ABSTRACT
Few studies on risk factors for and transmission of Clostridium difficile infection (CDI) in China have been reported. A cross-sectional study was conducted for 3 years in eastern China. Consecutive stool specimens from hospitalized patients with diarrhea were cultured for C. difficile. C. difficile isolates from these patients then were analyzed for toxin genes, genotypes, and antimicrobial resistance. A severity score for the CDI in each patient was determined by a blinded review of the medical record, and these scores ranged from 1 to 6. A total of 397 out of 3,953 patients (10.0%) with diarrhea were found to have CDI. Severity of CDI was mild to moderate, and the average (± standard deviation) severity score was 2.61 ± 1.01. C. difficile was isolated from stool specimens in 432 (10.9%) of all the patients who had diarrhea. C. difficile genotypes were determined by multilocus sequence analysis and PCR ribotyping; sequence type 37 (ST37)/ribotype 017 (RT017) (n = 68, 16.5%) was the dominant genotype. Eleven patients (16.2%) with this genotype had a CDI severity score of 5. Overall, three RTs and four STs were predominant; these genotypes were associated with significantly different antimicrobial resistance patterns in comparison to all genotypes (χ2 = 79.56 to 97.76; P < 0.001). Independent risk factors associated with CDI included age greater than 55 years (odds ratio [95% confidence interval], 26.80 [18.76 to 38.29]), previous hospitalization (12.42 [8.85 to 17.43]), previous antimicrobial treatment within 8 weeks (150.56 [73.11 to 310.06]), hospital stay more than 3 days before sampling (2.34 [1.71 to 3.22]), undergoing chemotherapy (3.31 [2.22 to 4.92]), and undergoing abdominal surgery (4.82 [3.54 to 6.55]). CDI is clearly a problem in eastern China and has a prevalence of 10.0% in hospitalized patients. Among risk factors for CDI, the advanced age threshold was younger for Chinese patients than that reported for patients in developed countries.
KEYWORDS: Clostridium difficile infection, risk factor, clinical features, cross-sectional study, molecular characterization
INTRODUCTION
The bacterium Clostridium difficile is a Gram-positive spore-forming anaerobic bacillus that is responsible for antibiotic-associated diarrhea. Patients with C. difficile infection (CDI) have clinical manifestations that range from asymptomatic carriage to severe life-threatening pseudomembranous colitis (1), toxic megacolon, and sepsis (2). In the United States, CDI is a frequent cause of gastrointestinal tract infection and can cause colitis-related death. CDI in the United States affects >300,000 hospitalized patients each year (3) and in 2007 resulted in 14,000 deaths (3, 4). In a survey of 183 hospitals in 2010, C. difficile was the most common health care-associated pathogen in the United States (5). In the United States in 2011, C. difficile caused almost 500,000 infections and was associated with approximately 29,000 deaths (4). CDI also is the most common gastrointestinal tract disease associated with nosocomial infection in Europe (6, 7).
Since the emergence of hypervirulent variants of toxigenic C. difficile in 2003, the incidence, severity, and fatality rates of CDI have increased dramatically (8, 9). Antimicrobial-associated treatment failure, frequent relapse, and a longer hospital stay now are more commonly seen with CDI (10). This hypervirulent C. difficile epidemic clone has been characterized as North American pulsed-field type 1 (NAP1) by pulsed-field gel electrophoresis, BI group by restriction endonuclease analysis, and ribotype 027 (RT027) by PCR ribotyping (10, 11). Notably, the BI/NAP1/RT027 clone is resistant to fluoroquinolones and produces significantly more TcdA and TcdB toxins than other strains. This property may increase its pathogenicity (11). This C. difficile clone has been transmitted rapidly and widely over the past decade, accounting for 50% of nonoutbreak hospital-associated CDI in some settings (12). CDI caused by this BI/NAP1/RT027 strain is conservatively estimated to cost $3.2 billion per year in the United States (4).
Since 2008, the BI/NAP1/RT027 strain has spread beyond the original North Atlantic domain. A few cases of CDI caused by a fluoroquinolone-resistant RT027 strain have been reported in Asian-Pacific and European countries (6, 13). However, few studies have examined risk factors and transmission in China, where CDI is an emerging problem (14–17). Previous studies in China focused on patients with specific clinical characteristics, including colorectal cancer (18), non-digestive tract cancer (18), hematological malignancies (19), pregnancy (17), and advanced age (15). Sequence types 35 (ST35), ST37, and ST54 are the predominant genotypes in China. A variant toxigenic A-negative/B-positive (A−B+) genotype of C. difficile in an ST37 strain is a potential cause of epidemics (14), although the BI/NAP1/RT027 C. difficile strain has been reported in China (20). Nevertheless, there is a paucity of data on CDI in China. For example, specific risk factors, the relationship between molecular characteristics and the severity of CDI, and antimicrobial susceptibility patterns for C. difficile isolates are not known. We conducted a cross-sectional study of CDI in eastern China in order to determine the clinical manifestations, prevalence, and molecular epidemiology of CDI in hospitalized patients in eastern China. Furthermore, the clinical features of patients with CDI were analyzed with respect to genotypes and risk factors.
(This study was presented in part at the 116th American Society for Microbiology Annual Meeting, Boston, Massachusetts, 15 to 21 June 2016.)
RESULTS
Collection of clinical C. difficile isolates.
A total of 3,953 hospitalized patients with diarrhea at the First Affiliated Hospital of Zhejiang University (FAHZU) (n = 1,820) or at a hospital belonging to the Hangzhou First People's Hospital Healthcare Facility Group (HFPH) (n = 2,133) were enrolled in this cross-sectional study over an approximately 3-year period. Standardized stool specimens were collected from each patient; 432 C. difficile isolates (10.9%) (FAHZU, n = 174; HFPH, n = 258) with the tpi gene were recovered from these stool specimens. Based on PCR amplification of tcdA and tcdB, 397 isolates (91.9%) carried one or both of these genes and were considered toxigenic, while the remaining 35 isolates (8.1%) were nontoxigenic. Twenty-one toxigenic C. difficile isolates were excluded from subsequent analyses because they became nonviable after storage (Fig. 1). The remaining 411 isolates were tested further for toxin gene identification, antimicrobial susceptibility patterns, PCR ribotyping, and multilocus sequence typing (MLST).
FIG 1.
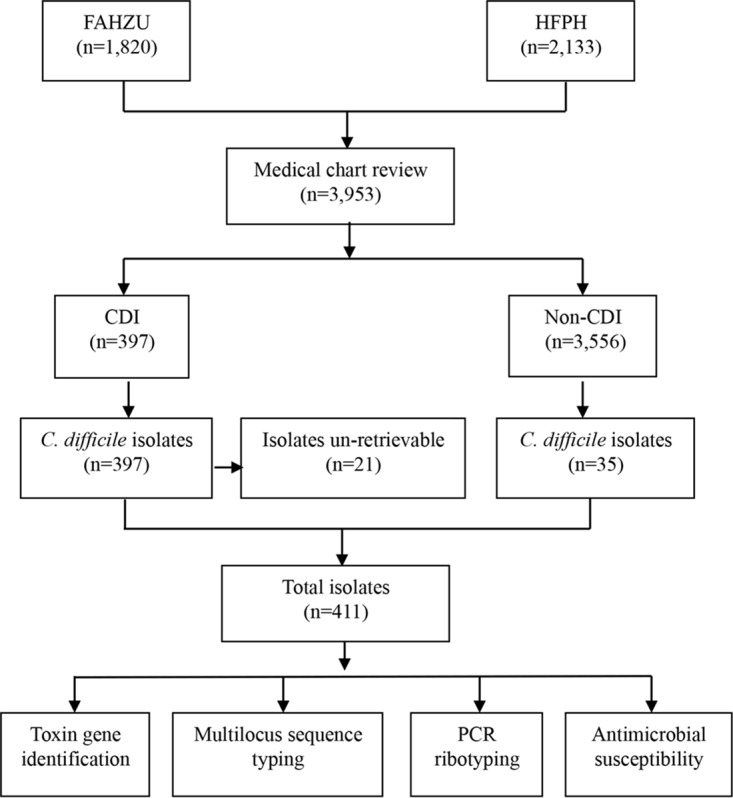
Flow diagram of data collected during the cross-sectional study (1 June 2012 to 30 September 2015).
Genotypes of C. difficile isolates.
Of the 411 C. difficile isolates, 282 (68.6%) tested positive for both tcdA and tcdB (A+B+), 94 (22.9%) tested negative for tcdA and positive for tcdB (A−B+), and 35 (8.5%) tested negative for both tcdA and tcdB (A−B−). There were no deletions found in tcdC genes from all toxigenic isolates examined. No isolate tested positive for the binary toxin genes cdtA and cdtB. They were assigned to 56 RTs; RT001 (n = 59, 14.4%), RT012 (n = 55, 13.4%), and RT017 (n = 68, 16.6%) were predominant. Similarly, 27 ST genotypes were assigned based on MLST; ST2 (n = 46, 11.2%), ST3 (n = 67, 16.3%), ST37 (n = 68, 16.6%), and ST54 (n = 53, 12.9%) were predominant. Exclusive correlations were found between toxigenic ST37/ST39/ST81 and toxin type A−B+ and between nontoxigenic ST15 and A−B− isolates.
The ST and RT genotypes had a general agreement of 55.2% (227/411), and exclusive correlations were found in three genotypes (i.e., ST37/RT017, ST39/RT085, and ST81/RT040). High correlations were revealed in ST54/RT012 (96.4 to 100.0%), ST48/RTAI-53 (81.8 to 100.0%), ST17/RT018 (76.5 to 100.0%), and ST3/RT001 (86.6 to 98.3%). However, no correlation was detected for ST2 strains, which exhibited high genetic diversity and included 10 RTs (007, 014, 020, 040, 404, 445, 452, 463, 477, and 563/1).
Antimicrobial susceptibility of isolates.
The antibiotic susceptibility patterns of 411 C. difficile isolates are presented in Table 1. The antimicrobial resistance rates were distinctly higher for fusidic acid, ciprofloxacin, clindamycin, levofloxacin, and erythromycin than for piperacillin-tazobactam (PIP-TAZ), metronidazole, rifampin, moxifloxacin, gatifloxacin, vancomycin, and tetracycline. While all isolates were sensitive to vancomycin, 64 (15.6%), including one nontoxigenic isolate, were revealed to be resistant to metronidazole (MIC values, >256 μg/ml). The remaining metronidazole-susceptible isolates had MIC values of <1.0 μg/ml. Four toxigenic and one nontoxigenic isolate were resistant to PIP-TAZ. Notably, 306 (74.5%) of these isolates were multidrug resistant (MDR); toxigenic isolates had a much higher MDR rate (295/376, 96.4%) than those of nontoxigenic strains (11/35, 31.4%) (odds ratio [OR], 7.95 [95% confidence interval (CI) 3.54 to 18.13]; P < 0.001). However, the MDR rate (217/282, 77.0%) in A+B+ isolates was not significantly different from that (78/94, 83.0%) in A−B+ strains (OR, 0.68 [95% CI, 0.36 to 1.30]; P = 0.218).
TABLE 1.
Correlations among MLST types, PCR ribotypes, and antimicrobial susceptibility patterns of C. difficile isolates
| Antimicrobial agenta | Total no. (%) of isolatesb (n = 411) | MLST type (no. [%] of nonsusceptible isolates) |
Analysis resultsc |
PCR ribotype (no. [%] of nonsusceptible isolates) |
Analysis resultsc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST2 (n = 46) | ST3 (n = 67) | ST37 (n = 68) | ST54 (n = 53) | Other STs (n = 177) | χ2 | P value | 001 (n = 59) | 012 (n = 55) | 017 (n = 68) | Others (n = 229) | χ2 | P value | ||
| Fusidic acid | 290 (70.6) | 36 (78.3) | 42 (62.7) | 55 (80.9) | 46 (86.8) | 111 (62.7) | 18.77 | 0.001 | 37 (62.7) | 48 (87.3) | 55 (80.9) | 150 (65.5) | 15.42 | 0.001 |
| Ciprofloxacin | 334 (81.3) | 36 (78.3) | 61 (91.0) | 54 (79.4) | 50 (94.3) | 133 (75.1) | 14.95 | 0.005 | 53 (89.8) | 52 (94.5) | 54 (79.4) | 175 (76.4) | 12.90 | 0.005 |
| PIP-TAZ | 5 (1.2) | 1 (2.2) | 0 | 0 | 0 | 4 (2.3) | 4.26 | 0.372 | 0 | 0 | 0 | 5 (2.2) | 4.01 | 0.26 |
| Metronidazole | 64 (15.6) | 9 (19.6) | 10 (14.9) | 5 (7.4) | 4 (7.6) | 36 (20.3) | 9.73 | 0.045 | 10 (17.0) | 6 (10.9) | 5 (7.4) | 43 (18.8) | 6.28 | 0.099 |
| Rifampin | 38 (9.3) | 6 (13.0) | 1 (1.5) | 8 (11.8) | 11 (20.8) | 12 (6.8) | 15.75 | 0.003 | 1 (1.7) | 11 (20.0) | 8 (11.8) | 18 (7.9) | 12.63 | 0.006 |
| Moxifloxacin | 104 (25.3) | 9 (19.6) | 25 (37.3) | 18 (26.5) | 2 (3.8) | 50 (28.3) | 19.77 | 0.001 | 22 (37.3) | 2 (3.6) | 18 (23.4) | 62 (27.1) | 18.57 | <0.001 |
| Gatifloxacin | 133 (32.4) | 10 (21.7) | 29 (43.3) | 36 (52.9) | 1 (1.9) | 57 (32.2) | 41.67 | <0.001 | 23 (39.0) | 3 (5.5) | 36 (50.7) | 71 (31.0) | 32.72 | <0.001 |
| Vancomycin | 0 | 0 | 0 | 0 | 0 | 0 | — | — | 0 | 0 | 0 | 0 | — | — |
| Clindamycin | 257 (62.5) | 30 (65.2) | 53 (79.1) | 37 (54.4) | 35 (66.0) | 102 (57.6) | 12.01 | 0.017 | 48 (81.4) | 35 (63.6) | 37 (52.1) | 137 (59.8) | 11.58 | 0.009 |
| Levofloxacin | 308 (74.9) | 36 (78.3) | 58 (86.6) | 52 (76.5) | 37 (69.8) | 125 (70.6) | 7.68 | 0.104 | 52 (88.1) | 39 (70.9) | 52 (73.2) | 165 (72.1) | 7.05 | 0.07 |
| Tetracycline | 149 (36.3) | 12 (26.1) | 21 (31.3) | 24 (35.3) | 35 (66.0) | 57 (32.2) | 24.38 | <0.001 | 21 (35.6) | 35 (63.6) | 24 (35.3) | 69 (30.1) | 21.60 | <0.001 |
| Erythromycin | 266 (64.7) | 20 (43.5) | 50 (74.6) | 57 (83.8) | 36 (67.9) | 103 (58.2) | 26.38 | <0.001 | 46 (78.0) | 38 (69.1) | 57 (83.1) | 125 (54.6) | 26.16 | <0.001 |
| MDR | 306 (74.5) | 30 (65.2) | 53 (79.1) | 57 (83.8) | 47 (88.7) | 119 (67.2) | 16.46 | 0.003 | 49 (83.1) | 48 (87.3) | 60 (83.8) | 149 (48.7) | 24.45 | <0.001 |
PIP-TAZ: piperacillin-tazobactam; MDR: multidrug resistant.
Of 411 isolates, 6 (1.5%), 14 (3.4%), 1 (0.2%), 1 (0.2%), 1 (0.2%), 6 (1.5%), 8 (1.9%), 0 (0%), 8 (1.9%), 12 (2.9%), 9 (2.2%), and 15 (3.6%) nontoxigenic isolates of nonsusceptible fusidic acid, ciprofloxacin, PIP-TAZ, metronidazole, rifampin, moxifloxacin, gatifloxacin, vancomycin, clindamycin, levofloxacin, tetracycline, and erythromycin were found, respectively. A total of 11 (2.7%) nontoxigenic MDR isolates were found.
—, data not calculated.
Genotypes and antimicrobial resistance.
Correlations between antimicrobial resistance and predominant genotypes (STs and RTs) were determined (Table 1). The antimicrobial patterns for ST54 isolates were significantly different from those of the other three major STs (χ2 = 30.40 to 59.05; P < 0.001), while a similar pattern was revealed for RT012 (χ2 = 40.56 to 47.92; P < 0.001). The MDR rate was noted to be high in all STs and RTs in this study. Higher resistance rates were found in ST2/ST3 (14.9 to 19.6%; χ2 = 4.876; P = 0.027) and RT001 (17.0%; χ2 = 2.504; P = 0.114) strains than in strains of other dominant genotypes (Table 1). Five PIP-TAZ-resistant strains were sporadically distributed in different STs/RTs. Among the five isolates, 1, 2, and 2 had a CDI severity score of 1, 2, or 4, respectively, resulting in an average severity score of 2.6. Overall, antimicrobial patterns were significantly different among predominant STs/RTs (χ2 = 79.56 to 97.76; P < 0.001) (Table 1).
Correlation between genotype and clinical manifestations.
The average (± standard deviation) severity score was 2.61 ± 1.01 for the 397 patients defined as having CDI. There were no CDI severity scores of 6. The correlation between clinical CDI severity and ST/RT genotypes for the 411 patients with retrievable C. difficile isolates was further analyzed (Table 2). All nontoxigenic type A[minus]B− C. difficile isolates were assigned a CDI severity score of 1 (Table 2). There were significant differences in CDI severity scores among different RTs and MLST types (χ2 = 19.35 to 21.58; P < 0.001) (Table 2). In patients with a CDI score of >3, ST37 was found more frequently than ST2 (χ2 = 4.25; P = 0.039), ST3 (χ2 = 5.67; P = 0.017), and other STs (χ2 = 6.97; P = 0.008). Similarly, in patients with a CDI score of >3, RT017 was found more frequently than RT001 (χ2 = 6.18; P = 0.013) and other RTs (χ2 = 8.08; P = 0.004). There was a total of 22 patients with a CDI severity score of 5, and half of their infections belonged to the ST37/RT017 genotype (Table 2).
TABLE 2.
Correlation between clinical CDI severities and genotypes of C. difficile isolates
| Molecular characterization | CDIa severity scoreb (%) |
Analysis results |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison among 5 CDI severity scores |
Trend χ2 |
|||||||||
| 1 (n = 35) | 2 (n = 193) | 3 (n = 103) | 4 (n = 58) | 5 (n = 22) | Mean ± SD | χ2 | P value | χ2 | P value | |
| Toxin gene pattern | ||||||||||
| A+B+ (n = 282) | 0 | 152 (53.9) | 79 (28.0) | 44 (15.6) | 7 (2.5) | 2.67 ± 0.05 | 113.8 | <0.001 | 9.28 | 0.002 |
| A−B+ (n = 94) | 0 | 41 (43.6) | 24 (25.5) | 14 (14.9) | 15 (16.0) | 3.03 ± 0.12 | ||||
| A−B− (n = 35) | 35 (100) | 0 | 0 | 0 | 0 | 1.00 | ||||
| MLST type | ||||||||||
| ST2 (n = 46) | 0 | 29 (63.0) | 10 (21.7) | 7 (15.2) | 0 | 2.52 ± 0.11 | 19.4 | 0.001 | 3.46 | 0.063 |
| ST3 (n = 67) | 0 | 36 (53.7) | 21 (31.3) | 9 (13.4) | 1 (1.5) | 2.63 ± 0.10 | 1.85 | 0.174 | ||
| ST37 (n = 68) | 0 | 30 (44.1) | 16 (23.5) | 11 (16.2) | 11 (16.2) | 3.04 ± 0.14 | 8.04 | 0.005 | ||
| ST54 (n = 53) | 0 | 24 (45.3) | 18 (34.0) | 11 (20.8) | 0 | 2.75 ± 0.11 | 0.001 | 0.978 | ||
| Others (n = 177) | 35 (19.7) | 74 (41.8) | 38 (21.5) | 20 (11.3) | 10 (5.7) | 2.41 ± 0.08 | 0.004 | 0.947 | ||
| PCR ribotype | ||||||||||
| RT001 (n = 59) | 0 | 33 (55.9) | 18 (30.5) | 7 (11.9) | 1 (1.7) | 2.59 ± 0.10 | 21.6 | <0.001 | 2.58 | 0.108 |
| RT012 (n = 55) | 0 | 25 (45.5) | 18 (32.7) | 11 (20.0) | 1 (1.8) | 2.78 ± 0.11 | 0.11 | 0.741 | ||
| RT017 (n = 68) | 0 | 30 (44.1) | 16 (23.5) | 11 (16.2) | 11 (16.2) | 3.04 ± 0.14 | 8.70 | 0.003 | ||
| Others (n = 229) | 35 (15.3) | 105 (45.9) | 51 (22.3) | 29 (12.7) | 9 (3.9) | 2.44 ± 0.07 | 1.967 | 0.160 | ||
CDI, Clostridium difficile infection.
CDI severity scores: 1, no clinical CDI; 2, mild CDI; 3, mild-to-moderate CDI; 4, moderate CDI; 5, moderate-to-severe CDI; 6, severe CDI (no cases observed).
Assessment of risk factors for CDI.
Risk factors for contracting CDI were assessed by a blinded review of the medical record of each patient. A bivariate analysis between 397 CDI cases and 3,556 controls without CDI was conducted, and the results are shown in Table 3. The following parameters were found statistically significant between cases of CDI and controls without CDI: hospital location, age, previous hospitalization, cumulative previous hospital stay exceeding 1 week, undergoing chemotherapy, undergoing abdominal surgery, previous antimicrobial treatment within 8 weeks, third- or fourth-generation cephalosporin use, fluoroquinolone use, carbapenem use, other antibiotic use, hospitalization stay over 3 days before sampling, and ward type (including oncology, gastrointestinal/anorectal diseases, infectious diseases, and others) (Table 3). A multivariate analysis including statistically significant parameters from the bivariate analysis was subsequently performed. Six parameters, including age, previous hospitalization, previous antimicrobial treatment within 8 weeks, hospitalization stay over 3 days before sampling, undergoing chemotherapy, and undergoing abdominal surgery, remained statistically significant between CDI cases and controls without CDI (Table 3). Notably, we found that an age of >55 years was a risk factor for patients in eastern China. In a bivariate analysis, the ORs for >45, >55, >65, and >75 years of age were 1.04, 9.62, 9.57, and 7.42, respectively (data not shown), indicating that the age threshold was much lower in eastern China than the age thresholds seen in developed countries.
TABLE 3.
Parameters and risk factors in 3,953 hospitalized patients with diarrhea
| Parameter | No. (%) of clinical samples from which C. difficile was isolated |
Analysis results |
||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate |
Multivariate logistic |
|||||||
| Toxigenic C. difficile isolated (n = 397) | No toxigenic C. difficile isolated (n = 3,556) | OR | 95% CI | P value | OR | 95% CI | P value | |
| Origin, HPFH | 244 (61.5) | 1889 (53.1) | 0.71 | 0.57–0.88 | 0.002 | 1.21 | 0.90–1.63 | 0.212 |
| Age > 55 yrs (median, 64 yrs) | 333 (83.9) | 1248 (35.1) | 9.62 | 7.30–12.68 | <0.001 | 26.80 | 18.76–38.29 | <0.001 |
| Gender, male | 205 (51.6) | 1854 (52.1) | 1.02 | 0.93-1.26 | 0.846 | |||
| Previous hospitalization, yes | 300 (75.6) | 1613 (45.4) | 3.73 | 2.94–4.73 | <0.001 | 12.42 | 8.85–17.43 | <0.001 |
| Cumulative previous hospital stay exceeding 1 wk, yes | 233 (58.7) | 711 (20.0) | 5.69 | 4.58–7.06 | <0.001 | |||
| Previous antimicrobial treatment within 8 wks, yes | 388 (97.7) | 2052 (57.7) | 31.60 | 16.26–61.39 | <0.001 | 150.56 | 73.11–310.06 | <0.001 |
| Previous antibiotic use | ||||||||
| Third- or fourth-generation cephalosporins | 167 (42.1) | 969 (27.3) | 1.94 | 1.57–2.40 | <0.001 | |||
| Fluoroquinolones | 104 (26.2) | 547 (15.4) | 1.95 | 1.53–2.49 | <0.001 | |||
| Carbapenems | 61 (15.4) | 312 (8.8) | 1.89 | 1.40–2.54 | <0.001 | |||
| Others | 56 (14.1) | 224 (6.3) | 2.44 | 1.79–3.34 | <0.001 | |||
| Hospital stay >3 days before sampling, yes | 132 (33.3) | 663 (18.6) | 2.17 | 1.74–2.73 | <0.001 | 2.34 | 1.71–3.22 | <0.001 |
| Ward type | ||||||||
| Oncology | 94 (23.7) | 311 (8.8) | 3.28 | 2.50–4.19 | <0.001 | |||
| Gastrointestinal disease | 190 (47.9) | 526 (14.8) | 5.29 | 4.25–6.57 | <0.001 | |||
| Infectious diseases | 63 (15.9) | 171 (4.8) | 3.73 | 2.74–5.09 | <0.001 | |||
| Othera | 50 (12.6) | 513 (14.4) | 0.86 | 0.63–1.17 | 0.322 | |||
| Chemotherapy, yes | 101 (25.4) | 432 (12.2) | 2.48 | 1.93–3.16 | <0.001 | 3.31 | 2.22–4.92 | <0.001 |
| Abdominal surgery, yes | 208 (52.4) | 993 (27.9) | 2.84 | 2.30–3.51 | <0.001 | 4.82 | 3.54–6.55 | <0.001 |
Other ward types included urology, transplantation, respiratory medicine, bone and joint, and neurology.
DISCUSSION
CDI is an increasing public health concern worldwide and is the leading cause of intestinal infection related to antimicrobial therapy. Since the emergence in recent decades of the epidemic NAP1 clone and subsequent outbreaks of C. difficile-associated disease in North America and Europe, there has been a dramatic increase in the number of severe cases of CDI (10, 11). To our knowledge, this is the first large active cross-sectional study of CDI in hospitalized patients in China to address both clinical features and characterization of isolates. In this study, we found that age, previous hospitalization, cumulative length of previous hospital stay, previous antimicrobial treatment within 8 weeks, undergoing chemotherapy, and undergoing abdominal surgery were risk factors in China. Age, previous hospitalization, and previous antimicrobial treatment within 8 weeks were the most prominent risk factors according to a multivariate logistic regression analysis.
Interestingly, we found the age threshold as a risk factor for CDI patients in eastern China to be lower than the age thresholds seen in developed countries. In a previous study, we found that cancer patients younger than 50 years were more likely to be C. difficile carriers (18). Similar results from a study of CDI in hospitalized cancer patients in Beijing, China, revealed that the mean age of cancer patients with CDI was 56 ± 16 years, which is significantly lower than the mean age reported in non-cancer hospitals in the United States (4, 21). Similarly, results of a study on factors associated with Staphylococcus aureus nasal carriage among healthy people in northern China indicated that younger people (≤24 years old) were more prone to S. aureus nasal carriage (22). Whether antimicrobial overuse in China is associated with a younger age of acquisition in patients with CDI merits further investigation.
There is still a paucity of antimicrobial resistance data for C. difficile strains in China. Based on limited data obtained in 2009, no C. difficile strains from Shanghai (eastern China) were resistant to metronidazole, vancomycin, PIP-TAZ, or meropenem. The rates of resistance to other antimicrobials ranged from 17.9 to 71.4%, and all the strains were resistant to ciprofloxacin. The antimicrobial resistance pattern observed in this study differed dramatically from that in previous studies. The resistance rate for fusidic acid (66.7%) was markedly higher than that reported previously (23). Even though the resistance rate for rifampin (9.3%) was lower than that described in Shanghai in 2009 (23), resistance to PIP-TAZ and metronidazole was noted in our study. Moreover, 15.6% of C. difficile strains in our study demonstrated high-level resistance to metronidazole. Multiple metronidazole resistance mechanisms in Bacteroides fragilis and Helicobacter pylori have been described (24). We are currently confirming the unusual resistance rate to metronidazole revealed in this study and also are investigating possible mechanisms for this resistance.
STs and RTs have been reported to have similar discriminatory abilities; multiple RTs for the same ST usually had very similar profiles, and multiple STs for the same RT generally had very closely related STs (25). Results of our study reveal that certain related strain genotypes assigned as STs and RTs were highly associated with the severity of CDI. Variant strains with the A−B+ genotype played a role in the epidemiology of CDI in China. Although the BI/NAP1/RT027 strain was reported recently in China (20), none of the 411 C. difficile isolates that we recovered in this study were found to be of that particular strain. In contrast, the A−B+ genotype accounted for 23.2% of the isolates from Shanghai in 2009 (23). Moreover, 13.5% of the variant strains were A−B+ (17), and ST37 was a dominant genotype in two surveys of Chinese hospitals in Beijing (16) and Hangzhou (26). ST37 has also been recognized as a potential epidemic strain in China (14). In our study, the A−B+ isolates were genetically diverse, but ST37/RT017 was the most prevalent type. Of 22 patients with a CDI severity score of 5, C. difficile strain ST37/RT017 was recovered from stool specimens in 50%. More than one half of these ST37/RT017 isolates were resistant to gatifloxacin, a fourth-generation fluoroquinolone. The ST37/RT017 strain, rather than A−B+ per se, seemed be the main driver of moderate-to-severe CDI in hospitals in eastern China. These results should be confirmed with more detailed quantitative clinical studies in which a multivariate analysis adjusted for confounding factors is conducted.
In conclusion, to our knowledge, this was the first large surveillance study in hospitalized patients in eastern China in which prevalence of and risk factors for CDI were determined. C. difficile clearly is present in hospitalized patients with diarrhea in eastern China. Significant risk factors for CDI in China appear to be advanced age (>55 years old), undergoing chemotherapy, undergoing abdominal surgery, previous hospitalization, exposure to antimicrobials, extended hospitalization (exceeding 1 week of cumulative hospitalization), and hospitalization in specific wards.
MATERIALS AND METHODS
Study design.
This cross-sectional study was conducted from June 2012 to September 2015, with two gaps from January 2013 to March 2013 and December 2014 to February 2015 due to the Chinese Spring Festival breaks. The study was conducted at the First Affiliated Hospital of Zhejiang University (FAHZU), which is a tertiary care academic medical center in eastern China with 2,500 beds in 30 wards, as well as at seven other hospitals belonging to the Hangzhou First People's Hospital Healthcare Facility Group (HFPH). These eight hospitals cover the entire Hangzhou area. The majority of hospitalized patients in these hospitals came from nine districts in Hangzhou and other cities in Zhejiang Province, while the rest of the patients were from the Shanghai city, Jiangsu, Jiangxi, Anhui, and Fujian provinces. Unformed stool specimens from selected patients with diarrheal illness during the study period were collected and transported to the Zhejiang Provincial Center for Disease Control and Prevention (ZJCDC) within 72 h for further testing (see below). To avoid overrepresentation, only the first stool specimen from each patient was included. This study was approved by the institutional review boards of the ZJCDC, FAHZU, and HFPH, and the informed consent requirement was waived.
Patient characteristics and definitions.
Hospitalized patients with diarrhea were included in this study. Parallel to stool collection, a standardized questionnaire was completed by clinicians for each patient to record patient gender, age, diagnosis-related disease, past hospitalization and duration, clinical data associated with CDI severity (e.g., abdominal pain, hypotension, shock, ileus, megacolon, leukocytosis, and creatinine levels), and risk factors (e.g., antimicrobial treatment within 8 weeks, undergoing abdominal surgery, and undergoing chemotherapy).
Diarrhea was defined as more than 3 loose, watery, or unformed stool passages within 24 h, based on the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America guidelines (27). A case of CDI was defined by a combination of clinical and laboratory results, which included the presence of diarrhea plus a positive stool test result for toxigenic C. difficile (culture and PCR) or clinical evidence of pseudomembranous colitis (27).
Clinical chart reviews were conducted by three independent investigators (Y.Z., P.Z., and W.F.) who were blinded to culture and genotype results. A CDI severity score was determined for each patient based on subjective interpretation of clinical manifestations, laboratory test findings, and attending physicians' clinical impression according to published guidelines (28, 29). Concordant scores from two or more reviewers of the results of toxigenic C. difficile culture, detection of C. difficile toxin genes, PCR ribotyping and multilocus sequence typing, and antimicrobial susceptibility testing were recorded. The severity of CDI in each patient was assigned one of six scores, 1 (no clinical CDI), 2 (mild), 3 (mild to moderate), 4 (moderate), 5 (moderate to severe), or 6 (severe) (28, 29).
Toxigenic C. difficile culture.
Prior to toxigenic C. difficile cultures, the stool samples were treated with alcohol, and the mixture was inoculated onto cefoxitin-cycloserine fructose agar plates prepared with a C. difficile agar base and selective supplement (Oxoid, Basingstoke, United Kingdom). After incubation for 48 h at 37°C in an anaerobic chamber with GENbag anaer (bioMérieux, Marcy l'Étoile, France), the isolates were confirmed to be C. difficile as described previously (10). All isolates were stored at −70°C in brain-heart infusion broth with 10% glycerol until subsequent analyses.
Detection of C. difficile toxin genes.
Bacterial genomic DNA was extracted using a QIAamp DNA blood minikit (Valencia, CA, USA) according to manufacturer instructions. The housekeeping gene tpi, toxin genes tcdA and tcdB, and binary toxin genes cdtA and cdtB were detected using previously described assays (25, 30). The tcdA primers amplified a 369-bp amplicon for toxin A-positive/B-positive (A+B+) strains and a 110-bp amplicon for toxin A-negative/B-positive (A−B+) strains (30). The standard C. difficile strains (ATCC 43255, 43598, BAA-1870, and BAA-1803) obtained from the American Type Culture Collection (Manassas, VA, USA) were used as positive controls for tcdA and tcdB and negative controls for the binary toxin genes. C. difficile ATCC BAA-1870 was used as a positive control for the binary toxin genes, and the C. difficile strains ATCC BAA-1801 and 700057 were chosen as negative controls for tcdA and tcdB and the binary toxin genes. The blank, positive, and negative controls were examined in parallel for each test. An analysis of partial tcdC deletions was performed as described elsewhere (31).
PCR ribotyping and multilocus sequence typing.
Six reference C. difficile strains (ATCC 43255, ATCC 43598, BAA-1870, BAA-1803, BAA-1801, and ATCC 700057) were used as controls. PCR ribotyping was performed by using PCR followed by capillary gel electrophoresis described previously (32). The 16S rRNA gene primers were labeled at the 5′ end with carboxyfluorescein. After PCR amplification, PCR fragments were analyzed using an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA) with a 36-cm capillary loaded with a POP4 gel (Applied Biosystems). The size of each peak was determined using GeneMapper ID-X 1.3 (Applied Biosystems). The data were deposited in the WEBRIBO database (https://webribo.ages.at/) for RT assignment. MLST was performed as described previously (25). In brief, seven loci (adk, atpA, dxr, glyA, recA, sodA, and tpi) were amplified by PCR. Amplicon sequences were determined by using a 3730 XL DNA analyzer (Applied Biosystems). Data for C. difficile alleles and STs were deposited in a public C. difficile MLST database (accessible at http://pubmlst.org/cdifficile).
Antimicrobial susceptibility testing.
C. difficile isolates were tested for susceptibility to fusidic acid, ciprofloxacin, piperacillin-tazobactam (PIP-TAX), metronidazole, rifampin, moxifloxacin, gatifloxacin, vancomycin, clindamycin, levofloxacin, tetracycline, and erythromycin by using Etest strips (AB Biodisk, Durham, NC, USA) and following the Clinical and Laboratory Standards Institute (CLSI) M100-S22 guideline recommendations published in 2012 (33). ATCC strains of Bacteroides fragilis (ATCC 25285) and C. difficile (ATCC 700057) were included in each run as controls. The interpretation of MIC results were done following the CLSI recommendations for PIP-TAZ, metronidazole, moxifloxacin, clindamycin, and tetracycline (33). The breakpoints for fusidic acid, vancomycin, rifampin, and erythromycin were determined according to a previous study (34, 35). For ciprofloxacin, gatifloxacin, and levofloxacin, breakpoints of ≤2 μg ml−1 (susceptible), 4 to 7 μg ml−1 (intermediate), and ≥8 μg ml−1 (resistant) were established on the basis of the CLSI guideline standard recommendations for C. difficile tested against trovafloxacin. A strain with resistance to at least three antimicrobial classes was defined as multidrug resistant (MDR) according to previously described standards (36).
Data analysis.
Data were analyzed using Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) version 16.0 and Epi Info version 3.5.1. Logistic regression analysis was used to identify independent risk factors. Kruskal-Wallis and χ2 tests were used to analyze correlations among STs, RTs, and antimicrobial susceptibility patterns of C. difficile strains. Kruskal-Wallis and linear trend χ2 tests were used to compare toxin gene patterns, STs, and RTs between CDI severity levels. Odds ratios (ORs), 95% confidence intervals (CIs), and P values were calculated to assess the differences between groups, and a P value of ≤0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant 81471998), the Key Research and Development Program of Zhejiang (grant 2015C03048), and the National Cancer Institute at the National Institutes of Health Support (grant P30 CA008748).
We thank Ruiting Lan, Kurt Reed, and Thomas Riley for critically reviewing the manuscript.
We declare no conflicts of interests.
REFERENCES
- 1.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 2.Bacci S, Molbak K, Kjeldsen MK, Olsen KE. 2011. Binary toxin and death after Clostridium difficile infection. Emerg Infect Dis 17:976–982. doi: 10.3201/eid/1706.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. 2012. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 4.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:2369–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KA, Ashwin H, Longshaw CM, Burns DA, Davis GL, Wilcox MH. 2016. Diversity of Clostridium difficile PCR ribotypes in Europe: results from the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID), 2012 and 2013. Euro Surveill 21:pii=30294 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22536. [DOI] [PubMed] [Google Scholar]
- 7.Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens M, Vaerenberg S, Hopkins S, Catry B, Monnet D, Goossens H, Suetens C. 2012. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill 17:pii=20316 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20316. [DOI] [PubMed] [Google Scholar]
- 8.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ. 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 9.Borgmann S, Kist M, Jakobiak T, Reil M, Scholz E, von Eichel-Streiber C, Gruber H, Brazier JS, Schulte B. 2008. Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro Surveill 13:pii=19057 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19057. [PubMed] [Google Scholar]
- 10.McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 11.Knight DR, Elliott B, Chang BJ, Perkins TT, Riley TV. 2015. Diversity and evolution in the genome of Clostridium difficile. Clin Microbiol Rev 28:721–741. doi: 10.1128/CMR.00127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. 2010. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins DA, Hawkey PM, Riley TV. 2013. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control 2:21. doi: 10.1186/2047-2994-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du P, Cao B, Wang J, Li W, Jia H, Zhang W, Lu J, Li Z, Yu H, Chen C, Cheng Y. 2014. Sequence variation in tcdA and tcdB of Clostridium difficile: ST37 with truncated tcdA is a potential epidemic strain in China. J Clin Microbiol 52:3264–3270. doi: 10.1128/JCM.03487-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Wu S, Chen R, Xu S, Fang H, Weintraub A, Nord CE. 2014. Risk factors of Clostridium difficile infections among patients in a university hospital in Shanghai, China. Anaerobe 30:65–69. doi: 10.1016/j.anaerobe.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Yan Q, Zhang J, Chen C, Zhou H, Du P, Cui Z, Cen R, Liu L, Li W, Cao B, Lu J, Cheng Y. 2013. Multilocus sequence typing (MLST) analysis of 104 Clostridium difficile strains isolated from China. Epidemiol Infect 141:195–199. doi: 10.1017/S0950268812000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye GY, Li N, Chen YB, Lv T, Shen P, Gu SL, Fang YH, Li LJ. 2016. Clostridium difficile carriage in healthy pregnant women in China. Anaerobe 37:54–57. doi: 10.1016/j.anaerobe.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Fang WJ, Jing DZ, Luo Y, Fu CY, Zhao P, Qian J, Tian BR, Chen XG, Zheng YL, Zheng Y, Deng J, Zou WH, Feng XR, Liu FL, Mou XZ, Zheng SS. 2014. Clostridium difficile carriage in hospitalized cancer patients: a prospective investigation in eastern China. BMC Infect Dis 14:523. doi: 10.1186/1471-2334-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu SL, Chen YB, Lv T, Zhang XW, Wei ZQ, Shen P, Li LJ. 2015. Risk factors, outcomes and epidemiology associated with Clostridium difficile infection in patients with haematological malignancies in a tertiary care hospital in China. J Med Microbiol 64:209–216. doi: 10.1099/jmm.0.000028. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JW, Xiao M, Kudinha T, Xu ZP, Hou X, Sun LY, Zhang L, Fan X, Kong F, Xu YC. 2016. The first two Clostridium difficile ribotype 027/ST1 isolates identified in Beijing, China—an emerging problem or a neglected threat? Sci Rep 6:18834. doi: 10.1038/srep18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han XH, Du CX, Zhang CL, Zheng CL, Wang L, Li D, Feng Y, DuPont HL, Jiang ZD, Shi YK. 2013. Clostridium difficile infection in hospitalized cancer patients in Beijing, China is facilitated by receipt of cancer chemotherapy. Anaerobe 24:82–84. doi: 10.1016/j.anaerobe.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Song Y, Yu X, Tao X, Yan J, Luo F, Zhang H, Zhang J, Li Q, He L, Li S, Meng F, Grundmann H. 2015. Factors associated with Staphylococcus aureus nasal carriage among healthy people in northern China. Clin Microbiol Infect 21:157–162. doi: 10.1016/j.cmi.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Wu S, Wang M, Zhang Y, Fang H, Palmgren AC, Weintraub A, Nord CE. 2009. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int J Antimicrob Agents 33:339–342. doi: 10.1016/j.ijantimicag.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Chong PM, Lynch T, McCorrister S, Kibsey P, Miller M, Gravel D, Westmacott GR, Mulvey MR, Canadian Nosocomial Infection Surveillance Program (CNISP). 2014. Proteomic analysis of a NAP1 Clostridium difficile clinical isolate resistant to metronidazole. PLoS One 9:e82622. doi: 10.1371/journal.pone.0082622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths D, Fawley W, Kachrimanidou M, Bowden R, Crook DW, Fung R, Golubchik T, Harding RM, Jeffery KJ, Jolley KA, Kirton R, Peto TE, Rees G, Stoesser N, Vaughan A, Walker AS, Young BC, Wilcox M, Dingle KE. 2010. Multilocus sequence typing of Clostridium difficile. J Clin Microbiol 48:770–778. doi: 10.1128/JCM.01796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YB, Gu SL, Wei ZQ, Shen P, Kong HS, Yang Q, Li LJ. 2014. Molecular epidemiology of Clostridium difficile in a tertiary hospital of China. J Med Microbiol 63:562–569. doi: 10.1099/jmm.0.068668-0. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 28.Huang B, Jin D, Zhang J, Sun JY, Wang X, Stiles J, Xu X, Kamboj M, Babady NE, Tang YW. 2014. Real-time cellular analysis coupled with a specimen enrichment accurately detects and quantifies Clostridium difficile toxins in stool. J Clin Microbiol 52:1105–1111. doi: 10.1128/JCM.02601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X, Tang YW. 2010. Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real-time cell analysis system. J Clin Microbiol 48:4129–4134. doi: 10.1128/JCM.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persson S, Torpdahl M, Olsen KE. 2008. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect 14:1057–1064. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 31.Curry SR, Marsh JW, Muto CA, O'Leary MM, Pasculle AW, Harrison LH. 2007. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol 45:215–221. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Indra A, Huhulescu S, Schneeweis M, Hasenberger P, Kernbichler S, Fiedler A, Wewalka G, Allerberger F, Kuijper EJ. 2008. Characterization of Clostridium difficile isolates using capillary gel electrophoresis-based PCR ribotyping. J Med Microbiol 57:1377–1382. doi: 10.1099/jmm.0.47714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. M100-S22. National Committee for Clinical and Laboratory Standards, Wayne, PA. [Google Scholar]
- 34.Bourgault AM, Lamothe F, Loo VG, Poirier L. 2006. In vitro susceptibility of Clostridium difficile clinical isolates from a multi-institutional outbreak in southern Quebec, Canada. Antimicrob Agents Chemother 50:3473–3475. doi: 10.1128/AAC.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutlu E, Wroe AJ, Sanchez-Hurtado K, Brazier JS, Poxton IR. 2007. Molecular characterization and antimicrobial susceptibility patterns of Clostridium difficile strains isolated from hospitals in south-east Scotland. J Med Microbiol 56:921–929. doi: 10.1099/jmm.0.47176-0. [DOI] [PubMed] [Google Scholar]
- 36.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]


