ABSTRACT
Chagas disease, caused by Trypanosoma cruzi, although endemic in many parts of Central and South America, is emerging as a global health threat through the potential contamination of blood supplies. Consequently, in the absence of a gold standard assay for the diagnosis of Chagas disease, additional antigens or strategies are needed. A proteomic analysis of the trypomastigote excreted-secreted antigens (TESA) associated with exosomal vesicles shed by T. cruzi identified ∼80 parasite proteins, with the majority being trans-sialidases. Mass spectrometry analysis of immunoprecipitation products performed using Chagas immune sera showed a marked enrichment in a subset of TESA proteins. Of particular relevance for diagnostic applications were the retrotransposon hot spot (RHS) proteins, which are absent in Leishmania spp., parasites that often confound diagnosis of Chagas disease. Interestingly, serological screens using recombinant RHS showed a robust immunoreactivity with sera from patients with clinical stages of Chagas ranging from asymptomatic to advance cardiomyopathy and this immunoreactivity was comparable to that of crude TESA. More importantly, no cross-reactivity with RHS was detected with sera from patients with malaria, leishmaniasis, toxoplasmosis, or African sleeping sickness, making this protein an attractive reagent for diagnosis of Chagas disease.
KEYWORDS: parasite, proteomics, TESA, Trypanosoma cruzi, trypomastigote
INTRODUCTION
Chagas disease is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi. Chagas disease is a major cause of morbidity and mortality in South and Latin America, where currently ∼9 million people are infected with T. cruzi. However, with increased globalization and immigration, Chagas disease has emerged as a health threat in Europe, Asia, and North America, due to transmission of T. cruzi through blood transfusions, organ transplants, or congenital infections associated with transplacental transfer of this parasite from mother to newborn (1–5). It is estimated that ∼300,000 infected individuals currently live in the United States, while in Canada the infection rate among ∼130, 000 Latino immigrants was ∼0.09% (6).
Chagas disease is characterized by an acute and chronic phase of infection. The acute stage of the disease develops after a short period (1 to 2 weeks) following transmission of the T. cruzi parasites, where it typically presents with the clinical signs of elevated fever, aches, and an acute inflammatory response that reduces the parasite burden (7–9). In some individuals, low levels of intracellular parasites continue to proliferate and persist in tissues for decades, remaining asymptomatic, which results in the establishment of a chronic infection (8, 10). In ∼30% of chronic disease cases, patients develop significant complications, which may include megacolon, neurological complications, and cardiomyopathy that is characterized by an enlargement of the heart, ventricular arrhythmias, and eventual death due to general heart failure (11, 12). Infants and newborns are the demographic group with the highest risk of developing a chronic infection (8, 13).
Approaches currently used for Chagas diagnosis include microscopy, which detects parasites in tissues, quantitative PCR (qPCR), which measures levels of parasite DNA in host tissues, and serological methods, such as enzyme-linked immunosorbent assays (ELISA) and immunoblotting, which detect circulating T. cruzi-specific antibodies. Microscopy and PCR-based methods are more effective for diagnosing acute or congenital forms of Chagas disease (14, 15), while serological tests using either parasite-derived antigens, recombinant proteins, or synthetic peptides are preferred for diagnosis of chronic infections (16). Despite the sensitivity of serological tests, current Chagas disease diagnostic tests may lack specificity due to cross-reactivity with the related parasites Leishmania spp. and Trypanosoma rangeli (17). Consequently, the Pan American Health Organization has recommended (18) the use of two different assays for a confirmatory diagnosis of Chagas infection (19–21). A typical serological method recommended for confirming Chagas disease uses the trypomastigote excretory-secretory antigens (TESA) either in an ELISA or immunoblotting format to detect antibodies that cross-react with proteins or glycoconjugates released by T. cruzi (22–25).
It is known that T. cruzi parasites, like many other cells, release extracellular vesicles that are postulated to be involved in cell-cell communication or in the modulation of the host immune responses to promote the establishment of an infection (26–29). These vesicles typically consist of a lipid bilayer membrane containing integral membrane proteins and a luminal cavity that is loaded with a variety of soluble proteins and nucleic acids (RNA and DNA). In T. cruzi parasites, two classes of vesicles, based on size, have been characterized. These include exovesicles (EVs; also referred to as ectosomes; 100 to 1,000 nm), which bud directly from the plasma membrane, and exosomes (30 to 100 nm), which are vesicles that are secreted into the extracellular environment following the fusion of multivesicular endosomes with the plasma membrane, typically occurring at the flagellar pocket membrane (28, 30–33). A proteomic analysis of extracellular vesicles released by metacyclic trypomastigotes and epimastigotes in culture demonstrated the presence of two populations of EVs containing plasma membrane and intracellular proteins, and also nucleic acids (26, 29, 32–34). Interestingly, treatment of mice with EVs shed by axenic trypomastigotes caused a downmodulation of the host immune response that was associated with higher parasitemia and an exacerbated inflammatory response that resulted in increased mortality following infection (26, 35). The T. cruzi small membrane proteins (TcSMP) family of proteins or phosphatases detected on T. cruzi EVs has been shown to trigger Ca2+ signaling and lysosome mobilization/exocytosis, events that promote formation of parasitophorous vacuoles and parasite invasion (36, 37). A similar modulation of macrophage responses was observed following exposure to purified Leishmania exosomes, a strategy that enhances intracellular parasite survival (38, 39). Mechanistic studies suggest that in the early stages of infection by T. cruzi, parasites promote the release of plasma membrane vesicles from the host cell, which may contribute to parasite survival in the circulatory system, an event thought to help mediate host cell invasion (40).
Although ELISAs and immunoblot serological assays using TESA are highly sensitive, there are some concerns due to the cross-reactivity for patients infected with Leishmania, which may lead to misdiagnosis. However, the identification of antigens that are only expressed by T. cruzi parasites would significantly increase the specificity of serological assays. In addition, the availability of diagnostic testing that would quantitatively detect the levels of T. cruzi antigens in body fluids, such as plasma or urine, could potentially be used to measure parasite burdens during acute and chronic phases of Chagas disease.
Metacyclic trypomastigotes released by the insect vector invade phagocytic and nonphagocytic cells and, once internalized, transform to the amastigote stage, which replicates in the cytosolic compartment. These amastigotes then convert back to the trypomastigote stage prior to rupturing the host cell. It is conjectured that the intracellular stages of T. cruzi, like those of the related parasite Leishmania spp. (38, 41), shed exosomes into the circulatory system by exocytosis (31) or when the host cell bursts. Indeed, EVs may account for T. cruzi antigens observed in the circulation and in urine of Chagas patients or infected animals with acute or chronic infections (42–44). However, no systematic analysis of T. cruzi antigens released from infected host cells has been reported, despite the use of TESA as a reagent in disease diagnosis (22, 25, 32, 45–47).
In this study, we selectively employed a purification strategy designed to isolate TESA EVs released by T. cruzi trypomastigotes and amastigotes in infected Vero cells and then conducted a proteomic analysis of these vesicles. Moreover, affinity columns generated from the immune sera of Chagas patients allowed the immunoprecipitation and proteomic characterization of numerous T. cruzi proteins that are likely released into the circulatory system during the chronic phase of infection
RESULTS
Trypomastigote excreted-secreted antigen preparations contain EVs.
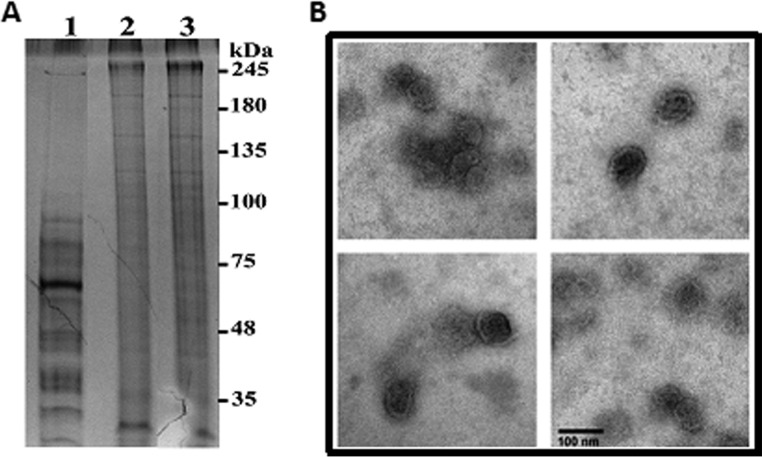
Vero cell cultures infected with T. cruzi trypomastigotes are known to spontaneously shed parasite antigens into the culture supernatant. To perform an in-depth characterization of parasite proteins associated with EVs in TESA preparations, these proteins were concentrated from the culture supernatant by ultracentrifugation sedimentation and then purified by sucrose density flotation centrifugation to eliminate proteins that were not encapsulated or associated with EV membranes. Analysis of high-speed centrifugation supernatants and the sucrose density-purified EVs by using silver-stained SDS-PAGE showed that the supernatant fraction contained predominantly proteins with masses of <100 kDa. In contrast, proteins partitioning with the EVs had masses ranging from ∼30 to 245 kDa (Fig. 1A). Moreover, the proteins cofractionating with EVs isolated from different batches of TESA exhibited a highly reproducible pattern, as shown by the similar SDS-PAGE profiles (Fig. 1A, lanes 2 and 3). The presence of EVs in the top fraction from the sucrose density gradient was validated by negative-stain electron microscopy analysis. The electron-dense vesicles had diameters of ∼60 to 100 nm (Fig. 1B), which is consistent with the size ranges previously reported for EVs (48).
FIG 1.
Isolation and characterization of TESA exovesicles. (A) Crude TESA isolated from infected Vero cell culture supernatant was concentrated by ultrafiltration, and a supernatant (lane 1) and EV pellets from two different batches (lanes 2 and 3) were isolated by ultracentrifugation as described in Materials and Methods. (B) The presence of EV in the sucrose density flotation fraction was determined by negative staining electron microscopy.
Proteomic analysis of TESA EVs.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) spectra obtained for purified TESA EV proteins were used to search T. cruzi and Macaque spp. genome databases. The latter genome database was included since it was speculated that the TESA EV preparations would contain a mixture of parasite and Vero cell-derived proteins. LC-MS/MS analysis generated 11,016 spectra corresponding to 766 proteins containing at least two high-confidence unique peptides at a protein identification confidence level of 95% (see Table S1 in the supplemental material). The bulk of these proteins were of Vero cell origin (Table S1), many of which have been previously detected in EVs isolated from mammalian cells (49). The remaining proteins were derived from either T. cruzi trypomastigotes or amastigotes (Tables S1 and S2). Quantification analysis performed using spectral counts indicated that only ∼10% of the total proteins detected in the EV preparations were of T. cruzi origin.
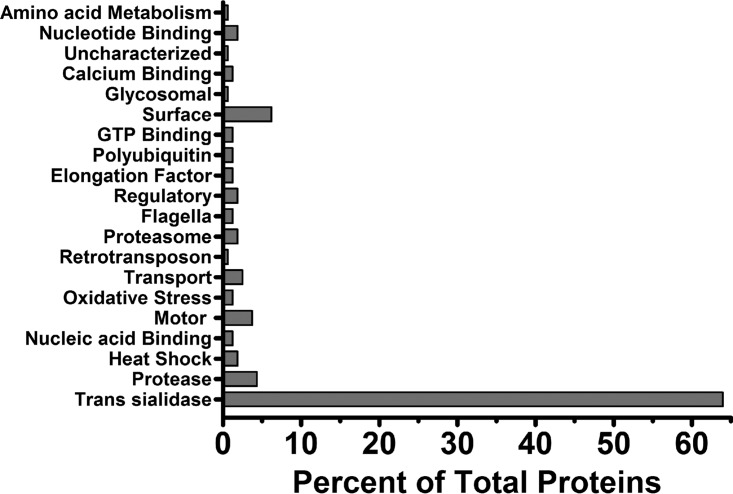
T. cruzi proteins detected in the TESA EVs encompassed a broad spectrum of biological processes that included production of proteases and trans-sialidases, intermediary metabolism, membrane transport, nucleotide binding, regulation of oxidative stress, heat shock responses, motor and cytoskeleton function, and protein turnover, as suggested by the presence of several proteasome subunits (Fig. 2). The most abundant group of T. cruzi proteins copurifying with TESA EVs was the multigene family trans-sialidases (Table S2), which accounted for ∼66% of the total parasite proteins in TESA EVs (Fig. 2). These EVs were enriched for a number of surface membrane proteins that included the protease gp63, TolT, mucin-associated surface proteins (MASPs), and mucin-like protein TASV-C (50). Interestingly, the amastigote surface proteins 2 and 3, which are polypeptides exclusively expressed by amastigotes, were also detected in TESA EVs (51, 52) (Fig. 2; Table S2). Several integral membrane proteins, such as the nucleoside transporter 1, an amino acid permease, and ABC transporters, were also detected in the EV proteome (Table S2). Other hallmark proteins typically associated with EVs were elongation factors 1 and 2, actin, heat shock proteins, and nucleotide binding proteins (Table S2) (53). Unexpectedly, TESA EVs did not appear to contain phosphatases, enzymes that have been implicated in parasite adhesion and infection (37).
FIG 2.
Biological function of protein in TESA EVs. Purified TESA EVs from at least two biological replicated were analyzed by nano-LC–MS/MS, and proteins were identified by searching the T. cruzi genome databases using the Mascot software. Proteins with various biological functions were grouped, and the presence of these groups is reported as a percentage of the total proteins identified with high confidence.
TESA EVs share considerable overlap with the proteome previously reported for metacyclic trypomastigote and epimastigote EVs (Table S2). However, TESA EVs released by trypomastigotes/amastigotes in Vero cell cultures contained a number of unique protein markers previously used, including the amastigote surface proteins 2 and 3, proteasome subunits, transporter proteins, a polyubiquitin protein, and complement regulatory proteins (Table S2).
Immunoreactivity of Chagas patient sera with TESA EV proteins.
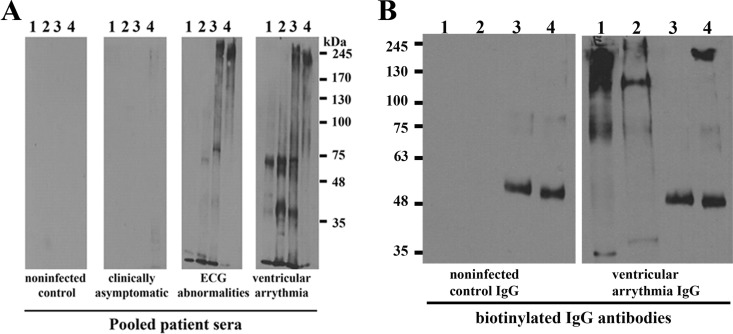
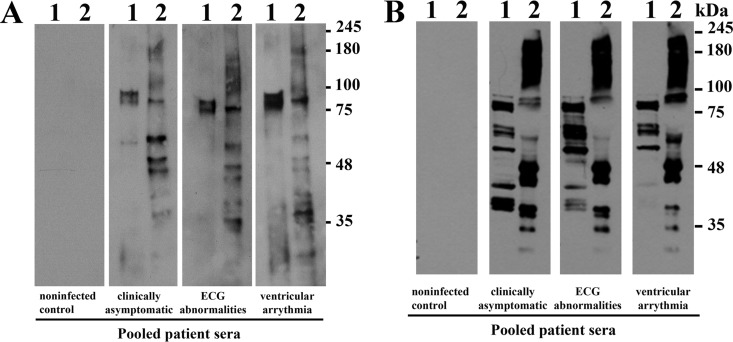
TESA is routinely used for serodiagnosis of Chagas disease (24, 25). However, a comprehensive analysis of antigens that cross-react with sera from Chagas patients with various degrees of cardiomyopathy (54) has not been performed. Western blot analysis using sera from uninfected control subjects exhibited no significant immunoreactivity with proteins from Leishmania donovani promastigotes, Trypanosoma brucei procyclics, or T. cruzi trypomastigotes. The sera from asymptomatic Chagas patients showed very weak immunoreactivity with high-molecular-weight proteins in the TESA EVs (Fig. 3), as previously reported with epimastigote antigens (55). In contrast, a robust cross-reactivity was detected with T. cruzi trypomastigotes and TESA EVs when blots were probed with sera from patients with electrocardiograph abnormalities or ventricular arrhythmia (Fig. 3A). While immunoreactive proteins ∼20 to 300 kDa in mass were observed in T. cruzi trypomastigote lysates, only proteins of >80 kDa were detected in the TESA EVs (Fig. 3A). This is consistent with the proteomic analysis, which showed that the most abundant proteins were trans-sialidases (Fig. 2). The sera from Chagas disease patients with cardiopathologies also exhibited cross-reactivity with whole-cell lysates from Leishmania spp. and T. brucei cultures (Fig. 3A).
FIG 3.
Immunoreactivity of TESA with Chagasic sera. (A) Western blots containing whole-cell extracts of L. donovani promastigotes (lane 1), T. brucei procyclics (lane 2), T. cruzi trypomastigotes (lane 3), or TESA EVs (lane 4) were probed with pooled sera from Colombian uninfected controls or Chagasic patients with various stages of cardiopathologies as previously detailed (54). (B) IgG antibodies isolated from uninfected controls or Chagas patients with ventricular arrhythmia were coupled to a protein G column, and reactive proteins were immunoprecipitated using these affinity matrices for Western blotting. Proteins in the total TESA EV fraction (lane 1), Triton X-100 extracts of TESA EVs (lane 2), precipitated by uninfected control IgG (lane 3), or IgG from Chagas patients (lane 4) were resolved by SDS-PAGE, and the membranes were probed with biotinylated IgG antibodies from uninfected subjects or Chagas-infected subjects. Primary antibodies were detected using streptavidin-horseradish peroxidase (Western blot membranes were exposed on film for 60 s).
To identify the immunoreactive antigens in the TESA EVs, extracts were incubated with immunoaffinity resins containing covalently coupled IgG antibodies from uninfected donors or Chagas patients with clinical symptoms of ventricular arrhythmia (54). Western blot analysis using biotinylated Chagasic IgG antibodies showed strong immunostaining of high-molecular-weight species in the total TESA EVs, Triton X-100 extracts of EVs, and proteins isolated on the immunoaffinity columns; however, only an ∼50-kDa protein was detected for the immunoaffinity resin IgG antibodies from uninfected controls (Fig. 3B).
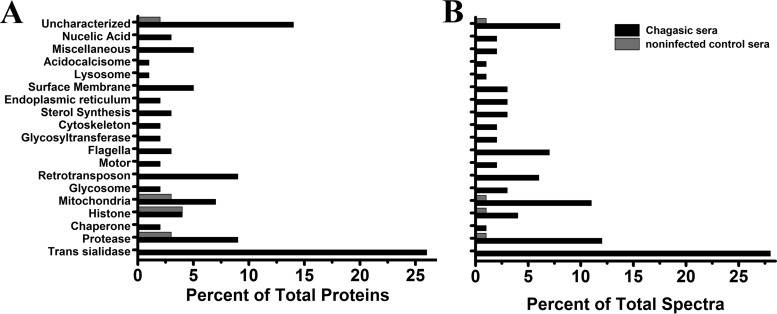
LC-MS/MS analysis of TESA EV immunoprecipitations performed with Chagasic IgG antibodies identified 111 T. cruzi proteins containing at least two unique high-confidence peptides with masses ranging from 11 to 519 kDa (Table 1; Table S3). Although trans-sialidases remained the most abundant group of proteins in the immunoprecipitation assay, Chagasic IgG affinity resin showed a significant enrichment of mitochondrial proteins, retrotransposon hot spot (RHS) proteins, proteases, and multiple uncharacterized proteins (Fig. 4A). A notable enrichment was also observed for paraflagellar rod proteins, which are preferentially expressed in trypomastigotes (56) and glycosomal proteins (Fig. 4B; Table 1).
TABLE 1.
Most abundant immunoaffinity-purified proteins from TESA extracellular vesiclesa
| TESA protein | Accession no. | Mass (kDa) | No. of peptides in: |
|
|---|---|---|---|---|
| Nonimmune serum | Chagas immune serum | |||
| Retrotransposon | ||||
| RHS protein | K4E7X9 | 77 | 0 | 29 |
| Paraflagellar rod | ||||
| Paraflagellar rod protein 3 | K4EBQ5 | 70 | 0 | 18 |
| Paraflagellar rod protein 3 | K2MCT5 | 69 | 0 | 17 |
| Mitochondrial | ||||
| ATP synthase subunit alpha | K4E934 | 63 | 2 | 17 |
| ATP synthase subunit beta | K2M741 | 56 | 0 | 9 |
| Enoyl coenzyme A hydratase, mitochondrial | K4DTD3 | 29 | 0 | 8 |
| Malic enzyme | K4E9P4 | 63 | 0 | 6 |
| trans-Sialidase | ||||
| Putative uncharacterized protein | Q7YZX6 | 95 | 0 | 16 |
| trans-Sialidase, group VIII, putative | TcCLB.506537.200 | 120 | 0 | 15 |
| trans-Sialidase, putative (fragment) | K4DRD9 | 35 | 0 | 13 |
| trans-Sialidase | Q9BHJ5 | 71 | 0 | 12 |
| trans-Sialidase, putative | K4E110 | 81 | 0 | 12 |
| trans-Sialidase, putative (fragment) | K4DRD9 | 35 | 0 | 11 |
| trans-Sialidase | P23253 | 120 | 0 | 10 |
| trans-Sialidase, putative (fragment) | Q4D3D0 | 50 | 0 | 10 |
| Glycosomal | ||||
| Glycosomal membrane protein (PEX11) | K4DY43 | 27 | 0 | 8 |
| Fructose-bisphosphate aldolase | K4DLE5 | 41 | 0 | 7 |
| Tubulin | ||||
| β-Tubulin (pseudogene) | TcCLB.509003.70 | 33 | 0 | 6 |
| α-Tubulin | Q26973 | 47 | 0 | 6 |
| Glycotransferase | ||||
| UDP-Gal/DP-GlcNAc-dependent glycosyltransferase | Q4CS30 | 43 | 0 | 9 |
| Proteases | ||||
| Cytoskeleton-associated protein CAP5.5,cysteine peptidase | K4E5Y1 | 88 | 0 | 13 |
| Serine carboxypeptidase S28, putative | K4DZV5 | 54 | 0 | 6 |
| Heat shock protein | ||||
| Heat shock protein 70 | B5U6T4 | 71 | 0 | 3 |
| Uncharacterized | ||||
| Uncharacterized protein | K4EDD2 | 34 | 0 | 8 |
| Uncharacterized protein | K4EBS5 | 88 | 0 | 7 |
| Cytoskeleton protein | ||||
| Clathrin heavy chain | K4E050 | 193 | 0 | 12 |
| Sterol synthesis | ||||
| Lanosterol synthase, putative | K4EA17 | 103 | 0 | 9 |
| Endoplasmic rectiulum | ||||
| Pretranslocation protein, alpha subunit, SEC61-like | TcCLB.506297.240 | 54 | 0 | 8 |
| Glucose-regulated protein 78 | K4DT97 | 71 | 0 | 6 |
| Lysosomal | ||||
| Lysosomal α-mannosidase, putative | K4E2E6 | 111 | 0 | 7 |
| Acidocalcisome | ||||
| Vacuolar-type proton translocating pyrophosphatase 1 | K2NKA3 | 85 | 0 | 7 |
| Miscellaneous proteins | ||||
| Surface protein TolT, putative | Q4CPM8 | 32 | 0 | 5 |
Amino acid sequences for the proteins may be found in the NCBI Protein, Tritryp, and Uniprot/TrEMBL databases using the accession numbers listed in the table.
FIG 4.
Enrichment of TESA proteins with immune sera. Proteins in TESA EVs were immunoprecipitated using affinity matrices containing IgG antibodies from uninfected control subjects or IgG from Chagas patients with ventricular arrhythmia. Bound proteins were analyzed by LC-MS/MS. Proteins were grouped on the basis of biological function (A), and the abundance levels of proteins in each group were determined for the total number of spectra obtained for each group (B).
LC-MS/MS analysis also detected a number of T. cruzi proteins that nonspecifically copurified with uninfected control and Chagas patient IgG immunoaffinity columns that included histones H2 and H4, calpains, mitochondrial proteins, and uncharacterized proteins (Table 1). Similarly, a number of Vero cell proteins also nonspecifically bound to the uninfected control and Chagas IgG affinity columns (Table S3).
Reactivity of Chagas sera with TESA EV proteins.
We next exploited the availability of the recombinant T. cruzi paraflagellar rod 3 (57) and RHS protein (K4E7X9) to assess the use of these proteins for the serological diagnosis of Chagas disease. Western blot assays with recombinant PFR1 and total TESA EV extracts probed with pooled sera from uninfected control subjects showed no detectable cross-reactivity with these antigens. In contrast, sera from Chagas patients who were clinically asymptomatic, exhibited electrocardiogram (ECG) abnormalities, or ventricular arrhythmia showed robust cross-reactivity with PFR1 and TESA EV antigens (Fig. 5A). Similarly, Chagasic sera, but not the sera from uninfected controls, showed a strong immunoreactivity with recombinant RHS and TESA EVs (Fig. 5B). Interestingly, Chagas immune sera, in addition to reacting with the full-length recombinant RHS protein (75 kDa), also detected multiple fragments arising from either aborted translation or proteolysis in the Escherichia coli expression system. These shorter fragments cross-reacted with anti-hexahistidine antibodies, which detected the N-terminal hexahistidine affinity tag (see Fig. S1 in the supplemental material). This result suggests that recombinant RHS is a relatively unstable protein that is subject to degradation, particularly within the C-terminal region of the protein.
FIG 5.
Immunoreactivity of Chagas patient sera with recombinant T. cruzi proteins. Western blots containing recombinant paraflagellar rod protein (lane 1) or total TESA EVs (lane 2) (A) or recombinant retrotransposon hot spot protein (lanes 1) or total TESA EVs (lane 2) (B) were probed with antisera from uninfected control subjects or with pooled sera from Chagas patients with different clinical stages of the infections (Western blot exposure to film was 2 min).
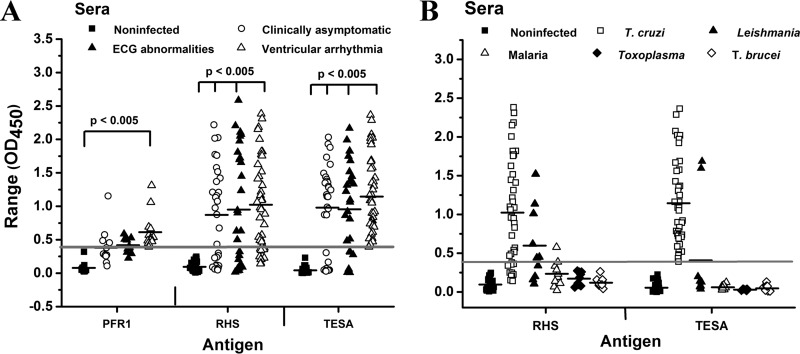
ELISAs performed on serum samples from individual Columbian and Venezuelan patients with various clinical manifestations of Chagas disease showed strong immune reactivity with TESA, with responses ranging from optical densities at 450 nm (OD450) from 0.04 to 2.4 (Fig. 6A), while serum from noninfected control subjects showed no notable signal development. With TESA, ∼31% of patients with Chagas disease who were classified as clinically asymptomatic or as exhibiting ECG abnormalities had responses on the ELISA that were below the cutoff value. This value decreased to ∼2.5% for patients exhibiting ventricular arrhythmia (Fig. 6A). A similar robust response was observed for RHS with sera from Chagas patients, with responses (OD450 values) ranging from 0.02 to 2.3. Although the mean values obtained for TESA and RHS were comparable, the responses with RHS alone were more variable, with a greater number of patients (24 to 40%) having responses below the cutoff value (Fig. 6A). This result is plausible, as not all patients may mount similar immune responses to RHS. A recent study showed that sera from Chagas patients exhibited a more robust humoral response to total parasite lysates, compared to the cross-reactivity with synthetic peptides corresponding to the conserved C-terminal region found in the family of MASPs, particularly in patients with cardiopathy (58). Surprisingly, although PFR1 exhibited notable immunoreactivity on Western blots with Chagasic pooled sera (Fig. 5), the responses in ELISAs were significantly dampened (OD450, 0.1 to 1.3) (Fig. 6A). Despite the reduced ELISA signal response of sera from Chagas patients, the response from Chagas patients with ventricular arrhythmia were significant (P < 0.005) (Fig. 6A). The difference in immunoreactivity may be attributed to the fact that PFR1 is more extensively denatured on Western blots than is antigen bound to the surfaces of microtiter plates. Previous studies have shown modest reactivity of Chagas immune sera with paraflagellar rod proteins (59).
FIG 6.
ELISA analysis of Chagas patients. (A) Microtiter plates were coated with crude TESA, recombinant RHS, or PFR1 protein, and ELISAs were performed with individual sera collected from Columbian and Venezuelan subjects, including noninfected controls or patients with various clinical degrees of Chagas disease. Positive-control sera were generated by pooling sera from Chagas patients with ventricular arrhythmia, and negative-control sera were obtained from a Quebec donor subject that had never traveled to South America. Sera were diluted 1:400. (B) The cross-reactivity of sera from patients infected with Leishmania, malaria parasites, Toxoplasma, or T. brucei with the T. cruzi RHS recombinant protein was examined by ELISA. All assays were performed in triplicate using a 1:400 dilution of sera. The thick gray line in the scatter plots represents the minimum cutoff value required for a positive response, and the black lines correspond to the mean OD450 values for each group of samples.
To examine the specificity of RHS, ELISAs were performed with sera from patients infected with Leishmania, malaria parasites, Toxoplasma gondii, Trypanosoma brucei, or noninfected control sera obtained from Canadian subjects who had never traveled to South America. A robust signal was observed for TESA and RHS when probed with sera from Chagas patients with ventricular arrhythmia (Fig. 6B). Analysis of the sera from malaria patients or Toxoplasma-infected or T. brucei-infected patients with TESA and RHS were below the cutoff value of the assay and indicated no significant cross-reactivity. In contrast, with the TESA and the RHS antigen, 2 out of 10 and 3 out of 10, respectively, serum samples from patients with leishmaniasis exhibited strong reactivity these antigens (Fig. 6B). The fact that the Leishmania genome does not encode RHS proteins suggests that the patients with a positive immune response were infected with T. cruzi parasites.
DISCUSSION
In this study, we performed for the first time a proteomic analysis of T. cruzi proteins that were associated with vesicles shed by trypomastigote and amastigote intracellular stages of this parasite. Proteomic analysis showed that the TESA used in a number of diagnostic assays (9, 22–24) contains a mixture of Vero cell and T. cruzi proteins (see Table S1 in the supplemental material). Purified TESA EVs, which by transmission electron microscopy had a diameter of ∼80 to 100 nm, were found to contain a preponderance of trans-sialidases, mucins, and MASPs, which may be anchored to the parasite cell surface via glycosyl phosphatidylinositol (GPI) lipid or inserted into the EV membrane via a conserved C-terminal region (58, 60, 61). This finding was in agreement with those of previous studies showing that trans-sialidases constitute the dominant group of proteins released by trypomastigotes in infected animals or cell cultures (32, 45, 62). The gp63 proteases, which are expressed by trypomastigotes and amastigotes (63), as well as several integral membrane proteins with transporter activity (Table S2), were also detected, which is consistent with the hypothesis that T. cruzi vesicles in the TESA preparation are derived primarily from the trypomastigote or amastigote plasma membrane (33, 37, 64). In addition, TESA EVs also contained a variety of proteins known to localize to the glycosome, flagella, mitochondria, nuclei, or cytosol (Table 1; Table S2), many of which have been previously detected in purified EVs from Leishmania and Trypanosoma brucei (38, 41, 65). Of note was the absence of glycolytic enzymes, which were previously observed in T. cruzi metacyclic trypomastigotes and epimastigotes and Leishmania EVs (33, 38, 41, 53).
Although TESA is a typical antigen used for serological diagnosis of Chagas disease, concerns regarding the specificity of this assay have been raised. TESA polypeptides with masses of 60 to 150 kDa have been implicated as a source of potentially cross-reactivity with leishmaniasis immune serum (24, 38). This is not surprising, since multiple sequence analyses showed that many of the T. cruzi proteins shed by trypomastigotes share a high degree of sequence homology with proteins from Leishmania and T. brucei.
More recently, a proteomic analysis of EVs isolated from axenic cultures of T. cruzi metacyclic trypomastigotes and epimastigotes (33) showed significant overlap with the TESA EV proteome, as reflected by the presence of membrane-bound proteins such as the flagellar calcium binding protein (FCaBP), trans-sialidases, the surface protease gp63, and elongation factor proteins (Table S2). Two of these proteins, FCaBP and gp63, have been previously suggested as potential candidates for diagnosis of T. rangeli infections (66). However, the latter EVs contain a number of additional proteins that were preferentially enriched in immunoprecipitates of the TESA EVs. Examples include the amastigote surface proteins ASP2 and ASP3 (51, 52), paraflagellar rod proteins, several mitochondrial membrane proteins (ATP synthase, ADP/ATP translocase, and cytochrome c1), the glycosome membrane protein Gim5A, and the Golgi apparatus protein UDP-galactose glycosyltransferase (Tables S2 and S3). Although our studies focused on EVs released into the culture supernatant following trypomastigote-mediated rupture of Vero cells, proteomic analysis revealed that EVs contained both Vero and T. cruzi proteins. However, it is unclear if host and parasite proteins segregate to separate vesicles or colocalize to the same EV. The latter possibility is supported by recent experiments showing that host cells infected with Leishmania spp. or T. cruzi released EVs contained both host and parasite proteins (38, 40, 41). Indeed, immunoelectron microscopy studies confirmed that EVs released by T. cruzi do not have a homogeneous composition, since some vesicles were found to contain the membrane-anchored MASPs while other vesicle populations contained clathrin (58). Both of the latter proteins were also detected in this study (Tables S2 and S3).
Previous attempts to characterize antigenic components in TESA preparations by using immunoaffinity purification (24) identified a pattern of ∼10 immunoreactive proteins, ranging in size from 25 to 220 kDa; however, the sequences of these antigenic proteins were not determined. Employing a combination of immunoprecipitation with affinity-purified IgG antibodies from Chagas patients and LC-MS/MS analysis, we detected a set of soluble, integral membrane, and peripheral membrane proteins that were enriched compared to the content of the total TESA EV proteome, indicating that these proteins induce a robust immune response. Notable among these were a family of retrotransposon hot spot and paraflagellar rod proteins (Table S3), the latter of which is more abundantly expressed in the motile trypomastigote stage of the parasite (67). It is interesting that posttranslational sumoylation modification has been detected with the latter two proteins (57, 68). RHS proteins have been localized to the nucleus and are encoded by a multigene family present in T. cruzi and T. brucei but not in the Leishmania genome, presumably because Leishmania spp. lack mobile retrotransposon elements (69). A recent proteomic analysis identified ∼39 RHS isoforms that were expressed by T. cruzi bloodstream trypomastigotes (70); however, the diversity of RHS proteins detected in EV preparations was much more restricted, as only 8 full-length RHS variants were observed (Table S3). In ELISAs, only the recombinant RHS showed a strong response with immune sera from patients with various clinical degrees of Chagas disease. It is noteworthy that proteomic analysis of T. brucei EVs also detected an RHS1 protein (71). However, multiple sequence alignments of RHS protein revealed that T. cruzi and T. brucei proteins share <33% sequence identity (data not shown). More importantly, no cross-reactivity was observed between T. cruzi RHS and immune sera from patients with African sleeping sickness or leishmaniasis, indicating that RHS may be used as an antigen to increase the specificity of Chagas disease diagnosis. Interestingly, recent reports have shown that MASPs trigger a rapid humoral IgM response but limited IgG class switching during infection (58); consequently, it is not surprising that our immunoaffinity LC-MS/MS strategy required a pool of IgG antibodies from Chagasic patients.
It is possible that EVs released from infected host cells may in part account for T. cruzi antigens previously detected in the circulatory system or urine of infected patients (72–75), making EVs an attractive and tractable biological tool for facilitating direct antigen detection in fluids of Chagas disease patients. Indeed, EVs are emerging as a unique mechanism for enriching low-level antigens for cancer diagnosis (76), and recent studies using T. cruzi-specific RNA aptamers detected antigens in TESA preparations or serum from mice with acute or chronic T. cruzi infection (77).
MATERIALS AND METHODS
Serum samples.
A total of 188 participants with various stages of Chagas disease or noninfected control patients were recruited in a cross-sectional study conducted in Bucaramanga, Colombia. The criteria for grouping the participants was based on a combination of the New York Heart Association (NYHA) classifications and other clinical information (serology, ECGs, echocardiograms, and chest X-rays), findings commonly used to classify the stage of chronic Chagas infection. These participants have been described previously (54).
Positive control sera represented a pool of sera from patients with clinically confirmed Chagas disease with ventricular arrhythmia. The negative-control serum was from a Quebec donor who had never traveled to South America. TESA and RHS antigen were screened for reactivity with sera collected from donors with confirmed Leishmania (n = 4), malaria parasite (n = 5), Toxoplasma gondii (n = 5), and T. brucei (n = 5) infections. The latter sera were obtained from the National Reference Centre for Parasitology/J. D. Maclean Centre for Tropical Diseases at McGill University Health Centre. All samples used in this study were anonymized.
Trypomastigote excreted-secreted antigen and EV isolation.
TESA proteins from T. cruzi strain Tulahuen were prepared as described previously (22, 24). Briefly, Vero cell monolayers at 75% confluence were infected for 4 days with trypomastigotes (1 × 109/ml) in Eagle's minimal essential medium supplemented with fetal bovine serum (FBS) at 37°C. Monolayers were stringently washed with Eagle's minimal essential medium without FBS and then incubated for an additional 18 to 20 h at 37°C in medium lacking both FBS and phenol red. The supernatant was collected, centrifuged at 2,800 × g to remove trypomastigotes or Vero cell debris, and then passed through a 0.22-μm filter to remove large membrane fragments (Millipore, Bradford, MA). The filtrates was centrifuged at 20,000 × g for 30 min at 4°C to remove membrane fragments, and the supernatant was centrifuged at 100,000 × g for 16 h at 4°C in a Beckman-Coulter type 70 Ti rotor to sediment EVs. The EV pellet was washed four times with 1.0 ml of phosphate-buffered saline (PBS) at 49,000 rpm for 1 h in a Beckman-Coulter tabletop ultracentrifuge equipped with a TLA 100.3 rotor to remove residual extravesicular proteins. TESA EVs were further purified by sucrose density flotation centrifugation. Briefly, EV pellets (100 μg of total protein) resuspended in 2.0 ml PBS containing 40% sucrose, 400 mM NaCl were overlaid with 2.5 ml of PBS containing 35% sucrose, 400 mM NaCl, and then 0.5 ml of PBS and the samples were centrifuged at 28,000 rpm for 18 h at 4°C in an SW55 Ti rotor. Fractions (1 ml) were collected from the top of the gradient, diluted 4-fold with PBS, and subjected to centrifugation at 200,000 × g for 1 h at 4°C in an SW55 Ti rotor to pellet EVs. Pellets were resuspended in 100 μl of PBS, aliquoted, and stored at −80°C. For crude TESA preparations used in the ELISAs, the filtered lysates were concentrated 32-fold using a 30,000-molecular-weight-cutoff (MWCO) ultrafiltration unit (EMD Millipore, Etobicoke, ON, Canada) as previously described (24).
Purification of IgG antibodies and immunoprecipitation.
Ten-milliliter samples of pooled sera from uninfected control subjects or Chagas patients with severe ventricular arrhythmia were clarified by centrifugation (3,000 × g, 10 min, 4°C) and then filtered through a 0.22-μm filter. The serum was diluted 2-fold with PBS and then passed through a protein G-Sepharose column (0.5 by 10 cm; GE Healthcare Life Sciences, Montreal, QC, Canada). The column was washed with 20 column volumes of 20 mM sodium phosphate (pH 7.0) to remove unbound proteins, and IgG antibodies were eluted with 100 mM glycine at pH 2.6. Column fractions were neutralized with 1.0 M Tris-HCl (pH 9.0), and proteins were quantified spectrophotometrically at 280 nm. Fractions containing antibodies were analyzed by SDS-PAGE, pooled and dialyzed against 100 mM NaHCO3, 500 mM NaCl (pH 8.5) for 24 h at 4°C, and then concentrated to ∼1.0 mg/ml using an Amicon ultracentrifugation device with a 30,000 MWCO.
An immunoaffinity resin was generated by loading purified human IgG antibodies (100 μg) onto 200 μl of packed protein G-Sepharose resin, and IgG antibodies were cross-linked to protein G by using disuccinimidyl suberate (2 mg/ml in PBS; ThermoFisher Scientific, Rockford, IL) for 60 min at 25°C on an end-on-end rotator. The resin was washed with 20 column volumes of PBS to remove the cross-linking reagent. Immunoreactive proteins were isolated by mixing purified TESA EVs (50 μg in 100 μl of PBS) treated with 0.1% Triton X-100 to release entrapped proteins, with 100 μl of packed affinity resin containing covalently coupled control or Chagas IgG antibodies. The mixture was incubated for 16 h at 4°C with end-on-end mixing, and unbound proteins were removed by washing resin 4 times with 1.0 ml of PBS containing 0.05% Triton X-100 and 4 times with 1.0 ml of PBS. Bound proteins were analyzed by mass spectrometry following an on-bead tryptic digestion.
Western blotting.
Western blot analysis of trypomastigote lysates (1 × 105 parasites/lane) or EVs (2 μg/lane) was performed by resolving proteins on a 6% or 8% SDS-PAGE gel, transferring proteins to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Mississauga, ON, Canada), and blocking with 2% milk powder in PBS containing 0.05% Tween 20 (PBST). Membranes were probed with primary antibodies diluted 1:2,000 in PBST containing 2% skimmed milk powder. Anti-human and anti-mouse antibodies conjugated to horseradish peroxidase were used as secondary antibodies. Membranes were developed with the ECL Western Lightening Plus detection system (PerkinElmer, Waltham, MA). Alternatively, Western blots were probed with either IgG antibodies isolated from uninfected control or Chagas patients with severe cardiomyopathy; samples were biotinylated with biotin–N-hydroxysuccinimide ester for 2 h at 20°C as described by the manufacturer (Invitrogen, Burlington, ON, Canada). Bound antibodies were detected using a streptavidin-horseradish peroxidase conjugate (Invitrogen).
For Western blot analysis using recombinant protein, ∼1.0 μg of PFR1 or RHS, or 10 μg of TESA EVs, was resolved on an 8% SDS-PAGE gel and transferred to a PVDF membrane, and membranes were blocked with 2% skimmed milk powder prior to probing with antisera (1:2,000 dilution) from uninfected controls or Chagas patients with asymptomatic clinical signs, ECG abnormalities, or ventricular arrhythmia. Bound antibodies were detected with anti-human antibodies conjugated to horseradish peroxidase.
ELISAs.
Immulon 2HB 96-well microtiter plates (ThermoFisher Scientific) were coated (100 μl/well) with 1 μg/ml of crude TESA preparation (24), recombinant paraflagellar rod, or retrotransposon hot spot proteins in 100 mM sodium carbonate solution (pH 9.6) at 4°C for 16 h. Plates were washed with PBST and blocked for 1 h at 37°C with 5% bovine serum albumin in PBST. Human control and immune sera were diluted 1:400 in PBST, and 100-μl aliquots were added to each well and incubated at 37°C for 1 h. Each serum sample was tested in duplicate against the three antigens, and assays were repeated at least twice. Plates were extensively washed with PBST and then probed with a 1:16,000 dilution of horseradish peroxidase-conjugated goat anti-human IgG (Perkin-Elmer Life Sciences, Boston, MA) in PBST for 30 min at 37°C. Plates were washed with PBST and developed using the substrate 3,3′,5,5′-tetramethylbenzidine (100 μl/well; Serologicals Corporation, MA) for 10 min at 20°C for 10 min. Reactions were terminated by the addition of 50 μl/well of 0.5 M H2SO4, and the optical density was measured at 450 nm using a Tecan ELISA reader. For this study, ELISAs were performed on individual serum samples from 35 noninfected control subjects, 29 patients with clinically asymptomatic Chagas disease, 29 patients with Chagas-associated ECG abnormalities, and 44 patients with ventricular arrhythmia. The cross-reactivity of sera from individuals infected with malaria parasites (5 patients), Toxoplasma gondii (5 patients), Trypanosoma brucei (5 patients), or Leishmania sp. (5 patients [collected in Venezuela]) were screened by ELISA using TESA and RHS antigen. The latter sera were obtained from the National Reference Centre for Parasitology, Research Institute of the McGill University Health Centre. These samples were collected as part of a previous study that examined the utility of matrix metalloproteases in the diagnosis of Chagas disease (54). The cutoff values for the ELISAs were calculated as previously described (25), and a one-way analysis of variance (ANOVA) was performed using the Origin 2016 graphing package to calculate the P values.
TEM.
TESA EVs isolated by sucrose density flotation centrifugation were absorbed onto Formvar/carbon-coated copper grids, washed in deionized water, and stained with 1% uranyl acetate for 1 min. Grids were allowed to air dry and viewed on a microscope. All samples were viewed on a JEOL 1200EX transmission electron microscope at an accelerating voltage of 80 kV. All transmission electron microscopy (TEM) experiments were performed at the Facility for Electron Microscopy Research, McGill University.
Proteomic analysis.
Sucrose density-purified EVs (20 μg) or proteins bound to immunoaffinity beads were diluted in 50 mM ammonium acetate (pH 7.5) with 6 M guanidinium hydrochloride buffer containing 0.5% octylglucoside, and proteins were reduced with 5 mM dithiothreitol and incubated at 80°C for 15 min. Samples were alkylated with 10 mM iodoacetamide at 20°C for 20 min and then quenched with 5 mM dithiothreitol. Modifying reagents, lipids, and detergent were removed with five 250-μl exchanges with 50 mM ammonium acetate (pH 7.5) buffer using a 10,000-MWCO ultrafiltration unit. Proteins concentrated to 25 μl were digested by adding 1 μg Promega sequencing-grade trypsin and incubating the sample for 16 h at 37°C. Peptides were purified from supernatant on stage tips (C18), vacuum dried, and then solubilized in 10 μl of 0.1% formic acid prior to mass spectrometry analysis.
Peptides were separated on a reversed-phase PicoFrit column (New Objective, Woburn, MA) packed with Michrom Magic C18 (100 Å, 5 μm; nanoLC) and coupled to a Velos Pro LTQ-Orbitrap mass spectrometer (ThermoFisher Scientific, Pittsburgh, PA). Peptide separation was performed with 2 to 50% solvent B (90% acetonitrile–10% water–0.1% formic acid) in a 120-min linear gradient at 300 nl/min. Mass spectra were acquired using a data-dependent acquisition mode and the Excalibur software version 1.6.2. Each full-scan mass spectrum (400 to 2,000 m/z) was followed by collision-induced dissociation of the 20 most intense ions. Dynamic exclusion was set for a period of 3 s and a tolerance of 100 ppm and then analyzed using the Protein Discover version 1.4.1 software (ThermoFisher Scientific). MGF format sample files were then analyzed using Mascot (version 2.4.0; Matrix Science, London, United Kingdom), and spectra were used to search the Trypanosoma cruzi version 13-03 and Brener databases, as well as the Macaque database to identify proteins derived from the Vero cells. All MS/MS samples were analyzed using Mascot with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 10 ppm. Mass spectrometry analysis was performed at the UVic-Genome BC Proteomic Centre, Victoria, BC, Canada, and The Proteomics Platform, Quebec Genomics Centre.
Production of T. cruzi PFR1 and retrotransposon hot spot proteins.
E. coli strain ER2566 cells transformed with the plasmid pET151/D-TOPO-TcPFR1 (57) were grown to an OD600 of 0.7 at 37°C in LB supplemented with 50 μg/ml ampicillin, and protein expression was induced with 0.5 mM isopropyl-β-d-isothiogalactopyranoside at 25°C for 5 h. Cultures (1.0-liter volumes) were harvested, and the cell pellets were resuspended in 22.5 ml of 40 mM phosphate buffer (pH 7.4) containing an EDTA-free protease inhibitor cocktail (Roche Molecular Biochemicals). Cells were lysed with three passes through a French press, and clarified lysates were made up to 500 mM NaCl prior to loading onto a 4-ml Ni2+-nitrilotriacetic acid–agarose column (Qiagen). The column was washed with 150 ml of 40 mM phosphate buffer (pH 7.4), 500 mM NaCl (buffer I), and then 100 ml buffer I containing 20 mM imidazole. Bound proteins were eluted with a 10-ml step gradient of buffer I containing 80 to 400 mM imidazole. Fractions containing an ∼70-kDa protein were pooled, concentrated to ∼0.5 mg/ml using a 3,000-MWCO Amicon ultrafiltration unit, flash-frozen in liquid nitrogen, and stored at −80°C.
For expression of the RHS protein (accession number EKG06703.1), the open reading frame was PCR amplified using the forward primer 5′-gtcacatATGTCTGGACGGCCCGAG-3′ and the reverse primer 5′-ttcgaggatccTTAGAGAACCACAGGAGTTTCTCG-3′ containing the restriction endonuclease sites NdeI and BamHI (lowercase text in the sequences). PCR mixtures containing 50 ng of genomic DNA isolated from T. cruzi strain Tulahuen trypomastigotes, 200 nM each primer, 200 μM deoxynucleoside triphosphates (dNTP), and 1 U of Q5 DNA polymerase (New England BioLabs) were run with an amplification program that included an initial denaturation at 98°C for 120 s followed by 25 cycles of denaturation at 98°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 2 min. The PCR product was cloned into the NdeI/BamHI sites of the pET15b vector to generate the pET-15b-RHS expression vector. The recombinant RHS protein was expressed using the protocol described for the production of PFR1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jeannie Mui (Facility for Electron Microscopy Research, McGill University, Montreal, Canada) for help with the TEM analysis and Abhishek Chatterjee, Ashley Tamming, and Anwer Kottarampatel (Institute of Parasitology, McGill University) for assistance in the cloning and production of recombinant proteins.
This project was funded by an operating grant from the Ministère de l'Économie, de l'Innovation et des Exportations of Quebec and an NSERC Discovery Grant (238249).
Footnotes
For a commentary on this article, see https://doi.org/10.1128/JCM.02353-16.
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01649-16.
REFERENCES
- 1.Bern C, Montgomery SP.. 2009. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 2.Martin DL, Goodhew B, Czaicki N, Foster K, Rajbhandary S, Hunter S, Brubaker SA. 2014. Trypanosoma cruzi survival following cold storage: possible implications for tissue banking. PLoS One 9:e95398. doi: 10.1371/journal.pone.0095398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huprikar S, Bosserman E, Patel G, Moore A, Pinney S, Anyanwu A, Neofytos D, Ketterer D, Striker R, Silveira F, Qvarnstrom Y, Steurer F, Herwaldt B, Montgomery S. 2013. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001-2011. Am J Transplant 13:2418–2425. doi: 10.1111/ajt.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gascon J, Bern C, Pinazo M-J. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery SP, Starr MC, Cantey PT, Edwards MS, Meymandi SK. 2014. Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg 90:814–818. doi: 10.4269/ajtmh.13-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmunis GA, Yadon ZE. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez FRS, Guedes PMM, Gazzinelli RT, Silva JS. 2009. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol 31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 8.Rassi A Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez JD, Guhl F, Umezawa ES, Morillo CA, Rosas F, Marin-Neto JA, Restrepo S. 2009. Evaluation of adult chronic Chagas' heart disease diagnosis by molecular and serological methods. J Clin Microbiol 47:3945–3951. doi: 10.1128/JCM.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Zulantay I, Apt P, Cortes P, Rodriguez J. 1994. Treatment of chronic human Chagas disease with itraconazole and allopurinol. Rev Med Chil 122:420–427. [PubMed] [Google Scholar]
- 11.Marin-Neto JA, Cunha-Neto EC, Maciel BC, Simoes MV. 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz AL, Raibagkar P, Pritt BS, Mateen FJ. 2015. Neurologic manifestations of the neglected tropical diseases. J Neurol Sci 349:20–32. doi: 10.1016/j.jns.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rendell VR, Gilman RH, Valencia E, Galdos-Cardenas G, Verastegui M, Sanchez L, Acosta J, Sanchez G, Ferrufino L, LaFuente C, Abastoflor MdC Colanzi R, Bern C. 2015. Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One 10:e0119527. doi: 10.1371/journal.pone.0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qvarnstrom Y, Schijman AG, Veron V, Aznar C, Steurer F, da Silva AJ. 2012. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Negl Trop Dis 6:e1689. doi: 10.1371/journal.pntd.0001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brasil P, De Castro L, Hasslocher-Moreno A, Sangenis L, Braga J. 2010. ELISA versus PCR for diagnosis of chronic Chagas disease: systematic review and meta-analysis. BMC Infect Dis 10:337. doi: 10.1186/1471-2334-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bern C, Kjos S, Yabsley MJ, Montgomery SP.. 2011. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev 24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes YM, Lorena VM, Luquetti AO. 2009. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz 104(Suppl 1):115–121. doi: 10.1590/S0074-0276200900090017. [DOI] [PubMed] [Google Scholar]
- 18.Jannin J, Salvatella R. 2006. Quantitative estimates of Chagas disease in the Americas. PAHO report OPS/HDM/CD/425-06. Pan American Health Organization, Washington, DC. [Google Scholar]
- 19.Hernandez P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, Beck E. 2010. Highly effective serodiagnosis for Chagas' disease. Clin Vaccine Immunol 17:1598–1604. doi: 10.1128/CVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira Gde A, Louzada-Neto F, Barbosa Vde F, Ferreira-Silva MM, de Moraes-Souza H. 2012. Performance of six diagnostic tests to screen for Chagas disease in blood banks and prevalence of Trypanosoma cruzi infection among donors with inconclusive serology screening based on the analysis of epidemiological variables. Rev Bras Hematol Hemoter 34:292–297. doi: 10.5581/1516-8484.20120074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Camargo CL, Albajar-Vinas P, Wilkins PP, Nieto J, Leiby DA, Paris L, Scollo K, Florez C, Guzmin-Bracho C, Luquetti AO, Calvo N, Tadokoro K, Saez-Alquezar A, Palma PP, Martin M, Flevaud L. 2014. Comparative evaluation of 11 commercialized rapid diagnostic tests for detecting Trypanosoma cruzi antibodies in serum banks in areas of endemicity and nonendemicity. J Clin Microbiol 52:2506–2512. doi: 10.1128/JCM.00144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umezawa ES, Nascimento MS, Kesper N, Coura JR, Borges-Pereira J, Junqueira AC, Camargo ME. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J Clin Microbiol 34:2143–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umezawa ES, Nascimento M, Stolf AM. 2001. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas' disease. Diagn Microbiol Infect Dis 39:169–176. doi: 10.1016/S0732-8893(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 24.Berrizbeitia M, Ndao M, Bubis J, Gottschalk M, Ache A, Lacouture S, Medina M, Ward BJ. 2006. Purified excreted-secreted antigens from Trypanosoma cruzi trypomastigotes as tools for diagnosis of Chagas' disease. J Clin Microbiol 44:291–296. doi: 10.1128/JCM.44.2.291-296.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakazawa M, Rosa D, Pereira V, Moura M, Furtado V, Souza W, Barros M, Abath F, Gomes Y. 2001. Excretory-secretory antigens of Trypanosoma cruzi are potentially useful for serodiagnosis of chronic Chagas' disease. Clin Diagn Lab Immunol 8:1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, E Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ. 2009. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 11:29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Bang C, Thum T. 2012. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Colombo M, Raposo G, Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Ann Rev Cell Develop Biol 30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Silva MR, das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernandez-Calero T, Souto-Padron T, de Souza W, Cayota A. 2014. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res 113:285–304. doi: 10.1007/s00436-013-3655-1. [DOI] [PubMed] [Google Scholar]
- 30.Schorey JS, Cheng Y, Singh PP, Smith VL. 2015. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncalves MF, Umezawa ES, Katzin AM, De Sousa W, Alves MJM, Zingales B, Colli W. 1991. Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol 72:43–53. doi: 10.1016/0014-4894(91)90119-H. [DOI] [PubMed] [Google Scholar]
- 33.Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC. 2013. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res 12:883–897. doi: 10.1021/pr300947g. [DOI] [PubMed] [Google Scholar]
- 34.Bayer-Santos E, Lima FM, Ruiz JC, Almeida IC, da Silveira JF. 2014. Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol Biochem Parasitol 193:71–74. doi: 10.1016/j.molbiopara.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaner S, Galiano A, Trelis M, Martin-Jaular L, del Portillo HA, Bernal D, Marcilla A. 2014. The role of extracellular vesicles in modulating the host immune response during parasitic infections. Front Immunol 5:433. doi: 10.3389/fimmu.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins NO, Souza RT, Cordero EM, Maldonado DC, Cortez C, Marini MM, Ferreira ER, Bayer-Santos E, Almeida IC, Yoshida N, Silveira JF. 2015. Molecular characterization of a novel family of Trypanosoma cruzi surface membrane proteins (TcSMP) involved in mammalian host cell invasion. PLoS Negl Trop Dis 9:e0004216. doi: 10.1371/journal.pntd.0004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neves RFC, Fernandes ACS, Meyer-Fernandes JR, Souto-Padrón T. 2014. Trypanosoma cruzi-secreted vesicles have acid and alkaline phosphatase activities capable of increasing parasite adhesion and infection. Parasitol Res 113:2961–2972. doi: 10.1007/s00436-014-3958-x. [DOI] [PubMed] [Google Scholar]
- 38.Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. 2010. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 39.Lambertz U, Silverman JM, Nandan D, McMaster WR, Clos J, Foster LJ, Reiner NE. 2012. Secreted virulence factors and immune evasion in visceral leishmaniasis. J Leukoc Biol 91:887–899. doi: 10.1189/jlb.0611326. [DOI] [PubMed] [Google Scholar]
- 40.Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI. 2012. Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol 188:1942–1952. doi: 10.4049/jimmunol.1102053. [DOI] [PubMed] [Google Scholar]
- 41.Hassani K, Olivier M. 2013. Immunomodulatory impact of Leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis 7:e2185. doi: 10.1371/journal.pntd.0002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araujo FG, Chiari E, Dias JCP. 1981. Demonstration of Trypanosoma cruzi antigen in serum from patients with Chagas disease. Lancet 317:246–249. doi: 10.1016/S0140-6736(81)92088-2. [DOI] [PubMed] [Google Scholar]
- 43.Araujo FG, Sharma SD, Tsai V, Cox P, Remington JS. 1982. Monoclonal antibodies to stages of Trypanosoma cruzi: characterization and use for antigen detection. Infect Immun 37:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freilij HL, Corral RS, Katzin AM, Grinstein S. 1987. Antigenuria in infants with acute and congenital Chagas' disease. J Clin Microbiol 25:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Affranchino JL, Ibanez CF, Luquetti AO, Rassi A, Reyes MB, Macina RA, Aslund L, Pettersson U, Frasch ACC. 1989. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol 34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 46.Umezawa ES, Shikanai-Yasuda MA, Stolf AM. 1996. Changes in isotype composition and antigen recognition of anti-Trypanosoma cruzi antibodies from acute to chronic Chagas disease. J Clin Lab Anal 10:407–413. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama-Yasunaka JK, Pral EM, Oliveira Júnior OC, Alfieri SC, Stolf AM. 1994. Trypanosoma cruzi: identification of proteinases in shed components of trypomastigote forms. Acta Trop 57:307–315. doi: 10.1016/0001-706X(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 48.Kowal J, Tkach M, Thery C. 2014. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Oldham KT, Ou J-S. 2008. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics 8:2430–2446. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernabó G, Levy G, Ziliani M, Caeiro LD, Sánchez DO, Tekiel V. 2013. TcTASV-C, a protein family in Trypanosoma cruzi that is predominantly trypomastigote-stage specific and secreted to the medium. PLoS One 8:e71192. doi: 10.1371/journal.pone.0071192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan AA, McMahon-Pratt D. 1989. Amastigote and epimastigote stage-specific components of Trypanosoma cruzi characterized by using monoclonal antibodies: purification and molecular characterization of an 83-kilodalton amastigote protein. J Immunol 143:1001–1008. [PubMed] [Google Scholar]
- 52.Boscardin SB, Kinoshita SS, Fujimura AE, Rodrigues MM. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental Infection. Infect Immun 71:2744–2757. doi: 10.1128/IAI.71.5.2744-2757.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D-K, Lee J, Simpson RJ, Lotvall J, Gho YS. 2015. EVpedia: a community Web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin Cell Develop Biol 40:4–7. doi: 10.1016/j.semcdb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Bautista-Lopez NL, Morillo CA, Lopez-Jaramillo P, Quiroz R, Luengas C, Silva SY, Galipeau J, Lalu MM, Schulz R. 2013. Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J 165:558–566. doi: 10.1016/j.ahj.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Cervantes-Landin AY, Martinez I, Schabib M, Espinoza B. 2014. High molecular weight proteins of Trypanosoma cruzi reduce cross-reaction with Leishmania spp. in serological diagnosis tests. Biomed Res Int 2014:365403. doi: 10.1155/2014/365403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quanquin NM, Galaviz C, Fouts DL, Wrightsman RA, Manning JE. 1999. Immunization of mice with a TolA-like surface protein of Trypanosoma cruzi generates CD4+ T-cell-dependent parasiticidal activity. Infect Immun 67:4603–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Annoura T, Makiuchi T, Sariego I, Aoki T, Nara T. 2012. SUMOylation of paraflagellar rod protein, PFR1, and its stage-specific localization in Trypanosoma cruzi PLoS One 7:e37183. doi: 10.1371/journal.pone.0037183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Pablos LM, Díaz Lozano IM, Jercic MI, Quinzada M, Giménez MJ, Calabuig E, Espino AM, Schijman AG, Zulantay I, Apt W, Osuna A. 2016. The C-terminal region of Trypanosoma cruzi MASPs is antigenic and secreted via exovesicles. Sci Rep 6:27293. doi: 10.1038/srep27293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooley G, Etheridge RD, Boehlke C, Bundy B, Weatherly DB, Minning T, Haney M, Postan M, Laucella S, Tarleton RL. 2008. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLoS Negl Trop Dis 2:e316. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agusti R, Couto AS, Campetella OE, Frasch AC, de Lederkremer RM. 1997. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology 7:731–735. doi: 10.1093/glycob/7.6.731. [DOI] [PubMed] [Google Scholar]
- 61.Queiroz RML, Charneau S, Bastos IMD, Santana JM, Sousa MV, Roepstorff P, Ricart CAO. 2014. Cell surface proteome analysis of human-hosted Trypanosoma cruzi life stages. J Proteome Res 13:3530–3541. doi: 10.1021/pr401120y. [DOI] [PubMed] [Google Scholar]
- 62.Ouaissi MA, Taibi A, Cornette J, Velge P, Marty B, Loyens M, Esteva M, Rizvi FS, Capron A. 1990. Characterization of major surface and excretory-secretory immunogens of Trypanosoma cruzi trypomastigotes and identification of potential protective antigen. Parasitol Res 100 115–124. [DOI] [PubMed] [Google Scholar]
- 63.Grandgenett PM, Coughlin BC, Kirchhoff LV, Donelson JE. 2000. Differential expression of GP63 genes in Trypanosoma cruzi Mol Biochem Parasitol 110:409–415. doi: 10.1016/S0166-6851(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 64.Da Silveira JF, Abrahamsohn PA, Colli W. 1979. Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi Biochim Biophys Acta 550:222–232. doi: 10.1016/0005-2736(79)90209-8. [DOI] [PubMed] [Google Scholar]
- 65.Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL. 2016. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164:246–257. doi: 10.1016/j.cell.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner G, Yamanaka LE, Moura H, Lückemeyer DD, Schlindwein AD, Stoco PH, Ferreira HB, Barr JR, Steindel M, Grisard EC. 2013. The Trypanosoma rangeli trypomastigote surfaceome reveals novel proteins and targets for specific diagnosis. J Proteomics 82:52–63. doi: 10.1016/j.jprot.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Paba J, Santana JM, Teixeira ARL, Fontes W, Sousa MV, Ricart CAO. 2004. Proteomic analysis of the human pathogen Trypanosoma cruzi Proteomics 4:1052–1059. doi: 10.1002/pmic.200300637. [DOI] [PubMed] [Google Scholar]
- 68.Bayona JC, Nakayasu ES, Laverrière M, Aguilar C, Sobreira TJP, Choi H, Nesvizhskii AI, Almeida IC, Cazzulo JJ, Alvarez VE. 2011. SUMOylation pathway in Trypanosoma cruzi: functional characterization and proteomic analysis of target proteins. Mol Cell Proteomics 10:M110.007369. doi: 10.1074/mcp.M110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bringaud F, Biteau N, Melville SE, Hez S, El-Sayed NM, Leech V, Berriman M, Hall N, Donelson JE, Baltz T. 2002. A new, expressed multigene family containing a hot spot for insertion of retroelements is associated with polymorphic subtelomeric regions of Trypanosoma brucei Eukarot Cell 1:137–151. doi: 10.1128/EC.1.1.137-151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunoro GV, Caminha MA, Ferreira AT, Leprevost FDV, Carvalho PC, Perales J, Valente RH, Menna-Barreto RF. 2015. Reevaluating the Trypanosoma cruzi proteomic map: the shotgun description of bloodstream trypomastigotes. J Proteomics 115:58–65. doi: 10.1016/j.jprot.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, Peltier J-B. 2010. Exocytosis and protein secretion in Trypanosoma BMC Microbiol 10:20. doi: 10.1186/1471-2180-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bongertz V, Hungerer KD, Galvao-Castro B. 1981. Trypanosoma cruzi: circulating antigens. Mem Inst Oswaldo Cruz 76:71–82. doi: 10.1590/S0074-02761981000100008. [DOI] [PubMed] [Google Scholar]
- 73.Corral RS, Orn A, Grinstein S. 1992. Detection of soluble exoantigens of Trypanosoma cruzi by a dot-immunobinding assay. Am J Trop Med Hyg 46:31–38. [DOI] [PubMed] [Google Scholar]
- 74.Umezawa ES, Shikanaiyasuda MA, Dasilveira JF, Cotrim PC, Paranhos G, Katzin AM. 1993. Trypanosoma cruzi: detection of a circulating antigen in urine of chagasic patients sharing common epitopes with an immunodominant repetitive antigen. Exp Parasitol 76:352–357. doi: 10.1006/expr.1993.1043. [DOI] [PubMed] [Google Scholar]
- 75.Petray P, Bonardello N, Clark R, Agranatti M, Corral R, Grinstein S. 1992. Evaluación del método de ELISA para detección de antígenos y complejos inmunes circulantes de Trypanosoma cruzi a través de un estudio de campo en una zona endémica de Argentina. Rev Instit Med Trop Sao Paulo 34:141–147. doi: 10.1590/S0036-46651992000200010. [DOI] [PubMed] [Google Scholar]
- 76.Boukouris S, Mathivanan S. 2015. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl 9:358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagarkatti R, de Araujo FF, Gupta C, Debrabant A. 2014. Aptamer based, non-PCR, non-serological detection of Chagas disease biomarkers in Trypanosoma cruzi infected mice. PLoS Negl Trop Dis 8:e2650. doi: 10.1371/journal.pntd.0002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.