ABSTRACT
During measles outbreaks, it is important to be able to rapidly distinguish between measles cases and vaccine reactions to avoid unnecessary outbreak response measures such as case isolation and contact investigations. We have developed a real-time reverse transcription-PCR (RT-PCR) method specific for genotype A measles virus (MeV) (MeVA RT-quantitative PCR [RT-qPCR]) that can identify measles vaccine strains rapidly, with high throughput, and without the need for sequencing to determine the genotype. We have evaluated the method independently in three measles reference laboratories using two platforms, the Roche LightCycler 480 system and the Applied Biosystems (ABI) 7500 real-time PCR system. In comparison to the standard real-time RT-PCR method, the MeVA RT-qPCR showed 99.5% specificity for genotype A and 94% sensitivity for both platforms. The new assay was able to detect RNA from five currently used vaccine strains, AIK-C, CAM-70, Edmonston-Zagreb, Moraten, and Shanghai-191. The MeVA RT-qPCR assay has been used successfully for measles surveillance in reference laboratories, and it could be readily deployed to national and subnational laboratories on a wide scale.
KEYWORDS: measles, PCR, genotyping, measles vaccine, molecular methods
INTRODUCTION
Endemic transmission of measles virus (MeV) was interrupted in the Americas in 2002 (1), but since then, importations of measles from areas of endemicity have caused frequent and sometimes large outbreaks (2–6) and a recent transitory suspension of the elimination status (7). An important component of the public health response to a measles outbreak is vaccination of unimmunized contacts (8). Since approximately 5% of recipients of measles virus-containing vaccine experience rash and fever which may be indistinguishable from measles (9), it is very important to identify vaccine reactions to avoid unnecessary isolation of the patient, as well as the need for contact tracing and other labor-intensive public health interventions. Recent measles outbreaks in the Canadian provinces of Alberta and British Columbia have emphasized the need for rapid differentiation of vaccine reactions (18, 19) from reactions to infection with the wild-type virus. During the measles outbreak in California in 2015, a large number of suspected cases occurred in recent vaccinees (3). Of the 194 measles virus sequences obtained in the United States in 2015, 73 were identified as vaccine sequences (R. J. McNall, unpublished data). In contrast, only 11 of 542 cases genotyped in the National Reference Center for Measles, Mumps, and Rubella in Germany were associated with the vaccine virus.
Genotyping is used to confirm the origin of an outbreak and to exclude endemic circulation, but it is also the only way to distinguish vaccine strains from wild-type viruses. Genetic characterization of MeV is accomplished by sequencing of the 450 nucleotides (nt) coding for the COOH terminal 150 amino acids of the nucleoprotein (N-450) (10). The WHO currently recognizes 24 genotypes of measles virus, and all of the vaccine strains are in a single genotype, genotype A. Wild-type viruses of genotype A are no longer circulating (11).
It is difficult, especially during outbreaks, to perform rapid confirmation of vaccine reactions by sequencing, and there is interest in developing rapid molecular tests to detect vaccine strains (12). Here, we describe a real-time reverse transcription-PCR (RT-PCR) method that detects the vaccine genotype (MeVA RT-quantitative PCR [RT-qPCR]) and that can provide rapid discrimination between wild-type-virus infections and vaccine reactions. The method was developed initially on the Roche LightCycler 480 platform at the Canadian National Microbiology Laboratory (NML) and then independently evaluated at the Robert Koch-Institute (RKI) in Germany using the same platform and at the US Centers for Disease Control (CDC) using the Applied Biosystems 7500 platform.
RESULTS
Assay development and evaluation at the NML.
The analytical sensitivity of the MeVA RT-qPCR on the Roche LightCycler 480 platform was established using the synthetic RNA standard, which was serially diluted from 103 to 10−1 copies per reaction and tested in triplicate in at least 6 separate assays in parallel with the MeV RT-qPCR. The lower limit of detection of the MeVA RT-qPCR was 10 to 100 copies per reaction, compared to a sensitivity of 1 to 10 copies per reaction for the MeV RT-qPCR (Table 1).
TABLE 1.
The lower limit of detection of MeVA RT-qPCR compared to MeV RT-qPCR was determined by testing serial dilutions of synthetic MeV RNA with a known copy number
| Assay | Copy no. | No. of samples with positive results/total no. of samples tested | % positive results |
|---|---|---|---|
| MeVA RT-qPCR | 103 | 18/18 | 100 |
| 102 | 18/18 | 100 | |
| 101 | 13/20 | 65 | |
| 100 | 1/18 | 6 | |
| 10−1a | 0/3 | 0 | |
| MeV RT-qPCR | 103 | 18/18 | 100 |
| 102 | 18/18 | 100 | |
| 101 | 18/18 | 100 | |
| 100 | 4/18 | 22 | |
| 10−1a | 0/3 | 0 |
This concentration was tested only 3 times since it is undetectable by both assays and therefore was not informative in the determination of the lower limit of detection.
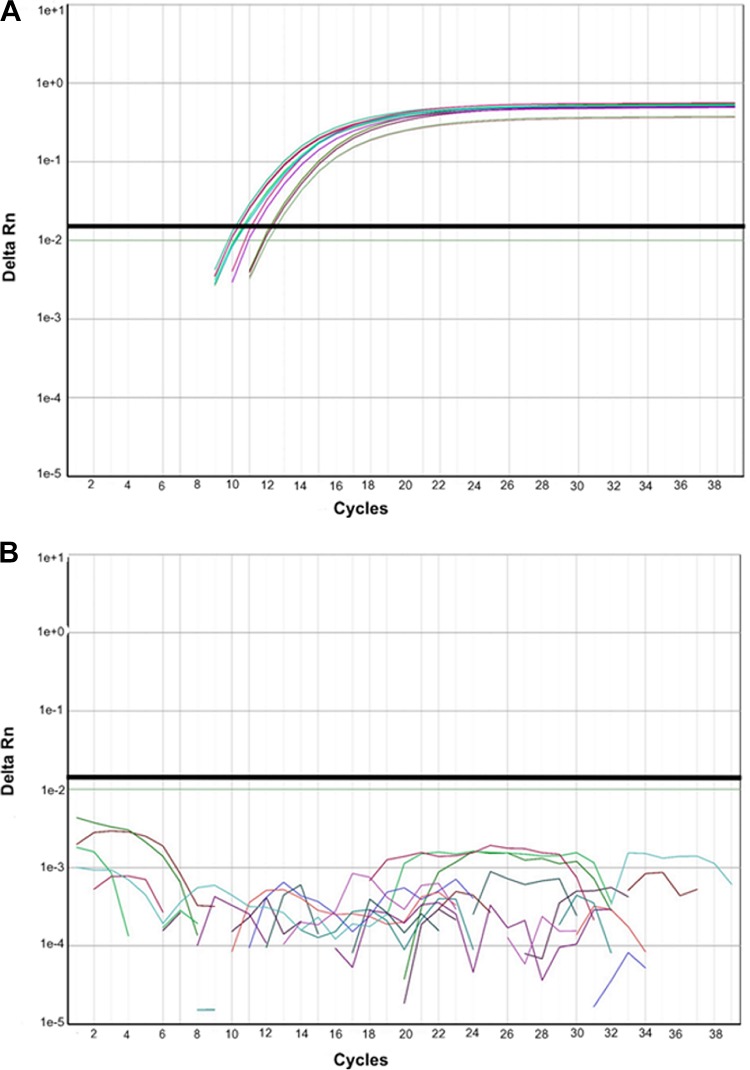
Eighty-eight surveillance specimens that were previously genotyped as genotype A, 96 specimens of nonvaccine measles virus genotypes (B3, C2, D3, D4, D6, D7, D8, D9, E, H1, and H2), and isolates for genotypes B2, C1, D2, D5, D6, D7, D10, G1, G2, and H2 (WHO Measles Strain Bank, US Centers for Disease Control, Atlanta, GA, USA) were tested with MeVA RT-qPCR and produced no false-positive results. The amplification curves of 33 wild-type measles virus samples, including all the genotypes listed above, did not rise significantly in comparison to the curves of samples containing vaccine strain RNA (Fig. 1). However, 3 of 88 genotype A specimens were not detected by the MeVA RT-qPCR (Table 2). These three specimens were near the lower limit of detection (crossing-point [Cp] value, >35) for the MeV RT-qPCR. The sensitivity of the MeVA RT-qPCR in relation to the MeV RT-qPCR was 97% (90% to 99%, 95% confidence interval [CI]), and the specificity was 100% (95% to 100%, 95% CI) (Table 3). Specificity was further evaluated by testing a panel of other viral agents from cell culture-derived material or clinical specimens (parvovirus B19, dengue virus serotypes 1 to 4, influenza virus H3N2, poliovirus Sabin 1 species C, enterovirus D68-2 [EV-D68-2] species D, Coxsackie virus, EV71, parechovirus, echovirus 18, herpes simplex virus 1 [HSV1], HSV2, Epstein-Barr virus [EBV], cytomegalovirus [CMV], human herpesvirus 6 [HHV-6], HHV7, varicella zoster virus [VZV], rubella virus, and mumps virus). All specimens were negative by MeVA RT-qPCR.
FIG 1.
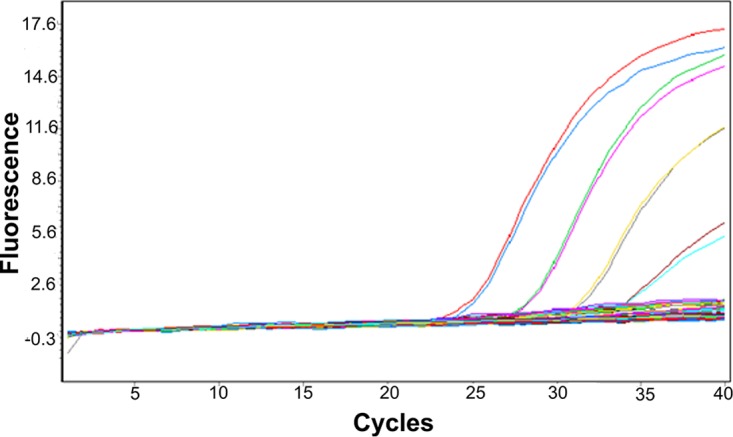
Amplification curve from the MeVA RT-qPCR on the Roche LightCycler 480 system. The bundle of the flat curves includes 33 wild-type measles virus specimens comprising the following genotypes: B2, B3, C1, C2, D2, D3, D4, D5, D6, D7, D8, D9, D10, G1, G2, E, H1, and H2. The amplification curves are from MeV vaccine RNA from 102 to 105 copy numbers, assessed in duplicate. The QuantiTect Probe RT-PCR kit was used for these reactions.
TABLE 2.
Comparison of MeVA RT-PCR and MeV RT-qPCR in three reference laboratories
| Reference laboratory and MeVA RT-qPCR result | No. of MeV RT-qPCR samples |
Total no. of samples | |
|---|---|---|---|
| Genotype A | Not genotype A | ||
| NML | |||
| Positive | 85 | 0 | 85 |
| Negative | 3 | 96 | 99 |
| Total | 88 | 96 | 184 |
| CDC | |||
| Positive | 12 | 0 | 15 |
| Negative | 1 | 12 | 13 |
| Total | 13 | 12 | 28 |
| RKI | |||
| Positive | 41 | 1 | 42 |
| Negative | 5 | 111 | 116 |
| Total | 46 | 112 | 158 |
TABLE 3.
Summary of sensitivity and specificity of MeVA RT-qPCR for the detection of MeV genotype A
| Center | No. of samples | % sensitivity (95% CI) | % specificity (95% CI) | Genotypes tested |
|---|---|---|---|---|
| NML | 184 | 97 (90–99) | 100 (95–100) | B3, B2, C1, D2, C2, D3, D4, D5 D6, D7, D8, D9, D10, G1, G2, E, H1, H2 |
| RKI | 158 | 89 (0.76–0.96) | 99 (94–100) | B3, D4, D5, D6, D8, D9, D10, G2, H1 |
| CDC | 28 | 92 (66–100) | 100 (70–100) | B3, D4, D8, D9, G3, H1, AIK, CAM-70, Edmonston-Zagreb, Moraten, Shanghai-191a |
| Overall | 370 | 94 (88–97) | 99 (97–100) |
Genotypes D4 and G3 and the non-Edmonston vaccine strains, tested using synthetic RNAs and culture lysate, respectively, were not included in the sensitivity and specificity calculations.
Fifty specimens that were positive for vaccine strain A were tested in parallel by MeVA RT-qPCR and MeV RT-qPCR, and there was a good correlation of the Cp values between the two methods, with a slope of 0.88 (0.82 to 0.94, 95% CI). The slope was significantly different from 1.00, and a y intercept of 4.1 (2.2 to 6.0, 95% CI) confirmed that the sensitivity and limit of detection of the MeVA RT-qPCR method were lower than those of the MeV RT-qPCR (Fig. 2).
FIG 2.
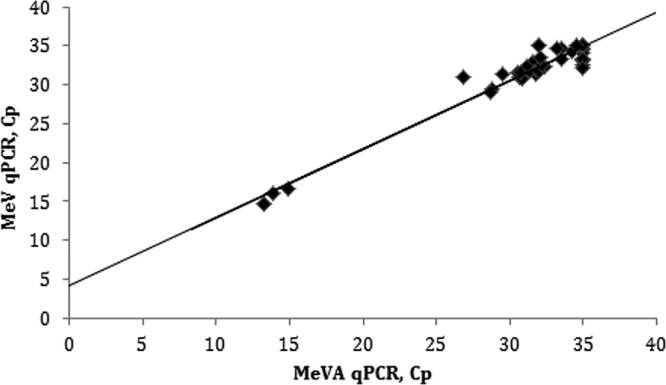
Correlation between Cp values of 50 genotype A measles virus specimens tested by MeVA RT-qPCR and the standard MeV RT-qPCR method. The regression line has a slope of 0.88 (0.82 to 0.94, 95% CI), a y intercept of 4.1 (2.2 to 6.0, 95% CI) and an R2 value of 0.949 (P < 0.0001). The QuantiTect Probe RT-PCR kit was used for these reactions.
Assay evaluation at RKI.
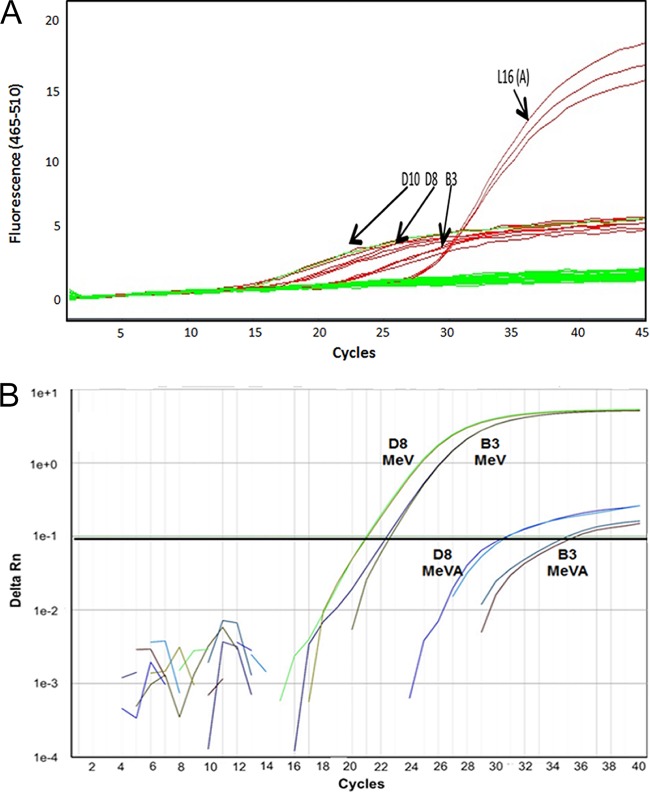
The MeVA qPCR was also independently evaluated at RKI by testing 46 archival measles virus specimens of genotype A and 112 samples containing wild-type MeV, including genotypes B3, D4, D5, D6, D8, D9, D10, G2, and H1. The same LightCycler 480 platform was used. The MeV RT-qPCR (16) includes the SuperScript III Platinum One-Step qRT-PCR kit (Invitrogen), so an evaluation was performed comparing the SuperScript III and QuantiTect reagent kits. The SuperScript III PCR kit produced suboptimal results, with significant increases of the amplification baseline of nonvaccine measles virus genotypes D10, D8, and B3 (Fig. 3A).
FIG 3.
Negative effect of the use of the SuperScript III Platinum One-Step quantitative RT-PCR kit on the specificity of the MeVA RT-qPCR for the vaccine genotype. (A) The use of SuperScript III on the Roche LightCycler 480 platform caused a significant rise in the baseline of the amplification curves for genotype D10, D8, and B3. (B) Results of the use of SuperScript III on an ABI 7500 platform in amplification curves from wild-type measles virus RNA.
When the QuantiTect Probe RT-PCR kit was used for the MeVA RT-qPCRs, the test was 89% sensitive and 99.5% specific for genotype A measles virus (Table 3), with amplification curves comparable to those shown in Fig. 1. There was a single false-positive result from a genotype D5 wild-type strain, which produced amplification with the MeVA RT-qPCR. The region targeted by the MeVA assay was sequenced, and the sequence differed from the vaccine strain sequence by a G at position 517 in the probe region (conserved in all wild-type genotypes listed in Fig. 4), by a C at position 538 in the reverse primer region (similar to genotypes D4, D7, and D8), and by a T at position 548, at the 5′ terminus of the reverse primer (similar to genotypes B3 and D6). These genotypes did not produce any cross-reactivity with the MeVA-specific assay, and the reason for the false-positive result for this D5 specimen is unclear.
FIG 4.
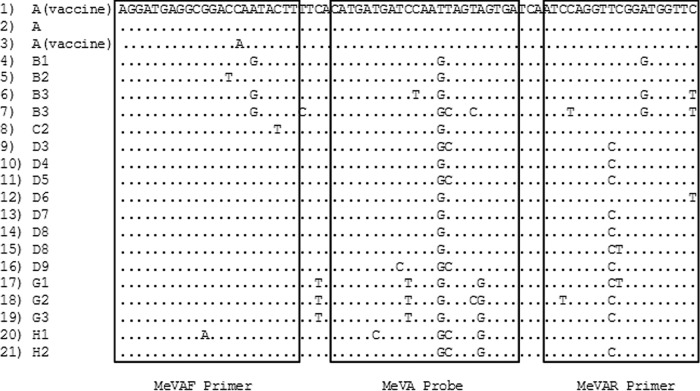
Alignment of the N gene region (positions 478 to 548) amplified by the MeVA RT-qPCR. The alignment includes examples of each genotype available on GenBank for this region, except for genotype D9, which was sequenced from one of our archival specimens. Row 1, 31 identical sequences from various vaccine strains; row 2, MVi/Maryland.USA/54 (A); row 3, two vaccine strains showing a 1-nt difference in the forward primer region; row 4, MVi/Yaounde.CMR/12.83 (B1); row 5, MVi/Libreville.GAB/84 (B2); row 6, MVi/Ibadan.NIE/971 (B3); row 7, MVi/New_York.USA/94 (B3); row 8, MVi/Maryland.USA/77 (C2); row 9, MVi/Illinois.USA/89/1 (D3); row 10, MVi/Montreal.CAN/89 (D4); row 11, Bankok.THA/12.93 (D5); row 12, MVi/New_Jersey.USA/94/1 (D6); row 13, MVs/Dundee.UNK/82 (D7); row 14, MVi/BritishColumbia.CAN/13.10/1 (D8); row 15, MVi/Manchester.GBR/30.94 (D8); row 16, MVs/Ontario.CAN/14.14 (D9); row 17, MVi/Berkeley.USA/83 (G1); row 18, MVi/Amsterdam.NLD/49.97 (G2); row 19, MVi/Gresik.IDN/17.02 (G3); row 20, MVi/Hunan.CHN/93/7 (H1); row 21, MVi/Beijing.CHN/94/1 (H2).
Assay evaluation at CDC on the ABI 7500 platform.
The MeVA RT-qPCR method was independently evaluated at the CDC on the ABI 7500 instrument, which is a commonly used instrument in state public health laboratories and is available in many laboratories in the WHO Measles Rubella Laboratory Network (14). Similarly to the results seen with the LightCycler 480 platform, the MeVA RT-qPCR assay performed suboptimally with the SuperScript III kit with respect to the resulting amplification curves for wild-type measles virus genotypes (Fig. 3B).
The MeVA RT-qPCR and MeV qPCR were compared using the ABI 7500 platform and the QuantiTect kit, and the samples included synthetic MeV RNAs serially diluted from 105 to 101 copies per reaction. The dilutions were tested in duplicate on at least four separate assays. The results were similar to those obtained from the Roche LightCycler 480 system (Table 1) in that the lower limit of detection of the MeVA RT-qPCR assay was approximately 1 Log10 higher than for the MeV RT-qPCR assay.
To assess the specificity of the MeVA RT-qPCR assay on the ABI 7500 platform and to compare it to the performance of the MeV RT-qPCR assay, three sets of samples were used, i.e., synthetic RNAs containing the entire N gene open reading frames from six currently circulating wild-type genotypes (B3, D4, D8, D9, G3, and H1) (B. Bankamp, unpublished data), RNA from cell culture lysates from five vaccine strains (AIK-C, CAM-70, Edmonston-Zagreb, Moraten, and Shanghai-191), and RNA extracted from 28 archival respiratory swabs and urine specimens that were submitted to the CDC for routine surveillance. Three archival specimens were negative by MeV RT-qPCR, and the other 25 were positive by MeV RT-qPCR and included clinical specimens from measles cases and vaccine reactions (with threshold cycle [CT] values ranging from 14 to 36).
Of the positive archival specimens, all specimens with wild-type genotypes (n = 12) were negative in the MeVA RT-qPCR assay but positive in the MeV RT-qPCR assay and 12 of 13 specimens from vaccine reactions were positive in both assays (Table 2). Three of the specimens from the vaccine reactions had CT values ranging from 38 to 40 in the MeV RT-qPCR assay and from 38 to 40 in the MeVA RT-qPCR.
The RNA from all five vaccine strains was detected in both assays with slightly lower sensitivity (CT value, 2 to 3) in the MeVA RT-qPCR assay than in the MeV RT-qPCR assay (data not shown). In addition, the MeVA RT-qPCR assay did not produce a positive signal in samples containing high copy numbers of synthetic RNA from the six commonly circulating wild-type genotypes (Fig. 5).
FIG 5.
Specificity of the MeVA RT-qPCR assay, using an Applied Biosystems 7500 platform. Synthetic RNA from the six active wild-type measles virus genotypes (B3, D4, D8, D9, G3, and H1) was tested. Panel A shows detection of 107 copies of RNA/reaction in the MeV qPCR assay, and panel B shows the lack of amplification of 107 copies of RNA/reaction in the MeVA RT-qPCR assay. The QuantiTect Probe RT-PCR kit was used for these reactions.
If we consider all samples that were amplified within 40 PCR cycles, as was done at NML and RKI for the LightCycler platform, the sensitivity of the MeVA test on the ABI 7500 platform was 94% and the specificity was 100% (Table 3).
DISCUSSION
In response to the need for prompt differentiation between vaccine reactions and wild-type measles virus infection cases, laboratories have been developing methods that do not require sequencing of N-450. A method targeting a region on the hemagglutinin gene has been described and tested with a small number of vaccine and wild-type specimens or isolates (15). Here, we describe the development and validation of a measles virus genotype A-specific RT-qPCR, MeVA RT-qPCR, that targets the N gene of MeV. This assay produces rapid results and is capable of high throughput. The MeVA RT-qPCR was thoroughly tested at three global reference laboratories. Two RT-qPCR platforms and over 300 samples were included in the evaluation. Overall, our data show very high (99.5%) specificity for the A genotype, albeit with lower (94%) sensitivity than the standard MeV RT-qPCR (16). Because of the lower sensitivity, the MeVA RT-qPCR is intended to be used as a tool for rapid detection of genotype A sequences and not as a primary diagnostic test. The MeVA RT-qPCR should be performed in parallel with the MeV RT-qPCR method. Multiplexing of the two tests is in progress to increase the efficiency of this method.
We have shown that the MeVA RT-qPCR can be used on both the Roche LightCycler 480 and the ABI 7500 platforms, which are available in a large number of laboratories around the world. We also demonstrated that the QuantiTect kit gave optimal performance on both platforms.
An alignment of the nt 478 to 548 region used for the MeVA RT-qPCR (Fig. 4) shows that some wild-type strains differ only by a single nucleotide in the probe region, a G at position 517, although other mismatches that may favor specificity are present in the primer regions. This point mutation may be stable, since it results in an amino acid change (serine in wild-type strains to isoleucine in vaccine strains), but it is conceivable that wild-type strains may arise with a mutation in this position that cross-reacts with the MeVA assay. Therefore, we currently still confirm every MeVA RT-qPCR result by WHO-recommended sequencing of the N-450 region.
The specificity of the test has been assessed on most of the measles virus genotypes currently circulating in the world (except D11 and G3) (11, 14), and there was only one genotype D5 specimen that gave a false-positive result. The sequence of the MeVA RT-qPCR target region of this genotype D5 virus differed from that of the vaccine strains by the G at position 517, conserved in all measles virus wild-type strains, and by two additional nucleotides in the reverse primer. Genotypes D4, D6, and D8 have the same sequence as this D5 strain in the probe region and one fewer difference in the reverse primer region (Fig. 4), but they did not cross-react with the MeVA RT-qPCR. Another genotype D5 strain, tested at the NML, and 12 additional D5 strains, tested at the RKI, were not amplified by the MeVA RT-qPCR. Therefore, the reasons for this false-positive result remain unclear.
During measles outbreak investigations, rapid detection of measles vaccine reactions is necessary to avoid unnecessary public health interventions. In Canada, the NML has been using the MeVA and MeV RT-qPCRs with a turnaround time of 2 days. Therefore, local health authorities can initiate appropriate public health responses without waiting for sequencing results, which often take several days to obtain. The MeVA RT-qPCR is especially useful during large measles outbreaks, when it is difficult for laboratories to perform sequencing on a large number of specimens in a timely manner. Similarly, recent measles outbreaks in the United States have reinforced the need for rapid confirmation of vaccine reactions. In countries such as Germany, which is still experiencing frequent measles outbreaks, this RT-PCR-based method has already proven to be a valuable tool for guiding the public health responses. The MeVA RTqPCR assay is a straightforward application of real-time RT-PCR methodology, and the two platforms evaluated here are available in many laboratories. This assay could be readily deployed to national and subnational laboratories on a wide scale.
MATERIALS AND METHODS
Primers, probes, and control RNA.
The primers and probe for the vaccine-specific assays were designed following analysis of 31 sequences available on GenBank from Edmonston-derived and non-Edmonston-derived vaccine strains. These sequences are identical in the target region of MeVA RT-qPCR (the 3′ region of the MeV N gene between nt 478 and nt 548 of the Edmonston strain [GenBank accession no. AF266288.2]), including the more divergent non-Edmonston-derived strains Shanghai-191 and CAM-70 (Fig. 4) (15). Two vaccine strains, Schwarz FF-8 (GenBank AB591381.1) and Edmonston AIK-C (GenBank S58435.1), have a 1-nt difference in the sequence of the forward primer, but they are identical to the other vaccine strains in the probe region (Fig. 4). The primers (Invitrogen) for reverse transcription and cDNA amplification were 5′-AGGATGAGGCGGACCAATACTT-3′ (MeVAF) and 5′-GAACCATCCGAACCTGGAT-3′ (MeVAR). Both primers were used at a concentration of 0.9 μM. Amplification was detected by a TaqMan probe (TIB Molbiol) with 6-carboxyfluorescein (FAM) as a fluorophore, at a concentration of 0.25 μM. The probe had the sequence 5′-FAM-CATGATGATCCAATTAGTAGTGA-BBQ-3′ (MeVA probe [BBQ, black berry quencher]), where the underlined characters indicate locked nucleic acid bases containing a 2′O,4-C methylene bridge which has the effect of increasing the melting temperature (Tm) and potentiating the destabilizing effect of a nucleotide mismatch (17).
As a standard for the measurement of MeV copy numbers, synthetic measles virus RNA was prepared by in vitro transcription, using a MEGAscript T7 transcription kit (Invitrogen, Life Technologies Inc.), either from a plasmid containing the open reading frame of the N gene of genotype A (16) or from PCR amplicons that included the T7 promoter in the forward primer (Bankamp, unpublished). DNase-treated RNA was purified with a MEGAclear transcription cleanup kit (Ambion, Life Technologies Inc.) and quantitated fluorometrically (Qubit, Life Technologies Inc.). The absence of residual DNA was verified by real-time RT-PCR (MeV RT-qPCR) (16) in the presence or absence of the reverse transcriptase.
Samples tested.
For this study, 370 samples were tested to evaluate the sensitivity and specificity of the MeVA RT-PCR. The majority of these were clinical samples that were submitted to NML, CDC, or RKI as part of routine surveillance activities for measles.
Roche LightCycler 480 platform.
Archival nasopharyngeal swabs and urine specimens sent to the NML for molecular surveillance were used. These specimens tested positive for measles virus by MeV RT-qPCR using a previously described method (16) and were genotyped using the N-450 target (10, 11). RNA was extracted using the QIAamp viral RNA minikit (Qiagen; catalog no. 52904) or the MagNA Pure liquid chromatograph (LC) total nucleic acid isolation kit—high performance (Roche Diagnostics; catalog no. 05323738001) on the MagNA Pure LC 2.0 instrument (Roche Diagnostics). For RT-PCR, 2 μl of extracted RNA was subjected to one-step reverse transcription and qPCR using the QuantiTect Probe RT-PCR kit (Qiagen; catalog no. 204443) according the instructions of the manufacturer. The RT-qPCR mixtures (total volume, 20 μl) were incubated at 50°C for 20 min (RT step) and 95°C for 15 min (activation of the polymerase) and subjected to 40 cycles of amplification (95°C for 5 s and 60°C for 1 min) on the Roche LightCycler 480 instrument. The RT-qPCR result was considered positive if there was amplification within 40 cycles, but crossing-point (Cp) values were recorded for only the first 35 cycles.
At the National Reference Center for Measles, Mumps, and Rubella at the RKI, archival surveillance specimens were extracted using the QIAamp viral RNA minikit (Qiagen; catalog no. 52906) and amplified by using the SuperScript III Platinum One-Step quantitative RT-PCR kit (Invitrogen; catalog no. 11732-088) or the QuantiTect Probe RT-PCR kit (Qiagen; catalog no. 20443). MeVA RT-qPCR, MeV RT-qPCR, and genotyping at the N-450 region were performed as described above. The RT-PCR result was considered positive if amplification was detected within 40 cycles.
Applied Biosystems 7500 platform.
At the CDC, RNA was extracted with the QIAamp viral RNA minikit as described above. The MeV RT-qPCR was performed using the same reaction conditions and primers and probes (16). As for the Roche LightCycler 480, the SuperScript III and QuantiTect reagent kits were evaluated as described in Results. For the comparisons described in this report, the RT-qPCR result was considered positive if there was amplification within 40 cycles; however, during routine use of this assay at the CDC, specimens with threshold cycle (CT) values between 38 and 40 are considered to represent equivocal results.
Statistical analyses.
Sensitivity and specificity of MeVA RT-qPCR were calculated using the VassarStats website (13). Linear regression and related statistics were calculated using an online calculator developed by GraphPad Software, Inc. (http://www.graphpad.com/quickcalcs/linear1) and graphed using Microsoft Excel.
ACKNOWLEDGMENTS
We thank Petra Kurzendörfer (RKI), Anne Wolbert (RKI), and Elena Lopareva (CDC) for technical assistance and Sabine Santibanez (RKI) for helpful discussions.
This study was supported by the Public Health Agency of Canada and the US Centers for Disease Control and Prevention.
The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Footnotes
For a commentary on this article, see https://doi.org/10.1128/JCM.02329-16.
REFERENCES
- 1.Strebel PM, Cochi SL, Hoekstra E, Rota PA, Featherstone D, Bellini WJ, Katz SL. 2011. A world without measles. J Infect Dis 204(Suppl 1):S1–S3. doi: 10.1093/infdis/jir111. [DOI] [PubMed] [Google Scholar]
- 2.De Serres G, Desai S, Shane A, Hiebert J, Ouakki M, Severini A. 2015. Measles in Canada between 2002 and 2013. Open Forum Infect Dis 2:ofv048. doi: 10.1093/ofid/ofv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K, Centers for Disease Control and Prevention (CDC). 2015. Measles outbreak–California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep 64:153–154. [PMC free article] [PubMed] [Google Scholar]
- 4.Jost M, Luzi D, Metzler S, Miran B, Mutsch M. 2015. Measles associated with international travel in the region of the Americas, Australia and Europe, 2001–2013: a systematic review. Travel Med Infect Dis 13:10–18. doi: 10.1016/j.tmaid.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS, Centers for Disease Control and Prevention (CDC). 2015. Measles - United States, January 4–April 2, 2015. MMWR Morb Mortal Wkly Rep 64:373–376. [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha HA, Correia LL, Campos JS, Silva AC, Andrade FO, Silveira DI, Machado MM, Leite AJ, Cunha AJ. 26 July 2015. Factors associated with non-vaccination against measles in northeastern Brazil: clues about causes of the 2015 outbreak. Vaccine doi: 10.1016/j.vaccine.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Leite RD, Barreto JL, Sousa AQ. 2015. Measles reemergence in Ceara, northeast Brazil, 15 years after elimination. Emerg Infect Dis 21:1681–1683. doi: 10.3201/eid2109.150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Measles and Rubella Elimination Working Group. 2013. Guidelines for the prevention and control of measles outbreaks in Canada. Can Commun Dis Rep 39:ACS-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berggren KL, Tharp M, Boyer KM. 2005. Vaccine-associated “wild-type” measles. Pediatr Dermatol 22:130–132. doi: 10.1111/j.1525-1470.2005.22208.x. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. 1998. Expanded Programme on Immunization (EPI). Standardization of the nomenclature for describing the genetic characteristics of wild-type measles viruses. Wkly Epidemiol Rec 73:265–269. [PubMed] [Google Scholar]
- 11.Anonymous. 2015. Genetic diversity of wild-type measles viruses and the global measles nucleotide surveillance database (MeaNS). Wkly Epidemiol Rec 90:373–380. [PubMed] [Google Scholar]
- 12.Xu CP, Li MH, He HQ, Lu YY, Feng Y. 19 November 2015. Laboratory diagnosis of vaccine-associated measles in Zhejiang Province, China. J Microbiol Immunol Infect doi: 10.1016/j.jmii.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Lowry R. VassarStats: website for statistical computation. http://vassarstats.net.
- 14.Anonymous. 2006. Global distribution of measles and rubella genotypes–update. Wkly Epidemiol Rec 81:474–479. [PubMed] [Google Scholar]
- 15.Rota JS, Wang ZD, Rota PA, Bellini WJ. 1994. Comparison of sequences of the H, F, and N coding genes of measles virus vaccine strains. Virus Res 31:317–330. doi: 10.1016/0168-1702(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 16.Hummel KB, Lowe L, Bellini WJ, Rota PA. 2006. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods 132:166–173. doi: 10.1016/j.jviromet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Petersen M, Wengel J. 2003. LNA: a versatile tool for therapeutics and genomics. Trends Biotechnol 21:74–81. doi: 10.1016/S0167-7799(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 18.Murti M, Krajden M, Petric M, Hiebert J , Hemming F , Hefford B , Bigham M , Van Buynder P. 2013. Case of vaccine-associated measles five weeks post-immunisation, British Columbia, Canada, October 2013. Euro Surveill 18:pii=20649. doi: 10.2807/1560-7917.ES2013.18.49.20649. [DOI] [PubMed] [Google Scholar]
- 19.Nestibo L, Lee BE, Fonseca K, Beirnes J , Johnson MM , Sikora CA. 2012. Differentiating the wild from the attenuated during a measles outbreak. Paediatr Child Health 17:e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]