomach pouch under the esophagus, and the gut anatomy is re-arranged such that nutrients are diverted from the upper to the middle part of the small intestine. RYGB not only induces significant weight loss but improves insulin resistance with remission of type 2 diabetes in many cases. Vertical sleeve gastrectomy (VSG) is an anatomically simpler operation, involving removing approximately 80% of the stomach along the greater curvature, leaving small intestinal anatomy unaltered. These procedures were formerly believed to be effective solely because of malabsorptive and restrictive properties; however, this paradigm has changed over the past decade as growing evidence supports that alterations in gut anatomy have profound effects on physiology including alterations in gut hormone secretion important for regulating feeding and metabolism. From the restrictive/malabsorptive point of view VSG would be expected to be inferior to RYGB because it involves a larger gastric reservoir and no intestinal bypass. Remarkably and counterintuitively, however, the efficacy of VSG is not far from RYGB (4).
Figure 1.
Bariatric surgery procedures. In the Roux-en-Y Gastric Bypass procedure a small stomach pouch is created under the esophagus. The jejunum is then attached to this pouch causing nutrient flow to bypass the proximal part of the duodenum. In the vertical sleeve gastrectomy procedure about 80% of the stomach is removed along the greater curvature. The intestines are left unaltered.
Adipose depot type and adipocyte size
The most obvious effect of bariatric surgery is loss of up to half of total adipose tissue mass within the first year after surgery along with improvements in systemic metabolism (5). These metabolic improvements associated with bariatric surgery do not correlate directly with reduction of adipose mass per se, but also relate to the extent different adipose tissue anatomic depots are affected. White adipose tissue may be divided into two broad categories: visceral adipose tissue (VAT) located in the peritoneal cavity, and subcutaneous adipose tissue (SAT), located under the skin. These two depots may be functionally subdivided even further: VAT includes omental (attached to the stomach), retroperitoneal (surrounding the kidneys) and mesenteric (attached to the intestines) subdepots, while SAT can be subdivided into deep and superficial as well as truncal and extremity compartments, each of which displays different functional characteristics (6).
Excess VAT is an independent risk factor for type 2 diabetes and cardiovascular disease and is more strongly correlated to these disease states than SAT (7–9). Adipose tissue depots manifest different physiologic profiles, with VAT demonstrating increased lipoytic capacity, inflammation, vascularization and secretion of specific adipokines. In addition, VAT drains its venous effluent directly to the liver via the portal venous system, thus exerting a disproportionate effect on hepatic and systemic metabolism (10). Despite the epidemiologic association of VAT with metabolic disease, studies of omental fat removal in humans, generally performed in combination with a bariatric procedure, have yielded conflicting results. Most of these studies demonstrate no change in metabolic disease relative to non-omentectomy controls, although one study reported greater weight loss (11), (11–16) and another found that omentectomy amplified the reduction in expression of inflammatory markers in skeletal muscle associated with RYGB (14). As omental fat only constitutes a minority of VAT, with mesenteric and retroperitoneal subdepots comprising a substantial proportion of total VAT mass, such findings emphasize the ability of distinct depots to not only store fat but also to impact metabolism.
Most studies of depot-specific fat mass find pronounced reductions in both VAT and SAT within the first few months after surgery (5,17–19). That said, magnetic resonance imaging of patients before and up to two years after bariatric surgery demonstrated that the vast majority of total fat mass is lost from SAT (20). However, studies with longer post-operative follow-up demonstrate further declines in VAT but not SAT, suggesting that late weight loss may disproportionately involve VAT (5,20–22). The particular significance of VAT was underscored in a study by Faria et al. (19) where metabolic parameters between subjects with persisting metabolic syndrome 1 year post RYGB were compared with patients that underwent type 2 diabetes remission and found that VAT mass was significantly decreased even though BMI and SAT area were similar. Finally, Toro-Ramos et al. (20) found that intramuscular adipose was highly reduced after surgery as well, suggesting that loss of mass from non-canonical adipose tissue depots may also contribute to the metabolic effects of bariatric surgery (23). These data highlight the fact that surgically induced weight loss involves different anatomic adipose tissue depots to different degrees, and suggest that the beneficial effects of bariatric surgery result not only from overall loss of fat mass but also a metabolically beneficial redistribution among different anatomic depots.
Reduction in adipocyte hypertrophy is a dominant feature of fat mass loss. Adipocyte size dramatically influences intracellular metabolic function. Larger adipocytes are associated with type 2 diabetes and metabolic disease in multiple studies (24–26). A proposed putative mechanism for the link between adipocyte hypertrophy and metabolic dysfunction involves induction of cellular hypoxia as adipocyte hypertrophy beyond the diffusion distance of oxygen, leading to inflammation and insulin resistance (27). Hypertrophy is also associated with a reduced capacity of adipose tissue to store energy in the form of triglycerides (TGs) in the fed state and to release free fatty acids (FFA) during fasting (10).
While the correlation between adipocyte hypertrophy and metabolic disease in the obese population is strong, this relationship is complex and context-dependent, which needs to be taken into account when interpreting adipocyte size changes after weight loss. Lean humans with smaller adipocytes have greater metabolic deterioration in response to overfeeding, suggesting that in the lean state, larger adipocytes are beneficial and a measure of nutrient buffering capacity (28). In obese patients, however, a hypertrophic threshold may be reached beyond which adipocyte buffering capacity is exceeded (29), leading to ectopic lipid deposition in peripheral tissues. Consistent with this concept extreme adipocyte hypertrophy in the obese state correlates positively with the degree of obesity and metabolic disease in humans and mice.
Studies investigating adipocyte size after bariatric surgery find that adipocytes become smaller (30,31) ultimately approaching diameters similar to lean controls (30), yet, total adipocyte number remains unchanged (32). These data are primarily restricted to SAT, because access to VAT samples in humans is limited after surgery. In line with these observations, Anderson et al. (32) reported that improvements in whole body insulin sensitivity 2 years after RYGB correlated strongly with a larger reduction in adipocyte size. Cotillard et al. (29) found significantly smaller adipocytes in subjects where type 2 diabetes risk was reverted 6 months post RYGB as compared to those patients where diabetes risk did not improve.
There are very few studies looking into adipocyte size in animal models. It has been shown that the mesenteric WAT (33) and the eWAT (34) contain smaller adipocytes after RYGB and VSG. One report observed that the weight loss induced by ileal interposition (a procedure where a portion of the ileum is moved to the jejunum) in diabetic rats was the result of decreases in mean adipocyte size in SAT as well as VAT (35). However, in another type of operation, the biliopancreatic diversion, SAT adipocytes shrink more than the cells found in the VAT (36). More clinical and pre-clinical research is needed to fully understand the intra-cellular changes in adipocytes after surgery. However, it is clear that overall bariatric surgery reduces both the size of the individual depots and adipocytes, and decreases the ratio of VAT to SAT. These changes are well known for their beneficial impact upon metabolic health and support a contributory role for improved adipose metabolic function after bariatric surgery.
Regulation of lipolysis
The physiologically most important function of adipose tissue is to act as an energy buffer. During positive energy balance, adipose tissue stores excess energy in a safe and accessible manner that allows for appropriate energy release primarily via lipolysis in times of negative energy balance. This balance between storage and release of lipids is regulated by a complex interplay of neurohumoral regulation for which insulin and plasma catecholamines play an integral role. A multitude of other factors regulate these processes as well, yet we will focus on (i) basal lipolysis; (ii) insulin inhibition of lipolysis; and (iii) catecholamine stimulation of lipolysis.
Basal lipolysis
Basal unstimulated lipolysis in isolated adipocytes has been shown to increase with obesity and seems to relate to the adipocytes being hypertrophic and thus dysfunctional (37–39). The information of ex vivo basal lipolysis with weight loss induced by reduced caloric intake is sparse with a few reports of no changes (37,40–42) and a single study showing a 50% reduction (43). To the best of our knowledge there are no studies reporting basal lipolysis rates in isolated adipocytes after bariatric surgery. A more clinically relevant measure of basal lipolysis is the outflow of FFA into the circulation during fasting. Yet, in the interpretation of data it has to be considered that systemic FFA levels not only are affected by lipolysis but also by clearance by muscle and liver. In general it is found that FFA levels increase systemically in the first few months after surgery after which they decrease (44–46). In studies where the follow-ups are performed at 6 months after surgery, the most common finding is decreased levels of FFA when compared to pre-surgery levels (12,21,47,48) but not as compared to the levels found in lean control subjects (46). A handful of studies report no significant changes after surgery (49–51).
Regulation by insulin
Even though insulin receptor stimulation of adipocytes promotes lipogenesis and uptake of fatty acids in addition to inhibiting lipolysis, this latter metabolic effect is by far the most important. The absence of insulin during fasting can relieve lipolytic inhibition to such an extent that it may cause intermittent fasting induced hepatic steatosis (52–54) and insulin resistance in this system manifests itself as a lack of ability to properly control the flow of FFAs in the transfer between fed and fasted states. The ability of the adipose tissue to respond to insulin can be measured by including plasma FFA in the hyperinsulinemic euglycemic clamp procedure, which has the distinct advantage of tightly controlling insulin levels. When compared to basal fasting levels of FFA as described above, the clamp studies report that insulin’s ability to suppress FFA outflow is impaired with obesity (45,55). Shortly after surgery FFA levels are increased compared to obese pre-surgery levels both in the absence and presence of insulin clamping (45,56), whereas insulin inhibition of lipolysis is fully comparable to lean control levels a few years after surgery (55). Interestingly this effect described by Curry et al. (55) seemed to depend on a higher dose of insulin.
In addition to evaluating the systemic effect of insulin upon adipose tissue by measuring FFA in the blood, insulin resistance within distinct adipose depots can also be investigated by quantification of downstream insulin receptor signaling. These types of studies are generally more widespread in animal models of bariatric surgery than in the clinic. Yet, studies that have investigated this in SAT biopsies after RYGB in humans and have found increased activation of the insulin receptor signaling pathway upon insulin stimulation (49,57). In the study by Carvalho et al. (49) this effect was shown to compare to lean control levels 6 months after surgery despite decreased insulin sensitivity prior to RYGB. Thus, at least in SAT, the obesity-induced insulin resistance is reduced after surgery. However, in rat models of RYGB few changes were observed in insulin receptor stimulation as validated by its phosphorylation or in expression of its downstream signaling molecules in adipose tissue (58,59) while one study found increased activation of downstream insulin receptor signaling via phosphorylation of Akt in the mesenteric depot (60). These apparently conflicting results most likely reflect that samples were obtained under conditions that were not optimal to reflect acute stimulation with a comparable level of insulin. The groups of animals differed in insulin levels and not all studies fasted the animals to down-regulate endogenous insulin prior to harvesting the samples.
Catecholamines
In contrast to insulin, plasma catecholamines play an important role in stimulation of lipolysis. Previous research has suggested that obesity causes ‘catecholamine resistance’ preventing adipose tissue from being appropriately catabolized when energy demand is high (e.g. fasting and/or exercise). Nonetheless this area of research has received relatively little attention over the past several decades. However, there is reason to revisit this phenomenon (61). Substantial evidence exists that adipose tissue in obese individuals indeed is resistant to catecholamine-induced lipolysis (62,63) which also explains why lipids accumulate in adipose depots despite obesity being linked to increased sympathetic activation (64). Catecholaminergic stimulation of lipolysis in adipocytes takes place via stimulation of adrenergic receptors of which the adipocyte contains several types. The best described receptor subtype is the beta-3 adrenergic receptor, which elicits a strong lipolytic response upon activation. In contrast, the alpha 2 receptor inhibits lipolysis. It has been hypothesized that the balance between these receptor sub-types changes with obesity and results in changes in the lipolytic response. Yet information about catecholaminergic responsiveness in adipose tissue after RYGB or VSG is sparse. Kaartinen et al. (65) isolated adipocyte membranes from obese subjects and patients, who had achieved substantial weight loss with bariatric surgery and found that lipolytic effects of pharmacological stimulation of beta adrenergic receptors were reduced with obesity as compared to lean subjects whereas the response after surgery was higher than in the lean controls despite no difference in receptor density between the groups. A mouse study examining beta-3 adrenergic receptor gene expression after RYGB found the levels to be increased in VAT (66). These findings match the increases in adrenergic response seen after weight loss in obese individuals (43,67).
In the setting of beta-adrenergic regulation of adipocytes it is worth mentioning the brown adipose tissue, which is highly metabolically active upon adrenergic stimulation and has a catabolic effect by converting fatty acids released by lipolysis to heat. Brown adipose tissue has only recently been proven active in human adults, and there is a great interest in exploring the therapeutic potential of its activation as it has been suggested as an explanation for the increased energy expenditure reported with RYGB (68–70). However, discrepancies have been reported between species with studies in rodents collectively failing to show such an effect (36,70–72) whereas data from the clinic suggests activation (68,69,73). As the function of this tissue in relation to metabolism, obesity and bariatric surgery is still not fully established, more information needs to be generated to understand its significance in these settings.
In general, the bulk of the evidence points towards surgery improving adipose tissue metabolic adaptability in terms of postprandial storage and fasting-induced release of fatty acids when appropriate. These changes do not occur immediately after surgery but rather take significant time to become evident. The profound hypocaloric state and attendant weight loss that follows surgery should be considered a significant confounder when interpreting changes in lipolytic capacity of adipose in the first weeks and months after surgery. More studies are needed to elucidate the molecular mechanisms behind depot specific long-term responsiveness towards insulin and catecholamines and also to clarify to which extent changes are bariatric surgery specific.
Adipokine secretion
Besides functioning as a storage depot, adipose tissue is also considered an endocrine organ secreting hundreds of different signaling proteins (adipokines) into the circulation (74). The actions of adipokines span autocrine signaling involved in lipid homeostasis and adipogenesis, crosstalk with the immune system and conveying information on energy status to the central nervous system (CNS) and other metabolic organs such as muscle and liver. The most well-known adipokine is the hormone leptin, which is the major contributor to the communication between adipose tissue and the CNS serving to suppress appetite when lipid storage is high. Similar to insulin, leptin responsiveness seems to be adversely affected by obesity such that despite increases in circulating leptin with adipose expansion, leptin is not able to successfully convey this surplus energy status to the brain (75). In addition to this leptin has been shown to stimulate proinflammatory immune responses (76). Adiponectin is another well-characterized adipokine, which acts on the peripheral metabolic tissues (liver and muscle). However, unlike leptin, plasma levels of adiponectin decrease, rather than increase with overall fat mass expansion, and as adiponectin is highly correlated to metabolic derangements of obesity and type 2 diabetes (77) the secretion of this adipokine is considered to be a hallmark of healthy adipocyte function. This is consistent with the observation that large dysfunctional adipocytes tend to decrease secretion of this adipokine to the circulation (78). Adiponectin exerts its effects via receptors expressed in muscle and liver and to some extent by autocrine actions causing improved insulin sensitivity as well as stimulating glucose utilization and fatty acid oxidation (79). Other less investigated adipokines that have been linked to metabolic function and obesity are visfatin and chemerin. Visfatin is produced primarily in VAT and has been linked to glucose usage, albeit the mechanism for this is still highly debated. Yet, several studies have shown a strong positive correlation between visfatin and impaired metabolic health (80). Chemerin has received interest for its autocrine actions in adipocyte. Especially because it is a necessary factor for adipogenesis and also it regulates adipocyte cellular metabolism (81). In addition to these there are a multitude inflammatory cytokines produced in adipose tissue, but these will be described in more detail in the inflammation section below.
As would be predicted based on fat mass changes, leptin decreases (82–87) whereas adiponectin increases (82,85,86,88–90) after bariatric surgery (see Table 1 for more references). These findings point strongly towards adipose tissue regaining its endocrine capacity after surgery. It has also been reported that SAT expression of leptin goes down after RYGB (91) whereas adiponectin gene expression in primarily SAT was reported to increase in only one out of five studies (84,91–94). In the context of these findings it is worth mentioning the novel hypothesis that adiponectin is produced in significant amounts by adipocytes in the bone marrow (95). In support of this hypothesis Coughlin et al. (96) found adiponectin expression to be highly upregulated in femoral adipose tissue after surgery. Both visfatin and chemerin have generally been found to decrease after surgery (11,90,97–102), and some studies report a correlation between reductions in the levels of these adipokines and improvements of other metabolic parameters such as insulin resistance, fatty liver and/or inflammation (90,98–100,102) Whether the secretion of adipokines plays a significant role in the improved metabolic state after surgery or is rather just a reflection of changes in adipose tissue mass is not fully elucidated and there are still mechanisms of action with many of the newly discovered adipokines, that are not well understood yet, but it has been described that adiponectin production in SAT after surgery is doubled after only 2 weeks (103) – before significant weight loss has occurred – suggesting weight loss independent adipokine responses.
Table 1.
Literature overview of clinical and animal studies investigating adipose tissue related end points after RYGB and VSG
| End point | Gastric bypass | VSG | Weight loss control group included | No effect with RYGB | No effect with VSG | |
|---|---|---|---|---|---|---|
| Clinical studies | ||||||
| Depot size | Decreased SAT size | (21); (17); (152); (48); (153); (19); (154); (20); (155) | (5); (156) | |||
| Decreased VAT size | (88); (157); (21); (17); (152); (48); (153); (19); (154); (20); (155) | (5); (156) | ||||
| Adipocyte size | Adipocyte size decreased | (32); (30); (31); (32); (85); (29); (116); (158) | (158) | (93) | ||
| Lipolysis | Improved insulin suppression | (55); (47); (158) | (158) | |||
| Catecholaminergic stimulation improved | (65) | |||||
| Adipokine secretion | Increased adiponectin in blood | (88); (82); (98); (11); (159); (160); (49); (31); (86); (93); (12); (85); (44); (161); (111); (84); (96); (162); (163); (164); (165); (57); (166) | (152); (167); (168); (169); (159); (89) | (88) | (103) | (170) |
| Decreased leptin in blood | (82); (11); (159); (160); (49); (31); (86); (93); (12); (85); (44); (161); (87); (55); (111); (84); (163); (171); (172); (164); (166) | (152); (167); (168); (169); (159); (89); (170) | ||||
| Inflammation | Decrease in inflammatory markers in blood/adipose tissue | (88); (173); (174); (152); (82); (48); (115); (175); (108); (11); (159); (49); (31); (86); (93); (12); (112); (85); (19); (116); (154); (163); (171); (172); (164); (176); (166); (177); (178); (179) | (169); (115); (180); (108); (15); (159); (89); (156) | (88) | (82); (111); (110); (165); (103) | |
| Decreased amounts of inflammatory cells | (30); (31); (33); (118); (117) | |||||
| In vivo studies | ||||||
| Depot size | Decreased SAT size | (34); (181) | (34); (182); (181) | (34); (182) | ||
| Decreased VAT size | (33) | (124); (34);(182); (181) | (124); (34); (182); (181) | |||
| Adipocyte size | Adipocyte size decreased | (33) | (34) | (34) | ||
| Lipolysis | Improved insulin suppression | |||||
| Catecholaminergic stimulation improved | ||||||
| Adipokine secretion | Increased adiponectin in blood | (59); (183) | (184); (185) | (185) | (186) | |
| Decreased leptin in blood | (187); (188); (185); (189); (34) | (185); (182) | (183) | (182); (181) | ||
| Inflammation | Decrease in inflammatory markers in blood/adipose tissue | (190) | (190) | |||
| Decreased amounts of inflammatory cells | (191) | (191) | ||||
Adipose inflammation
Low grade chronic inflammation within adipose tissue is associated with obesity. The fact that adipose mass in the obese may constitute as much as 50% of bodyweight and contain more than 1 million immune cells/g accentuates the significance of this tissue as an immunological organ with capacity to influence systemic immune function (104). Adipose inflammation has been hypothesized to be an important contributor to systemic insulin resistance and multiple other metabolic derangements (105). Despite its potential importance, the precipitating events for this inflammatory process are still being debated. Hypertrophic adipocytes increase production of pro-inflammatory adipokines (106) and also saturated fatty acids in the extracellular space have the capability to initiate a direct inflammatory response in macrophages through activation of pattern recognition receptors such as Toll-like receptors (107).
Chronic low-grade inflammation in adipose tissue contributes to levels of inflammatory markers in the circulation and for this reason bariatric surgery follow-up studies frequently apply measurements of common biomarkers such as C-Reactive Protein (CRP), TNF-alpha and/or IL-6. IL-6 is mostly consistently reported to decrease after surgery (15,31,49,85,108) albeit there are reports of no change as well (82). There is less consensus with TNF-alpha levels as they have been reported to decrease (108,109), stay unaltered (110) or even increase (111) in patients after surgery as compared with levels before surgery and between groups of obese versus operated patients. In addition the presence of the inflammatory adipokine Monocyte Chemotactic Protein-1 (MCP-1) in the blood has likewise been reported to decrease (11). Inflammation has also been evaluated within the adipose depots by protein or gene expression of these pro-inflammatory cytokines, and the results resemble the findings from blood with decreased expression of IL-6, TNF-alpha and MCP-1 (31,46,93,112–114).
CRP originates primarily from the liver. Still it is considered a marker of adipose inflammation as the liver is highly affected by obesity and CRP has consistently been shown to be upregulated with obesity. After bariatric surgery CRP levels show rapid (11) and large (115) declines that persist for up to 10 years (109) post-surgery.
Another aspect of inflammation is the abundance and inflammatory phenotype of immune cells residing within the adipose tissues. SAT, relative to VAT, contains not only fewer immune cells/mm (3) in general (116) but also the macrophage population (31), their assembly into crown like structures (117) and the balance of pro-inflammatory over anti-inflammatory macrophages (118) decrease after surgery (118). These findings are supported by a recent comprehensive RNAseq analysis of gene expression in SAT showing that 3 months after surgery clusters of genes related to specific immune populations all decreased (119).
With inflammation comes fibrotic remodeling and potential excessive synthesis of extracellular matrix components (120) and accordingly studies within animals models have shown that adipose fibrosis is reduced when macrophages are depleted (121). One of the major consequences of fibrosis is that the adipose tissue loses the plasticity to expand or contract with metabolic demands such that fibrosis in the obese state negatively affects the ability to lose weight after surgery (82,122). Yet whether surgery improves the ability of the fibrotic adipose to heal better than with weight loss induced by calorie restriction has not been examined.
To the best of our knowledge, very few studies have successfully measured local inflammation within adipose tissues after surgery in animal studies. However, these limited findings do indicate that inflammation decreases within the distinct adipose depots as assessed by TNF-alpha and IL-6 mRNA expression as well as number of macrophages and T-cells residing within the mesenteric depot in particular (33,60,82,123). These observations support the contention that bariatric surgery reduces inflammation associated with obesity (Fig. 2).
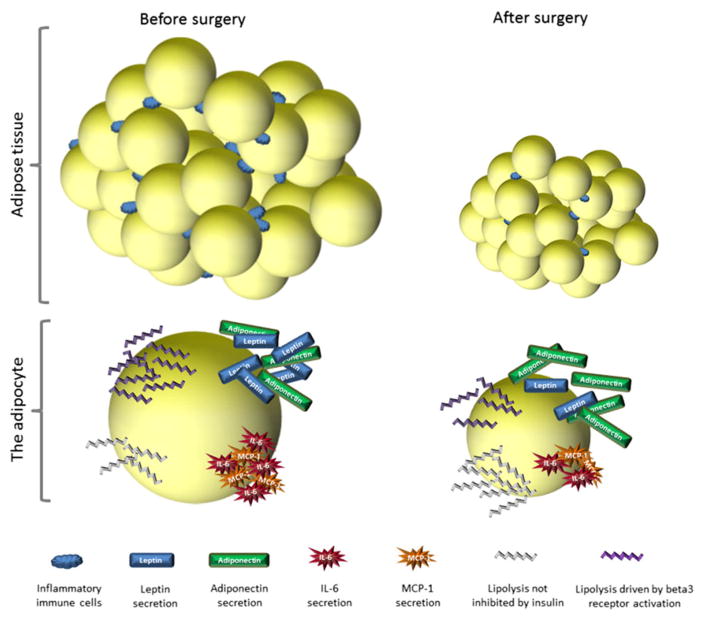
Figure 2.
Changes in adipocytes after surgery. After surgery the number of adipocytes stays the same but adiposity is decreased by a reduction in the lipid content/size of the individual cells. The amount of inflammatory immune cells residing within the adipose tissue also decreases. Individual adipocytes respond differently to lipolytic stimuli before and after surgery. In the obese state insulin is not able to suppress lipolysis causing leakage of FFA in the fed state and catecholamine stimulated lipolysis is hampered. Leptin secretion is high causing hyperleptinemia whereas adiponectin production is low. Also the adipocyte secretes pro-inflammatory adipokines such as MCP-1 and Il-6. After surgery the response towards lipolytic signals improves with insulin inhibiting FFA release and responsiveness towards catecholamines being restored. Leptin secretion decreases whereas adiponectin is upregulated. Proinflammatory adipokines are downregulated.
Future directions
Bariatric surgery is by far the most effective treatment for obesity, yet the resources required to treat the obesity epidemic with surgery outstrip our ability to deliver these surgical interventions to a large percentage of those impacted. An understanding of the mechanisms that underlie the potent effects of bariatric surgery on systemic metabolism will lead to novel targets for the development of therapeutics for obesity and metabolic disease. Towards this goal it is crucial to distinguish between physiologic changes resulting from weight loss secondary to reduced caloric intake and those that are a direct and independent effect of surgery per se as many responses will overlap. In animal studies, weight loss-independent effects are confirmed with weight-matched or pair-fed control groups. As seen in Table 1, few clinical studies include a weight loss control group because of the challenging if not impossible task of inducing weight loss of the same magnitude by diet restrictions in humans. Also this discrepancy in bodyweight outcome makes it difficult to compare studies with surgery alone to those studies where other interventions are investigated. Alternative approaches include comparing different types of surgery in the same study or avoiding pooling results from several different surgeries (a significant number of studies were excluded from Table 1 for this reason) to better define the distinct effects of different operations. In addition physiological changes that occur before significant weight loss might be detected by studying subjects in the early post-operative period. So far the studies that have used these approaches have shown that bariatric surgery independently reduces the mass of VAT, improves the circulating adipokines (123) as well as reduces lipid accumulation in the liver and blood (123–127). We propose that the following mechanisms could be responsible but require more investigation to be fully elucidated (see Fig. 3):
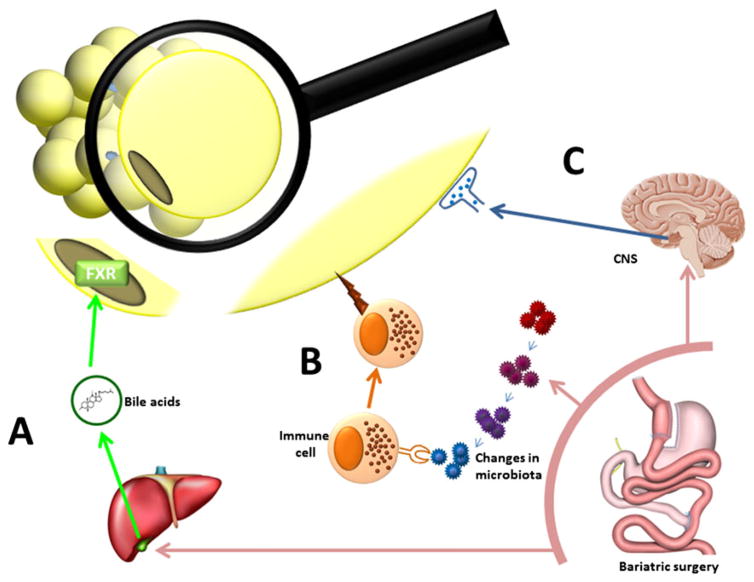
Figure 3.
Proposed novel mechanisms for adipose improvement after surgery. A) It is persistently reported that plasma bile acid levels go up after bariatric surgery. Bile acids can act upon the FXR receptor causing effects in metabolic tissues, and genetic deletion of the FXR receptor eliminates the effect of sleeve gastrectomy in mice. We propose that changes in bile acid signaling induced by bariatric surgery have the capacity to improve the function of the adipocyte. B) With surgery the composition of the bacteria in the gut changes. There is a tight interaction between the microbiota and the immune system. With the recent discoveries that immune cells have ability to regulate metabolic outcomes and that immune cell populations known to respond to microbiota changes are found in the adipose tissues, there is the possibility that cells of the immune system can significantly impact adipose function and that these changes can be initiated by bariatric surgery. C) Adipose tissue receives innervation from the CNS, and this has physiological impact as denervation of specific depots causes hypertrophy. As bariatric surgery causes metabolic changes related to central regulation of metabolism this draws attention to the possibility that neural output to the adipose tissues are altered with surgery causing physiological changes.
One of the current candidates for weight loss independent mechanisms is changes in bile acid levels. Plasma bile acids have been shown to increase after surgery in humans (128) and animals (126) – an effect that is weight independent and has been hypothesized to underlie the dramatic effects of bariatric surgery on metabolism (129). Bile acids act as endogenous ligands for several receptors of which FXR (Farnesoid-X Receptor) and TGR5 (G protein-coupled bile acid receptor 1) have received particular attention. FXR is a nuclear receptor that regulates lipid metabolism and trafficking as it is expressed in the intestine, liver, and adipose tissue (130). FXR and its target genes have been shown to modulate adipocyte phenotype and function (131). Interestingly, in our search to identify molecular mechanisms behind the metabolic effects of sleeve gastrectomy, we found that deletion of FXR abolished the effect of VSG in high fat fed mice (132). TGR5 is a cell-surface receptor which is expressed in BAT but not WAT and deletion of this receptor does accordingly not affect weight loss after VSG in mice, yet it reduces the glucoregulatory improvements that occur after surgery (133). One of the potential mechanisms by which bile acid receptor activation is hypothesized to exert beneficial effects is by reducing endoplasmic reticulum (ER) stress – a stress response proven to be increased in adipose tissue with obesity in animal models as well as in clinical studies (134,135) having impact on insulin sensitivity and inflammation. Recent work from Cummings et al. has confirmed this as a possible mechanism of action (60). In addition to neural and hormonal input, adipose tissues also receive metabolic regulatory input by other mechanisms. Fibroblast Growth Factor 21 (FGF21) and Fibroblast Growth Factor 19 (FGF19) are two endocrine FGFs that have both been shown to be upregulated by bariatric surgery in humans (136) (137). FGF21 controls the adipose metabolic phenotype and has been shown to hold great therapeutic potential for treating type 2 diabetes and obesity (138,139). Interestingly, Lips et al. (136) compared RYGB directly to gastric banding and weight loss by calorie restriction, and found that FGF21 levels were robustly upregulated with RYGB only. An additional aspect of interest with FGF21 is that it is differentially expressed in type 2 diabetes, and furthermore gene expression in the liver after RYGB differs between patients with diabetes remission and those without (140). However, a caveat with FGF21 is that it appears to differ between rodents and humans, such that translational extrapolations may be hard to establish.
Also it is worth considering the impact of bariatric surgery on the function of adipose tissue as an immunological organ capable of modulating not only immune populations in other tissues but also metabolic outcomes. This aspect of immune function has not been discovered until very recently but adds to our understanding of how immune cells directly influence the regulation of metabolism (141). Recently discovered key players of interest in this setting are distinct sub population of T-cells, namely invariant Natural Killer T (iNKT) cells and Mucosal Associated Invariant T (MAIT) cells. Magalhaes et al. (142) studied these two T-cell populations in obese and diabetic patients and found that both these types of T cells decreased in the circulation with obesity and type 2 diabetes. The MAIT cells were found to recruit to the adipose tissues in obesity and type 2 diabetes where they shifted to a distinct IL-17 cytokine profile. With bariatric surgery the abundance of these cells in the circulation increased (albeit not to the level of the lean controls), and they produced less IL-17. What makes these cell populations of specific interest in the setting of bariatric surgery are that (i) in contrast to the inflammatory component of the immune system these cells maintain a healthy homeostasis (143,144); (ii) iNKT seems to be able to interact directly with adipose metabolism by the adipocytes presenting lipid antigens to these T-cells, which in return regulate insulin sensitivity in the adipocytes (145); and (iii) MAIT are known to be associated to the gut mucosa and thus provide a potential missing link to the potent effects of surgery to alter the microbiota can directly impact the adipose tissue function. Such findings emphasize that the immune system could be involved in the metabolic benefits observed after surgery.
Alterations in the gut–brain axis may contribute to weight loss-independent effects of bariatric surgery such as gut signaling to the CNS regarding postprandial status via humoral and neural signals. The CNS, in turn, can regulate metabolic function via efferent neuronal activity of target metabolic organs, including adipose tissue which has been shown to become hypertrophic when denervated because of lack of sympathetic nervous system stimulation of lipolysis (146–148). In addition, sympathetic stimulation not only affects the release of fatty acids through lipolysis but also induces ‘beige’ or ‘brite’ adipocyte differentiation (149) with a concomitant increase in thermogenesis and fatty acid catabolism. Accordingly, it has been demonstrated that animals with more beige adipocytes are protected from obesity and diabetes (150) even though it is not yet fully understood if this effect relates to increased thermogenesis alone or whether there are secretory factors at play as well (151). The presence of beige adipocytes within SAT upon stimulation after bariatric surgery has not yet been fully investigated, but we hypothesize that induction of beige adipocytes might be one of the mechanisms by which bariatric surgery improves metabolism as catecholaminergic responsiveness seems to be increased after surgery. Evidence for this comes from Neinast et al. who reported upregulation of genes involved in beigeing after RYGB in mice (66). The CNS has also been shown to be a regulator of beige fat and so could be a mediator of any such surgical effects to increase the number of beige adipocytes after surgical intervention.
Conclusion
To advance our understanding of obesity and why bariatric surgery is superior to other treatment options we will have to strive for a deeper understanding of how different physiological processes and organs interact in the setting of metabolism. Current literature holds tantalizing hints that bariatric surgery affects adipose tissue far beyond mere reduction in lipid content. We believe this is important given the crucial role of adipose tissue in organism survival and energy usage. Adipose tissue communicates directly with multiple metabolic target organs regarding acute and chronic energy status and is likely a key component to the success of surgery.
Acknowledgments
HFS drafted the manuscript and prepared the figures. RWO, CNL, DS and RJS provided critical review of the manuscript and figures. The work of HFS and RJS is funded by NIH grant DK093848, the work of RWO is funded by NIH grant DK097449, the work of CNL is funded by NIH grant DK090262 and the work of DS is funded by NIH grant DK082480.
Abbreviations
- CRP
C Reactive Protein
- FFA
Free Fatty Acids
- FGF19
Fibroblast Growth Factor 19
- FGF21
Fibroblast Growth Factor 21
- FXR
Farnesoid X Receptor
- iNKT
invariant Natural Killer T cells
- MAIT
Mucosal Associated Invariant T cells
- RYGB
Roux-en-Y gastric bypass
- SAT
Subcutaneous Adipose Tissue
- TG
Triglycerides
- VAT
Visceral Adipose Tissue
- VSG
Vertical Sleeve Gastrectomy
Footnotes
Conflicts of interest
R.J.S. has received research support from Novo Nordisk, Boerhinger Ingelheim, Sanofi and Ethicon Endo-Surgery. He has also served as a paid consultant for Novo Nordisk, Boehringer Ingelheim, Ethicon Endo-Surgery, Sanofi, Novartis, Circuit Therapeutics, Nestle, Daichii Synkyo and Takeda. DS has received research support from Novo Nordisk, Ethicon Endo Surgery and Boehringer Engelheim. DS has also served as paid speaker for Novo Nordisk. HFS, CNL and RWO have nothing to declare.
References
- 1.Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2015;58:211–20. doi: 10.1007/s00125-014-3433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822–32. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 4.Carlin AM, Zeni TM, English WJ, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257:791–7. doi: 10.1097/SLA.0b013e3182879ded. [DOI] [PubMed] [Google Scholar]
- 5.Galanakis CG, Daskalakis M, Manios A, Xyda A, Karantanas AH, Melissas J. Computed tomography-based assessment of abdominal adiposity changes and their impact on metabolic alterations following bariatric surgery. World J Surg. 2014;39:417–23. doi: 10.1007/s00268-014-2826-2. [DOI] [PubMed] [Google Scholar]
- 6.Di Taranto G, Cicione C, Visconti G, et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy. 2015;17:1076–89. doi: 10.1016/j.jcyt.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher D, Kelley DE, Yim JE, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–14. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Int J Assoc Study Obes. 2010;11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 11.Lima MM, Pareja JC, Alegre SM, et al. Visceral fat resection in humans: effect on insulin sensitivity, beta-cell function, adipokines, and inflammatory markers. Obesity (SilverSpring) 2013;21:E182–E89. doi: 10.1002/oby.20030. [DOI] [PubMed] [Google Scholar]
- 12.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–55. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–42. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamboli RA, Hajri T, Jiang A, et al. Reduction in inflammatory gene expression in skeletal muscle from Roux-en-Y gastric bypass patients randomized to omentectomy. PLoS One. 2011;6:e28577. doi: 10.1371/journal.pone.0028577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sdralis E, Argentou M, Mead N, Kehagias I, Alexandridis T, Kalfarentzos F. A prospective randomized study comparing patients with morbid obesity submitted to sleeve gastrectomy with or without omentectomy. Obes Surg. 2013;23:965–71. doi: 10.1007/s11695-013-0925-z. [DOI] [PubMed] [Google Scholar]
- 16.Andersson DP, Thorell A, Lofgren P, et al. Omentectomy in addition to gastric bypass surgery and influence on insulin sensitivity: a randomized double blind controlled trial. Clin Nutr. 2014;33:991–6. doi: 10.1016/j.clnu.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Keidar A, Appelbaum L, Schweiger C, et al. Baseline abdominal lipid partitioning is associated with the metabolic response to bariatric surgery. Obes Surg. 2014;24:1709–16. doi: 10.1007/s11695-014-1249-3. [DOI] [PubMed] [Google Scholar]
- 18.Gaborit B, Abdesselam I, Kober F, et al. Ectopic fat storage in the pancreas using H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond) 2014;39:480–7. doi: 10.1038/ijo.2014.126. [DOI] [PubMed] [Google Scholar]
- 19.Faria G, Pestana D, Aral M, et al. Metabolic score: insights on the development and prediction of remission of metabolic syndrome after gastric bypass. Ann Surg. 2014;260:279–86. doi: 10.1097/SLA.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 20.Toro-Ramos T, Goodpaster BH, Janumala I, et al. Continued loss in visceral and intermuscular adipose tissue in weight-stable women following bariatric surgery. Obesity. 2015;23:62–9. doi: 10.1002/oby.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MK, Kim W, Kwon HS, Baek KH, Kim EK, Song KH. Effects of bariatric surgery on metabolic and nutritional parameters in severely obese Korean patients with type 2 diabetes: a prospective 2-year follow up. J Diabetes Investig. 2014;5:221–27. doi: 10.1111/jdi.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon DY, Kim HK, Kim JA, et al. Changes in the abdominal fat distribution after gastrectomy: computed tomography assessment. ANZ J Surg. 2007;77:121–25. doi: 10.1111/j.1445-2197.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- 23.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. Journal of magnetic resonance imaging : JMRI. 2009;29:1340–5. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 24.Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One. 2014;9:e105262. doi: 10.1371/journal.pone.0105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bays HE, Gonzalez-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–68. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell J, Lynch L, Cawood TJ, et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5:e9997. doi: 10.1371/journal.pone.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 28.Johannsen DL, Tchoukalova Y, Tam CS, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care. 2014;37:2789–97. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotillard A, Poitou C, Torcivia A, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99:E1466–70. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- 30.Cancello R, Zulian A, Gentilini D, et al. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int J Obes (Lond) 2013;37:867–73. doi: 10.1038/ijo.2013.7. [DOI] [PubMed] [Google Scholar]
- 31.Aghamohammadzadeh R, Greenstein AS, Yadav R, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62:128–35. doi: 10.1016/j.jacc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson DP, Eriksson HD, Thorell A, et al. Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care. 2014;37:1831–36. doi: 10.2337/dc13-2395. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–69. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez-Gimenez L, Becerril S, Moncada R, et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes Surg. 2015;25:1723–34. doi: 10.1007/s11695-015-1612-z. [DOI] [PubMed] [Google Scholar]
- 35.Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–46 46. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30:419–29. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 37.Mauriege P, Prud’homme D, Lemieux S, Tremblay A, Despres JP. Regional differences in adipose tissue lipolysis from lean and obese women: existence of postreceptor alterations. Am J Physiol. 1995;269:E341–50. doi: 10.1152/ajpendo.1995.269.2.E341. [DOI] [PubMed] [Google Scholar]
- 38.Arner P, Ostman J. Relationship between the tissue level of cyclic AMP and the fat cell size of human adipose tissue. J Lipid Res. 1978;19:613–8. [PubMed] [Google Scholar]
- 39.Sancho V, Trigo MV, Martin-Duce A, et al. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med. 2006;17:1133–7. [PubMed] [Google Scholar]
- 40.Imbeault P, Chevrier J, Dewailly E, et al. Increase in plasma pollutant levels in response to weight loss in humans is related to in vitro subcutaneous adipocyte basal lipolysis. Int J Obes Relat Metab Disord : journal of the International Association for the Study of Obesity. 2001;25:1585–91. doi: 10.1038/sj.ijo.0801817. [DOI] [PubMed] [Google Scholar]
- 41.Berman DM, Nicklas BJ, Ryan AS, Rogus EM, Dennis KE, Goldberg AP. Regulation of lipolysis and lipoprotein lipase after weight loss in obese, postmenopausal women. Obes Res. 2004;12:32–9. doi: 10.1038/oby.2004.6. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez TL, Sutherland JP, Wolfe P, et al. Lack of suppression of circulating free fatty acids and hypercholesterolemia during weight loss on a high-fat, low-carbohydrate diet. Am J Clin Nutr. 2010;91:578–85. doi: 10.3945/ajcn.2009.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynisdottir S, Langin D, Carlstrom K, Holm C, Rossner S, Arner P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin Sci. 1995;89:421–9. doi: 10.1042/cs0890421. [DOI] [PubMed] [Google Scholar]
- 44.Kullberg J, Sundbom M, Haenni A, et al. Gastric bypass promotes more lipid mobilization than a similar weight loss induced by low-calorie diet. J Obes. 2011;2011:959601. doi: 10.1155/2011/959601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 46.Pardina E, Ferrer R, Baena-Fustegueras JA, et al. Only C-reactive protein, but not TNF-alpha or IL6, reflects the improvement in inflammation after bariatric surgery. Obes Surg. 2012;22:131–39. doi: 10.1007/s11695-011-0546-3. [DOI] [PubMed] [Google Scholar]
- 47.Soriguer F, Garcia-Serrano S, Garcia-Almeida JM, et al. Changes in the serum composition of free-fatty acids during an intravenous glucose tolerance test. Obesity (SilverSpring) 2009;17:10–15. doi: 10.1038/oby.2008.475. [DOI] [PubMed] [Google Scholar]
- 48.Kim MK, Jang EH, Hong OK, et al. Changes in serum levels of bone morphogenic protein 4 and inflammatory cytokines after bariatric surgery in severely obese korean patients with type 2 diabetes. Int J Endocrinol. 2013;2013:681205. doi: 10.1155/2013/681205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carvalho BM, Oliveira AG, Ueno M, et al. Modulation of double-stranded RNA-activated protein kinase in insulin sensitive tissues of obese humans. Obesity (SilverSpring) 2013;21:2452–57. doi: 10.1002/oby.20410. [DOI] [PubMed] [Google Scholar]
- 50.Maymo-Masip E, Fernandez-Veledo S, Garcia EA, et al. The rise of soluble TWEAK levels in severely obese subjects after bariatric surgery may affect adipocyte-cytokine production induced by TNFalpha. J Clin Endocrinol Metab. 2013;98:E1323–E33. doi: 10.1210/jc.2012-4177. [DOI] [PubMed] [Google Scholar]
- 51.Dharuri H, ’t Hoen PA, van Klinken JB, et al. Downregulation of the acetyl-CoA metabolic network in adipose tissue of obese diabetic individuals and recovery after weight loss. Diabetologia. 2014;57:2384–92. doi: 10.1007/s00125-014-3347-0. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Donepudi AC, Moscovitz JE, Slitt AL. Keap1-knockdown decreases fasting-induced fatty liver via altered lipid metabolism and decreased fatty acid mobilization from adipose tissue. PLoS One. 2013;8:e79841. doi: 10.1371/journal.pone.0079841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambers KT, Chen Z, Crawford PA, et al. Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding. PLoS One. 2012;7:e52645. doi: 10.1371/journal.pone.0052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–8. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 55.Curry TB, Roberts SK, Basu R, et al. Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab. 2011;300:E746–E51. doi: 10.1152/ajpendo.00596.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Weijer BA, Aarts E, Janssen IM, et al. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity. 2013;21:1143–7. doi: 10.1002/oby.20220. [DOI] [PubMed] [Google Scholar]
- 57.Albers PH, Bojsen-Moller KN, Dirksen C, et al. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2015;309:R510–24. doi: 10.1152/ajpregu.00228.2014. [DOI] [PubMed] [Google Scholar]
- 58.Li SQ, Zhou Y, Wang Y, Liu Y, Geng DH, Liu JG. Upregulation of IRS-1 expression in Goto–Kakizaki rats following Roux-en-Y gastric bypass surgery: resolution of type 2 diabetes? Tohoku J Exp Med. 2011;225:179–86. doi: 10.1620/tjem.225.179. [DOI] [PubMed] [Google Scholar]
- 59.Bonhomme S, Guijarro A, Keslacy S, et al. Gastric bypass upregulates insulin signaling pathway. Nutrition. 2011;27:73–80. doi: 10.1016/j.nut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Cummings BP, Bettaieb A, Graham JL, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6:443–56. doi: 10.1242/dmm.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins P, Williams G. Drug treatment of obesity: from past failures to future successes? Br J Clin Pharmacol. 2001;51:13–25. doi: 10.1046/j.1365-2125.2001.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koppo K, Siklova-Vitkova M, Klimcakova E, et al. Catecholamine and insulin control of lipolysis in subcutaneous adipose tissue during long-term diet-induced weight loss in obese women. Am J Physiol Endocrinol Metab. 2012;302:E226–32. doi: 10.1152/ajpendo.00240.2011. [DOI] [PubMed] [Google Scholar]
- 63.Bairras C, Mauriege P, Bukowiecki L, Atgie C. Regulation of lypolysis in white adipose tissues of lean and obese Zucker rats. J Physiol Biochem. 2007;63:287–96. doi: 10.1007/BF03165760. [DOI] [PubMed] [Google Scholar]
- 64.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome—causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–72. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Kaartinen JM, LaNoue KF, Martin LF, Vikman HL, Ohisalo JJ. Beta-adrenergic responsiveness of adenylate cyclase in human adipocyte plasma membranes in obesity and after massive weight reduction. Metabolism. 1995;44:1288–92. doi: 10.1016/0026-0495(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 66.Neinast MD, Frank AP, Zechner JF, et al. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab. 2015;4:427–36. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mauriege P, Imbeault P, Langin D, et al. Regional and gender variations in adipose tissue lipolysis in response to weight loss. J Lipid Res. 1999;40:1559–71. [PubMed] [Google Scholar]
- 68.Bucerius J, Vijgen GH, Brans B, et al. Impact of bariatric surgery on carotid artery inflammation and the metabolic activity in different adipose tissues. Medicine. 2015;94:e725. doi: 10.1097/MD.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijgen GH, Bouvy ND, Teule GJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–E33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 70.Hankir MK, Bronisch F, Hintschich C, Krugel U, Seyfried F, Fenske WK. Differential effects of Roux-en-Y gastric bypass surgery on brown and beige adipose tissue thermogenesis. Metabolism. 2015;64:1240–9. doi: 10.1016/j.metabol.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Baraboi ED, Li W, Labbe SM, et al. Metabolic changes induced by the biliopancreatic diversion in diet-induced obesity in male rats: the contributions of sleeve gastrectomy and duodenal switch. Endocrinology. 2015;156:1316–29. doi: 10.1210/en.2014-1785. [DOI] [PubMed] [Google Scholar]
- 72.Lindqvist A, de la Cour CD, Hakanson R, Erlanson-Albertsson C. Ghrelin affects gastrectomy-induced decrease in UCP1 and beta3-AR mRNA expression in mice. Regul Pept. 2007;142:24–8. doi: 10.1016/j.regpep.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Rachid B, van de Sande-Lee S, Rodovalho S, et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int J Obes (Lond) 2015;39:1515–22. doi: 10.1038/ijo.2015.94. [DOI] [PubMed] [Google Scholar]
- 74.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 75.Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 76.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med. 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 77.Mohan V, Deepa R, Pradeepa R, et al. Association of low adiponectin levels with the metabolic syndrome—the Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54:476–81. doi: 10.1016/j.metabol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, Phillips SA. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013;2:217–26. doi: 10.4161/adip.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 80.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–27. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 81.Ferland DJ, Watts SW. Chemerin: a comprehensive review elucidating the need for cardiovascular research. Pharm Res: the official journal of the Italian Pharmacological Society. 2015;99:351–61. doi: 10.1016/j.phrs.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdennour M, Reggio S, Le NG, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99:898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 83.Lee YJ, Heo YS, Park HS, Lee SH, Lee SK, Jang YJ. Serum SPARC and matrix metalloproteinase-2 and metalloproteinase-9 concentrations after bariatric surgery in obese adults. Obes Surg. 2014;24:604–10. doi: 10.1007/s11695-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 84.Chen J, Pamuklar Z, Spagnoli A, Torquati A. Serum leptin levels are inversely correlated with omental gene expression of adiponectin and markedly decreased after gastric bypass surgery. Surg Endosc. 2012;26:1476–80. doi: 10.1007/s00464-011-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim MJ, Marchand P, Henegar C, et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119:377–83. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tschoner A, Sturm W, Engl J, et al. Plasminogen activator inhibitor 1 and visceral obesity during pronounced weight loss after bariatric surgery. NutrMetab Cardiovasc Dis. 2012;22:340–46. doi: 10.1016/j.numecd.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 88.Oberbach A, Schlichting N, Neuhaus J, et al. Establishing of a reliable multiple reaction monitoring-based method for the quantification of obesity associated comorbidities in serum and adipose tissue requires intensive clinical validation. J Proteome Res. 2014;13:5784–800. doi: 10.1021/pr500722k. [DOI] [PubMed] [Google Scholar]
- 89.Haluzikova D, Lacinova Z, Kavalkova P, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (SilverSpring) 2013;21:1335–42. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 90.Auguet T, Terra X, Hernandez M, et al. Clinical and adipocytokine changes after bariatric surgery in morbidly obese women. Obesity (SilverSpring) 2014;22:188–94. doi: 10.1002/oby.20470. [DOI] [PubMed] [Google Scholar]
- 91.Ferrer R, Pardina E, Rossell J, et al. Decreased lipases and fatty acid and glycerol transporter could explain reduced fat in diabetic morbidly obese. Obesity (SilverSpring) 2014;22:2379–87. doi: 10.1002/oby.20861. [DOI] [PubMed] [Google Scholar]
- 92.Chen J, Spagnoli A, Torquati A. Omental gene expression of adiponectin correlates with degree of insulin sensitivity before and after gastric bypass surgery. Obes Surg. 2012;22:472–77. doi: 10.1007/s11695-011-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sideleva O, Suratt BT, Black KE, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Savu MK, Phillips SA, Oh DK, et al. Response of adiponectin and its receptors to changes in metabolic state after gastric bypass surgery: dissociation between adipose tissue expression and circulating levels. Surg Obes Relat Dis. 2009;5:172–80. doi: 10.1016/j.soard.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coughlin CC, Finck BN, Eagon JC, et al. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity (SilverSpring) 2007;15:640–45. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 97.Terra X, Auguet T, Quesada I, et al. Increased levels and adipose tissue expression of visfatin in morbidly obese women: the relationship with pro-inflammatory cytokines. Clin Endocrinol(Oxf) 2012;77:691–98. doi: 10.1111/j.1365-2265.2011.04327.x. [DOI] [PubMed] [Google Scholar]
- 98.Shrestha C, He H, Liu Y, Zhu S, Xiong J, Mo Z. Changes in adipokines following laparoscopic Roux-en-Y gastric bypass surgery in Chinese individuals with type 2 diabetes mellitus and BMI of 22–30 kgm(−2.) Int J Endocrinol. 2013;2013:240971. doi: 10.1155/2013/240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–8. doi: 10.1007/s11695-013-1033-9. [DOI] [PubMed] [Google Scholar]
- 100.Sell H, Divoux A, Poitou C, et al. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–6. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 101.Ress C, Tschoner A, Engl J, et al. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Invest. 2010;40:277–80. doi: 10.1111/j.1365-2362.2010.02255.x. [DOI] [PubMed] [Google Scholar]
- 102.Parlee SD, Wang Y, Poirier P, et al. Biliopancreatic diversion with duodenal switch modifies plasma chemerin in early and late post-operative periods. Obesity. 2015;23:1201–8. doi: 10.1002/oby.21084. [DOI] [PubMed] [Google Scholar]
- 103.Sams VG, Blackledge C, Wijayatunga N, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc. 2015 doi: 10.1007/s00464-015-4638-3. [DOI] [PubMed] [Google Scholar]
- 104.Grant R, Youm YH, Ravussin A, Dixit VD. Quantification of adipose tissue leukocytosis in obesity. Methods Mol Biol. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 107.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–9. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 108.Viana EC, Araujo-Dasilio KL, Miguel GP, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-alpha. Prospective clinical trial. Obes Surg. 2013;23:1252–61. doi: 10.1007/s11695-013-0894-2. [DOI] [PubMed] [Google Scholar]
- 109.Kardassis D, Schonander M, Sjostrom L, Karason K. Carotid artery remodelling in relation to body fat distribution, inflammation and sustained weight loss in obesity. J Intern Med. 2014;275:534–43. doi: 10.1111/joim.12171. [DOI] [PubMed] [Google Scholar]
- 110.Catalan V, Gomez-Ambrosi J, Ramirez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- 111.Appachi S, Kelly KR, Schauer PR, et al. Reduced cardiovascular risk following bariatric surgeries is related to a partial recovery from “adiposopathy”. Obes Surg. 2011;21:1928–36. doi: 10.1007/s11695-011-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toubal A, Clement K, Fan R, et al. SMRT-GPS2 corepressor pathway dysregulation coincides with obesity-linked adipocyte inflammation. J Clin Invest. 2013;123:362–79. doi: 10.1172/JCI64052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eissing L, Scherer T, Todter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iannelli A, Anty R, Schneck AS, Tran A, Hebuterne X, Gugenheim J. Evolution of low-grade systemic inflammation, insulin resistance, anthropometrics, resting energy expenditure and metabolic syndrome after bariatric surgery: a comparative study between gastric bypass and sleeve gastrectomy. J Visc Surg. 2013;150:269–75. doi: 10.1016/j.jviscsurg.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 116.Poitou C, Dalmas E, Renovato M, et al. CD14dimCD16+ and CD14+ CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–30. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 117.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 118.Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–23. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 119.Poitou C, Perret C, Mathieu F, et al. Bariatric surgery induces disruption in inflammatory signaling pathways mediated by immune cells in adipose tissue: a RNA-Seq study. PLoS One. 2015;10:e0125718. doi: 10.1371/journal.pone.0125718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vila IK, Badin PM, Marques MA, et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7:1116–29. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 122.Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu C, Zhang G, Sun D, Han H, Hu S. Duodenal–jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes Surg. 2013;23:1436–44. doi: 10.1007/s11695-013-0976-1. [DOI] [PubMed] [Google Scholar]
- 124.Lancha A, Moncada R, Valenti V, et al. Effect of sleeve gastrectomy on osteopontin circulating levels and expression in adipose tissue and liver in rats. Obes Surg. 2014;24:1702–08. doi: 10.1007/s11695-014-1240-z. [DOI] [PubMed] [Google Scholar]
- 125.Stefater MA, Sandoval DA, Chambers AP, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:939–49. doi: 10.1053/j.gastro.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He B, Liu L, Yu C, Wang Y, Han P. Roux-en-Y gastric bypass reduces lipid overaccumulation in liver by upregulating hepatic autophagy in obese diabetic rats. Obes Surg. 2014;25:109–18. doi: 10.1007/s11695-014-1342-7. [DOI] [PubMed] [Google Scholar]
- 128.De Giorgi S, Campos V, Egli L, et al. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: a cross-sectional pilot study. Clin Nutr. 2014;34:911–7. doi: 10.1016/j.clnu.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 129.Kohli R, Myronovych A, Tan BK, et al. Bile acid signaling: mechanism for bariatric surgery, cure for NASH? Dig Dis. 2015;33:440–6. doi: 10.1159/000371699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gadaleta RM, Cariello M, Sabba C, Moschetta A. Tissue-specific actions of FXR in metabolism and cancer. Biochim Biophys Acta. 1851;2015:30–9. doi: 10.1016/j.bbalip.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism. Trends in endocrinology and metabolism: TEM. 2011;22:458–66. doi: 10.1016/j.tem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–8. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2015 doi: 10.1136/gutjnl-2015-309871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma NK, Das SK, Mondal AK, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–41. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang YC, Hee SW, Hsieh ML, Jeng YM, Chuang LM. The role of organelle stresses in diabetes mellitus and obesity: implication for treatment. Anal Cell Pathol. 2015;2015:972891. doi: 10.1155/2015/972891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lips MA, de Groot GH, Berends FJ, et al. Calorie restriction and Roux-en-Y gastric bypass have opposing effects on circulating FGF21 in morbidly obese subjects. Clin Endocrinol (Oxf) 2014;81:862–70. doi: 10.1111/cen.12496. [DOI] [PubMed] [Google Scholar]
- 137.Jansen PL, van Aarts WJE, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
- 138.Adams AC, Kharitonenkov A. FGF21: the center of a transcriptional nexus in metabolic regulation. Curr Diabetes Rev. 2012;8:285–93. doi: 10.2174/157339912800840505. [DOI] [PubMed] [Google Scholar]
- 139.Jahansouz C, Xu H, Hertzel AV, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 140.Roesch SL, Styer AM, Wood GC, et al. Perturbations of fibroblast growth factors 19 and 21 in type 2 diabetes. PLoS One. 2015;10:e0116928. doi: 10.1371/journal.pone.0116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferrante AW., Jr Macrophages, fat, and the emergence of immunometabolism. J Clin Invest. 2013;123:4992–3. doi: 10.1172/JCI73658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Magalhaes I, Pingris K, Poitou C, et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest. 2015;125:1752–62. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Magalhaes I, Kiaf B, Lehuen A. iNKT and MAIT cell alterations in diabetes. Front Immunol. 2015;6:341. doi: 10.3389/fimmu.2015.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schipper HS, Rakhshandehroo M, van de Graaf SF, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cantu RC, Goodman HM. Effects of denervation and fasting on white adipose tissue. Am J Physiol. 1967;212:207–12. doi: 10.1152/ajplegacy.1967.212.1.207. [DOI] [PubMed] [Google Scholar]
- 147.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–93. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- 148.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R514–R20. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 149.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 152.Umemura A, Sasaki A, Nitta H, Otsuka K, Suto T, Wakabayashi G. Effects of changes in adipocyte hormones and visceral adipose tissue and the reduction of obesity-related comor-bidities after laparoscopic sleeve gastrectomy in Japanese patients with severe obesity. Endocr J. 2014;61:381–91. doi: 10.1507/endocrj.ej13-0524. [DOI] [PubMed] [Google Scholar]
- 153.Pardina E, Baena-Fustegueras JA, Catalan R, et al. Increased expression and activity of hepatic lipase in the liver of morbidly obese adult patients in relation to lipid content. Obes Surg. 2009;19:894–904. doi: 10.1007/s11695-008-9739-9. [DOI] [PubMed] [Google Scholar]
- 154.Torriani M, Oliveira AL, Azevedo DC, Bredella MA, Yu EW. Effects of Roux-en-Y gastric bypass surgery on visceral and subcutaneous fat density by computed tomography. Obes Surg. 2015;25:381–5. doi: 10.1007/s11695-014-1485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pardina E, Baena-Fustegueras JA, Fort JM, et al. Hepatic and visceral adipose tissue 11betaHSD1 expressions are markers of body weight loss after bariatric surgery. Obesity. 2015;23:1856–63. doi: 10.1002/oby.21173. [DOI] [PubMed] [Google Scholar]
- 156.Dadson P, Landini L, Helmio M, et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care. 2016;39:292–9. doi: 10.2337/dc15-1447. [DOI] [PubMed] [Google Scholar]
- 157.Bazzocchi A, Ponti F, Cariani S, et al. Visceral Fat and body composition changes in a female population after RYGBP: a two-year follow-up by DXA. Obes Surg. 2014 doi: 10.1007/s11695-014-1422-8. [DOI] [PubMed] [Google Scholar]
- 158.Hansen M, Lund MT, Gregers E, et al. Adipose tissue mitochondrial respiration and lipolysis before and after a weight loss by diet and RYGB. Obesity. 2015;23:2022–9. doi: 10.1002/oby.21223. [DOI] [PubMed] [Google Scholar]
- 159.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–82. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis : official journal of the American Society for Bariatric Surgery. 2013;9:306–14. doi: 10.1016/j.soard.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 161.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (SilverSpring) 2009;17:1671–77. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Domienik-Karlowicz J, Rymarczyk Z, Dzikowska-Diduch O, et al. Emerging markers of atherosclerosis before and after bariatric surgery. Obes Surg. 2015;25:486–93. doi: 10.1007/s11695-014-1407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Netto BD, Bettini SC, Clemente AP, et al. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2015;25:1010–8. doi: 10.1007/s11695-014-1484-7. [DOI] [PubMed] [Google Scholar]
- 164.Lindegaard KK, Jorgensen NB, Just R, Heegaard PM, Madsbad S. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr. 2015;7:12. doi: 10.1186/s13098-015-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu XJ, Apovian C, Hess D, Carmine B, Saha A, Ruderman N. Improved insulin sensitivity 3 months after RYGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes. 2015;64:3155–9. doi: 10.2337/db14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond) 2015;40:275–80. doi: 10.1038/ijo.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Urbanova M, Dostalova I, Trachta P, et al. Serum concentrations and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type 2 diabetes mellitus: the effect of very-low-calorie diet, physical activity and laparoscopic sleeve gastrectomy. Physiol Res. 2014;63:207–18. doi: 10.33549/physiolres.932530. [DOI] [PubMed] [Google Scholar]
- 168.Bruna M, Gumbau V, Guaita M, et al. Prospective study of glucolipidic hormone and peptide levels in morbidly obese patients after sleeve gastrectomy. Cir Esp. 2014;92:175–81. doi: 10.1016/j.ciresp.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 169.Trachta P, Dostalova I, Haluzikova D, et al. Laparoscopic sleeve gastrectomy ameliorates mRNA expression of inflammation-related genes in subcutaneous adipose tissue but not in peripheral monocytes of obese patients. Mol Cell Endocrinol. 2014;383:96–102. doi: 10.1016/j.mce.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 170.Costa Justus JF, Ligocki Campos AC, Figueroa AL, et al. Early effect of bariatric surgery on the circadian rhythms of adipokines in morbidly obese women. Metab Syndr Relat Disord. 2016;14:16–22. doi: 10.1089/met.2015.0051. [DOI] [PubMed] [Google Scholar]
- 171.Montecucco F, Lenglet S, Quercioli A, et al. Gastric bypass in morbid obese patients is associated with reduction in adipose tissue inflammation via N-oleoylethanolamide (OEA)-mediated pathways. Thromb Haemost. 2015;113:838–50. doi: 10.1160/TH14-06-0506. [DOI] [PubMed] [Google Scholar]
- 172.Butte NF, Brandt ML, Wong WW, et al. Energetic adaptations persist after bariatric surgery in severely obese adolescents. Obesity. 2015;23:591–601. doi: 10.1002/oby.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chang EJ, Lee SK, Song YS, et al. IL-34 is associated with obesity, chronic inflammation, and insulin resistance. J Clin Endocrinol Metab. 2014;99:E1263–E71. doi: 10.1210/jc.2013-4409. [DOI] [PubMed] [Google Scholar]
- 174.Moreno-Navarrete JM, Novelle MG, Catalan V, et al. Insulin resistance modulates iron-related proteins in adipose tissue. Diabetes Care. 2014;37:1092–100. doi: 10.2337/dc13-1602. [DOI] [PubMed] [Google Scholar]
- 175.Iannelli A, Martini F, Rodolphe A, et al. Body composition, anthropometrics, energy expenditure, systemic inflammation, in premenopausal women 1 year after laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2014;28:500–07. doi: 10.1007/s00464-013-3191-1. [DOI] [PubMed] [Google Scholar]
- 176.Barazzoni R, Palmisano S, Gortan Cappellari G, et al. Gastric bypass-induced weight loss alters obesity-associated patterns of plasma pentraxin-3 and systemic inflammatory markers. Surg Obes Relat Dis : official journal of the American Society for Bariatric Surgery. 2016;12:23–32. doi: 10.1016/j.soard.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 177.Mocanu AO, Mulya A, Huang H, et al. Effect of Roux-en-Y gastric bypass on the NLRP3 inflammasome in adipose tissue from obese rats. PLoS One. 2015;10:e0139764. doi: 10.1371/journal.pone.0139764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vaittinen M, Walle P, Kuosmanen E, et al. FADS2 genotype regulates delta-6 desaturase activity and inflammation in human adipose tissue. J Lipid Res. 2016;57:56–65. doi: 10.1194/jlr.M059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.da Oliveira CS, Beserra BT, Cunha RS, et al. Impact of Roux-en-Y gastric bypass on lipid and inflammatory profiles. Rev Col Bras Cir. 2015;42:305–10. doi: 10.1590/0100-69912015005007. [DOI] [PubMed] [Google Scholar]
- 180.Gjessing HR, Nielsen HJ, Mellgren G, Gudbrandsen OA. Energy intake, nutritional status and weight reduction in patients one year after laparoscopic sleeve gastrectomy. Springer plus. 2013;2:352. doi: 10.1186/2193-1801-2-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moncada R, Rodriguez A, Becerril S, et al. Sleeve gastrectomy decreases body weight, whole-body adiposity, and blood pressure even in aged diet-induced obese rats. Obes Surg. 2015 doi: 10.1007/s11695-015-1919-9. [DOI] [PubMed] [Google Scholar]
- 182.Moncada R, Becerril S, Rodriguez A, et al. Sleeve gastrectomy reduces body weight and improves metabolic profile also in obesity-prone rats. Obes Surg. 2015 doi: 10.1007/s11695-015-1915-0. [DOI] [PubMed] [Google Scholar]
- 183.Wen Y, Lin N, Yan HT, et al. Down-regulation of renal gluconeogenesis in type II diabetic rats following Roux-en-Y gastric bypass surgery: a potential mechanism in hypoglycemic effect. Obes Facts. 2015;8:110–24. doi: 10.1159/000381163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawano Y, Ohta M, Hirashita T, Masuda T, Inomata M, Kitano S. Effects of sleeve gastrectomy on lipid metabolism in an obese diabetic rat model. Obes Surg. 2013;23:1947–56. doi: 10.1007/s11695-013-1035-7. [DOI] [PubMed] [Google Scholar]
- 185.Cummings BP, Bettaieb A, Graham JL, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–32. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rideout DA, Peng Y, Rakita SS, et al. Roux-en-Y gastric bypass alters tumor necrosis factor-alpha but not adiponectin signaling in immediate postoperative period in obese rats. Surg Obes Relat Dis. 2010;6:676–80. doi: 10.1016/j.soard.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 187.Nausheen S, Shah IH, Pezeshki A, Sigalet DL, Chelikani PK. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol EndocrinolMetab. 2013;305:E507–E18. doi: 10.1152/ajpendo.00130.2013. [DOI] [PubMed] [Google Scholar]
- 188.Rodriguez A, Becerril S, Valenti V, et al. Short-term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet-induced obese rats. Obes Surg. 2012;22:1481–90. doi: 10.1007/s11695-012-0702-4. [DOI] [PubMed] [Google Scholar]
- 189.Bielohuby M, Stemmer K, Berger J, et al. Carbohydrate content of post-operative diet influences the effect of vertical sleeve gastrectomy on body weight reduction in obese rats. Obes Surg. 2012;22:140–51. doi: 10.1007/s11695-011-0528-5. [DOI] [PubMed] [Google Scholar]
- 190.Oberbach A, Neuhaus J, Schlichting N, Kugler J, Baumann S, Till H. Sleeve gastrectomy reduces xanthine oxidase and uric acid in a rat model of morbid obesity. Surg Obes Relat Dis. 2014;10:684–90. doi: 10.1016/j.soard.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 191.Schneck AS, Iannelli A, Patouraux S, et al. Effects of sleeve gastrectomy in high fat diet-induced obese mice: respective role of reduced caloric intake, white adipose tissue inflammation and changes in adipose tissue and ectopic fat depots. Surg Endosc. 2014;28:592–602. doi: 10.1007/s00464-013-3211-1. [DOI] [PubMed] [Google Scholar]