ABSTRACT
OXA-48 is the most prevalent carbapenemase in Enterobacteriaceae in Europe and the Middle East, but it is frequently missed because many isolates display low MICs for carbapenems. Furthermore, in contrast to metallo-β-lactamases or Klebsiella pneumoniae carbapenemases (KPC), no specific inhibitor is available for the phenotypic detection of OXA-48. Molecular detection of blaOXA-48 is the “gold standard” but is not available in many laboratories. A few phenotypic assays have been described but have not been independently evaluated. The aim of this study was the systematic comparison of phenotypic tests and an immunochromatographic assay (ICT) for the detection of OXA-48/OXA-48-like carbapenemases and the development of an algorithm for reliable phenotypic detection of OXA-48. Four phenotypic tests (temocillin disk test, faropenem disk test, OXA-48 disk test, and high-inoculum [HI] OXA-48 disk test) and a new ICT (OXA-48 K-SeT) were compared by using a set of 166 Enterobacteriaceae isolates, including isolates producing OXA-48/OXA-48-like carbapenemases (n = 84) or Ambler class A and B carbapenemases (n = 41) and carbapenemase-negative isolates (n = 41). The sensitivity and specificity for the different assays were 100% and 43.9% for temocillin, 57.1% and 98.8% for faropenem, 53.6% and 100% for the OXA-48 disk test, 98.8% and 97.6% for the HI OXA-48 disk test, and 100% and 100% for the ICT, respectively. The ICT displayed the highest sensitivity and specificity and was the most rapid assay, but it is more costly than phenotypic assays. Based on these results, a new algorithm incorporating temocillin, faropenem, and ICT which allows cost-effective detection of OXA-48 with 100% sensitivity and specificity was developed.
KEYWORDS: OXA-48, OXA-48 K-SeT, carbapenemase, faropenem, temocillin
INTRODUCTION
OXA-48 is an Ambler class D carbapenem-hydrolyzing oxacillinase and now the most prevalent carbapenemase in Europe and the Middle East (1, 2). It mediates resistance to penicillins and reduced susceptibility to carbapenems but spares oxyimino-cephalosporins (3). However, most clinical isolates are resistant to all β-lactams because they coexpress extended-spectrum β-lactamase (ESBL) (4). Numerous variants of OXA-48, frequently referred to as OXA-48-like carbapenemases, have been described which differ from OXA-48 by several amino acids and have different hydrolytic activities, e.g., OXA-162, OXA-163, OXA-181, and OXA-204 (5).
The phenotypic detection of OXA-48 is difficult, as there is a considerable variation of MICs for carbapenems, ranging from <0.5 mg/liter to >32 mg/liter. Usually, the highest MICs are recorded for ertapenem, with meropenem and/or imipenem MICs that are lower and frequently even in the susceptible range (≤1 mg/liter [CLSI] and ≤2 mg/liter [EUCAST] [6, 7]). With automated systems or manual susceptibility testing, OXA-48-like carbapenemases can be easily missed, especially if ertapenem is not routinely tested and if isolates do not coexpress ESBLs. In contrast to metallo-β-lactamases (MBLs) or K. pneumoniae carbapenemases (KPC), there are currently no specific inhibitors which can be used for the phenotypic detection of OXA-48-like carbapenemases.
PCR is the “gold standard” for the detection of blaOXA-48-like, but it is costly and usually available only in larger laboratories or academic institutions (8). Several phenotypic assays have been described, but they have not been systematically compared in larger studies. An inhibition zone of <11 mm around a temocillin 30-μg disk has been reported to be a highly sensitive marker of OXA-48-producing Enterobacteriaceae (9). Day et al. described the faropenem disk test, which showed a double inhibition zone in 76.2% of all OXA-48 producers (10). Another phenotypic assay is the OXA-48 disk test, which has been reported to be highly sensitive and specific for OXA-48 producers (11). Recently, a highly sensitive and specific immunochromatographic lateral flow test (ICT) which detects two epitopes specific to OXA-48 and OXA-48-like carbapenemases has been described (12).
In order to systematically assess the available nonmolecular assays for OXA-48 detection, four phenotypic tests (temocillin inhibition zone, OXA-48 disk test, high-inoculum [HI] OXA-48 disk test, and faropenem disk test) and the ICT were compared on a set of carbapenemase-producing and carbapenemase-negative Enterobacteriaceae isolates.
RESULTS AND DISCUSSION
A total of 166 Enterobacteriaceae isolates were tested for the presence of OXA-48-like carbapenemase using four different phenotypic assays and the ICT. Of these isolates, 84 were positive for an OXA-48-like carbapenemase, 41 were positive for other carbapenemases, and 41 were carbapenemase negative (Table 1). Among blaOXA-48-like genes, blaOXA-48 was most frequent (n = 59), followed by blaOXA-181 (n = 8), blaOXA-162 (n = 7), blaOXA-232 (n = 7), blaOXA-244 (n = 2), and blaOXA-204 (n = 1). The 41 isolates with other carbapenemases were positive for either KPC (n = 14) or MBLs (n = 27) (Table 1; see also Tables S1 and S2 in the supplemental material). By PCR and CARBA-NP test, 41 isolates were negative for carbapenemases. Elevated carbapenem MICs in these isolates were most commonly caused by ESBL and/or AmpC production and/or decreased permeability (Table S3).
TABLE 1.
Results of the phenotypic tests and the immunochromatographic OXA-48 testa
| Carbapenemase | No. with indicated temocillin disk test result |
No. with indicated faropenem disk test result |
No. with indicated OXA-48 disk test result |
No. with indicated HI OXA-48 disk test result |
No. with indicated ICT result |
MIC range (mg/liter) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | N | P | N | P | N | P | N | P | N | ETP | IPM | MEM | DOR | |
| Ambler class D (n = 84) | ||||||||||||||
| OXA-48 (n = 59) | 59 | 45 | 14 | 35 | 24 | 59 | 59 | 0.5 to >32 | 0.5 to >32 | 0.25 to >32 | 0.25 to >32 | |||
| OXA-162 (n = 7) | 7 | 7 | 5 | 2 | 7 | 7 | 2 to >32 | 1 to 8 | 0.5 to >32 | 0.25 to >32 | ||||
| OXA-181 (n = 8) | 8 | 2 | 6 | 3 | 5 | 8 | 8 | 1 to >32 | 0.25 to >32 | 0.25 to >32 | 0.25 to >32 | |||
| OXA-204 (n = 1) | 1 | 1 | 1 | 1 | 1 | >32 | 8 | >32 | >32 | |||||
| OXA-232 (n = 7) | 7 | 7 | 1 | 6 | 7 | 7 | 2 to >32 | 0.5 to 32 | 0.25 to >32 | 0.25 to >32 | ||||
| OXA-244 (n = 2) | 2 | 2 | 2 | 1 | 1 | 2 | 2 to >32 | 0.5 to 32 | 0.25 to >32 | 0.12 to >32 | ||||
| Total (%) | 84 (100) | 0 (0) | 48 (57.1) | 36 (42.9) | 45 (53.6) | 39 (46.4) | 83 (98.8) | 1 (1.2) | 84 (100) | 0 (0) | ||||
| Ambler class A (n = 14) | ||||||||||||||
| KPC-2 (n = 13) | 8 | 5 | 0 | 13 | 0 | 13 | 2 | 11 | 0 | 13 | 16 to >32 | >32 | >32 | 8 to >32 |
| KPC-3 (n = 1) | 1 | 1 | 1 | 1 | 1 | >32 | >32 | >32 | >32 | |||||
| Total (%) | 9 (64.3) | 5 (35.7) | 0 (0) | 14 (100) | 0 (0) | 14 (100) | 2 (14.3) | 12 (85.7) | 0 (0) | 14 (100) | 16 to >32 | >32 | >32 | 8 to >32 |
| Ambler class B (n = 27) | ||||||||||||||
| GIM-1 (n = 2) | 2 | 2 | 2 | 2 | 2 | >32 | >32 | >32 | >32 | |||||
| NDM-1 (n = 4) | 4 | 4 | 4 | 4 | 4 | >32 | 8 to >32 | >32 | >32 | |||||
| NDM-7 (n = 1) | 1 | 1 | 1 | 1 | 1 | >32 | >32 | >32 | >32 | |||||
| VIM-1 (n = 16) | 15 | 1 | 0 | 16 | 0 | 16 | 0 | 16 | 0 | 16 | 0.5 to >32 | 1 to >32 | 0.25 to >32 | 0.12 to >32 |
| VIM-26 (n = 2) | 2 | 2 | 2 | 2 | 2 | >32 | >32 | >32 | >32 | |||||
| VIM-27 (n = 1) | 1 | 1 | 1 | 1 | 1 | >32 | >32 | >32 | >32 | |||||
| VIM-29 (n = 1) | 1 | 1 | 1 | 1 | 1 | >32 | >32 | >32 | >32 | |||||
| Total (%) | 26 (96.3) | 1 (3.7) | 0 (0) | 27 (100) | 0 (0) | 27 (100) | 0 (0) | 27 (100) | 0 (0) | 27 (100) | 0.25 to >32 | 1 to >32 | 0.25 to >32 | 0.12 to >32 |
| No carbapenemases (n = 41) | ||||||||||||||
| Total (%) | 11 (26.8) | 30 (73.2) | 1 (2.4) | 40 (97.6) | 0 (0) | 41 (100) | 0 (0) | 41 (100) | 0 (0) | 41 (100) | 0.12 to >32 | 0.25 to >32 | 0.016 to >32 | 0.016 to >32 |
HI OXA-48 disk test, high-inoculum OXA-48 disk test; P, positive; N, negative; ETP, ertapenem; IPM, imipenem; MEM, meropenem; DOR, doripenem.
Temocillin disk test.
All isolates producing OXA-48-like carbapenemases (84/84) had a temocillin inhibition zone of 6 mm, which was well below the recommended cutoff of 11 mm. However, most isolates with MBLs (26/27) or KPC (9/14) and 11/41 isolates without carbapenemase had inhibition zones below the cutoff, resulting in a poor specificity, 43.9%.
An inhibition zone of <11 mm for temocillin is often used as a marker and is recommended by EUCAST as an indirect confirmation test in case combination testing with EDTA or PBA is negative (8). In the present study, it was an excellent marker for OXA-48 production, with a sensitivity of 100% across all OXA-48 variants. A diameter of temocillin of ≥11 mm effectively ruled out OXA-48 production, but the poor specificity precludes its use as a single test, as observed in other studies (13, 14).
Faropenem disk test.
Using the faropenem disk test, 48/84 isolates producing OXA-48 or OXA-48-like carbapenemases showed a double inhibition zone (Fig. S1c), resulting in an overall sensitivity of 57.1%. Marked differences between the different OXA-48 variants were recorded: 45/59 OXA-48-producing isolates were positive (sensitivity, 76.3%), but only 3/25 isolates producing OXA-48 variants were positive (sensitivity, 12.0%; P < 0.0001). No isolate producing OXA-162 (0/7), OXA-232 (0/7), or OXA-244 (0/2) was positive, but 1/1 OXA-204-producing isolate and 2/8 OXA-181-producing isolates tested positive. The sensitivity was higher in ESBL-negative isolates (8/12 producing OXA-48 or OXA-48-like carbapenemases) than in ESBL-harboring isolates (40/72 producing OXA-48 or OXA-48-like carbapenemases), but differences were not statistically significant. No correlation of false-negative results with the species or coproduction of CTX-M, SHV, or TEM β-lactamases was observed. A false-positive result was observed in a single Serratia marcescens isolate with an AmpC β-lactamase (specificity 98.8%).
Day et al. reported a double inhibition zone in 16/21 OXA-48-producing isolates (sensitivity 76.2%) and a specificity of 98.0% (201/205) (10). Only a few OXA-48-producing isolates and no producers of OXA-48 variants were tested, which likely explains the overall higher sensitivity observed in the aforementioned study.
In our study, all isolates with other carbapenemases (27/27 with MBL and 14/14 with KPC) showed an inhibition zone of 6 mm, compared to only 4/41 carbapenemase-negative isolates and 30/84 isolates with OXA-48-like carbapenemases (P < 0.0001). A faropenem inhibition zone of 6 mm can therefore be used as a highly sensitive screening test for KPC or MBL, confirming results of previous studies (10, 15).
OXA-48 disk test.
The OXA-48 disk test was positive for only 45/84 isolates producing OXA-48-like carbapenemases (sensitivity, 53.6%) when the test was performed as previously published, compared to 83/84 isolates (sensitivity 98.8%) with the HI OXA-48 disk test. One isolate producing OXA-244 tested negative (Table 1 and Table S1), and one false-positive test result was obtained with a K. pneumoniae isolate with a KPC-2 and the HI OXA-48 test.
Overall, the OXA-48 disk test demonstrated a modest sensitivity and is cumbersome, difficult to standardize, and highly dependent on the inoculum used; we think it is therefore less suitable for diagnostic laboratories. With the high-inoculum variation (HI OXA-48 disk test), the sensitivity can be improved to 98.8%, which is similar to the sensitivity of 96.3% reported in the original publication (11). In that study, 95.1% of isolates produced OXA-48 and only four isolates produced OXA-48 variants. No isolates with OXA-244 were tested in the original study; this was the only variant which remained negative in our setting, even when a high inoculum was used. Similar to our observation with a KPC-2-producing isolate, false-positive results in KPC-producing isolates have been reported with this assay (11).
OXA-48 ICT.
The ICT was positive for all isolates producing OXA-48 and OXA-48-like carbapenemases and no false-positives were recorded, resulting in a sensitivity and specificity of 100%. The test was equally sensitive and specific regardless of the species or coproduction of any β-lactamase. It worked equally well for producers of OXA-48 and OXA-48-like carbapenemases and was the most rapid of all tests, yielding results after 15 min. It is a robust test: in all cases the correct result was obtained on the first attempt, and no test had to be repeated. In contrast to the other tests, the OXA-48 protein is detected directly by immunological capture of OXA-48 specific epitopes in the ICT. It is therefore less influenced by the coexpression of ESBLs or other β-lactamases, which can render phenotypic tests difficult to interpret.
Our results are in accordance with those of other studies, which also demonstrated sensitivity and specificity of 100% with this assay (12, 16–18). Recently, a new version of the ICT has been reported which includes the OXA-48 and the OXA-163 subfamilies (19), which are not detected by the current assay.
Overall, the ICT is an excellent test in areas with a high prevalence of OXA-48-like carbapenemases or in an outbreak situation, where rapid results are indispensable. However, the ICT is more costly ($9.50/test in Germany; $1,577.00 for the 166 isolates analyzed in this study) than simple disk tests and might therefore not be an ideal test in areas with a high prevalence of other carbapenemases, e.g., MBL or KPC.
In this setting, inhibitor-based tests (e.g., meropenem with PBA or EDTA) in combination with a temocillin disk are frequently recommended for routine identification of isolates suspected of producing carbapenemase (8, 9). Given the low specificity of decreased temocillin diameters, it is mandatory to confirm OXA-48 by PCR (8). The inclusion of both temocillin (highly sensitive) and faropenem (highly specific) in the panel with meropenem/inhibitor disks could be a useful and less costly alternative for improved detection of OXA-48 producers (∼$0.25/test) (Fig. 1). In the first step of the algorithm, the temocillin inhibiton zone is read and in isolates with a temocillin inhibition zone of ≥11 mm, OXA-48-like carbapenemases can be definitely ruled out. Isolates with a temocillin inhibition zone of <11 mm are regarded as potential producers of OXA-48-like carbapenemases. In the second step, the faropenem result is read and isolates with a double inhibition zone are definitely identified as OXA-48 without the need for further testing (48/84 producers of OXA-48-like carbapenemases were detected at this point, resulting in a sensitivity of 57.1% and specificity of 100% for combined temocillin and faropenem). In the third step, isolates with a temocillin inhibition zone of <11 mm and no faropenem double inhibition zone are then subjected to the ICT, which detects all remaining OXA-48-like isolates. Using this algorithm, all isolates producing OXA-48-like carbapenemases would have been correctly identified with a sensitivity and specificity of 100% (cost, $820.50 for all 166 isolates tested in this study).
FIG 1.
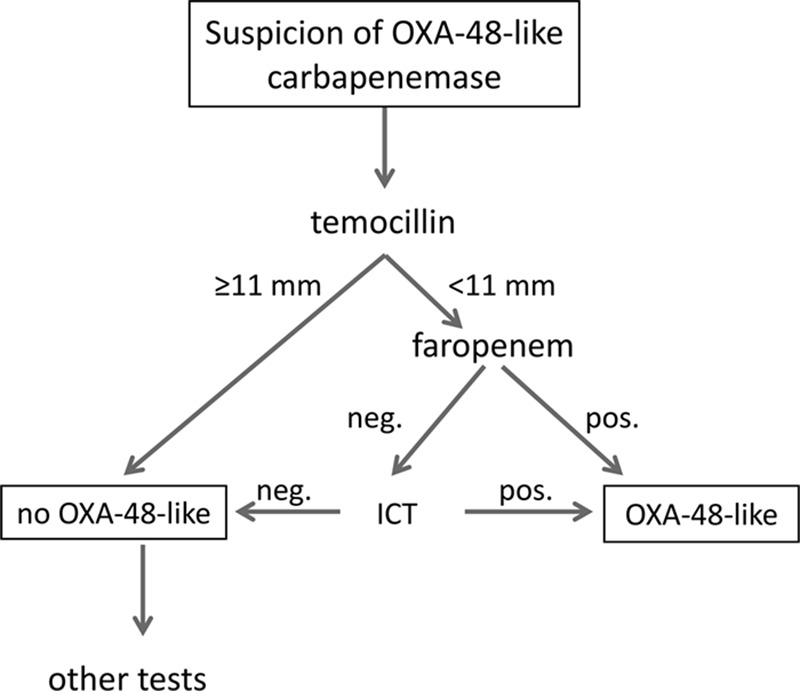
Proposed algorithm for the phenotypic detection of OXA-48-like carbapenemases. A temocillin and faropenem disk test is carried out simultaneously in all isolates suspected of OXA-48 production (e.g., resistant to piperacillin-tazobactam; susceptible, intermediate, or resistant to 3rd-generation cephalosporins; elevated MICs for ertapenem). When a temocillin inhibition zone of ≥11 mm is recorded, OXA-48 can be definitely ruled out. In isolates with a temocillin inhibition zone of <11 mm and a positive faropenem test, production of an OXA-48-like carbapenemase is confirmed without the need for further testing. In the remaining cases, the ICT is carried out to confirm or exclude OXA-48.
The proposed algorithm can be used in laboratories where PCR is not available, in addition to PCR, e.g., when PCR for carbapenemases is not performed daily or in cases where the PCR result is inconclusive. A limitation of this approach is the longer duration, 18 to 20 h, due to incubation and that the algorithm was validated only on preselected isolates and not prospectively.
This is the first study to systematically compare different phenotypic methods and an immunochromatographic assay for the detection of OXA-48. The study encompasses well-characterized clinical isolates from different centers with different OXA-48 variants and coexpression of different β-lactamases.
The detection of OXA-48 remains unsatisfactory with phenotypic tests alone but can be improved by the use of the proposed algorithm incorporating temocillin and faropenem disk tests in combination with the ICT. The algorithm could substantially improve the detection of OXA-48 producers in laboratories without access to molecular methods for carbapenemase detection at low additional costs.
MATERIALS AND METHODS
Clinical isolates.
A total of 166 isolates were included in the analysis (165 of clinical origin and a quality control strain); 115 isolates were from three large German tertiary care hospitals (University Hospital Cologne, University Hospital Frankfurt, and University Hospital Essen) and 50 from the National Reference Centre for Nosocomial Pathogens, Bochum, Germany.
All isolates were tested for the presence of OXA-48-like carbapenemases with a total of four different phenotypic tests (temocillin disk test, faropenem disk test, OXA-48 disk test, and HI OXA-48 disk test) and the ICT. Results were compared to those obtained by PCR and subsequent Sanger sequencing as the gold standard.
Molecular and phenotypic detection of beta-lactamases.
All strains were analyzed for the presence of the carbapenemase genes blaOXA-48-like, blaVIM, blaIMP, blaNDM, blaGIM, and blaKPC and of the ESBL genes blaCTX-M, blaSHV, and blaTEM by PCR and subsequent DNA Sanger sequencing (3, 20–22). Phenotypic detection of ESBL production was performed with the CLSI combination disk test (6, 8). MICs of ertapenem, meropenem, imipenem, and doripenem were determined using MIC test strips (Liofilchem, Roseto degli Abruzzi, Italy). For phenotypic identification of metallo-β-lactamases, KPC, or AmpC, a combination disk test was used with meropenem and one of the following β-lactamase inhibitors: EDTA, dipicolinic acid, cloxacillin, and boronic acid (Liofilchem). Results were interpreted according to the manufacturer's recommendations.
Furthermore, all isolates which were carbapenemase negative in the inhibitor-based assay and by PCR were additionally tested for carbapenemase production using the Carba NP test (23).
Temocillin and faropenem disk test.
A 30-μg temocillin disk (MAST Diagnostica, Reinfeld, Germany) and a 10-μg faropenem disk (carbapenemase activity test [CAT]; MAST Diagnostica) were placed on a Mueller-Hinton plate inoculated with a fresh bacterial suspension equivalent to a 0.5 McFarland standard. Plates were incubated for 16 to 20 h under aerobic conditions at 35 ± 2°C. An inhibition zone diameter of <11 mm for temocillin was considered positive for OXA-48, as recommended by EUCAST (Fig. S1a) (8). The faropenem test was interpreted according to the manufacturer's recommendation as follows: zone of inhibition present, no carbapenemase; no zone of inhibition, MBL or KPC); or single colonies in the zone of inhibition/double zone of inhibition, OXA-48 (Fig. S1c and d).
OXA-48 disk test.
The OXA-48 disk test was carried out as previously described (11). Briefly, a Mueller-Hinton plate was inoculated with a suspension of Escherichia coli ATCC 25922 equivalent to a 0.5 McFarland standard. A blank disk was prepared with either 10 μl of 0.1 M EDTA alone or 10 μl of 60 μg/μl of PBA (both Sigma-Aldrich, Steinheim, Germany). Adjacent to a 10-μg imipenem disk, blank disks with EDTA and with EDTA-PBA were placed on the agar, after they had been inoculated with 2 or 3 colonies of the isolate to be tested. Results were read after an incubation of 16 to 20 h at 35 ± 2°C. An indentation of growth of E. coli ATCC 25922 toward the imipenem disk at both sides was interpreted as a positive result (Fig. S1e). However, the OXA-48 disk test was positive for only 45/84 isolates with OXA-48-like carbapenemases (sensitivity, 53.6%) when the test was performed as previously published. After using several variations of the test conditions, we noted that increasing the inoculum from 2 or 3 colonies to 5 or 6 colonies improved the performance of the test significantly. The test was carried out on all isolates as previously published and with a high inoculum (here referred to as the HI OXA-48 disk test).
OXA-48 ICT (OXA-48 K-SeT).
The OXA-48 immunochromatographic test (ICT) (OXA-48 K-SeT; Coris Bioconcept, Gembloux, Belgium) was performed according to the manufacturer's recommendations. The result was recorded after 15 min, with a single upper line indicating a valid test (control, OXA-48-like negative). The presence of two lines indicated a positive test for OXA-48/OXA-48-like carbapenemases.
Statistical analysis.
The chi-square test with Yates correction or the two-sided Fisher's exact test was used where appropriate. P values of <0.05 were considered statistically significant.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01929-16.
REFERENCES
- 1.Cantón R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 2.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirel L, Heritier C, Tolun V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamprecht A, Göttig S. 2014. Treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Curr Treat Options Infect Dis 6:425–438. doi: 10.1007/s40506-014-0029-x. [DOI] [Google Scholar]
- 5.Oueslati S, Nordmann P, Poirel L. 2015. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother 70:1059–1063. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.xls.
- 8.Giske CG, Martinez-Martinez L, Canton R, Stefani S, Skov R, Glupczynski Y, Nordmann P, Wootton M, Miriagou V, Skov Simonsen G, Zemlickova H, Cohen-Stuart J, Gniadkowski M. 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 1.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf.
- 9.van Dijk K, Voets GM, Scharringa J, Voskuil S, Fluit AC, Rottier WC, Leverstein-Van Hall MA, Cohen Stuart JW. 2014. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin Microbiol Infect 20:345–349. doi: 10.1111/1469-0691.12322. [DOI] [PubMed] [Google Scholar]
- 10.Day KM, Pike R, Winstanley TG, Lanyon C, Cummings SP, Raza MW, Woodford N, Perry JD. 2013. Use of faropenem as an indicator of carbapenemase activity in the Enterobacteriaceae. J Clin Microbiol 51:1881–1886. doi: 10.1128/JCM.00720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsakris A, Poulou A, Bogaerts P, Dimitroulia E, Pournaras S, Glupczynski Y. 2015. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J Clin Microbiol 53:1245–1251. doi: 10.1128/JCM.03318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wareham DW, Shah R, Betts JW, Phee LM, Momin MHFA. 2016. Evaluation of an immunochromatographic lateral flow assay (OXA-48 K-SeT) for rapid detection of OXA-48-like carbapenemases in Enterobacteriaceae. J Clin Microbiol 54:471–473. doi: 10.1128/JCM.02900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang TD, Poirel L, Bogaerts P, Berhin C, Nordmann P, Glupczynski Y. 2014. Temocillin and piperacillin/tazobactam resistance by disc diffusion as antimicrobial surrogate markers for the detection of carbapenemase-producing Enterobacteriaceae in geographical areas with a high prevalence of OXA-48 producers. J Antimicrob Chemother 69:445–450. doi: 10.1093/jac/dkt367. [DOI] [PubMed] [Google Scholar]
- 14.Woodford N, Pike R, Meunier D, Loy R, Hill R, Hopkins KL. 2014. In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp and Enterobacter spp, and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. J Antimicrob Chemother 69:564–567. doi: 10.1093/jac/dkt383. [DOI] [PubMed] [Google Scholar]
- 15.Hu F, Ahn C, O'Hara JA, Doi Y. 2014. Faropenem disks for screening of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 52:3501–3502. doi: 10.1128/JCM.02837-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dortet L, Jousset A, Sainte-Rose V, Cuzon G, Naas T. 10 March 2016. Prospective evaluation of the OXA-48 K-SeT assay, an immunochromatographic test for the rapid detection of OXA-48-type carbapenemases. J Antimicrob Chemother doi: 10.1093/jac/dkw058. [DOI] [PubMed] [Google Scholar]
- 17.Meunier D, Vickers A, Pike R, Hill RL, Woodford N, Hopkins KL. 2016. Evaluation of the K-SeT R.E.S.I.S.T. immunochromatographic assay for the rapid detection of KPC and OXA-48-like carbapenemases. J Antimicrob Chemother 71:2357–2359. doi: 10.1093/jac/dkw113. [DOI] [PubMed] [Google Scholar]
- 18.Glupczynski Y, Evrard S, Ote I, Mertens P, Huang T-D, Leclipteux T, Bogaerts P. 2016. Evaluation of two new commercial immunochromatographic assays for the rapid detection of OXA-48 and KPC carbapenemases from cultured bacteria. J Antimicrob Chemother 71:1217–1222. doi: 10.1093/jac/dkv472. [DOI] [PubMed] [Google Scholar]
- 19.Pasteran F, Denorme L, Ote I, Gomez S, De Belder D, Glupczynski Y, Bogaerts P, Ghiglione B, Power P, Mertens P, Corso A. 17 August 2016. Rapid identification of OXA-48 and OXA-163 subfamily in carbapenem resistant gram-negative bacilli with a novel immunochromatographic lateral flow assay. J Clin Microbiol doi: 10.1128/jcm.01175-16. [DOI] [PMC free article] [PubMed]
- 20.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 50:3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamprecht A, Poirel L, Gottig S, Seifert H, Kaase M, Nordmann P. 2013. Detection of the carbapenemase GIM-1 in Enterobacter cloacae in Germany. J Antimicrob Chemother 68:558–561. doi: 10.1093/jac/dks447. [DOI] [PubMed] [Google Scholar]
- 22.Hamprecht A, Rohde AM, Behnke M, Feihl S, Gastmeier P, Gebhardt F, Kern WV, Knobloch JK, Mischnik A, Obermann B, Querbach C, Peter S, Schneider C, Schroder W, Schwab F, Tacconelli E, Wiese-Posselt M, Wille T, Willmann M, Seifert H, Zweigner J, DZIF-ATHOS Study Group. 2016. Colonization with third-generation cephalosporin-resistant Enterobacteriaceae on hospital admission: prevalence and risk factors. J Antimicrob Chemother 71:2957–2963. doi: 10.1093/jac/dkw216. [DOI] [PubMed] [Google Scholar]
- 23.Dortet L, Poirel L, Nordmann P. 2012. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 56:6437–6440. doi: 10.1128/AAC.01395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


