ABSTRACT
Coccidioidomycosis is a common cause of community-acquired pneumonia in areas of the southwestern United States in which the disease is endemic. Clinical presentations range from self-limited disease to severe disseminated disease. Therefore, early and accurate diagnosis is essential to ensure appropriate treatment and monitoring. Currently available diagnostic tests have variable accuracy, particularly in certain patient populations, and new tests may offer improved accuracy for the diagnosis of coccidioidomycosis. Serum samples from 103 cases of coccidioidomycosis and 373 controls were tested for IgG and IgM antibodies using the MVista anti-Coccidioides antibody enzyme immunoassay. Serum specimens from 170 controls from areas in which the disease is endemic and 44 cases were tested by immunodiffusion at MiraVista Diagnostics. The sensitivity of the MVista antibody assay was 88.3%, and the specificity was 90%. The sensitivity was maintained in the presence of immunocompromising conditions or immunosuppressive therapies. The sensitivity of immunodiffusion was 60.2%, and the specificity was 98.8%. The sensitivity of complement fixation (62 cases) was 66.1%, but the specificity could not be determined. The MVista anti-Coccidioides antibody enzyme immunoassay offers improved sensitivity, compared with immunodiffusion and complement fixation, is not impaired in immunocompromised patients, and permits highly reproducible semiquantification.
KEYWORDS: coccidioidomycosis, endemic mycoses, fungal infections, serology
INTRODUCTION
Coccidioidomycosis is an endemic mycosis caused by the fungi Coccidioides immitis and Coccidioides posadasii (1). The disease can range from a subclinical self-resolving illness to a life-threatening pulmonary or disseminated disease requiring intensive medical intervention. Some estimates indicate that coccidioidomycosis may be responsible for 15 to 29% of cases of community-acquired pneumonia in areas in which the disease is endemic (2, 3). The number of cases among residents of and visitors to areas in which the disease is endemic has been increasing over the past decade (4).
Diagnosis is challenging because of nonpathognomonic clinical findings (5). Culture is the “gold standard,” but culture results are positive in only about one-half of cases among immunocompromised patients and usually do not provide the initial basis for diagnosis. Additionally, cytopathology or histopathology results are positive for only 20 to 30% of immunocompromised patients (6–9). Serological methods, including immunodiffusion (ID), complement fixation (CF), and enzyme immunoassay (EIA), provide the laboratory basis for diagnosis in most cases (10) but often yield falsely negative results for immunocompromised patients (8, 9, 11) and during the first few months following acute infection (12, 13).
EIA often is used as an initial diagnostic test, followed by ID and/or CF tests if the EIA results are positive. EIA also may be used as a screening test for subclinical or past infection prior to the initiation of immunosuppression (5, 11). The primary objectives of this study were to determine the sensitivity and specificity of the MVista anti-Coccidioides antibody EIA and to compare the test with ID.
(Some of these data were presented in abstract form at IDWeek 2015, San Diego, CA, 7 to 11 October 2015 [14], and at IDWeek 2016, New Orleans, LA, 26 to 30 October 2016 [15].)
RESULTS
Overview of cases.
Of the 103 patients with coccidioidomycosis, 72 (69.9%) had pulmonary coccidioidomycosis and 31 (30.1%) had disseminated coccidioidomycosis. Seventy-eight patients (75.7%) were receiving antifungal therapy, including fluconazole for 68 patients, itraconazole for 5 patients, voriconazole for 3 patients, and combination therapy for 2 patients. Thirty patients (29.1%) had underlying immunocompromising conditions, including HIV infection for 5 patients, solid organ transplants for 4 patients, autoimmune or inflammatory diseases requiring immunosuppressive agents for 19 patients, and chronic prednisone therapy for 2 patients.
Receiver operating characteristic curve analysis.
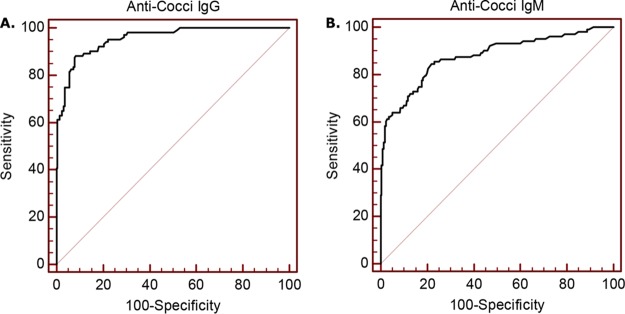
The optimal cutoff value for optical density at 450 nm with 620-nm correction (OD450/620) for IgG detection was 0.133, which was assigned a value of 10 EIA units (EU). The sensitivity was 87.4%, as determined with 103 cases, and the specificity was 92.3%, as determined with 220 controls (170 from areas in which the disease is endemic and 50 from an area in which is it not). The area under the curve (AUC) was 0.957 (95% confidence interval [CI], 0.928 to 0.977), and the standard error was 0.011 (P < 0.0001) (Fig. 1). The corresponding Youden J index was 0.802. The optimal OD450/620 cutoff value for IgM detection was 0.137, which was assigned a value of 10 EU. The sensitivity was 61.2%, and the specificity was 96.4%. The AUC was 0.887 (95% CI, 0.846 to 0.921), and the standard error was 0.0227 (P < 0.0001) (Fig. 1). The corresponding Youden J index was 0.627. The sensitivity and specificity were 88.3% and 91.8%, respectively, for detection of IgG and/or IgM antibodies at ≥10 EU.
FIG 1.
ROC curves for determination of anti-Coccidioides IgG and IgM antibody cutoff values. (A) The ROC curve recommended an OD450/620 cutoff value of 0.133 for IgG detection. The sensitivity with this cutoff value was 87.4% (n = 103) and the specificity was 92.3% (n = 220), with an AUC of 0.957 (95% CI, 0.928 to 0.977) and a standard error of 0.0109 (P < 0.0001). (B) The ROC curve recommended an OD450/620 cutoff value of 0.137 for IgM detection. The sensitivity with this cutoff value was 61.2% and the specificity was 96.4%, with an AUC of 0.887 (95% CI, 0.846 to 0.921) and a standard error of 0.0227 (P < 0.0001).
MVista antibody EIA results for cases and controls.
IgG results were positive for 90 cases (87.4%), indeterminate for 10 (9.7%), and negative for 3 (2.8%) (Table 1). The sensitivity and specificity (Table 1) and predictive values (Table 2) are reported. EIA IgG and IgM antibody levels for cases and controls are shown in Fig. 2 and Fig. 3, respectively. IgG results were negative for 100% of healthy blood donors from an area in which the disease is not endemic; among control subjects from areas in which the disease is endemic, results were positive for 17 (10%), indeterminate for 26 (15.3%), and negative for 127 (74.7%). Positive results for the control subjects from areas in which the disease is endemic were between 10.0 and 20.0 EU for 16 (94.1%) and 26.7 EU for 1.
TABLE 1.
Comparison of antibody detection using different antibody assaysa
| Test | Sensitivity (%) using cases | Specificity (%) using controlsb |
|---|---|---|
| MVista IgG | 87.4 | 90.0 |
| MVista IgM | 61.2 | 95.3 |
| MVista IgG or IgM | 88.3 | 90.0 |
| IDCF | 53.4 | 98.8 |
| IDTP | 33.0 | 100 |
| IDCF or IDTP | 60.2c | 98.8c |
| CF | 64.5c | NA |
The numbers of cases and controls were as follows: MVista EIA, 103 and 170; ID, 103 and 164; CF, 62 and 0. IDCF, immunodiffusion with the CF antigen; IDTP, immunodiffusion with the TP antigen; CF, complement fixation; NA, not available.
Controls from areas in which the disease is endemic included controls from Bakersfield, California, and Tucson, Arizona, and clinical controls from Tucson, Arizona.
P < 0.001, in comparison with the MVista assay.
TABLE 2.
Comparison of sensitivities, specificities, likelihood ratios, and predictive values determined by ID and the MVista anti-Coccidioides antibody EIA at different cutoff values and estimates of prevalence
| Test and cutoff value | Sensitivity (%) | Specificity (%) | LR+a | LR− | Prevalence of 5% |
Prevalence of 10% |
||
|---|---|---|---|---|---|---|---|---|
| PPV | NPV | PPV | NPV | |||||
| EIA, IgG | ||||||||
| 10 EU | 87.4 | 90.0 | 8.74 | 0.14 | 31.5 | 99.3 | 49.3 | 98.5 |
| 20 EU | 60.2 | 99.4 | 100.3 | 0.40 | 84.1 | 97.9 | 91.8 | 95.7 |
| EIA, IgM | ||||||||
| 10 EU | 61.2 | 95.3 | 13.0 | 0.41 | 40.7 | 97.9 | 59.1 | 95.7 |
| 20 EU | 30.1 | 99.4 | 50.2 | 0.70 | 72.5 | 96.4 | 84.8 | 92.8 |
| EIA, IgG or IgM | ||||||||
| 10 EU | 88.3 | 90.0 | 8.83 | 0.13 | 31.7 | 99.3 | 49.5 | 98.6 |
| 20 EU | 60.2 | 99.4 | 100.3 | 0.40 | 84.1 | 97.9 | 91.8 | 95.7 |
| IDCF | 53.3 | 98.8 | 44.5 | 0.47 | 70.0 | 97.6 | 83.1 | 95.0 |
| IDTP | 33.0 | 100 | ∞ | 0.67 | 100 | 96.6 | 100 | 93.1 |
| IDCF or IDTP | 60.1 | 98.8 | 50.1 | 0.40 | 72.5 | 97.9 | 84.8 | 95.7 |
LR+, positive likelihood ratio; LR−, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; IDCF, immunodiffusion with the CF antigen; IDTP, immunodiffusion with the TP antigen; ∞, perfect specificity results with an infinite positive likelihood ratio.
FIG 2.
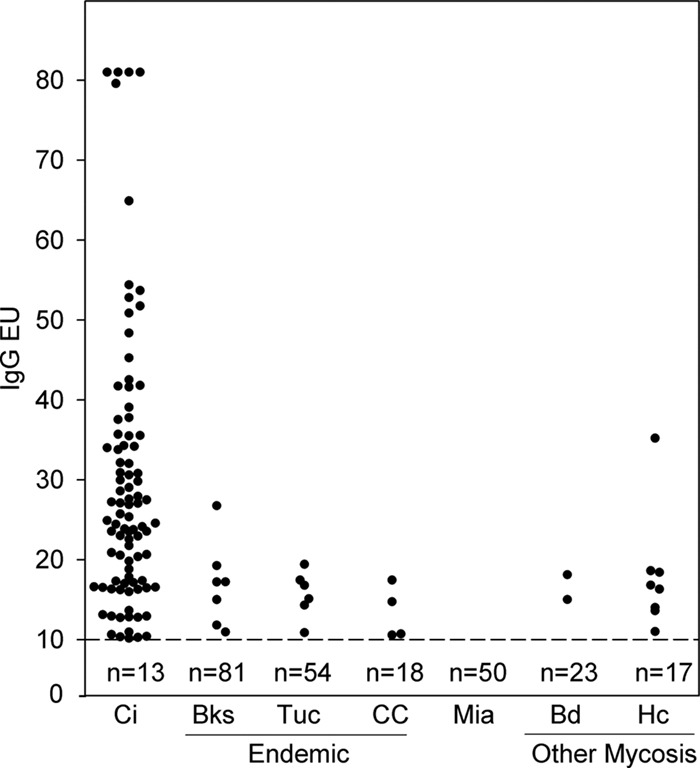
IgG antibody levels determined with the MVista anti-Coccidioides antibody EIA. IgG antibody levels were measured in samples from patients with coccidioidomycosis (Ci) (n = 103), individuals from Bakersfield, California (Bks) (n = 88), or Tucson, Arizona (Tuc) (n = 60) (where the disease is endemic), clinical controls from Tucson, Arizona (CC) (n = 22), controls from Miami, Florida (Mia) (n = 50), and individuals with blastomycosis (Bd) (n = 25) or histoplasmosis (Hc) (n = 25). The cutoff value of 10 EU is indicated by the dashed line. The numbers under the line represent the numbers of serum samples with negative results.
FIG 3.
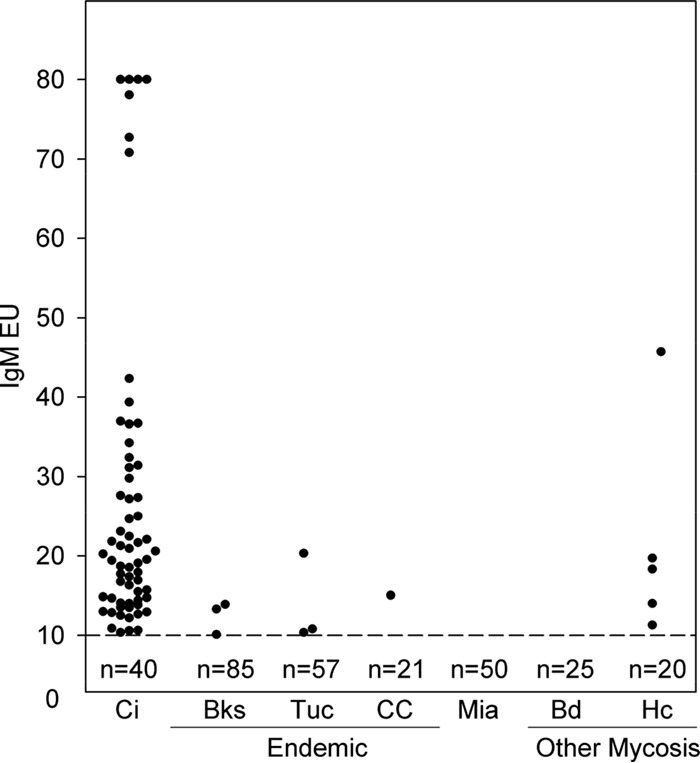
IgM antibody levels determined with the MVista anti-Coccidioides antibody EIA. See the legend to Fig. 2 for explanations.
IgM results were positive for 63 cases (61.2%), indeterminate for 5 (4.9%), and negative for 35 (34.0%). IgM results were negative for 100% of control subjects from an area in which the disease is not endemic; among control subjects from areas in which the disease is endemic, results were positive for 7 (4.1%), indeterminate for 21 (12.4%), and negative for 142 (83.5%). Positive results for the control subjects from areas in which the disease is endemic were between 10.0 and 20.0 EU for 7 of 8 subjects (87.5%) and 20.3 EU for 1.
Serum samples from 8 (32%) of 25 patients with histoplasmosis were cross-reactive for IgG antibody and those from 5 (20%) were cross-reactive for IgM antibody. Only 2 serum samples from patients with blastomycosis were cross-reactive for IgG antibody, and no cross-reactivity was demonstrated for IgM antibody. The anti-Histoplasma IgG levels were more than 5 EU higher than the anti-Coccidioides level for 6 (75%) of the 8 patients, while the reverse was true for 1 patient and the results were not different for another patient. The anti-Histoplasma IgM antibody level was more than 5 EU higher than the anti-Coccidioides IgM antibody level for 3 patients, the reverse was true for 1 patient, and the results were not different for another patient.
Comparison of MVista EIA, ID, and CF results for coccidioidomycosis cases and controls from areas in which the disease is endemic.
The sensitivity for IgG or IgM antibodies was 88.3%, compared to 60.2% for ID (P < 0.0001). Both ID and EIA results were positive for 60 cases (58.3%), EIA results alone for 31 (30.0%), ID results alone for 2 (2.0%), and neither for 10 (9.8%). The 2 cases with positive results by ID but not by EIA had indeterminate results by EIA (8.4 and 9.9 EU). The specificity was 98.8% for ID and 90% for EIA (P < 0.0001).
Assuming a prevalence of coccidioidomycosis of 5% and using a cutoff value of 10 EU, ID demonstrated a higher positive predictive value (PPV) than did EIA (72.5% versus 31.7%) but a lower negative predictive value (NPV) (97.9% versus 99.3%) (Table 2). With a cutoff value of 20 EU, EIA exhibited a higher PPV (84.1%) and an equivalent NPV (97.9%). If the prevalence was assumed to be 10%, then the PPVs were higher and the NPVs were lower.
The sensitivity of the MVista EIA was 93.5%, compared to 64.5% for CF (P = 0.0001). Both EIA and CF results were positive for 38 cases (61.3%), EIA results alone for 20 (32.2%), CF results alone for 2 (3.2%), and neither for 2 (3.2%). The case with a positive result by CF but not by EIA had a CF titer of 1:2 and an indeterminate EIA result (9.9 units).
Assessment of some conditions affecting antibody responses.
The sensitivity for detection of IgG antibodies was 90.2% for patients with pulmonary disease and 80.6% for those with disseminated disease (P = 0.308) (Table 3). The sensitivity for detection of IgM antibodies was 66.7% for patients with pulmonary disease and 48.4% for those with disseminated disease (P = 0.127).
TABLE 3.
Analysis of parameters potentially affecting the sensitivity of anti-Coccidioides antibody detection
| Clinical factor and immunoglobulin | Sensitivity (%) |
P | |
|---|---|---|---|
| MVista EIA | IDa | ||
| Pulmonary disease (n = 72) | |||
| IgG | 90.2 | 45.8 | <0.0001 |
| IgM | 66.7 | 34.7 | <0.0001 |
| IgG or IgM | 90.2 | 54.2 | <0.0001 |
| Disseminated disease (n = 31) | |||
| IgG | 80.6 | 71.0 | 0.289 |
| IgM | 48.4 | 25.8 | 0.016 |
| IgG or IgM | 83.9 | 71.0 | 0.180 |
| Immunocompromised (n = 30) | |||
| IgG | 83.3 | 40.0 | 0.002 |
| IgM | 56.7 | 30.0 | 0.022 |
| IgG or IgM | 83.3 | 50.0 | 0.012 |
| Immunocompetent (n = 73) | |||
| IgG | 90.4 | 58.9 | <0.0001 |
| IgM | 63.0 | 32.9 | <0.0001 |
| IgG or IgM | 90.4 | 63.0 | <0.0001 |
| Antifungal treated (n = 78) | |||
| IgG | 87.2 | 57.7 | <0.0001 |
| IgM | 60.3 | 29.5 | <0.0001 |
| IgG or IgM | 88.5 | 62.3 | <0.0001 |
| Untreated (n = 25) | |||
| IgG | 88.0 | 40.0 | 0.005 |
| IgM | 64.0 | 40.0 | 0.109 |
| IgG or IgM | 88.0 | 52.0 | 0.004 |
IgG and IgM results refer to ID with the CF antigen and ID with the TP antigen, respectively.
The sensitivity for detection of IgG antibodies was 90.4% for immunocompetent patients and 83.3% for immunocompromised patients (P = 0.493). The sensitivity for detection of IgM antibodies was 63.0% for immunocompetent patients and 56.7% for immunocompromised patients (P = 0.708). The sensitivity for detection of IgG or IgM antibodies among immunocompromised patients was 83.3% for EIA, compared to 50.0% for ID (P = 0.012).
The sensitivity for detection of IgG antibodies was 87.2% for patients receiving antifungal treatment and 88.0% for untreated patients (P = 0.760). The sensitivity for detection of IgM antibodies was 60.3% for treated patients and 64.0% for untreated patients (P = 0.969). The sensitivity for detection of IgG or IgM antibodies in treated patients was 88.5% by EIA and 62.3% by ID (P < 0.0001).
Reproducibility.
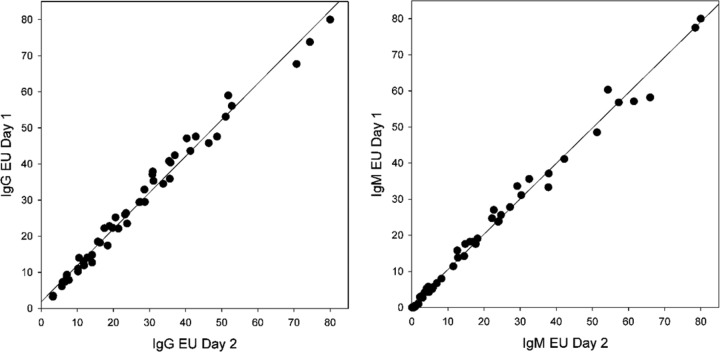
Specimens from the cases and controls were tested on two occasions. IgG results were reproducibly positive or negative for 372 (99.7%) of 373 subjects, and IgM antibodies were reproducible for 364 (97.6%) of 373 subjects (Fig. 4). Antibody levels were determined on two occasions with specimens from 51 patients. The coefficient of determination (R2) for IgG antibody levels was 0.984 (slope, 1.01 [95% CI, 1.00 to 1.12]; residual standard deviation, 1.76). The R2 for IgM antibody levels was 0.993 (slope, 0.981 [95% CI, 0.957 to 1.00]; residual standard deviation, 1.58). Repeatability, between-day precision, and within-laboratory precision were determined for the high, moderate, low, and negative calibrators. As an example of within-laboratory precision, the mean of 60 observations for the moderate IgG calibrator was 40.9 EU (standard deviation, 2.01 EU [95% CI, 1.62 to 2.65 EU]; coefficient of variation [CV], 4.9%). The mean for the moderate IgM calibrator was 30.0 EU (standard deviation, 0.84 EU [95% CI, 0.66 to 1.17 EU]; CV, 2.8%). CVs were similar for the high and low calibrators.
FIG 4.
Reproducibility determined by linear regression. Interassay agreement of IgG unit values obtained from repeat testing of coccidioidomycosis patient samples (n = 51) demonstrated a strong correlation, with a R2 of 0.984 (slope 95% CI, 1.00 to 1.12 [P < 0.0001]). Interassay agreement of IgM unit values obtained from repeat testing of coccidioidomycosis patient samples demonstrated a strong correlation, with a R2 of 0.993 (slope 95% CI, 0.985 to 1.02 [P < 0.0001]).
DISCUSSION
The MVista anti-Coccidioides antibody EIA offered several advantages, compared to ID. The primary advantage was improved sensitivity (88.3% for EIA and 60.2% for ID). EIA results were positive for all except two patients with positive ID results, who had indeterminate EIA results (8.4 EU and 9.9 EU). Conversely, ID results were negative for 34.1% of patients with positive EIA results. Furthermore, immunocompromising conditions and antifungal treatment did not reduce the sensitivity of the EIA, which is another important advantage.
Comparison of the sensitivity of EIA and CF was hampered by incomplete CF testing, which was performed in 62 cases. However, EIA demonstrated superior sensitivity, compared to CF. IgG antibodies were positive by EIA for all but two patients with positive CF results, for whom the CF titers were marginally positive and the IgG level was 9.9 EU, at the upper end of the cutoff range for indeterminate. CF results were negative for 32.8% of patients with positive EIA results.
The MVista EIA is semiquantitative, made possible by incorporation of a 5-point standard curve. The EIA demonstrates excellent interassay reproducibility, which is important to allow comparisons of antibody levels in specimens obtained at different times and tested in different assays. Within-laboratory imprecision was less than 5% with the MVista EIA; a 5-EU difference is statistically significant. CF, which is currently recommended for quantification, requires testing of current and prior specimens in the same assay to adjust for interassay imprecision (16) and demonstration of a ≥4-fold (400%) difference in titers to adjust for intraassay imprecision (17).
EIA offers higher throughput and shorter turnaround times than ID and CF, is less labor-intensive, and can be automated. Hundreds of specimens can be tested in the same EIA, and results can be reported the same day. ID assays must be read for up to 3 days before a negative result is reported, and CF requires overnight incubation. Furthermore, these tests may not be performed every day in some laboratories, further increasing turnaround time.
The MVista EIA was less specific than ID, which is expected, given its greater analytical sensitivity. However, the test allows for improved PPVs with increasing EU. In areas in which the disease is endemic, where coccidioidomycosis is highly prevalent (15 to 29%) among patients with community-acquired pneumonia (2, 3), the PPV is further increased.
Low positive (10.0 to 19.9 EU) and indeterminate (8.0 to 9.9 EU) results for healthy controls from areas in which the disease is endemic may be caused by asymptomatic or recently resolved coccidioidomycosis. IgG antibodies determined by CF and IgM antibodies determined by ID with the tube precipitin (TP) antigen may persist for years following the initial infection (16). Furthermore, reexposure is common and could “boost” antibody production. Assessment of epidemiological factors, clinical findings, and additional diagnostic tests may help differentiate active from past infections in patients with EIA results between 8.0 and 20 EU. Demonstration of increases in IgG or IgM levels or no change in IgG levels but decreases in IgM levels may suggest acute coccidioidomycosis. This hypothesis requires further investigation but was documented for histoplasmosis (18).
CF cross-reactivity is common among patients with histoplasmosis and blastomycosis (16, 19). Cross-reactions occurred for 32% of patients with histoplasmosis and 8% with blastomycosis. In the case of histoplasmosis, the cross-reactions were between 10 and 19.9 EU for IgG antibodies in 9 of 10 cases and for IgM antibodies in 4 of 5 cases. Only IgG was cross-reactive with blastomycosis, and the EU values were below 20 in both cases. Therefore, an EU result of ≥20 is more likely to be caused by coccidioidomycosis. Specific antibody testing for these endemic mycoses may aid in further establishing a correct diagnosis.
This study has several limitations. First, only some patients enrolled in the study had acute coccidioidomycosis; others had more remote disease. Specifically, a delay of more than 1 month between diagnosis and enrollment occurred in 80% of cases. Second, testing performed for diagnosis was not standardized, as 40% of patients were not tested by CF and 50% were not tested by commercial EIA. However, diagnosis of coccidioidomycosis commonly requires a multifaceted approach incorporating clinical, laboratory, and radiographic data. Third, the number of controls was inadequate to establish a specificity value with precision. Fourth, serial samples were not available, precluding assessment of the time course for antibody production in acute infections and of antibody clearance with spontaneous recovery or treatment. Lastly, we do not provide comparisons with other commercially available EIAs that are commonly used for the diagnosis of coccidioidomycosis. Given the strong performance of this EIA relative to ID and CF and the ongoing complexity of attempting to diagnose coccidioidomycosis with currently available assays, a future comparison with such EIAs is warranted.
In conclusion, the MVista EIA demonstrated higher sensitivity than ID and CF and was not affected by immune status or antifungal therapy. Higher EU results may better differentiate among individuals with active versus prior disease or other endemic mycoses. The EIA is reproducibly semiquantitative, permitting comparison of antibody levels in specimens obtained at different times.
MATERIALS AND METHODS
Serum samples.
Serum samples from 103 patients with coccidioidomycosis who were evaluated at the University of Arizona between 2007 and 2014 were obtained with the informed consent of the patients and were stored frozen until the time of testing. Cases were classified as definite based on detection of Coccidioides spp. by culture or spherule visualization by histopathology or cytology. Individuals with positive results from any commercially available anti-Coccidioides antibody test were classified as probable cases. CF results were regarded as positive at any titer, including neat specimens. The results of antibody testing performed at the hospital laboratory or its national reference laboratory were used to make the diagnosis. Cases were verified by chart reviews performed by J.M., T.Z., and C.S.
Control samples included serum specimens from 88 healthy donors at the Houchin Blood Bank (Bakersfield, CA), 60 healthy donors from the University of Arizona (Tucson, AZ), and 22 clinical controls with acute respiratory illnesses other than coccidioidomycosis who were evaluated at the University of Arizona Medical Center (Tucson, AZ). Serum samples from 50 healthy individuals from an area in which the disease is not endemic were purchased from a biorepository (SeraCare, Miami, FL). Additionally, samples from 25 individuals with culture- and/or histopathology-proven blastomycosis and 25 individuals with probable histoplasmosis were included.
Anti-Coccidioides antibody testing by immunodiffusion.
ID testing was not performed at the time of initial diagnosis for 44 cases and 214 controls. ID testing was performed at MiraVista Diagnostics (Indianapolis, IN), a Clinical Laboratory Improvement Amendments (CLIA)- and College of American Pathologists (CAP)-certified laboratory, using Meridian Biosciences reagents (Meridian Biosciences, Cincinnati, OH), and results were validated using Clinical and Laboratory Standards Institute (CLSI) protocols.
Anti-Coccidioides IgG and IgM antibody EIA.
Nunc MaxiSorp 96-well plates (Thermo Fisher) were coated with a proprietary MVista Coccidioides antigen, and the assay was performed as described previously (18, 20). A 5-point standard curve created from serum pools from patients with coccidioidomycosis, spanning the linear range of the assay, was used for semiquantification. OD450/620 values extrapolated from the standard curve were converted to EU values, ranging from 0 to 80 EU. The optimal cutoff values for positive results were determined by receiver operating characteristic (ROC) curve analysis. Results of at least 10 EU were considered positive, between 8.0 and 9.9 EU as indeterminate, and below 8.0 EU as negative. For analysis of sensitivity and specificity, indeterminate results were analyzed as negative. Reproducibility was determined by testing specimens on two separate days, and precision was determined with the appropriate CLSI protocols.
Statistics.
SigmaPlot statistical analysis software (Systat Software, San Jose, CA) was used for transformation of OD450/620 values from individual serum samples into EU values, based on the standard curve. Statistical analysis was performed using MedCalc for Windows version 12.3.0 (MedCalc Software, Ostend, Belgium). Linear regression analysis according to the Passing-Bablok method was used to analyze reproducibility and precision. The McNemar test was used to compare diagnostic assays utilizing paired samples, and chi-square analysis was used to compare subgroups using MedCalc software. P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
We thank Janko Nikolich-Zugich for providing some of the control serum samples used in this study.
E.H., M.D., and L.J.W. are employees of MiraVista Diagnostics and developed the MVista EIA.
REFERENCES
- 1.Fisher MC, Koenig GL, White TJ, Taylor JW. 2002. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94:73–84. doi: 10.2307/3761847. [DOI] [PubMed] [Google Scholar]
- 2.Valdivia L, Nix D, Wright M, Lindberg E, Fagan T, Lieberman D, Stoffer T, Ampel NM, Galgiani JN. 2006. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis 12:958–962. doi: 10.3201/eid1206.060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang DC, Anderson S, Wannemuehler K, Engelthaler DM, Erhart L, Sunenshine RH, Burwell LA, Park BJ. 2008. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis 14:1053–1059. doi: 10.3201/eid1407.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2013. Increase in reported coccidioidomycosis: United States, 1998–2011. MMWR Morb Mortal Wkly Rep 62:217–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Malo J, Luraschi-Monjagatta C, Wolk DM, Thompson R, Hage CA, Knox KS. 2014. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc 11:243–253. doi: 10.1513/AnnalsATS.201308-286FR. [DOI] [PubMed] [Google Scholar]
- 6.Woods CW, McRill C, Plikaytis BD, Rosenstein NE, Mosley D, Boyd D, England B, Perkins BA, Ampel NM, Hajjeh RA. 2000. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994–1997: incidence, risk factors, and prevention. J Infect Dis 181:1428–1434. doi: 10.1086/315401. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom L, Yocum DE, Ampel NM, Villanueva I, Lisse J, Gluck O, Tesser J, Posever J, Miller M, Araujo J, Kageyama DM, Berry M, Karl L, Yung CM. 2004. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum 50:1959–1966. doi: 10.1002/art.20454. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza N, Noel P, Blair JE. 2015. Diagnosis, treatment, and outcomes of coccidioidomycosis in allogeneic stem cell transplantation. Transpl Infect Dis 17:380–388. doi: 10.1111/tid.12372. [DOI] [PubMed] [Google Scholar]
- 9.Mendoza N, Blair JE. 2013. The utility of diagnostic testing for active coccidioidomycosis in solid organ transplant recipients. Am J Transplant 13:1034–1039. doi: 10.1111/ajt.12144. [DOI] [PubMed] [Google Scholar]
- 10.Ampel NM. 2010. The diagnosis of coccidioidomycosis. F1000 Med Rep 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair JE, Coakley B, Santelli AC, Hentz JG, Wengenack NL. 2006. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 162:317–324. doi: 10.1007/s11046-006-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CE, Saito MT, Simons SA. 1956. Pattern of 39,500 serologic tests in coccidioidomycosis. JAMA 160:546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- 13.Blair JE, Chang YH, Cheng MR, Vaszar LT, Vikram HR, Orenstein R, Kusne S, Ho S, Seville MT, Parish JM. 2014. Characteristics of patients with mild to moderate primary pulmonary coccidioidomycosis. Emerg Infect Dis 20:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holbrook ED, Zangeneh T, Malo J, Strawter C, Oren E, Robey I, Erickson H, Chahal R, Thompson C, Ampel NM, Wheat LJ, Knox KS. 2015. Development of an improved antibody detection EIA for use in detection of coccidioidomycosis, abstr 251 IDWeek 2015, San Diego, CA, 7 to 11 October 2015. [Google Scholar]
- 15.Holbrook ED, Malo J, Zangeneh T, Strawter C, Oren E, Robey I, Erickson H, Chahal R, Durkin M, Thompson C, Hoover SE, Ampel NM, Wheat LJ, Knox K. 2016. Development of an improved antibody detection EIA for use in diagnosis of coccidioidomycosis, abstr 1553 IDWeek 2016, New Orleans, LA, 26 to 30 October 2016. [Google Scholar]
- 16.Pappagianis D, Zimmer BL. 1990. Serology of coccidioidomycosis. Clin Microbiol Rev 3:247–268. doi: 10.1128/CMR.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappagianis D. 2001. Serologic studies in coccidioidomycosis. Semin Respir Infect 16:242–250. doi: 10.1053/srin.2001.29315. [DOI] [PubMed] [Google Scholar]
- 18.Richer SM, Smedema ML, Durkin MM, Herman KM, Hage CA, Fuller D, Wheat LJ. 2016. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 62:896–902. doi: 10.1093/cid/ciw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huppert M, Krasnow I, Vukovich KR, Sun SH, Rice EH, Kutner LJ. 1977. Comparison of coccidioidin and spherulin in complement fixation tests for coccidioidomycosis. J Clin Microbiol 6:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richer SM, Smedema ML, Durkin MM, Brandhorst TT, Hage CA, Connolly PA, Leland DS, Davis TE, Klein BS, Wheat LJ. 2014. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin Vaccine Immunol 21:143–146. doi: 10.1128/CVI.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]