ABSTRACT
The Aptima Combo 2 (AC2) and Aptima CT (ACT) (Hologic Inc., San Diego, CA) are nucleic acid amplification tests (NAATs) that detect Chlamydia trachomatis. AC2 also detects Neisseria gonorrhoeae. Storage and temperature conditions may impact the utility of NAATs in some settings and screening programs. We evaluated specimen stability for use beyond the Aptima package insert specifications for temperature and duration of storage (between 2°C and 30°C and 60 days, respectively) in two studies: (i) dry C. trachomatis-seeded swabs were used with ACT after storage at 4°C, 23°C, or 36°C for up to 84 days and (ii) swabs seeded with C. trachomatis and N. gonorrhoeae and then placed in transport medium were tested with AC2, after being mailed via the U.S. Postal Service to three different sites. Prolonged storage of samples had no effect, and samples stored at 4°C, 23°C, and 36°C for up to 84 days yielded comparable ACT positivities, although there was a drop in signal intensity for virtually all specimens under all storage/shipping conditions after day 21. In the mailing study, 80%, 52% and 29% of seeded swabs were exposed to temperatures of >30°C during three rounds in transit, and 2% reached temperatures of >40°C. No evidence of signal degradation in the AC2 assay for detection of C. trachomatis or N. gonorrhoeae was observed, although some mailed swabs took more than 5 weeks to reach the laboratory site. These two studies support the potential use of swabs at temperatures above 36°C and storage beyond 60 days and provide confidence regarding this commercially available NAAT for testing of specimens after mailing.
KEYWORDS: Chlamydia trachomatis, Neisseria gonorrhoeae, detection, nucleic acid amplification test, stability, swabs
INTRODUCTION
Nucleic acid amplification tests (NAATs) are highly sensitive and specific for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae (1–3). These tests are now widely available and used routinely for diagnosis and screening of men and women for genital tract infections (4). Each NAAT has package insert (PI) instructions that detail the requirements for specimen type and handling. However, off-label use of these NAATs is permissible if the laboratory performs validation studies demonstrating results comparable to those of standard tests. For example, NAATs are not currently FDA cleared for use as tests for pharyngeal and rectal C. trachomatis or N. gonorrhoeae, but numerous laboratories have evaluated these specimens, verifying their suitability for testing (5).
The Aptima Combo 2 (AC2; Hologic Inc., San Diego, CA) is a NAAT that detects both C. trachomatis and N. gonorrhoeae using a single specimen (6). The PI specifies that specimens may be stored for up to 60 days at temperatures of 2°C to 30°C (7). While these parameters are suitable for most current use, if specimens could be held longer and/or could tolerate higher temperatures, then they would become more attractive for use in developing countries or under conditions likely to be encountered when specimens are mailed. This could broaden current screening programs.
The stability of the specimens under nonstandard, harsh conditions is a real concern in developing countries. In areas with intense heat, specimen transport is periodically done above the set temperature limit of 30°C. It is difficult and sometimes impossible to maintain a cold chain when specimens are collected in the field (an all-day process) and then brought to the laboratory. Under these circumstances, the ability to collect dry swabs and to transport them in a warm environment (often >36°C) would make NAAT use more practical. In addition, there are instances where specimens must be shipped to another country for testing.
Our laboratory was engaged in a follow-up study on the use of azithromycin in control of trachoma (8). For this study, the Aptima CT (ACT; Hologic Inc., San Diego, CA) test was used for C. trachomatis detection on specimens collected in Niger. We had delays in getting the specimens shipped back to San Francisco, CA. Being concerned about specimen stability, we evaluated the feasibility of using dry swabs with ACT.
There is some literature on the use of dry swabs in NAATs for C. trachomatis and N. gonorrhoeae. An in-house study showed that dry swabs spiked with C. trachomatis and tested by AC2 remained stable at 4°C for up to 90 days (9). In another study, Gaydos et al. found that dry swabs were appropriate for use in PCR testing, (10), and they later expanded that observation to evaluate an Internet-based screening program for C. trachomatis and N. gonorrhoeae using dry swabs (11).
In another study, we evaluated the use of home versus clinic self-obtained vaginal swabs (SOVS). When the CDC recommended SOVS as the specimen of choice for NAATs (12), this presented the possibility of at-home collection of specimens for screening. However, SOVS specimens are FDA cleared for use only when collected in a clinic setting. The ability to self-collect at home and mail in specimens could have a significant impact on sexually transmitted disease (STD) control efforts (13–15). To assess such use in screening programs, we initially performed a pilot study to determine specimen stability and monitor the shipping temperatures during transit.
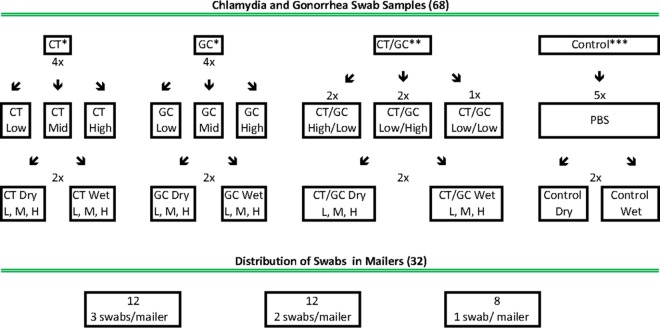
In this study, we evaluated the performance of the ACT and AC2 under conditions outside the storage and temperature conditions specified in the package insert. These studies included (i) evaluation of dry, C. trachomatis-inoculated swabs stored for up to 84 days at temperatures of 4°C, 23°C, and 36°C and (ii) evaluation of C. trachomatis- and N. gonorrhoeae-inoculated swabs stored dry or placed in AC2 transport medium and then transported to other laboratories using the U.S. Postal Service (USPS). The distribution of swab samples in the latter study is illustrated in Fig. 1.
FIG 1.
Seeded samples for mailed transport. *, four C. trachomatis (CT) and 4 N. gonorrhoeae (GC) strains seeded with low (L), medium (M), and high (H) titers (each strain had a dry and wet state), total of 48 samples. **, five combinations of C. trachomatis/N. gonorrhoeae, two seeded with a C. trachomatis high/N. gonorrhoeae low titer, two with a C. trachomatis low/N. gonorrhoeae high titer, and one with a C. trachomatis low/N. gonorrhoeae low titer (each had a dry and wet state), total of 10 samples. ***, five controls (negative for C. trachomatis and N. gonorrhoeae) containing PBS (each had a dry and wet state), total of 10 samples.
RESULTS
For the dry swab study, there were no differences in the results from the dry and wet swabs (Table 1). The test results with the dry swabs were similar to results with the wet swabs, even though samples tested were more dilute (dry swabs were rehydrated, and then a 1/5 volume was tested). The relative light unit (RLU) readings for both swab types remained relatively high (∼8,700 RLU) through 21 days. Beyond the 21-day time point, an approximately 35% reduction (to ∼5,500 RLU) in signal was observed and then the signal remained consistently positive through day 84, and thus well beyond the manufacturer's stated 60-day limit. Samples held at 36°C did not deteriorate at rates different from those of samples held at 4 or 23°C, and ACT results were comparable to those obtained with swabs stored at 4°C and 23°C.
TABLE 1.
Stability of dry versus wet swabs seeded with C. trachomatis antigens determined by Aptima CT test
| Storage condition/samplea | Storage temp (°C) | RLU at day: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 56 | 63 | 69 | 77 | 84 | ||
| Wetb | |||||||||||
| 50 | 4 | 9,328 | 8,843 | 8,999 | 8,440 | 5,848 | 5,707 | 5,171 | 4,502 | 4,498 | 5,557 |
| 23 | 9,271 | 8,426 | 9,177 | 8,499 | 5,382 | 5,432 | 5,481 | 4,715 | 4,418 | 5,378 | |
| 36 | 9,098 | 8,810 | 8,911 | 8,416 | 5,315 | 5,550 | 5,166 | 4,806 | 4,287 | 5,576 | |
| 486 | 4 | 9,337 | 8,888 | 8,848 | 8,252 | 5,509 | 5,751 | 5,287 | 4,286 | 4,479 | 5,429 |
| 23 | 9,196 | 8,502 | 9,165 | 8,441 | 5,448 | 5,637 | 5,679 | 4,759 | 4,352 | 5,332 | |
| 36 | 8,929 | 8,823 | 9,332 | 8,332 | 5,270 | 5,475 | 5,318 | 4,761 | 4,329 | 5,494 | |
| 39 | 4 | 9,675 | 8,846 | 8,787 | 8,422 | 5,451 | 5,716 | 5,367 | 4,758 | 4,367 | 5,499 |
| 23 | 9,412 | 8,902 | 9,568 | 8,308 | 5,644 | 5,623 | 5,363 | 4,782 | 4,450 | 5,315 | |
| 36 | 9,169 | 8,449 | 9,273 | 8,297 | 5,565 | 5,629 | 5,400 | 4,750 | 4,354 | 5,200 | |
| Neg | 4 | 1 | 1 | 1 | 1 | 1 | NDd | 0 | 1 | 0 | 0 |
| 23 | 1 | 1 | 1 | 1 | 1 | 1 | ND | 1 | 0 | 0 | |
| 36 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| Dryc | |||||||||||
| 50 | 4 | 9,005 | 8,842 | 8,653 | 8,524 | 5,453 | 5,649 | 5,671 | 4,889 | 4,338 | 5,401 |
| 23 | 9,429 | 8,877 | 8,897 | 8,338 | 5,458 | 5,389 | 5,264 | 4,594 | 4,367 | 5,395 | |
| 36 | 9,364 | 8,474 | 8,584 | 8,275 | 5,704 | 5,644 | 5,432 | 4,647 | 4,463 | 5,671 | |
| 486 | 4 | 9,231 | 9,148 | 8,740 | 8,678 | 5,631 | 5,470 | 5,511 | 4,804 | 4,242 | 5,322 |
| 23 | 9,292 | 8,672 | 9,069 | 8,135 | 5,430 | 5,311 | 5,177 | 4,663 | 4,384 | 5,628 | |
| 36 | 9,309 | 8,339 | 9,013 | 8,568 | 5,468 | 5,644 | 5,432 | 4,647 | 4,463 | 5,671 | |
| 39 | 4 | 9,243 | 8,373 | 8,734 | 8,455 | 5,423 | 5,496 | 5,514 | 4,779 | 4,377 | 5,357 |
| 23 | 9,264 | 9,031 | 8,413 | 8,369 | ND | 5,704 | 5,080 | 4,718 | 4,466 | 5,332 | |
| 36 | ND | 8,422 | 9,057 | 8,618 | 5,539 | 5,580 | 5,425 | 4,546 | 4,482 | 5,491 | |
| Neg | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 23 | 1 | 1 | 1 | ND | 1 | 1 | 1 | 1 | 0 | 1 | |
| 36 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
C. trachomatis isolates (isolates 50, 486, and 39) and negative (Neg) sample (phosphate-buffered saline).
Dacron swabs spiked with 50 μl of sample and placed into Aptima transport tubes.
Dacron swabs spiked with 50 μl of sample and stored dry (without medium).
ND, not done.
For the mailed swab study (Table 2), each medium and high concentration of organism was positive by all tests over the course of specimen transport from lab to lab with the exception of a single midlevel N. gonorrhoeae specimen missed that was then positive again in 2 subsequent tests. As expected, the low-level-positive specimens were positive at a rate slightly below 100%. This dilution was included to provide specimens that would be most sensitive to degradation and result in loss of signal. The majority of these negative results occurred at the start and middle of the testing. Thus, there was no suggestion of degradation of signal throughout this mailing process (a negative result on the final test would have suggested degradation, but at low titers, the first and last tests had similar numbers of negative results). There were 13 N. gonorrhoeae low-level false-negative (FN) results in specimens containing C. trachomatis/N. gonorrhoeae. For positive samples, dry and wet swab results were similar. However, some false positives (FP) were observed in our negative controls, and these were from dry swab samples only.
TABLE 2.
Aptima Combo 2 results for all mailed samples tested at the three sites
| Inoculum | No. positive/total no. |
|
|---|---|---|
| C. trachomatis | N. gonorrhoeae | |
| C. trachomatis low | 77/80 | |
| C. trachomatis medium | 80/80 | |
| C. trachomatis high | 80/80 | |
| N. gonorrhoeae low | 79/80 | |
| N. gonorrhoeae medium | 79/80 | |
| N. gonorrhoeae high | 80/80 | |
| C. trachomatis low/N. gonorrhoeae low | 20/20 | 16a/20 |
| C. trachomatis high/N. gonorrhoeae low | 40/40 | 31b/40 |
| C. trachomatis low/N. gonorrhoeae high | 40/40 | 40/40 |
| Negative | 7c/100 | 0/100 |
Four false-negative results are from 2 different samples.
Nine false-negative results are from 5 different samples.
Seven false-positive results are from 4 different samples.
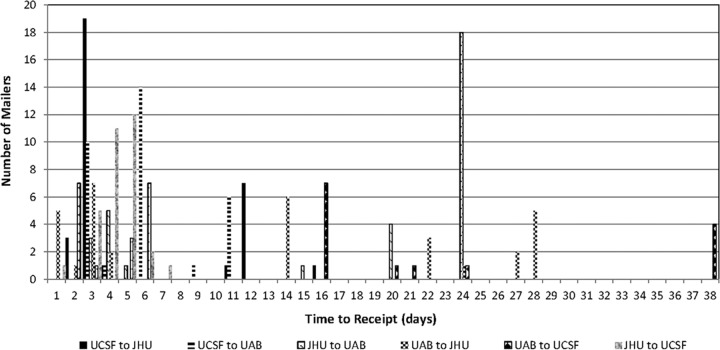
The duration of transit between laboratories (University of California, San Francisco [UCSF]-Johns Hopkins University [JHU]-University of Alabama, Birmingham [UAB]-UCSF and UCSF-UAB-JHU-UCSF) is shown in Fig. 2. For each mailer, we recorded the transport time from one site to the next site (UCSF-JHU, JHU-UAB, etc.). Although the majority of mailers (121/192) were received within 7 days, use of first-class mail for specimen transport between laboratories led to substantial variation in transport time, ranging from 2 to 39 days. Four (approximately 2%) of mailers were in transit between laboratories for more than 5 weeks.
FIG 2.
Transit time for mailers.
The temperatures (Table 3) experienced by specimens in transport were monitored using TagAlert devices, which trigger an alarm if a temperature threshold is crossed (<0°C, <2°C, >30°C, and >40°C). There were some failures (2%) of these devices which may have been due to an improperly functioning unit or start-up error by the laboratory technician. Many specimens were exposed to temperatures outside the PI specifications (between 2°C and 30°C) for the AC2 assay with 80%, 52%, and 29% exposed to >30°C (for rounds 1, 2, and 3, respectively) and an additional 2% exposed to >40°C. This was not unexpected with the study being started in late summer (September through October) and going through the mail in the southeastern United States.
TABLE 3.
Temperature ranges recorded in mailers
| Temp (°C) | % of samples mailed from lab: |
||
|---|---|---|---|
| UCSF | UAB | JHU | |
| Over 40a | 2 | 0 | 0 |
| 30–40a | 80 | 52 | 29 |
| 2–30 | 17 | 48 | 70 |
| 0–2 | 0 | 0 | 0 |
| Below 0 | 0 | 0 | 0 |
Beyond package insert specifications.
DISCUSSION
Our results show that the RNA target detected using the ACT is very stable on dry swabs under relatively high temperatures for an extended period of time, as previous studies had demonstrated (10, 11, 15). These findings suggest that the combination of the dry swab and the Aptima test may be suitable for use when specimens are collected in unfavorable settings, where ambient temperatures may be high and transport difficult. Based on our stability results using seeded specimens, the use of dry swabs may be appropriate for the Hologic NAATs as the results were virtually identical to results obtained with Hologic transport medium. Specimens stored above the recommended temperatures were not compromised, and the C. trachomatis target could be detected for up to 84 days. While we were studying trachoma isolates, our conclusions are also relevant to diagnosis of chlamydial infection of the genital tract.
The mailed swab study confirmed our lab findings. Positive dry and wet swab results were similar. There were only four samples that were negative on the final AC2 test run, regardless of temperature extremes (Table 3) or duration of transit (Fig. 2). We concluded that this was not evidence of target degradation, as they all occurred with the lowest target concentration, and 3 at this titer were negative at the first test. These results are likely a reflection of testing variability. The majority of low-level false negatives occurred in specimens containing both C. trachomatis and N. gonorrhoeae. Here, target competition during the amplification phase could have caused some misses. But, a more likely explanation is that our effort to make a dilution of N. gonorrhoeae that would approach the lowest detectable level was a success. Adding C. trachomatis to that low-level N. gonorrhoeae created a further 1:2 dilution. In that instance, repeat testing of specimens will produce some negative results (16, 17). A similar proportion of FN N. gonorrhoeae results was seen in the presence of high and low C. trachomatis levels (9/40 and 4/20, respectively), further supporting the contention that these misses were due to dilution, not competition. We had 7 negative-control samples that tested C. trachomatis positive (low RLU) with 4 different samples, an indication of laboratory contamination at all test sites. Since these false positives were seen only with dry swabs, it is possible that some aerosols were created during the transfer of sample to the AC2 transport tube. Processing samples with such a large proportion of positives (93% [63/68]) coupled with very high target concentrations (35% [24/68]) allowed a high-risk environment for contamination to occur. Under normal conditions, laboratories rarely test numerous high-titer specimens.
We found the USPS to be an inefficient method for specimen delivery over the long distances that we used. While the majority of samples were received within a week, 37% were received between 8 and 39 days. This time was well within the PI cutoff of 60 days. Presumably, shorter transit times would be seen when mailing is done to local testing sites.
Although this is a relatively small experiment, when coupled with data from other studies (10, 11, 14, 15) that have shown the maintenance of signal with specimens kept at 35°C for 2 months or longer, it seems reasonable to conclude that from the test viewpoint results should be reliable with specimens mailed from the client to the laboratory. These studies may support the promise of home- and self-collected and mailed vaginal specimens for testing for chlamydia and gonorrhea.
Our swab stability studies indicate that ACT and AC2 can be used well beyond the manufacturer's specifications for temperature and storage. Further studies are needed with matched clinical specimens to confirm our preliminary findings.
MATERIALS AND METHODS
In-house dry swab stability studies.
For the initial, in-house dry swab stability studies, we cultured three C. trachomatis isolates from Ethiopian trachoma cases (isolate 486 [serovar A] and isolates 50 and 39 [both serovar Ba]) using a modification of the procedure of Ripa and Mardh (18). Isolates were passed in shell vials until >75% of the McCoy cell monolayers contained inclusions when stained with the MicroTrak C. trachomatis culture confirmation reagent (Trinity Biotech Plc, Wicklow, Ireland), a species-specific fluorescent antibody test. Cultures of each isolate were then pooled separately, quantified, and diluted in phosphate-buffered saline (PBS) prior to inoculation of swabs. Individual Dacron AC2 specimen collection swabs from Hologic transport kits were then inoculated with 50 μl of each chlamydial isolate pool and contained approximately 16, 28, and 138 inclusions/swab for isolates 39, 50, and 486, respectively. As negative controls, Dacron swabs were inoculated with 50 μl of PBS. Following inoculation, swabs were placed into individual sterile screw-cap tubes for storage. Groups of 40 test swabs each (10 of each isolate × 3 and 10 negative controls) were placed at room temperature (23°C), in a refrigerator (4°C), or in a 36°C incubator. For comparison to the dry swabs, and more closely reflecting standard collection conditions, similar sets of inoculated swabs were placed in AC2 transport tubes containing the manufacturer's transport medium (wet controls) and also placed at 23°C, 4°C, and 36°C. At day 0 and weekly through day 84, samples and controls stored at the three different temperatures were tested by the ACT assay according to the manufacturer's instructions. Testing was not performed on days 35, 42, and 49. Prior to testing, dry swabs were rehydrated with the addition of 1.0 ml of M4 medium (Remel Inc., Lenexa, KS) to the tube and vortexed. A 200-μl aliquot (one-fifth of the swab volume) was then transferred to an AC2 transport tube for testing. This amount of sample maintains the concentration of buffers and lysis reagents in the transport tube for adequate DNA extraction. At each time interval, we ran four swab samples (3 positives and 1 negative) from both the dry and wet groups.
Specimen stability following mail transport.
To further evaluate the stability of specimens transported using the U.S. mail, 4 different strains of C. trachomatis (ATCC strain VR 885 [UW-3Cx] and three recent endocervical isolates) and four of N. gonorrhoeae (ATCC strain 19424 [NCTC 8375] and three recent endocervical isolates) were cultured to high titers. The C. trachomatis and N. gonorrhoeae suspensions were then diluted into PBS to yield 3 different concentrations. For each of the 4 C. trachomatis strains, the inoculum was diluted to result in approximately 5 to 20 inclusions/swab (low), 500 to 2,000 inclusions/swab (medium), and 2,000 to 5,000 inclusions/swab (high). For each of the 4 N. gonorrhoeae strains, culture suspension was diluted to result in three different concentrations of approximately 7.5 × 102 CFU/ml (low), 7.5 × 104 CFU/ml (medium), and 7.5 × 106 CFU/ml (high). Both dry and wet swab sets were prepared for all suspensions of each organism as described above. For the dry set, AC2 Dacron swabs were inoculated with 50 μl of positive samples and placed in sterile empty screw-cap tubes, and for the wet set, AC2 Dacron swabs were inoculated in the same manner and placed into AC2 transport tubes containing 2.9 ml of the Hologic transport medium. Thus, for each set of swabs (dry or wet) 4 were inoculated with low-, medium-, and high-titer C. trachomatis and 4 were inoculated with low-, medium-, and high-titer N. gonorrhoeae. In addition, 2 were inoculated with C. trachomatis (high)/N. gonorrhoeae (low), 2 were inoculated with C. trachomatis (low)/N. gonorrhoeae (high), 1 was inoculated with C. trachomatis (low)/N. gonorrhoeae (low), and 5 were inoculated with PBS as negative controls. This yielded a total of 68 inoculated swabs (34 wet and 34 dry) in the groups (Fig. 1).
Specimens were mailed between the three participating laboratories between September and October. To prepare the mailers, a group of mock samples were randomly packaged into 32 commercial mailer envelopes (STP-210xs, category B; Saf-T-Pak, Edmonton, AB, Canada) (12 mailers contained 3 tubes, 12 mailers contained 2 tubes, and 8 mailers contained 1 tube). Each sample was barcoded and had a mailer identification (ID) number. All samples were matched to their respective mailer and had index cards labeled with the departure and arrival location. A TagAlert monitor (Sensitech Inc., Beverly, MA) was activated and inserted into the mailer to monitor the temperature ranges to which the specimen was exposed during transport. All samples (a group of 32 mailers each) were sent blind to two testing sites: Johns Hopkins University in Baltimore, MD (JHU), and the University of Alabama at Birmingham, AL (UAB). At 48 h (following swab inoculation), staff deposited mailers in various USPS blue collection mailboxes (first-class service) within a 25-mile radius of San Francisco, CA.
The specimens were tested with AC2 according to PI instructions as follows. (i) One group of mock specimens was tested in duplicate with AC2 after 24 h of seeding the samples at the University of California, San Francisco (UCSF). For dry mock specimens, the swabs were aseptically transferred into AC2 transport tubes for testing. (ii) At each testing site, the date of mailer receipt and temperature data on the TagAlert monitor were recorded. Swabs were then tested by AC2 within 24 h of receipt and then placed back in the mailer with a new TagAlert monitor and sent via first-class mail to the other site (UAB to JHU and JHU to UAB) for a fourth round of testing. Again, these specimens were tested within 24 h of receipt and then placed back in the mailer with a TagAlert monitor and sent to UCSF for the fifth and final round of testing.
Thus, in summary, each specimen had 5 results: the first set of duplicate tests at UCSF, two more at the collaborating laboratory sites, and the fifth test upon receipt back at UCSF. There were a total of 340 results for C. trachomatis, N. gonorrhoeae, C. trachomatis and N. gonorrhoeae and controls.
ACKNOWLEDGMENTS
This work was partially supported by the donation of test kits by Hologic, Inc., and NIH grant STI CTG A100420.
REFERENCES
- 1.Taylor SN, Van Der Pol B, Lillis R, Hook EW III, Lebar W, Davis T, Fuller D, Mena L, Fine P, Gaydos CA, Martin DH. 2011. Clinical evaluation of the BD ProbeTec Chlamydia trachomatis Qx amplified DNA assay on the BD Viper system with XTR technology. Sex Transm Dis 38:603–609. doi: 10.1097/OLQ.0b013e31820a94d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernesky MA, Martin DH, Hook EW III, Willis D, Jordan J, Wang S, Lane JR, Fuller D, Schachter J. 2005. Ability of new APTIMA CT and APTIMA GC assays to detect Chlamydia trachomatis and Neisseria gonorrhoeae in male urine and urethral swabs. J Clin Microbiol 43:127–131. doi: 10.1128/JCM.43.1.127-131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Der Pol B, Liesenfeld O, Williams JA, Taylor SN, Lillis RA, Body BA, Nye M, Eisenhut C, Hook EW III. 2012. Performance of the Cobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 50:2244–2249. doi: 10.1128/JCM.06481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(RR-03):1–137. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. 2008. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 35:637–642. doi: 10.1097/OLQ.0b013e31817bdd7e. [DOI] [PubMed] [Google Scholar]
- 6.Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW III, Martin DH, Ferrero DV, Schachter J. 2003. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol 41:304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hologic Inc. 2012. APTIMA unisex swab specimen collection kit for endocervical and male urethral swab specimens. Package insert. Hologic Inc, San Diego, CA. [Google Scholar]
- 8.Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, Vitale S, Quinn TC, Sheta A, Sallam S, Mkocha H, Mabey D, Faal H. 1999. Azithromycin in control of trachoma. Lancet 354:630–635. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- 9.Dize L, Gaydos CA, Quinn TC, West SK. 2015. Stability of Chlamydia trachomatis on storage of dry swabs for accurate detection by nucleic acid amplification tests. J Clin Microbiol 53:1046–1047. doi: 10.1128/JCM.03218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaydos CA, Crotchfelt KA, Shah N, Tennant M, Quinn TC, Gaydos JC, McKee KT Jr, Rompalo AM. 2002. Evaluation of dry and wet transported intravaginal swabs in detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in female soldiers by PCR. J Clin Microbiol 40:758–761. doi: 10.1128/JCM.40.3.758-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masek BJ, Arora N, Quinn N, Aumakhan B, Holden J, Hardick A, Agreda P, Barnes M, Gaydos CA. 2009. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an Internet-based screening program. J Clin Microbiol 47:1663–1667. doi: 10.1128/JCM.02387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs MM, Van Der Pol B, Totten P, Gaydos CA, Wald A, Warren T, Winer RL, Cook RL, Deal CD, Rogers E, Schachter J, Holmes KK, Martin DH. 2008. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis 35:8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloomfield PJ, Kent C, Campbell D, Hanbrook L, Klausner JD. 2002. Community-based chlamydia and gonorrhea screening through the United States mail, San Francisco. Sex Transm Dis 29:294–297. doi: 10.1097/00007435-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Gaydos CA, Farshy C, Barnes M, Quinn N, Agreda P, Rivers CA, Schwebke J, Papp J. 2012. Can mailed swab samples be dry-shipped for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by nucleic acid amplification tests? Diagn Microbiol Infect Dis 73:16–20. doi: 10.1016/j.diagmicrobio.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schachter J, Chow JM, Bolan G, Howard H, Moncada J. 2006. Detection of Chlamydia trachomatis by nucleic acid amplification testing: our evaluation suggests that CDC-recommended approaches for confirmatory testing are ill-advised. J Clin Microbiol 44:2512–2517. doi: 10.1128/JCM.02620-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moncada J, Donegan E, Schachter J. 2008. Evaluation of CDC-recommended approaches for confirmatory testing of positive Neisseria gonorrhoeae nucleic acid amplification test results. J Clin Microbiol 46:1614–1619. doi: 10.1128/JCM.02301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripa KT, Mardh P-A. 1977. Cultivation of Chlamydia trachomatis in cycloheximide-treated McCoy cells. J Clin Microbiol 6:328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]