ABSTRACT
The diagnosis of Mycobacterium avium complex pulmonary disease (MAC-PD) is sometimes complicated and time-consuming. A serodiagnostic kit that measures the serum levels of IgA antibodies against the glycopeptidolipid (GPL) core is commercially available and has good diagnostic accuracy for MAC-PD. However, the significance of measurement of GPL core IgA antibody levels in monitoring for chemotherapy response in patients with MAC-PD was not well investigated. Thirty-four treatment naive MAC-PD patients who were started on multidrug chemotherapy were enrolled. Their antibody levels were prospectively measured at regular intervals. The relationships between their antibody levels and the therapeutic outcomes were examined. The patients were classified into three groups (conversion, recurrence, and nonconversion) based on the bacteriological outcomes after chemotherapy. There were no significant differences in the antibody levels before treatment between the culture conversion (n = 19), recurrence (n = 7), and nonconversion (n = 8) groups (P = 0.9881). The levels decreased significantly after the chemotherapy (P < 0.0001). Recurrence and/or worsening of chest radiography findings were observed in cases whose antibody levels subsequently increased after cessation of the chemotherapy. No significant difference in the percent decrease in antibody levels by the chemotherapy was observed between the culture conversion and recurrence groups (P = 0.9338). The initial antibody levels are not a predictor of therapeutic outcomes, and also the percent decrease in antibody levels is not a sufficient indicator of the cessation of chemotherapy. However, serial measurements of antibody levels may allow objective monitoring of disease activity in individual MAC-PD patients.
KEYWORDS: enzyme immunoassay, nontuberculous mycobacteria, serodiagnosis
INTRODUCTION
The prevalence of nontuberculous mycobacterial pulmonary disease is increasing in Japan and worldwide (1, 2), and the importance of proper diagnosis and management of the disease has been recently recognized. Mycobacterium avium complex (MAC) is the most common and important causative agent of pulmonary disease among nontuberculous mycobacteria (3). A bacterial culture positive for MAC is insufficient for diagnosis because MAC is ubiquitous in the natural environment and can easily contaminate respiratory specimens. The diagnosis of MAC pulmonary disease (MAC-PD), therefore, is usually made using the diagnostic criteria advocated by the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) (4). Although the criteria have been revised and simplified, their application remains complicated and time-consuming in clinical practice.
We have developed a serodiagnosis kit specific for MAC disease (5, 6), and it has been commercially available since 2011 in Japan. This enzyme immunoassay measures the serum levels of IgA antibodies against the glycopeptidolipid (GPL) core that is a major cell wall antigen in MAC, but not in M. tuberculosis complex or M. kansasii. The serodiagnostic kit has been reported to be highly specific and sensitive (6–16). A meta-analysis revealed that the summary estimates of sensitivity and specificity were 69.6% (95% confidence interval [CI] = 62.1 to 76.1) and 90.6% (95% CI = 83.6 to 0.95.1) with a cutoff value of 0.7 U/ml, respectively (17). The positive and negative likelihood ratios were 7.4 (95% CI = 4.1 to 13.8) and 0.34 (95% CI = 0.26 to 0.43), respectively. The serodiagnostic kit had good diagnostic accuracy and was useful for ruling in MAC-PD with the cutoff value. Together with the current guidelines of the ATS/IDSA, the serodiagnosis may allow more rapid, simple, and accurate diagnosis of MAC-PD.
The current chemotherapy options for MAC-PD are not sufficiently strong to eliminate mycobacteria from the lungs. Although the initial sputum culture conversion rate achieved by multidrug chemotherapy is high, the recurrence rate is high as well (18–20). Moreover, we cannot predict the efficacy of chemotherapy in advance. Currently, the goal of the therapy is not to cure but rather to control the disease progression in order to slow or prevent the development of respiratory failure. Therefore, an objective marker is important to manage the disease effectively.
Several studies have shown that the serum antibody levels reflect the disease activity to some extent (21). Other studies found a weak correlation between the GPL core antibody levels and the extent of the disease on chest computed tomography (CT) (9, 22). In addition, the antibody levels were found to be decreased after surgical resection in patients with MAC pulmonary disease (21). However, it is unclear whether the pretreatment levels of IgA antibody toward GPL core components are a predictor of treatment outcome or whether they are a useful marker for monitoring the chemotherapy response and deciding the timing of cessation of chemotherapy in patients with MAC-PD. To clarify these points, we conducted a prospective cohort study in patients who received initial chemotherapy for MAC-PD.
RESULTS
Enrollment of study subjects and their microbiological outcomes.
The initial enrollment consisted of 39 treatment naive patients who had been started on multidrug chemotherapy. Five patients were excluded: three because of adverse reactions to the therapy, and two because of missed follow-ups. Finally, 34 patients were investigated. The mean observation period and duration of multidrug chemotherapy were 4.6 ± 1.2 and 1.8 ± 1.1 years, respectively. The sputum culture conversion rate was 76.5% (26 of 34), and the recurrence rate was 26.9% (7 of the 26 with negative results). At the end of the observation period, 19 patients were included in the sputum culture conversion group, 7 in the recurrence group, and 8 in the nonconversion group. The chemotherapy drugs, duration, and outcomes are shown in Table 1. There were no significant differences in the dosage of clarithromycin or the number of drugs among the patient groups. Four patients died during the study period; 3 of them belonged to the nonconversion group, who died of respiratory failure, and 1 belonged to the culture conversion group, who died because of exacerbation of complicated interstitial pneumonia.
TABLE 1.
Chemotherapy drugs, duration, and outcomes in the study patientsa
| Parameter | Mean ± SD |
Pb | |||
|---|---|---|---|---|---|
| Total (n = 34) | Culture conversion (n = 19) | Recurrence (n = 7) | Nonconversion (n = 8) | ||
| No. of drugs | 3.6 ± 0.6 | 3.5 ± 0.6 | 3.7 ± 0.5 | 3.4 ± 0.7 | 0.6165* |
| Drug combinations (no. of patients tested) | |||||
| RIF+EB+CAM | 13 | 6 | 3 | 4 | |
| RIF+EB+CAM+FQ | 6 | 5 | 0 | 1 | |
| RIF+EB+CAM+AMG | 8 | 5 | 2 | 1 | |
| RIF+CAM+FQ+AMG | 4 | 2 | 2 | 0 | |
| RIF+CAM+FQ | 3 | 1 | 0 | 2 | |
| Dose of clarithromycin (mg/day) | 535.3 ± 127.6 | 557.9 ± 126.1 | 542.9 ± 97.6 | 475 ± 148.8 | 0.4219* |
| Use of aminoglycoside (no. of subjects) | 12 | 7 | 4 | 1 | 0.1917‡ |
| Treatment period (yrs) | 1.8 ± 1.1 | 1.6 ± 0.2 | 2.3 ± 0.4 | 2.2 ± 0.4 | 0.1795* |
| Observation period (yrs) | 4.6 ± 1.2 | 4.5 ± 1.1 | 4.9 ± 0.9 | 4.3 ± 1.6 | 0.5358* |
| Time to culture conversion (yrs) | 0.4 ± 0.8 | 0.4 ± 0.2 | 0.3 ± 0.3 | NA | 0.7766† |
| Time to recurrence (yrs) | 2.0 ± 0.6 | NA | 2.0 ± 0.6 | NA | NA |
Data are presented as means ± the SD except as noted otherwise in column 1. NA, not applicable; RIF, rifampin; EB, ethambutol; CAM, clarithromycin; FQ, fluoroquinolones (levofloxacin [n = 7], moxifloxacin [n = 5], sitafloxacin [n = 1]); AMG, aminoglycoside (amikacin [n = 4], streptomycin [n = 8]).
P values were calculated using either the Kruskal-Wallis test (*) or the Mann-Whitney U test (†) for continuous values or the Fisher exact test (‡) for categorical variables.
Characteristics of study subjects.
The baseline characteristics of the study subjects are shown in Table 2. There were no significant differences in age, sex, body mass index, drinking habits, previous lung disease, disease type, extent of disease on chest CT, serum albumin, total cholesterol, erythrocyte sedimentation rate, and C-reactive protein among the three patient groups. The proportion of ex-smokers was significantly higher in the recurrence and nonconversion groups than in the culture conversion group (P = 0.0439). Patients infected with M. intracellulare showed a significant increase in the rate of culture conversion (57.9%) and nonconversion (66.7%) compared to M. avium (conversion, 42.1%; nonconversion, 33.3% [P = 0.0378]).
TABLE 2.
Clinical characteristics of patients with M. avium complex pulmonary diseasea
| Parameter | Mean ± SD |
Pb | |||
|---|---|---|---|---|---|
| Total (n = 34) | Culture conversion (n = 19) | Recurrence (n = 7) | Nonconversion (n = 8) | ||
| Age (yrs) | 66.8 ± 9.1 | 67.9 ± 8.4 | 61.4 ± 8.9 | 68.6 ± 9.9 | 0.2426* |
| Female (no. of patients) | 30 | 18 | 6 | 6 | 0.3385† |
| Body mass index (kg m−2) | 18.3 ± 2.6 | 18.3 ± 2.0 | 19.6 ± 3.0 | 17.2 ± 3.4 | 0.3498* |
| No. of patients | |||||
| Former smokers | 7 | 1 | 3 | 3 | 0.0439‡ |
| Daily alcohol intake | 2 | 0 | 2 | 0 | 0.0533‡ |
| Underlying lung disease | 5 | 3 | 0 | 2 | 0.5181‡ |
| Comorbidity (no. of patients) | |||||
| Diabetes mellitus | 3 | 3 | 0 | 0 | |
| Cardiovascular disease | 3 | 1 | 1 | 1 | |
| Hyperlipidemia | 3 | 1 | 0 | 2 | |
| Other | 4 | 2 | 1 | 1 | |
| Disease type (no. of patients) | |||||
| NB disease | 32 | 18 | 7 | 7 | 0.5187‡ |
| FC disease | 2 | 1 | 0 | 1 | |
| Extent of diseasec | 7.5 ± 3.6 | 7.4 ± 0.8 | 7.0 ± 1.4 | 8.5 ± 1.3 | 0.6956* |
| MAC species (no. of strains) | |||||
| M. avium | 20 | 11 | 7 | 2 | |
| M. intracellulare | 12 | 8 | 0 | 4 | 0.0378‡ |
| Both Mycobacterium species | 2 | 0 | 0 | 2 | |
| Albumin level (g/dl) | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.6 ± 0.4 | 3.5 ± 0.4 | 0.3672* |
| Total cholesterol level (mg/dl) | 193.6 ± 35.1 | 199.9 ± 40.5 | 189.1 ± 25.1 | 182.5 ± 28.6 | 0.4199* |
| Erythrocyte sedimentation rate (mm/h) | 38.5 ± 30.7 | 30.5 ± 7.0 | 19.7 ± 7.5 | 45.1 ± 40.3 | 0.9103* |
| C-reactive protein level (mg/dl) | 0.6 ± 1.2 | 0.6 ± 1.2 | 0.2 ± 0.4 | 1.2 ± 1.3 | 0.2376* |
Data are presented as means ± the SD, except as noted otherwise in column 1. FC disease, fibrocavitary disease; NB disease, nodular bronchiectatic disease.
P values were calculated using either the Kruskal-Wallis test (*) for continuous values or the chi-square test (†) or Fisher exact test (‡) for categorical variables.
The number of CT segments in which MAC lesions were present.
GPL core antibody levels before chemotherapy.
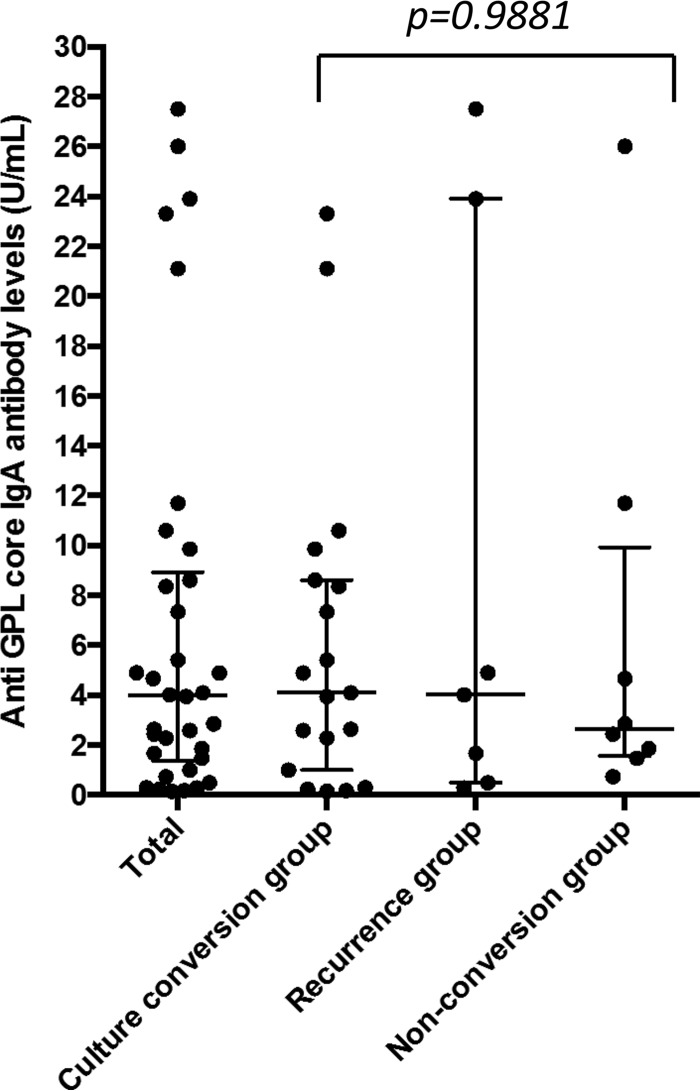
The median GPL core serum antibody level before treatment was 3.985 U/ml (interquartile range [IQR] = 1.360 to 8.930 U/ml) in all patients. When the cutoff value was set as 0.7 U/ml, as indicated by the manufacturer, 85.3% of patients were seropositive. There were no significant differences in the antibody levels before treatment with 4.1 U/ml (IQR = 1.000 to 8.620) in the culture conversion group, 4.02 U/ml (IQR = 0.5 to 23.90 U/ml) in the recurrence group, and 2.645 U/ml (IQR = 1.575 to 9.943 U/ml) in the nonconversion group (P = 0.9881) (Fig. 1). In addition, there was no significant relationship between the antibody levels and extent of disease on chest CT (r = 0.1385, P = 0.4346).
FIG 1.
Pretreatment levels of IgA antibody toward GPL core components in the entire study population and three subgroups based on treatment outcomes (the culture conversion, recurrence, and nonconversion groups). All results are expressed as individual data; horizontal bars indicate the medians and interquartile ranges. The P value was calculated by using the Kruskal-Wallis test.
Change in GPL core antibody levels by chemotherapy.
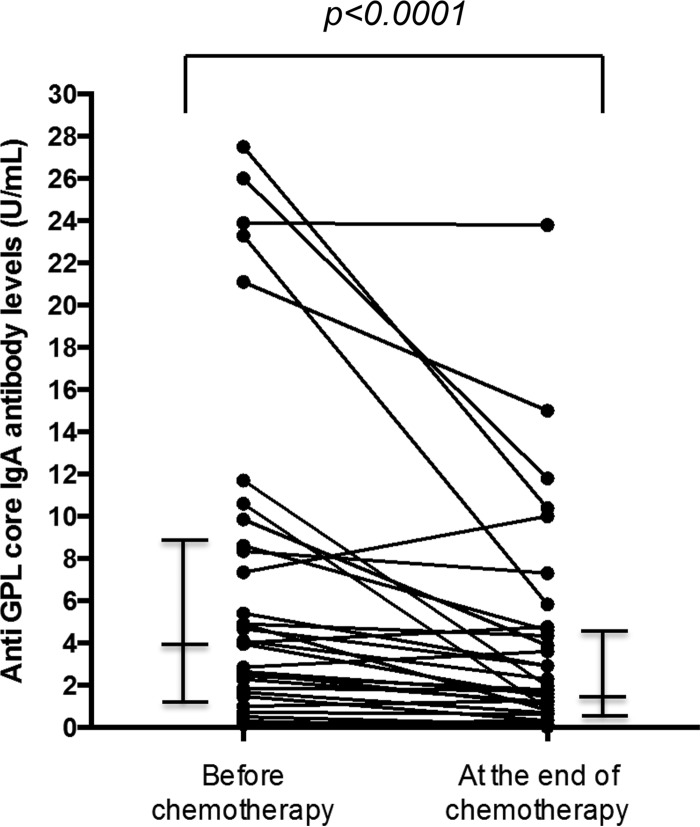
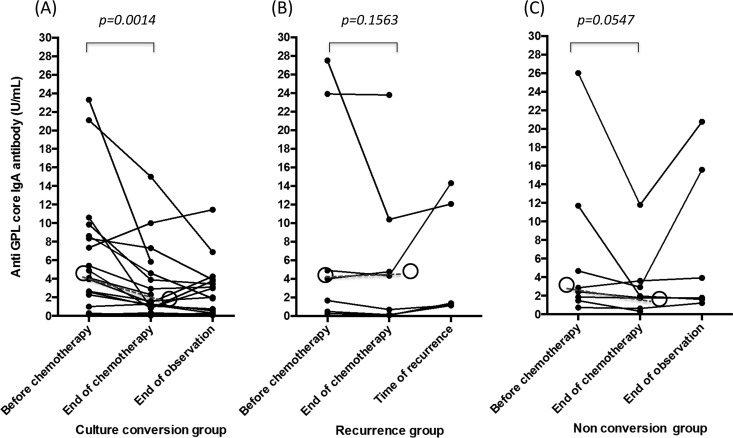
The antibody levels at the start and at the end of chemotherapy were compared. The overall median GPL core serum antibody level (n = 34) at the start and at the end of treatment was 3.985 U/ml (IQR = 1.360 to 8.930 U/ml) and 1.765 U/ml (IQR = 0.6825 to 4.650 U/ml), respectively, and the difference was statistically significant (P < 0.0001) (Fig. 2). In the culture conversion group (n = 19, Fig. 3A), the median reduction was significant (P = 0.0014). In contrast, the median reduction was not significant in the recurrence group (n = 7, P = 0.1563) and the nonconversion group (n = 8, P = 0.0547) (Fig. 3B and C). However, a decrease in the antibody levels between the start and end of chemotherapy was observed in 8 (53.3%) of the 15 patients in the recurrence and nonconversion groups. In addition, of the 20 patients who showed a decrease in antibody levels after chemotherapy in all three groups, 11 showed a transient increase of antibody levels 1 or 2 months after the chemotherapy.
FIG 2.
Overall median levels of GPL core serum antibody (n = 34) at the start and at the end of treatment. All results are expressed as individual data; horizontal bars indicate medians and interquartile ranges. A P value was calculated using the Wilcoxon matched-pair signed-rank test.
FIG 3.
Levels of individual patient serum IgA antibody against glycopeptidolipid core antigen before chemotherapy, at the end of chemotherapy, and at the end of observation or time of recurrence. Data are expressed as individual data (●) and shown separately for the three groups based on the outcome of therapy. (A) Culture conversion group; (B) recurrence group; (C) nonconversion group. In the recurrence group, the data are lacking at the time of recurrence in two patients because their recurrence occurred during the chemotherapy. Open circles represent the median values, and dashed lines represent the change in median values for the group. P values were calculated based on the median antibody levels before and after chemotherapy using the Wilcoxon matched-pair signed-rank test.
Regarding the antibody levels in the culture conversion group, we observed an increase in 5 patients, no change in 10 patients, and a decrease in 10 patients after the end of chemotherapy (Fig. 3A). Although none of these patients met the criteria for bacteriological recurrence, a single positive culture for MAC with deterioration in their plain chest radiographic scans was observed in 4 patients. The remaining patient showed only deterioration in the plain chest radiographic scans. One patient had indications of possible recurrence because of a single positive culture for MAC after conversion, but she did not show an increase in antibody levels after the cessation of chemotherapy. Her antibody levels were 0.16 U/ml before treatment and 0.08 U/ml at the end of observation.
In the recurrence group, the recurrence occurred during chemotherapy in two patients. In the remaining five patients, whose recurrence occurred after the cessation of chemotherapy, the increase in the antibody levels was observed at the time of recurrence, although the difference was statistically insignificant (P = 0.0625). Among the 11 patients with recurrence or possible recurrence, 10 (90.9%) showed increased antibody levels.
The percent decrease in antibody levels between the start and end of chemotherapy was 40.1 ± 36.0% in the culture conversion group, 39.5 ± 34.1% in the recurrence group, and 34.1 ± 37.1% in the nonconversion group. There was no significant difference in the decrease among the three groups (P = 0.9029) and between the culture conversion and the recurrence groups (P = 0.9338). In addition, the antibody levels (above cutoff levels before chemotherapy) were not reduced into the normal range (<0.7 U/ml) in any of the patients.
DISCUSSION
This is the first prospective long-term study of the changes in the levels of GPL core IgA serum antibody in patients with MAC-PD who underwent a multidrug chemotherapy regimen. The clinical characteristics of the patients with MAC disease in this present study were consistent with those in previous reports (4), i.e., the patients were predominantly slim, middle-aged to elderly women with nodular bronchiectatic (NB) disease. The initial culture conversion rate of 76.5% in response to the chemotherapy was also similar to the results from previous reports (18, 20, 23). The observed GPL core IgA seropositive rate of 85.3% was higher than the 69.6% (95% CI = 62.1 to 76.1%) reported in the meta-analysis (17). This may due to the fact that all patients with MAC-PD in this study obviously needed antimicrobial chemotherapy, i.e., their disease was more severe than among the general population of MAC-PD patients, who do not always require chemotherapy.
Based on the findings of this study, the antibody levels before treatment were not a predictor of therapeutic outcomes. In other words, high levels of antibodies before treatment do not lead to a poor therapeutic response, nor do low levels predict a favorable response. Because serum antibody levels are determined not only by the disease severity but also by an individual's immune response, the absolute levels may vary widely from person to person. Indeed, in this study, there was no relationship between the extent of disease and the antibody levels before treatment (r = 0.1385, P = 0.4346). Therefore, measurements of the antibody level at a single time point are not useful for a comparison of disease activity among individuals.
On the other hand, the changes in antibody levels over time may be useful for assessing the disease activity in individual patient. The antibody levels decreased in response to the multidrug chemotherapy. Recurrence and/or worsening assessed by chest radiography findings were observed in cases whose antibody levels subsequently increased after cessation of the chemotherapy. This indicates that the antibody levels may reflect the disease progression and/or activity. A transient increase of antibody levels after chemotherapy suggests that antimicrobials that can lyse MAC and release GPL antigen might increase the antibody response/titer via lysis. Currently, a combination of the clinical symptoms, mycobacteriological examination, and radiographic findings are generally used to assess the disease progression/activity in clinical practice. However, the symptoms are nonspecific and difficult to assess objectively, and it is often difficult to obtain good, homogeneous sputum samples. Furthermore, a deterioration observed in the radiographic findings need not necessarily be associated with MAC-PD. Patients are often coinfected with bacterial pneumonia or chronic necrotizing pulmonary aspergillosis (24, 25). Together with the radiographic and microbiologic findings, measurements of the changes in antibody levels over time will help the objective evaluation of disease activity in individual MAC-PD patients. In particular, an increase in the antibody levels after culture conversion could serve as an early warning sign of recurrence, allowing physicians to take the appropriate precautionary steps.
The optimal duration of therapy is one of the important questions in the treatment of MAC-PD. Unfortunately, we found that serial monitoring of the antibody levels was not useful for the prediction of recurrence in patients who achieved sputum culture conversion. Although a significant reduction of antibody levels was observed in the culture conversion group, a decrease in antibody levels was also observed in more than half of the patients in recurrence group. There was no significant difference in the percent decrease between the culture conversion group and the recurrence group (P = 0.9029). In addition, there were no patients whose antibody levels decreased from above the cutoff value to below it. Therefore, it is difficult to make the decision to discontinue chemotherapy based on the antibody levels. One reason for this may be that the currently available therapeutic agents are unable to eradicate MAC, which thus persists in the host. This may suggest that the host constantly produces the antibodies due to being chronically exposed to MAC antigens even in a small amount. Therefore, the antibody levels in MAC-PD patients did not reduce sufficiently, even in the culture conversion group.
Obviously, the EIA serodiagnosis kit requires validation in a larger number of patients, at diverse geographic locations, and among other races. To clarify the usefulness of the commercially available EIA kit (Tauns Laboratory, Inc.) for MAC-GPL, we compared the cutoff levels, sensitivity, and specificity among reports from Japan, Taiwan, South Korea, and the United States (Table 3). The performance in Taiwan (sensitivity, 61%; specificity, 91%) (11) was relatively low compared to Japan (sensitivity, 84%; specificity, 100%) (6), the United States (sensitivity, 77%; specificity, 94%) (8), and South Korea (sensitivity, 78%; specificity, 100%). Possible reasons for the low sensitivity and specificity in the Taiwanese study include a relatively high incidence of diseases due to rapidly growing mycobacteria possessing GPL and the presence of immunocompromised hosts in the study population, although the studies in Japan, South Korea, and the United States analyzed the sera of patients with immunocompetent but not immunocompromised MAC-PD. Similarly, a relatively low sensitivity (78%) was reported in the South Korean study, where disease due to rapidly growing mycobacteria possessing GPL rather than MAC is common (13), although the serodiagnostic performance for differentiating MAC-PD and TB was high (100%). A cutoff value of 0.7 U/ml was used in the studies from Japan (6), Taiwan (11), and South Korea (13), but 0.3 U/ml was used in the United States (8), based on the best performance calculated by the respective receiver operating characteristic curves. As a result, the cutoff value in the United States was low compared to the others. Taken together, these data suggest the global availability of the test kit for the serodiagnosis of MAC-PD.
TABLE 3.
Comparison of serodiagnostic performance among patients in Japan, Taiwan, South Korea, and the United States determined using a commercially available EIA kit
This single-center study has some limitations. The major limitation was that the number of patients with MAC-PD, especially that in the recurrence and nonconversion groups, was small. It is difficult to perform a long-term observation using a large number of patients, such as a multicenter study. Further investigations are required to confirm the results of this study in large-scale studies. In addition, there is no standard for evaluating the severity of MAC by plain chest radiography. Therefore, the deterioration of the chest radiographic findings was evaluated by two pulmonologists according to the original definition.
In conclusion, this study has demonstrated that the changes in antibody levels reflect the disease activity during multidrug chemotherapy, and it has potential as a useful marker. This allows for simple monitoring of the disease activity within an individual patient, and therefore contributes to better management of MAC-PD.
MATERIALS AND METHODS
Study subjects.
We enrolled consecutive patients who visited the NHO National Toneyama Hospital between September 2008 and November 2010, based on the following criteria: (i) diagnosed with MAC-PD based on the criteria advocated by the ATS/IDSA (4) and (ii) started on the initial multidrug chemotherapy regimen recommended by the ATS/IDSA guidelines (4). According to the guideline, the diagnosis of MAC-PD is based on clinical and bacteriologic criteria. Patients should have symptoms and radiographic findings suggestive of MAC-PD, and cultures of multiple sputum samples or a bronchial wash sample must be positive for MAC. For treatment of MAC-PD, the guideline recommends a regimen of rifamycin, ethambutol, and macrolide for the initial treatment of noncavitary nodular bronchiectatic disease, with the addition of aminoglycoside for patients with fibrocavitary type and cavitary nodular bronchiectatic disease. Tuberculosis and diseases due to rapidly growing mycobacteria possessing GPL, such as M. abscessus, M. chelonae, and M. fortuitum, were excluded in this study using biochemical and genetic characterization of cultured bacteria from patients. We characterized rapidly growing mycobacteria within 7 days and slowly growing mycobacteria, such as M. tuberculosis and MAC, within 30 days. All subjects examined were vaccinated with bacillus Calmette-Guerin (BCG), in accordance with the Infectious Diseases Control Law, of Japan. The study was approved by the National Hospital Organization (NHO), National Toneyama Hospital Review Board (approval 7888), Osaka, Japan. Written informed consent was obtained from all participants at enrollment.
The respective attending physicians determined the timing of the initiation of chemotherapy. We excluded patients who were unable to continue to receive multidrug chemotherapy for more than 6 months at the hospital. The following information was provided: underlying lung disease, comorbidities, smoking history, alcohol intake habits, height, and body weight. Plain chest radiography and chest CT, including high resolution CT (HRCT), were performed. A sputum culture examination for acid-fast bacilli (AFB) was performed using the conventional methods of 2% Ogawa egg medium (Japan BCG, Tokyo, Japan) or a mycobacteria growth indicator tube (Japan Becton, Dickinson and Company, Tokyo Japan) (26). GPL core IgA antibody levels of sera were measured using a commercial EIA kit (Tauns Laboratory, Inc., Shizuoka, Japan) according to the manufacturer's instructions. No subjects were known to be seropositive for human immunodeficiency viruses 1 and 2. The disease type was determined on the basis of the HRCT results as previously described (6). The extent of the disease was defined based on the number of CT segments in which MAC lesions were present, as described previously (6, 22).
Multidrug chemotherapy and therapy evaluation.
Multidrug chemotherapy was defined as the use of 2 or more anti-mycobacterial drugs. The attending physicians decided when to discontinue the multidrug chemotherapy, based on the ATS/IDSA guidelines and their own individual assessment of the patient. The evaluation of therapy outcome was based on the results of sputum cultures for MAC. Sputum culture conversion was defined as six consecutive negative sputum AFB cultures, with the time of conversion defined as the date of the first negative culture. Two or more positive AFB cultures after sputum conversion constituted recurrence, with the time of recurrence defined as the date of the second positive culture. Based on the outcome of the therapy, the participants were classified into three patient groups: a culture conversion group, a recurrence group, and a nonconversion group.
Follow-up examinations.
Chest radiography and measurement of the GPL core antibody levels were performed at 1, 2, and 3 months after the initiation of chemotherapy and thereafter at 3-month intervals until 24 months and at 6-month intervals until the end of the observation period. Sputum culture examinations were performed every month until 24 months after the initiation of therapy and then at 3-month intervals until the end of the observation period. The longest observation period was 5 years. Deterioration of chest radiography findings was defined as an increase in the size of preexisting abnormal shadows or emergence of new abnormal shadows. The radiographic findings were evaluated independently by two pulmonologists, and the final diagnosis was decided by consensus.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, Inc., San Diego, CA). Continuous variables were reported as means ± standard deviations (SD) or median and IQR. Patient groups were compared using the Kruskal-Wallis test or the Mann-Whitney U test for continuous variables and the chi-square test or the Fisher exact test for categorical variables. The Kruskal-Wallis test was used to compare the medians of pretreatment antibody levels among three subgroups based on treatment outcomes. A Wilcoxon matched-pair signed-rank test was used to compare the anti-GPL core antibody levels before and after treatment. Spearman's correlation coefficient by rank was used to determine the relationship between the antibody levels and the extent of disease as assessed by CT. Probability values (P) of <0.05 were regarded as statistically significant.
ACKNOWLEDGMENTS
This study was supported by a grant (Research on Emerging and Re-emerging Infectious Diseases) from the Ministry of Health, Labour and Welfare, Japan, and the Research Program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and Development.
S.K. received nonfinancial support from the Tauns Laboratory, Inc., during the conduct of the study.
We thank Editage (Chiyoda-ku, Tokyo) for English language editing.
REFERENCES
- 1.Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, Mitarai S. 2016. Epidemiology of pulmonary nontuberculous mycobacterial disease. Jpn Emerg Infect Dis 22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, et al. . 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Kitada S, Maekura R, Toyoshima N, Fujiwara N, Yano I, Ogura T, Ito M, Kobayashi K. 2002. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex with an enzyme immunoassay that uses a mixture of glycopeptidolipid antigens. Clin Infect Dis 35:1328–1335. doi: 10.1086/344277. [DOI] [PubMed] [Google Scholar]
- 6.Kitada S, Kobayashi K, Ichiyama S, Takakura S, Sakatani M, Suzuki K, Takashima T, Nagai T, Sakurabayashi I, Ito M, Maekura R, MAC Serodiagnosis Study Group. 2008. Serodiagnosis of Mycobacterium avium complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med 177:793–797. doi: 10.1164/rccm.200705-771OC. [DOI] [PubMed] [Google Scholar]
- 7.Kitada S, Kobayashi K, Nishiuchi Y, Fushitani K, Yoshimura K, Tateishi Y, Miki K, Miki M, Hashimoto H, Motone M, Fujikawa T, Hiraga T, Maekura R. 2010. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex proven by bronchial wash culture. Chest 138:236–237. doi: 10.1378/chest.10-0248. [DOI] [PubMed] [Google Scholar]
- 8.Kitada S, Levin A, Hiserote M, Harbeck RJ, Czaja CA, Huitt G, Kasperbauer SH, Daley CL. 2013. Serodiagnosis of Mycobacterium avium complex pulmonary disease in the U S A. Eur Respir J 42:454–460. doi: 10.1183/09031936.00098212. [DOI] [PubMed] [Google Scholar]
- 9.Kitada S, Yoshimura K, Miki K, Miki M, Hashimoto H, Matsui H, Kuroyama M, Ageshio F, Kagawa H, Mori M, Maekura R, Kobayashi K. 2015. Validation of a commercial serodiagnostic kit for diagnosing pulmonary Mycobacterium avium complex disease. Int J Tuberc Lung Dis 19:97–103. doi: 10.5588/ijtld.14.0564. [DOI] [PubMed] [Google Scholar]
- 10.Komazaki Y, Miyazaki Y, Fujie T, Sakashita H, Tsuchiya K, Tamaoka M, Sumi Y, Maruyama Y, Nanki T, Inase N. 2014. Serodiagnosis of Mycobacterium avium complex pulmonary disease in rheumatoid arthritis. Respiration 87:129–135. doi: 10.1159/000354791. [DOI] [PubMed] [Google Scholar]
- 11.Shu CC, Ato M, Wang JT, Jou R, Wang JY, Kobayashi K, Lai HC, Yu CJ, Lee LN, Luh KT. 2013. Sero-diagnosis of Mycobacterium avium complex lung disease using serum immunoglobulin A antibody against glycopeptidolipid antigen in Taiwan. PLoS One 8:e80473. doi: 10.1371/journal.pone.0080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobashi Y, Mouri K, Obase Y, Kato S, Oka M. 2013. Serological assay by use of glycopeptidolipid core antigen for Mycobacterium avium complex. Scand J Infect Dis 45:241–249. doi: 10.3109/00365548.2012.714904. [DOI] [PubMed] [Google Scholar]
- 13.Jeong BH, Kim SY, Jeon K, Lee SY, Shin SJ, Koh WJ. 2013. Serodiagnosis of Mycobacterium avium complex and Mycobacterium abscessus complex pulmonary disease by use of IgA antibodies to glycopeptidolipid core antigen. J Clin Microbiol 51:2747–2749. doi: 10.1128/JCM.00702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M, Banno S, Sasaki K, Naniwa T, Hayami Y, Ueda R. 2011. Serodiagnosis of Mycobacterium avium complex pulmonary disease with an enzyme immunoassay kit that detects anti-glycopeptidolipid core antigen IgA antibodies in patients with rheumatoid arthritis. Mod Rheumatol 21:144–149. doi: 10.3109/s10165-010-0368-5. [DOI] [PubMed] [Google Scholar]
- 15.Numata T, Araya J, Yoshii Y, Shimizu K, Hara H, Nakayama K, Kuwano K. 2015. Clinical efficacy of anti-glycopeptidolipid-core IgA test for diagnosing Mycobacterium avium complex infection in lung. Respirology 20:1277–1281. doi: 10.1111/resp.12640. [DOI] [PubMed] [Google Scholar]
- 16.Hirose W, Uchiyama T, Nemoto A, Harigai M, Itoh K, Ishizuka T, Matsumoto M, Yamaoka K, Nanki T. 2015. Diagnostic performance of measuring antibodies to the glycopeptidolipid core antigen specific to Mycobacterium avium complex in patients with rheumatoid arthritis: results from a cross-sectional observational study. Arthritis Res Ther 17:273. doi: 10.1186/s13075-015-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata Y, Horita N, Yamamoto M, Tsukahara T, Nagakura H, Tashiro K, Watanabe H, Nagai K, Nakashima K, Ushio R, Ikeda M, Narita A, Kanai A, Sato T, Kaneko T. 2016. Diagnostic test accuracy of anti-glycopeptidolipid-core IgA antibodies for Mycobacterium avium complex pulmonary disease: systematic review and meta-analysis. Sci Rep 6:29325. doi: 10.1038/srep29325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. 1996. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med 153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka E, Kimoto T, Tsuyuguchi K, Watanabe I, Matsumoto H, Niimi A, Suzuki K, Murayama T, Amitani R, Kuze F. 1999. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med 160:866–872. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 20.Kobashi Y, Matsushima T. 2007. The microbiological and clinical effects of combined therapy according to guidelines on the treatment of pulmonary Mycobacterium avium complex disease in Japan, including a follow-up study. Respiration 74:394–400. [DOI] [PubMed] [Google Scholar]
- 21.Kitada S, Maekura R, Toyoshima N, Naka T, Fujiwara N, Kobayashi M, Yano I, Ito M, Kobayashi K. 2005. Use of glycopeptidolipid core antigen for serodiagnosis of Mycobacterium avium complex pulmonary disease in immunocompetent patients. Clin Diagn Lab Immunol 12:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitada S, Nishiuchi Y, Hiraga T, Naka N, Hashimoto H, Yoshimura K, Miki K, Miki M, Motone M, Fujikawa T, Kobayashi K, Yano I, Maekura R. 2007. Serological test and chest computed tomography findings in patients with Mycobacterium avium complex lung disease. Eur Respir J 29:1217–1223. doi: 10.1183/09031936.00061806. [DOI] [PubMed] [Google Scholar]
- 23.Dautzenberg B, Piperno D, Diot P, Truffot-Pernot C, Chauvin JP, Clarithromycin Study Group of France. 1995. Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Chest 107:1035–1040. [DOI] [PubMed] [Google Scholar]
- 24.Kobashi Y, Fukuda M, Yoshida K, Miyashita N, Niki Y, Oka M. 2006. Chronic necrotizing pulmonary aspergillosis as a complication of pulmonary Mycobacterium avium complex disease. Respirology 11:809–813. doi: 10.1111/j.1440-1843.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujita K, Ito Y, Hirai T, Kubo T, Togashi K, Ichiyama S, Mishima M. 2014. Prevalence and risk factors for chronic coinfection in pulmonary Mycobacterium avium complex disease. BMJ Open Respir Res 1:e000050. doi: 10.1136/bmjresp-2014-000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Thoracic Society/Centers for Disease Control and Prevention. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]