CASE
A 39-year-old man presented to the Bellevue Hospital emergency department in November with 1 week of fevers and cough. On the previous day, he had been assaulted, developing right buttock and thigh pain.
His medical history was notable for alcohol use disorder treated with the opioid antagonist naltrexone (depot formulation), injected intramuscularly into the gluteal region monthly. He received left and right gluteal injections 1 and 6 weeks prior to presentation, respectively. He was homeless and denied travel or animal contact. He drank up to two dozen beer cans daily and smoked cigarettes but denied drug use.
On admission, he appeared ill and disheveled. His temperature was 38.3°C, his pulse was 124 beats per minute, and oxygen saturation was 94%; blood pressure and respiratory rate were normal. He had ecchymoses on the right buttock and thigh. His white blood cell count was 2.3 × 103/μl (differential of 87% polymorphonuclear leukocytes and 8% band leukocytes), hemoglobin was 8.4 g/dl, and platelets were 45 × 103/μl. Transaminases, alkaline phosphatase, and creatine kinase were elevated; albumin was low. Radiographs of the chest, pelvis, and femur were unremarkable. Direct antigen tests for influenza viruses were negative.
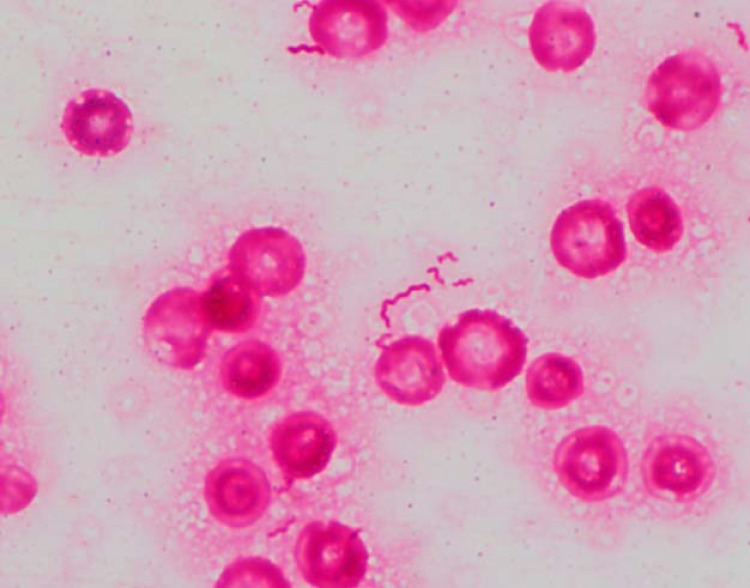
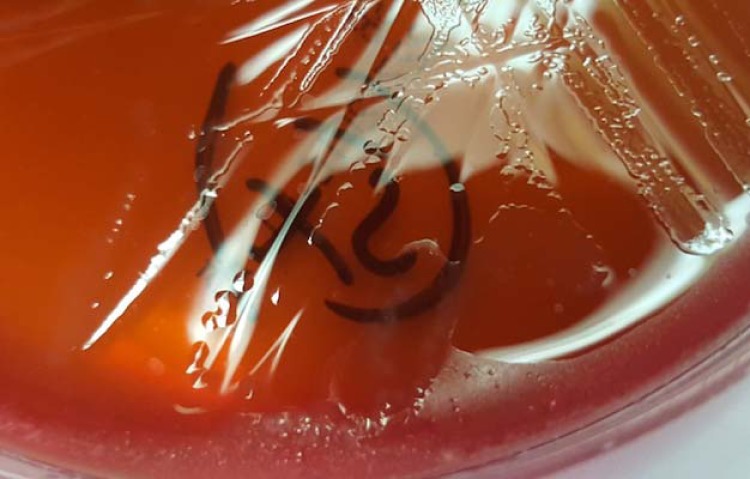
After blood cultures were obtained, ceftriaxone and azithromycin were administered for treatment of pneumonia; however, fevers persisted, and hypotension and altered mentation developed. On day two, antibiotics were empirically changed to vancomycin and cefepime due to clinical deterioration. An anaerobic blood culture bottle (Bactec 9240 System; Becton, Dickinson Inc., Franklin Lakes, NJ) became positive after 3 days of incubation, and Gram staining showed spiral-shaped Gram-negative organisms (Fig. 1). Broth was subcultured onto Trypticase soy agar with 5% sheep blood (blood agar plate [BAP]) and MacConkey, chocolate, and Campylobacter blood agar plates (BBL prepared plated media; Becton, Dickinson Inc., Franklin Lakes, NJ). BAP incubated anaerobically yielded small, translucent, spreading, nonhemolytic colonies that were oxidase and catalase negative (Fig. 2). The organism was identified as Anaerobiospirillum succiniciproducens by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using the FDA-approved Vitek MS version MS-CE CLI 2.0.0 on intact cells without extraction (bioMérieux Inc., Durham, NC). Although the species did not have a claimed or validated identification in the instrument database, the confidence value was 99.9, and its identification was subsequently confirmed by 16S rRNA gene sequencing performed at the New York State Department of Health Wadsworth Center.
FIG 1.
Gram stain of Anaerobiospirillum succiniciproducens from blood culture bottle, counterstained with fuchsin, demonstrating Gram-negative, spiral-shaped organisms.
FIG 2.
Colonies of Anaerobiospirillum succiniciproducens, which were small, translucent, spreading, and nonhemolytic, on 5% sheep blood agar.
Due to the patient's persistent fever, metronidazole was started on day 6. Computed tomography (CT) done on day 7 revealed at least two intramuscular fluid collections with enhancing rims in the right buttock, one in the posteromedial gluteus medius and another in the gluteus maximus. On the same day, 150 ml of purulent fluid was drained from the gluteus maximus collection. Gram staining this fluid showed many polymorphonuclear leukocytes but no microorganisms; aerobic and anaerobic cultures were negative. Subsequent blood cultures were negative, and the patient clinically improved. Once the organism was identified, antibiotics were changed on day 11 to intravenous ampicillin-sulbactam for six additional days, followed by 2 weeks of oral amoxicillin-clavulanic acid. Repeat pelvis CT showed resolution of abscesses.
DISCUSSION
Bacteremia is a common clinical condition with high rates of mortality. A significant minority of bloodstream isolates are obligate anaerobic organisms, which are similarly associated with poor clinical outcomes. Early diagnosis and appropriate antimicrobial therapy are crucial to the outcome of bacteremic patients. Identification systems based on panels of biochemical or enzymatic reactions, though widely available and inexpensive, can be limited in their ability to identify infrequently encountered anaerobic organisms, and some require relatively long incubation times (1). Recognizing the importance of rapid identification of bloodstream isolates, clinical microbiology laboratories have adopted commercially available molecular tests based on nucleic acid amplification and use of fluorescent probes, though these products are not currently available to identify obligate anaerobes.
MALDI-TOF MS has emerged as a rapid diagnostic tool for accurate identification of diverse bacterial isolates, including anaerobes. It can identify anaerobic isolates more rapidly than conventional biochemical systems and provides more reliable identification than rapid enzymatic panel systems do (2). Studies have also explored MALDI-TOF MS for identifying bacterial species directly from blood culture bottles, which would further shorten time to identification. This case is the first published in which Anaerobiospirillum succiniciproducens was successfully identified by using Vitek MS.
A. succiniciproducens is a motile, Gram-negative, spiral-shaped, obligate anaerobe first reported in 1976, and it is one of two species in the genus Anaerobiospirillum, along with Anaerobiospirillum thomasii. The organism has been isolated from the feces of asymptomatic pet cats and dogs, where it seems to constitute normal flora.
A. succiniciproducens has been reported as an uncommon cause of human illness in the form of bacteremia. To date, 43 cases of A. succiniciproducens bacteremia have been reported, some of which may be represented among the 11 blood culture isolates of A. succiniciproducens identified by the Centers for Disease Control and Prevention since 2012, all using 16S rRNA gene sequencing (D. Lonsway, personal communication). Cases consisted of persons mostly over 40 years of age with chronic medical conditions, including malignancy, diabetes mellitus, liver disease, alcoholism, and cardiovascular disease. Clinical manifestations included fever, leukocytosis, and constitutional symptoms, such as malaise or weight loss. Gastrointestinal manifestations, such as abdominal pain, diarrhea, nausea, and emesis, were common. While one patient presented with an infected upper extremity wound thought to represent a primary source of infection, only one patient, a woman with a tubo-ovarian abscess with secondary peritonitis, had a clear pyogenic focus of infection along with bacteremia. Several patients died despite receiving adequate antimicrobial therapy.
A. succiniciproducens can grow in commercial blood culture media as well as in blood agar media incubated under anaerobic conditions. While Gram stain morphology of A. succiniciproducens may appear similar to that of Campylobacter, the former organism can be distinguished by growth under anaerobic conditions only and negative reactions to oxidase and catalase, while Campylobacter grows under microaerophilic conditions and is oxidase and catalase positive (3). Since both organisms are thin, counterstain with fuchsin instead of safranin might be necessary for Gram staining. A. succiniciproducens displays corkscrew motility and contains bipolar tufts of flagella that can be seen using a stain of crystal violet in ethanol combined with tannic acid and aluminum potassium sulfate or under electron microscopy. Attempts to identify A. succiniciproducens using the RapID ANA II System (Thermo Fisher Scientific, Waltham, MA) have been unsuccessful, since the organism is not included in the system's database (3). The Rapid ID 32A system (bioMérieux, Durham, NC) has been used to successfully identify A. succiniciproducens in a few cases (4). In a recent publication, another MALDI-TOF MS system, MALDI Biotyper (Bruker Daltonics, Bremen, Germany), failed to identify A. succiniciproducens (5). That Vitek MS successfully identified the organism in our case may reflect differences in the databases or other features of commercially available systems. In recent cases, 16S rRNA gene sequencing has been used to confirm organism identification or provide initial identification after other attempts have failed.
A. succiniciproducens is frequently resistant to metronidazole and clindamycin but is generally susceptible to combined beta-lactam and beta-lactamase inhibitors, cephalosporins other than those in the first generation, carbapenems, and fluoroquinolones (6). Penicillin, aminopenicillins, and tetracyclines are variably active (6).
The patient described in this case had an alcohol use disorder, a frequently cited risk factor for A. succiniciproducens infection. The pathophysiology of this patient's infection is unclear though he may have developed primary bacteremia via translocation of the organism from his gastrointestinal tract, a frequently proposed though unproven mechanism. While A. succiniciproducens was not cultured from the patient's gluteal abscess, likely due to prolonged antibiotic administration before sampling, the presence of abscesses coincident with bacteremia, one of which resolved on antibiotics without drainage, makes A. succiniciproducens very likely the etiologic agent of his pyomyositis. Testing culture-negative isolates with universal 16S rRNA gene primers and sequencing was not widely used in this institution at that time. Risk factors for this unusual pyogenic manifestation of A. succiniciproducens bacteremia may have included prior depot naltrexone injections to the site and more recent trauma sustained during an assault. The patient's slight elevation of creatine kinase may have been due to pyomyositis, though it may also have reflected skeletal muscle injury from his assault or a nonspecific response to severe sepsis. His cough and mild hypoxemia may have been due to a coincidental, unrelated respiratory illness or may also have been a nonspecific manifestation of sepsis. Antimicrobial susceptibility testing was not performed, according to this hospital's policy on the workup of anaerobic isolates.
Clinicians should consider pyogenic complications of A. succiniciproducens bacteremia when patients with bacteremia have localizing signs or symptoms, as in this case. Additionally, as more bacterial species are added to proprietary and open-access MALDI-TOF MS databases, the utility of this method of identification to identify uncommon organisms rapidly will likely expand. Prompt species identification of bloodstream isolates is important in facilitating early selection of appropriate antimicrobials.
SELF-ASSESSMENT QUESTIONS
- What is the most well-established risk factor for Anaerobiospirillum succiniciproducens bacteremia?
-
A.Chronic disease, including alcoholism
-
B.Contact with dogs or other canine species
-
C.Recent travel to Southeast Asia
-
D.Recent abdominopelvic surgery, including hysterectomy
-
A.
- Which of the following antimicrobial agents is likely the most reliably active against A. succiniciproducens?
-
A.Ceftriaxone
-
B.Metronidazole
-
C.Clindamycin
-
D.Doxycycline
-
A.
- A. succiniciproducens is characterized by which of the following properties, which allows it to be distinguished from Campylobacter?
-
A.Beta-hemolytic colonies on blood agar plates
-
B.Growth in chocolate agar but not in standard blood agar media
-
C.Microscopy showing spiral-shaped, Gram-negative organisms
-
D.Oxidase- and catalase-negative reactions
-
A.
For answers to the self-assessment questions and take-home points, see page 986 in this issue (https://doi.org/10.1128/JCM.01358-16).
ACKNOWLEDGMENTS
We thank Tatyana Datiashvili, Rajinder Kalsi, and Kenneth Inglima for their assistance in the microbiologic workup of this isolate.
REFERENCES
- 1.Blairon L, Maza ML, Wybo I, Pierard D, Dediste A, Vandenberg O. 2010. Vitek 2 ANC card versus BBL Crystal Anaerobe and RapID ANA II for identification of clinical anaerobic bacteria. Anaerobe 16:355–361. doi: 10.1016/j.anaerobe.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Barba MJ, Fernandez A, Oviano M, Fernandez B, Velasco D, Bou G. 2014. Evaluation of MALDI-TOF mass spectrometry for identification of anaerobic bacteria. Anaerobe 30:126–128. doi: 10.1016/j.anaerobe.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Tee W, Korman TM, Waters MJ, Macphee A, Jenney A, Joyce L, Dyall-Smith ML. 1998. Three cases of Anaerobiospirillum succiniciproducens bacteremia confirmed by 16S rRNA gene sequencing. J Clin Microbiol 36:1209–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadzilah MN, Faizatul LJ, Hasibah MS, Sam IC, Bador MK, Gan GG, Abubakar S. 2009. Anaerobiospirillum succiniciproducens bacteraemia in a patient with acute lymphoblastic leukaemia. J Med Microbiol 58:142–143. doi: 10.1099/jmm.0.004622-0. [DOI] [PubMed] [Google Scholar]
- 5.Decroix V, Pluquet E, Choquet M, Ammenouche N, Castelain S, Guiheneuf R. 2016. Place of diagnostic tools in the identification of Anaerobiospirillum succiniciproducens bacteraemia. Anaerobe 39:28–30. doi: 10.1016/j.anaerobe.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Kelesidis T. 2011. Bloodstream infection with Anaerobiospirillum succiniciproducens: a potentially lethal infection. South Med J 104:205–214. doi: 10.1097/SMJ.0b013e318200c8d7. [DOI] [PMC free article] [PubMed] [Google Scholar]