ABSTRACT
Infection by Leishmania (Viannia) panamensis, the predominant etiologic agent for cutaneous leishmaniasis in Colombia, is characterized by a chronic mixed inflammatory response. Current treatment options are plagued by toxicity, lengthy treatment regimens, and growing evidence of drug resistance. Immunotherapy, modulating the immune system to mount a protective response, may provide an alternate therapeutic approach. We investigated the ability of the Toll-like receptor 9 (TLR9) ligand CpG to modulate established disease in the L. (V.) panamensis mouse model. Treatment of established infection with a high dose (50 μg) of CpG ameliorated disease and lowered parasite burden. Interestingly, immediately after treatment there was a significant increase in transforming growth factor β (TGF-β) and concomitantly an increase in T regulatory cell (Treg) function. Although a general reduction in cell-mediated immune cytokine and chemokine (gamma interferon [IFN-γ], interleukin 10 [IL-10], IL-13, IL-6, granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-4, and MIP-1α) responses of the treated mice was observed, certain chemokines (RANTES, monocyte chemoattractant protein 1[MCP-1], and IP-10) were increased. Further, in peripheral blood mononuclear cells (PBMCs) from patients with cutaneous leishmaniasis, CpG treatment similarly exhibited a dose-response effect on the production of IFN-γ, IL-17, IL-10, and IL-13, with reductions observed at higher doses. To further understand the underlying mechanisms and cell populations driving the CpG mediated response, we examined the ex vivo dose effects mediated by the TLR9+ cell populations (dendritic cells, macrophages, and B cells) found to accumulate labeled CpG in vivo. Notably, B cells altered the production of IL-17, IL-13, and IFN-γ, supporting a role for B cells functioning as antigen-presenting cells (APCs) and/or regulatory cells during infection. Interestingly, B cells have been previously demonstrated as a primary type of APC in patients infected with L. (V.) panamensis and thus may be useful targets of immunotherapy. Collectively, our results show that CpG-induced immune regulation leads to a dampening of the host immune response and healing in the mouse model, and it may provide an alternate approach to treatment of cutaneous leishmaniasis caused by L. (V.) panamensis.
KEYWORDS: CpG, Leishmania, Treg, immunomodulation, inflammation
INTRODUCTION
Leishmaniasis, caused by infection with the protozoan parasite Leishmania, affects approximately 12 million people worldwide (1). Infection may manifest as cutaneous, mucocutaneous, or visceral disease dependent upon both parasite and host intrinsic factors (2). In Colombia, the species Leishmania (Viannia) panamensis is responsible for the majority of leishmaniasis cases and typically results in the cutaneous form of disease (3). Despite its public health importance, there is a lack of effective treatment options for cutaneous leishmaniasis (CL). Current first-line treatments, antimonial compounds, are impeded by lengthy treatment regimens, severe toxicity, and growing evidence of drug resistance (4, 5). Consequently, new approaches to treatment are desperately needed.
Rather than a classical chemotherapeutic strategy to directly kill the parasite, methods of initiating or redirecting the immune system toward a protective response are now being considered aas n alternate approach to treatment for leishmaniasis and other diseases (6, 7). This approach could be especially appropriate for leishmaniasis, as the immune response is directly linked to outcome of infection. Indeed, immunotherapy has been tried in different modalities for several Leishmania species, with various results (8–15). Understanding the immune response that leads to healing is important for the development of appropriate modulation methods. Based on studies in both human and mouse models of L. (V.) panamensis infection, it is apparent that an unregulated, mixed cytokine response is induced by infection. Specifically, interleukin 13 (IL-13) as well as IL-10 favors parasite persistence and disease; mice are resistant to disease in the absence of these cytokines or the signaling receptor of IL-13, IL-4Rα (16). Additionally, among infections by species in the Leishmania (Viannia) subgenus, heightened inflammation is associated with more severe forms of disease (13). Recently, we discovered a beneficial role of T regulatory cells (Tregs) in controlling excessive inflammation and promoting amelioration of disease in the mouse model (17) and during the course of human disease (18). Therefore, immunotherapy directed toward downregulation of the cytokines IL-13 and IL-10 and/or increasing Treg numbers/functional capacity could potentially be effective in promoting disease resolution.
Toll-like receptors (TLRs) are pattern recognition receptors that induce cellular signaling and activation in response to pathogen-associated molecular patterns (PAMPs) (19). TLR9 is specific for unmethylated CpG oligonucleotides generally found in viral or bacterial DNA. In humans, TLR9 expression is primarily restricted to B cells, plasmacytoid dendritic cells (pDC), and polymorphonuclear leukocytes (PMNs) and may be expressed on some activated monocytes. However, in mice, expression is expanded to include monocytes and macrophages (Mϕ) (20, 21). Given the role of these cells in antigen presentation and the ongoing immune response, TLR9 ligands as well as other TLR (TLR4 and TLR7) ligands have been employed for immunotherapy for several diseases, including leishmaniasis (9–11, 14, 20, 22–25).
Generally, treatment of leishmaniasis (vaccine immunotherapy or adjuvant alone) with CpG or other TLR ligands directed the host immune response toward Th1-like (higher levels of IFN-γ) or multifunctional T cell responses, which were associated with healing (14, 24, 26, 27). However, ligation of TLRs can activate or downregulate the immune response; studies indicate that the precise effect can be dose dependent. For example, the TLR9 ligand CpG initiates a Th1-mediated response at lower doses; however, experimental evidence has also shown that TLR9 signaling in response to high doses of CpG can induce a regulatory response (28–33). CpG activation of TLR9 has led to healing in experimental autoimmune diabetes (34) and amelioration of asthma and inflammatory arthritis through downregulation of Th2 cytokines (35, 36). We therefore hypothesized, in light of recent results concerning the potential role of Tregs in leishmaniasis caused by L. (V.) panamensis infection and the role of inflammation in the pathology caused by L. (Viannia) parasites (37–40), that CpG under the appropriate conditions could ameliorate pathology/disease.
Herein we show that CpG can downregulate cytokine production in human peripheral blood mononuclear cells (PBMCs) from patients with CL and murine PBMCs and draining lymph node (dLN) cells from infected mice in a dose-dependent fashion. We found that high-dose CpG effectively ameliorates disease in the murine model and is characterized by increased Treg function, decreased cytokine responses (Th1 and Th2), and chemokine modulation. In vitro cell depletion studies demonstrated a role for B cells in antigen presentation and cytokine responses and CpG-mediated immunomodulation. Taken together, the results of these studies provide support for further clinical investigation of CpG therapy for cutaneous disease caused by L. (V.) panamensis.
RESULTS
CpG downregulates the cytokine response in lymphocytes from infected patients and mice.
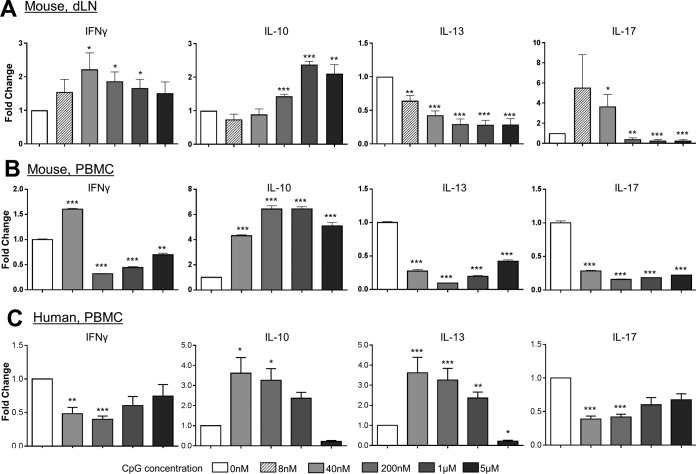
To initially evaluate CpG as an immunomodulator for the immune response caused by L (V.) panamensis infection, studies were performed in vitro using peripheral blood mononuclear cells (PBMCs) from infected patients and cells (PBMCs and dLN cells) from chronically infected BALB/c mice. As dichotomous, dose-dependent outcomes of CpG-mediated therapy have been reported, we examined whether this holds true in cells from mice and patients infected with L. (V.) panamensis. dLN cells from L. (V.) panamensis-infected mice were stimulated with promastigote Leishmania antigen (pLAg) in combination with various concentrations of CpG (8 nM to 5 μM). Consistent with the description by Mellor et al. (41) of a biphasic gamma interferon (IFN-γ) response (42), lower concentrations of CpG induced the production of IFN-γ; however, the response then decreased at higher concentrations of CpG (1 and 5 μM) from a peak response at 8 to 40 nM CpG (Fig. 1A and B). Notably, in vitro CpG stimulation appeared to promote IL-10 production in both the mouse and human responses (Fig. 1); hence, the balance of the immune response would appear to be shifted with the higher CpG stimulation concentrations. Also of note is difference between PBMCs and dLN cells in their responses to antigen in the presence of CpG. While the IL-10 and IL-13 responses were comparably affected in the PBMCs or dLN cells by a given concentration of CpG, the dampening of the maximum IFN-γ and IL-17 responses became evident at lower CpG concentrations for PBMCs. These differences potentially reflect the number/balance of circulatory T effector cells and/or antigen-presenting cell (APC) populations expressing TLR9.
FIG 1.
CpG modulates the human and mouse response to parasite antigen in vitro. Draining lymph node cells (A) or PBMCs (B and C) from infected BALB/c mice (A and B) or human patients (C) were incubated with CpG 1826 (A and B) or CpG ODN 2006 (C) at the given concentrations and leishmanial antigen (pLAg) for 96 h. The modulation of the IFN-γ, IL-10, IL-13, or IL-17 cytokine response to pLAg is indicated. Data represent means and SEM of the fold change from the combined results from 3 independent experiments (A), PBMCs pooled from 5 infected mice (B), and PBMCs from 10 patients with acute cutaneous leishmaniasis (C). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to values for untreated cells).
PBMCs were isolated from infected patients and cultured with pLAg in the presence or absence of CpG (40 nM to 5 μM). Although the specific responses were somewhat variable between individual patients, treatment with CpG paralleled the responses found for the murine PBMCs. Notably, there was a reduction in the antigen-specific production of IFN-γ, IL-17, and IL-13 cytokine responses associated with infection (Fig. 1B and C) generally over the range examined (40 nM to 1 μM). However, IL-10 production (as also found for the mouse) increased (40 nM to 1 μM) with CpG stimulation. Overall, these results demonstrate that the response to CpG is dose dependent and that CpG is capable of dampening the ongoing human inflammatory immune response to leishmanial antigens.
As the human and mouse PBMC cytokine response patterns appear to be similar, this allowed for the mouse model to be used for screening of the effectiveness of CpG in modulating the immune response and parasite burden in disease caused by L. (V.) panamensis infection in vivo.
CpG treatment reduces lesion size and parasite numbers in vivo.
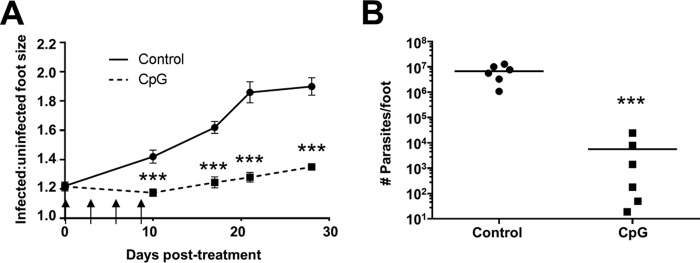
The ability of CpG to induce immunotherapeutic healing was evaluated in the L. (V.) panamensis chronic infection mouse model. In alignment with the overall goal of immunotherapy to treat established disease, treatment of the L. (V.) panamensis-infected mice was initiated following lesion development (3 to 5 weeks postinfection) rather than simultaneously with or relatively immediately after infection (15, 43, 44). Additionally, mice were treated perilesionally, as local rather than systemic treatment would be a less toxic approach and reduce side effects. Local treatment is considered preferable and acceptable for single, small, localized uncomplicated lesions in the absence of risk factors for mucocutaneous disease (45, 46). The relatively high dose of 50 μg of CpG was used, as it had been previously demonstrated to elicit the induction of Tregs after either systemic (28, 34, 47) or dermal (48, 49) delivery of CpG. Notably, the downregulation of the immune and inflammatory responses and the induction of Treg activity have been recently shown in this model to ameliorate disease (17).
Local CpG administration successfully reduced lesion size compared to that in the control-treated mice (Fig. 2A). This corresponded to a >1,000-fold reduction in parasite burden (Fig. 2B). This healing response could potentially be through different, but not mutually exclusive, mechanisms. It was possible that the high level of CpG administered, as expected based upon in vitro results, downregulated the ongoing inflammatory and mixed cytokine responses that promote disease. Alternatively, as murine macrophages express TLR9, it was possible that CpG activation of Leishmania-infected macrophages led to induction of parasite killing.
FIG 2.
CpG treatment of chronically infected mice results in decreased lesion size and parasite burden. Following the development of lesions (∼4 weeks), BALB/c mice were injected perilesionally with 50 μg of CpG 1826, twice a week for 2 weeks for a total of four doses, indicated by arrows. Lesions were measured throughout the course of infection (A), and parasite burden was analyzed as described in Materials and Methods at the termination of the experiment (B). Data represent results from at least two independent experiments. ***, P ≤ 0.001.
CpG acts independently of direct parasite killing.
To further evaluate the mechanism by which CpG mediated lesion resolution and parasite clearance, we first determined whether CpG directly triggered macrophage killing of Leishmania. Murine bone marrow-derived macrophages (BMMs) were infected with L. (V.) panamensis. High doses of CpG (1 or 5 μM) were employed to approximate the in vivo conditions. Infection was monitored for 24 to 72 h after addition of CpG and/or culture supernatants. No changes were observed in the percentage of parasitized cells in CpG-treated L. (V.) panamensis-infected macrophages (Fig. 3A) or in the number of parasites/macrophage (Fig. 3B) when infected cells were treated with CpG alone (1 or 5 μM; 24 to 72 h). As expected, miltefosine-induced parasite killing was effective under all conditions (50, 51).
FIG 3.
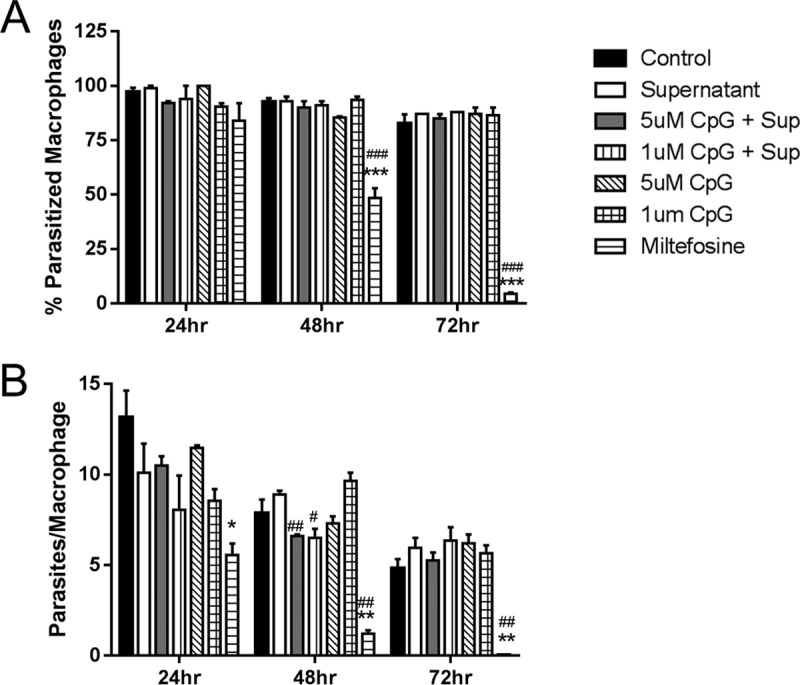
CpG does not induce parasite killing within infected mouse bone marrow-derived macrophages. Bone marrow-derived macrophages were infected with late-stationary-phase L. (V.) panamensis promastigotes (isolated from Percoll gradients as indicated) for 4 h, washed, and then incubated for 24, 48, or 72 h with (i) CpG 1826 (1 or 5 μM), (ii) miltefosine (20 μM, positive control; Sigma), (iii) 72-h supernatants from dLN cell cultures (as described in the text), or (iv) 72-h supernatants from dLN cell cultures together with CpG 1826 (1 or 5 μM). Following staining with Giemsa (Fluke Analytical, Sigma-Aldrich), the mean number of parasites per macrophage (B) and the percentage of parasitized macrophages (A) were calculated by counting 100 macrophages per sample, in triplicate per determination. Data represent those from one of three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001 compared to control. #, P ≤ 0.05; ##, P ≤ 0.01; and ###, P ≤ 0.001 compared to supernatant alone.
Previous reports have indicated reduced survival of Leishmania donovani or Leishmania major in CpG 1826-treated macrophages (11, 26), although intracellular killing mediated through TLR9 appeared to require the additional activation by IFN-γ. To take into account the lesion microenvironment in vivo, we used culture supernatants from pLAg-stimulated dLN cells of L. (V.) panamensis-infected mice, which produced the mixture of cytokines (IFN-γ, IL-10, and IL-13) made in response to infection (16, 17). In general, under these conditions, no evidence of macrophage-activated killing was obtained with infected macrophages either treated with culture supernatants alone or treated with CpG (1 or 5 μM) together with culture supernatants. Even though high levels of IFN-γ were present (39 ng/ml), parasite killing was not consistently observed; although some effect was observed at 48 h, this was not evident at 24 or 72 h of treatment. It should be noted that Leishmania (Viannia) species are less susceptible to IFN-γ-mediated macrophage killing than other Leishmania species (52); further, the immune response to infection is mixed Th1-Th2, with IL-13 and IL-10 being present concomitantly with IFN-γ (16). Although these results do not exclude the possibility that CpG might facilitate some minimal parasite clearance in vivo, it appears likely that CpG would not primarily contribute to disease control through the induction of macrophage-mediated parasite destruction. These results suggest that in this experimental model of L. (V.) panamensis, CpG did not primarily act through enhanced macrophage-mediated killing.
CpG treatment generally induces downregulation of the immune response in vivo.
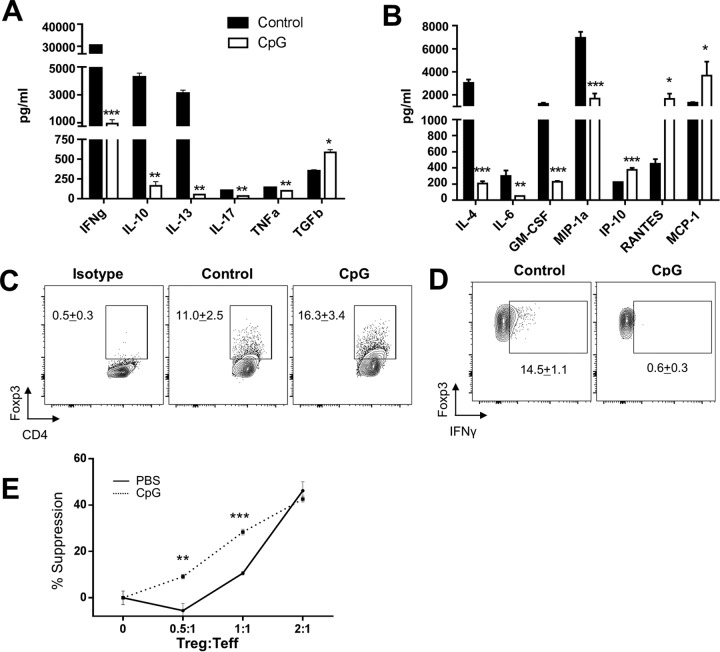
Given the known role of high-dose CpG to induce a regulatory response and the results of the ex vivo analyses (Fig. 1), we hypothesized that CpG might impart a therapeutic response through immune manipulation of inflammatory responses in vivo. Similar to our ex vivo stimulation findings, in vivo CpG treatment downregulated the mixed cytokine response to L. (V.) panamensis infection, with a significant reduction in IFN-γ, IL-10, IL-4, IL-13, tumor necrosis factor alpha (TNF-α), IL-6, and IL-17 (Fig. 4A and B); further, a reduction in certain chemokines (MIP-1α and granulocyte-macrophage colony-stimulating factor [GM-CSF]) but not others (monocyte chemoattractant protein 1 [MCP-1]/CCL2, RANTES/CCL5, and IP-10/CXCL10) was observed. In general, it appeared that the CpG treatment resulted in lowering of the immune responses.
FIG 4.
CpG treatment of chronically infected BALB/c mice results in decreased cytokine responses and the increased function of T regulatory cells. The immune response was evaluated 2 days following the last injection of CpG 1826 treatment (4 × 50 μg/dose) of established L. (V.) panamensis-infected BALB/c mice. Cytokine (IFN-γ, IL-10, IL-13, IL-17, TNF-α, and TGF-β) production was measured by ELISA in response to pLAg in draining lymph node cells from control (PBS)-treated and CpG 1826-treated mice (A). Chemokine and cytokine levels were measured using Luminex as described in Materials and Methods in response to pLAg in draining lymph node cells from control (PBS)- and CpG-treated mice (B). FACS analyses of Foxp3+ cells gated on CD4+ cells from the draining lymph nodes of infected mice (control and CpG treated) (C). FACS analyses of CD4+ Foxp3+ T cells expressing IFN-γ in draining lymph node cells from control- or CpG-treated infected mice (D). Levels of Foxp3 expression were comparable between CD4+ CD25+ cells isolated from naive and infected mice. The ability of CD4+ CD25+ cells from the draining lymph nodes of control- or CpG-treated infected mice to suppress proliferation of CD4+ CD25− cells from naive mice in response to anti-CD3 and irradiated APC stimulation was assessed (E). Results represent data from two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Interestingly, the reduced cytokine response corresponded with an increase in the regulatory cytokine transforming growth factor β (TGF-β) (Fig. 4A). We therefore hypothesized that CpG impacted cytokine responses in part through upregulation of Tregs. Indeed, there was an approximately 50% increase in the percentage of total CD4+ Foxp3+ cells in the dLNs of treated mice (Fig. 4C). In addition, CpG treatment resulted in a decrease of CD4+ Foxp3+ cells producing IFN-γ (Fig. 4D), which has also been noted to occur after treatment of L. (V.) panamensis-infected mice with anti-IL-2–rIL-2 complexes (17). In addition, Treg plasticity within inflammatory microenvironments has been well documented (53, 54). Production of IFN-γ by Foxp3+ cells is indicative of a dysregulated phenotype, with Tregs having impaired suppressive capacity (55). As increased functional capacity of Tregs was found after successful treatment in patients infected with L. (V.) panamensis (18), we assessed whether Tregs had increased suppressive function following CpG treatment in addition to an increase in the percentage of Tregs. Indeed, CD4+ CD25+ cells from CpG-treated mice had a 3-fold increase in their ability to suppress proliferative responses compared to that of CD4+ CD25+ cells from infected control mice (Fig. 4E). That this induced regulatory response would lead to healing is not surprising, as the importance of regulatory T cells in healing in L. (V.) panamensis infection has been shown (18) for human infection and, more recently, in the murine model of disease (17).
CpG cellular targets and consequent mode of action.
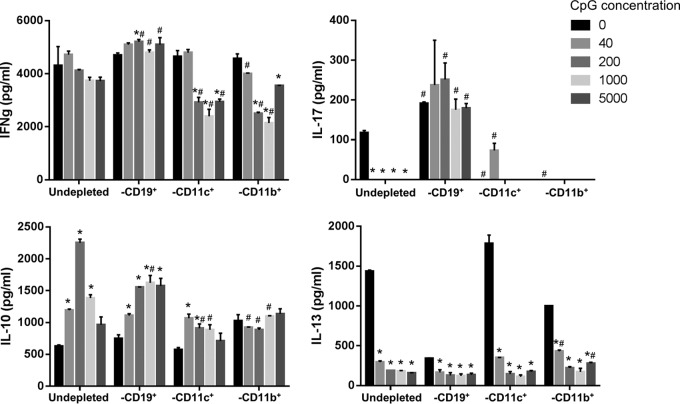
To further delineate the underlying mechanisms leading to immunomodulation by CpG, we first examined the TLR9+ cells that took up APC-labeled CpG after perilesional injection. Not unexpectedly, macrophages, dendritic cells, and B cells in the draining lymph node and lesion site were all highly (∼90%) and proportionally equally labeled with CpG (data not shown). Consequently, we sought to determine the effect of each TLR9-expressing population using cellular depletion (B cell, dendritic cell, and macrophage) and in vitro stimulation assays employing splenocytes from chronically infected mice (Fig. 5) and examined the CpG treatment effect on IFN-γ, IL-10, IL-13, and IL-17 production.
FIG 5.
CD19+ B cells are critical targets of CpG immunomodulation. Pooled splenocytes (whole or CD19, CD11b, or CD11c cell depleted) from L. (V.) panamensis chronically infected mice were cultured with CpG 1826 at the given concentrations and leishmanial antigen (pLAg) for 72 h. Cytokine (IFN-γ, IL-10, IL-13, and IL-17) production was measured in culture supernatants by ELISA. Data are representative of those from 3 independent experiments. *, P ≤ 0.05 compared to the value for cells incubated in the absence of CpG; #, P ≤ 0.05 compared to the value for nondepleted cells incubated with the same concentration of CpG.
The cytokine responses without CpG (basal levels) did not significantly decrease or change for the depleted cell cultures (B cells, dendritic cells, and macrophages) compared to the nondepleted cell populations in the case of the IL-10 and IFN-γ responses; in contrast, the IL-13 response was significantly diminished in B cell-deficient cultures, while IL-17 production was reduced in the absence of either macrophages or dendritic cells and increased in the absence of B cells. The lack of marked changes in the basal IL-10 and IFN-γ responses in cell-depleted cultures may reflect compensatory increases in the various cells in these cultures that can produce these cytokines or present antigen. The increased IL-17 and decreased IL-13 responses found in the absence of B cells are consistent with observed functions of B cells (56, 57). Notably, an investigation of Mycobacterium tuberculosis (58) demonstrated that B cells modulated IL-17 levels and PMN recruitment during infection and Mycobacterium bovis BCG vaccination. Further, recent studies of L. (V.) panamensis-infected patients (59) indicated that B cells can act as APCs to induce T cell IL-13.
In response to TLR9 stimulation, modulations of cytokine responses were observed that appeared to be associated with the different populations of APCs. Cultures without B cells failed to modulate IFN-γ; in fact, IFN-γ levels appeared to increase in response to CpG stimulation in the absence of B cells. In contrast, cultures depleted of either dendritic cells or macrophages had reduced levels of IFN-γ with increasing amounts of CpG; further, the decreases in IFN-γ production was more pronounced in the dendritic cell- and macrophage-depleted populations than for the undepleted cells. These results suggest that TLR9 stimulation of B cells is responsible for decreased IFN-γ and that this is opposed by dendritic cells and macrophages. This is consistent with other studies that indicate that B cells stimulated with CpG can develop regulatory function (60, 61) especially in the context of BCR signaling. Similarly, IL-17 production significantly increases and little modulation is observed in the absence of B cells, suggesting that these cells are important in the regulation of IL-17.
Although dendritic cells and macrophages can instruct and induce T cell production of IL-13, B cells in the murine model of L. (V.) panamensis infection also appear to be important for the modulation of IL-13; in the absence of B cells, little IL-13 is produced. These results are consistent with other studies showing the importance of B cells in IL-13 production (56, 57, 62). However, only in cultures that contained B cells was IL-13 significantly downmodulated, suggesting that targeting this cell population could be critical to reductions of this cytokine.
In the case of IL-10, cultures without B cells behaved similarly to undepleted cells, showing increased IL-10 production with increasing CpG stimulation, whereas cultures depleted of dendritic cells or macrophages produced significantly less IL-10 in response to CpG. These results suggest that macrophages and/or dendritic cells are responsible for the increased IL-10 observed with increasing CpG stimulation. Consequently, different cells appear to act to regulate different cytokines during infection. The importance of each cell population in relation to specific immunomodulation of cytokines affecting disease may provide for selective targeting and might be directed to improve treatment.
DISCUSSION
A hyperimmune (mixed Th1-Th2) response is associated with a more severe disease outcome in Leishmania (Viannia) infections (13, 37–40, 63–65). Recently, it has been found in human L. (V.) panamensis infection that increased Treg capacity correlates with healing (18). In the murine model of L. (V.) panamensis, the transfer of Tregs or their induction through the use of IL-2–anti-IL-2 complexes leads to significant disease amelioration and reduction of parasite burden and cytokine responses (Th1/Th2) (17). However, Treg transfer and IL-2 complex treatment are expensive and not practical treatment approaches for leishmaniasis. Consequently, the development of alternate methods for modulating the immune response is of interest.
CpG treatment has been shown to induce a regulatory response (34, 41, 66) when relatively high levels of CpG (≥50 μg/mouse) are employed (41). Initiation of the Treg response is reportedly due to the induction of indoleamine 2,3-deoxygenase (IDO) (34, 41, 66) and the regulatory cytokines IL-10 (31) and TGF-β (67). The high-dose-mediated regulatory effects of CpG have been ascribed to TRIF/TRAF6-dependent signal transduction in dendritic cells (67). Thus, high-dose CpG might have immunotherapeutic potential for local treatment of cutaneous leishmaniasis caused by L. (V.) panamensis infection. Previous studies have utilized CpG alone (15) or in combination with other adjuvants or L. major antigen for both treatment and prevention of experimental leishmaniasis (68). However, therapeutic treatment by CpG alone of L. major is controversial, and both healing and the absence of an effect have been reported (14, 15); where healing was observed, however, this was accompanied by a Th1 response. In the current study, we employed a high dose of CpG, administered locally after the development of lesions, which induces a regulatory response and effectively ameliorates L. (V.) panamensis infection. As different species of Leishmania establish distinct inflammatory milieu in the host (69), it is not surprising that the effects of CpG may be dependent on the inherently different environments created by infection. Not only are opposing immune responses elicited by CpG with different species of Leishmania but also both activating and regulatory effects of CpG have been observed during different inflammatory disease states, including asthma, infectious disease, and cancer models (20, 70, 71). Thus, CpG ligation of TLR9 may have different downstream effects during steady state and in the presence of inflammation. The mechanism behind this deserves further exploration, as it could provide insight into CpG mechanistic responses and also the Leishmania species-specific interactions with the host immune system.
In the mouse model, the role of IL-13 or IL-10 in L. (V.) panamensis infection development has been established (16) using genetically deficient mice. However, IL-10 does not likely sustain infection primarily via Tregs, as Treg expansion or transfer (17) led to disease amelioration and concomitant reduction in IL-10 as well as IL-13, IFN-γ, and IL-17. These cytokines were also reduced in CpG-treated mice, which likely contributed to the disease resolution observed. In addition to a reduction in these cytokines, CpG treatment led to a reduction in GM-CSF and MIP-1α (CCL3). GM-CSF is important for the development and regulation of granulocytic and monocytic cells and their activation (72); PMNs and macrophages act as host cells for Leishmania, which is critical for parasite persistence. MIP-1α is broadly chemotactic (granulocytes, myeloid dendritic cells [mDC], Mϕ, memory T cells, Th1 cells, Tregs, NK cells, and pDC) and has been implicated in both disease exacerbation and healing in leishmaniasis, dependent upon the infective species (73, 74). L. (V.) panamensis induces MIP-1α production by infected macrophages, which is associated with parasites causing chronic disease and suggestive of a role in disease exacerbation.
The alteration in cytokines and chemokines was not a global downregulation of immune responses by CpG but rather a more balanced modulation of responses, with an increase in RANTES (CCL5) and MCP-1 (CCL2). RANTES is associated with the recruitment of T cells, basophils, and eosinophils. Studies with RANTES neutralization suggest that the chemokine may ameliorate disease caused by L. major, although other studies indicate that CCR5, the RANTES receptor, is critical for Treg homing and parasite persistence (74–76). The role of RANTES in infection caused by L. (V.) panamensis remains to be determined; however, it has been noted that RANTES/CCL5 levels are reduced in macrophages infected with L. (V.) panamensis parasites causing chronic disease (77), while infection with parasites isolated from self-healing lesions do not modulate RANTES expression. MCP-1/CCL2 has been linked to monocyte, NK cell, DC, and T cell recruitment and has been reported to induce macrophage-mediated killing of Leishmania (78, 79). However, in genomic studies of human leishmaniasis caused by L. (V.) braziliensis, mucocutaneous disease was correlated with higher MCP-1 expression (80), although the chemokine was found to have little impact on cutaneous disease. Further, macrophages infected with L. (V.) panamensis have been demonstrated to express higher levels of MCP-1 when infected with parasites from chronic versus acute infection (77, 81), suggesting that MCP-1 expression may facilitate the inflammation associated with disease. The recruitment and activation of innate immune cells would enable the activation of adaptive immune cell responses, potentiating chronic inflammation and immunopathology; however, this could be counterbalanced by the role of RANTES in recruitment of Treg cells. Presently, it is not clear what precise impact increases in specific chemokines might have on the outcome of L. (V.) panamensis infection; further work is required to determine their role in L. (V.) panamensis infection.
High-dose CpG, injected perilesionally, is nonpreferentially taken up by cells in the feet and dLNs. We examined the contribution of TLR9-expressing cell types to the CpG response by ex vivo cell depletion. Although it is known that there are interactions between these various potential APCs and some effects could in part be indirect, the approach taken was to examine the effect of depleting one of these populations on the overall ongoing immune response as a method for approximating which cells (if any) might be useful CpG targets to elicit a healing response. Overall, these data show the complexity of the immune response to infection in the case of L. (V.) panamensis and also demonstrate a critical role of B cells in the modulation of the immune response with CpG treatment. The roles of B cells in leishmaniasis are not fully understood and can vary dependent upon the strain and species (69, 82). B cell-derived antibody may either exacerbate (83, 84) or ameliorate (85) disease; B cell-derived IL-10 (82) is related to disease progression. Further, B cells can be key antigen-presenting cells during disease (59, 86). Notably, B cell levels in lesions of L. (V.) panamensis have been correlated with disease severity (87). Further, it is of interest that B cells as well as pDC have been shown to be important in the regulation and maintenance of T regulatory cells (88–90). Selective targeting of CpG to B cells in principle would potentially result in lowered IFN-γ, IL-10, and IL-13 as well as IL-17, a response observed in this study and previously (17) to result in amelioration of disease. In future studies, it would be of interest to examine the selective targeting of B cells in the treatment of disease.
Here, we have demonstrated the utility of in vitro evaluation of the immunomodulator CpG to assess an in vivo immune responses to L. (V.) panamensis infection. By employing both patient samples and the mouse model, we have shown evidence that the TLR9 ligand CpG ameliorates the disease-associated mixed-cytokine response as well as disease and parasite burden in vivo in the mouse model of infection. The downregulation in vivo corresponded to increased Treg percentages, with improved suppressive capacity and downregulation of both Th1 and Th2 responses. In vitro analyses in the mouse model indicated an important role of B cells as APCs during infection; these are consistent observations of the responses in human patients (59). For in vivo experiments, a local rather than systemic route of administration of CpG was used, and this resulted in disease amelioration. Cutaneous leishmaniasis is well suited for topical treatment, and this approach has been recently undertaken in various drug formulations (91). Development of topical treatments would potentially minimize the need for health center visits required for administration of injections, minimize drug side effects (particularly of systemically delivered drugs), and enhance adherence to treatment. Further, the use of immunomodulators in conjunction with topical drugs has the potential to reduce the amount of drug required to achieve healing. Lipid-based or other transcutaneous delivery systems have been reported for administration of CpG and may have potential for treatment in patients (48, 49, 92). CpG may prove to be an effective alternative treatment or coadjuvant that could be used either independently or in combination with lower doses of standard chemotherapeutic drugs for the treatment of cutaneous leishmaniasis caused by L. (V.) panamensis.
MATERIALS AND METHODS
Patient samples and analyses.
Peripheral blood samples (100 ml) were obtained from cutaneous leishmaniasis patients from the southwestern region of Colombia (departments of Valle del Cauca and Nariño). Participants included 9 males and 1 female 22 to 54 years of age (median = 32 years) who had typical cutaneous leishmaniasis lesions that had been parasitologically diagnosed by smear, culture, or biopsy and had not received antileishmanial treatment before enrollment. Parasites were isolated from 6 of these 10 patients; all strains were typed as L. (V.) panamensis. All subjects had negative serology for HIV and human T cell leukemia virus type 1 (HTLV-1) and provided written informed consent. The study protocol, consent forms, and all procedures were approved by the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) Institutional Review Board.
Analysis of the immune response of human PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). Promastigote Leishmania antigen (pLAg) was prepared by suspending promastigotes of L. (V.) panamensis strain MHOM/COL/03/3594 at a concentration of 1 × 107/ml, followed by 6 cycles of freezing in liquid nitrogen and thawing at 37°C. Cells were suspended in RMPI 1640 (Sigma-Aldrich) with 10% fetal bovine serum (FBS; GIBCO, Carlsbad, CA), 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml). A total of 4 × 105 PBMCs were distributed in 96-well plates in 200 μl per well with or without pLAg (8 μl to reach a parasite/cell ratio of 0.2:1) and/or 0.04, 0.2, 1, and 5 μM CpG ODN 2006 (InvivoGen, San Diego, CA; human class B). The cells were incubated for 5 days at 37°C with 5% CO2. Cytokines were measured in culture supernatants using a Luminex screening assay (R&D Systems, Minneapolis, MN). Luminex assays were performed using 50-μl volumes of culture supernatants in duplicate according to the manufacturer's specifications.
Animals.
Female BALB/c mice were obtained from the National Cancer Institute (Frederick, MD) and housed at Yale School of Medicine animal facilities under the approval of the American Association for Accreditation of Laboratory Animal Care. All mouse procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (93) and were approved by the Yale University Committee for the Care and Use of Animals.
Leishmania culture, infection, and parasite burden analysis.
L. (V.) panamensis (MHOM/1995/CO/1989) was cultured in Schneider's Drosophila medium supplemented with 20% heat-inactivated fetal calf serum (FCS) and 17.5 μg/ml of gentamicin. Infective parasites were isolated from low-passage-number late-stationary-phase cultures off the 45%/60% Percoll gradient interface, as previously described (16). Parasites (5 × 104) were injected intradermally into the hind foot of a mouse. Lesion development was monitored by measuring the thickness of the infected and uninfected feet using a dial gauge caliper (Starrett Thickness Gauge). At the termination of experiments, parasites were quantified by the limiting-dilution assay as previously described (94).
CpG treatment in vivo.
Mice with established lesions were injected subcutaneously, adjacent to the lesion (perilesionally), with 50 μg of CpG 1826 (mouse class B [Trilink Biotechnologies, San Diego, CA] or oligonucleotide synthesized by Keck Center, Yale University), or phosphate-buffered saline (PBS) as a vehicle control, twice a week for 2 weeks for a total of four doses. Two days following the final treatment, analysis of cytokines, as well as the percentages of CD4+ Foxp3+ cells and IFN-γ intracellular cytokine production, was carried out for the dLNs. Parasite load was calculated at the termination of the experiment, as previously described (94). Lesion size was monitored as described above.
CpG treatment in vitro.
For in vitro experiments, PBMCs or dLN cells (5 × 106 per ml) from L. (V.) panamensis-infected mice were cocultured with CpG 1826 at the doses indicated for the respective experiments, in the presence of pLAg (equivalent to 1 × 106 or 5 × 106 organisms/ml) for 3 to 5 days prior to cytokine analysis.
Mouse model cytokine analyses.
For the analysis of mouse cytokine production, PBMCs or single-cell suspensions made from the dLNs were cultured at 5 × 106 cells/ml in RPMI medium supplemented with 10% FCS in the presence of pLAg for 72 h. Supernatants were collected and IFN-γ, IL-10, IL-13, IL-17, IL-6, TNF-α, and TGF-β were measured by enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer's protocol (eBioscience) or using the Luminex system employing either R&D Systems or EMD Millipore specific cytokine/chemokine sets.
For intracellular cytokine analysis following pLAG incubation, cells were cultured for an additional 4 h in the presence of brefeldin A (BD Golgi Plug), Fc receptors were blocked (anti-CD16/CD32; BD Pharmingen), and surface markers were stained with anti-CD3 (145-2C11; BD Biosciences) and anti-CD4 (RM4-5; BD Biosciences). Cells were permeabilized with a Foxp3/transcription factor staining buffer set (eBioscience) and stained with anti-Foxp3 (FJK-16S; eBioscience) and anti-IFN-γ (XMG1.2; BD Pharmingen). Fluorescence-activated cell sorting (FACS) data were acquired on an LSRII flow cytometer (BD Biosciences), using FACSDiva software, and collected data were analyzed on FlowJo software (Tree Star, Inc.).
In vitro murine macrophage-Leishmania killing assays.
Bone marrow-derived BALB/c mouse macrophages were generated in vitro by culturing cells harvested from mouse femurs and tibiae in L cell-conditioned medium (RPMI 1640 supplemented with 30% L929 cell supernatant, 20% heat-inactivated FBS, and 1% penicillin-streptomycin) for 4 days. On the fourth day, cell cultures were replenished with L cell-conditioned medium; on day 6, BMMs were then harvested, counted, and used for experiments. Macrophages were cocultured at a 20 to 30 to 1 ratio with late-stationary-phase L. (V.) panamensis promastigotes (isolated from Percoll gradients as indicated above) for 4 h, washed, and then incubated for 24, 48, or 72 h with (i) CpG 1826 (1 and 5 μM), (ii) miltefosine (20 μM, positive control; Sigma), (iii) 72-h culture supernatants from dLN cell cultures (as described above), or (iv) 72-h culture supernatants from dLN cell cultures together with CpG 1826 (1 and 5 μM). Following staining with Wright-Giemsa SureStain (Fisher Scientific, Pittsburgh, PA), the number of parasites per macrophage and the percentage of parasitized macrophages were calculated by counting 100 macrophages per sample, in triplicate per determination.
T regulatory cell analysis.
CD4+ CD25− and CD4+ CD25+ cells were isolated from the spleens or draining lymph nodes of BALB/c mice using the CD4+ CD25+ regulatory T cell isolation kit (MACS Miltenyi Biotec) according to the manufacturer's protocol. Isolated CD4+ CD25+ cells were of >90% purity. For suppression assays, 5 × 104 isolated naive CD4+ CD25− cells (Teffs) were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE; eBisoscience) and cocultured with CD4+ CD25+ cells (Tregs) and 2 × 105 T cell-depleted irradiated splenocytes as APCs. Cells were stimulated with 0.5 μg/ml of anti-CD3 (145-2C11; eBioscience). Treg suppressive capacity was assessed by examining CFSE dilution using flow cytometry. The percent suppression was calculated as (percent proliferation of Teffs alone − percent proliferation of Tregs and Teffs)/percent proliferation of Teffs. Levels of Foxp3 expression were comparable between CD4+ CD25+ cells isolated from naive and infected mice. For Treg quantification, cells were isolated from the dLNs and directly stained, as described above, without prior stimulation. FACS data were acquired on a Stratedigm flow cytometer (Stratedigm Inc.), using CellCapTure software, and collected data were analyzed on FlowJo software (Tree Star, Inc.).
In vitro cell depletion studies.
Pooled spleens from infected mice were made into single-cell suspensions, and Fc receptors were blocked (anti-CD16/CD32; eBioscience) and labeled with APC-labeled antibodies (eBioscience) against CD19 (MB19-1), CD11c (N418), or CD11b (M170). CD19, CD11c, or CD11b cells were depleted using anti-APC magnetic microbeads and column separation (MACS Miltenyi Biotec). Depletion efficacy was evaluated by flow cytometry at >98% depletion for the specific cell populations. Further, FACS analysis indicated that CD19 or CD11c depletion removed only the selected cell populations; however, CD11b depletion also depleted 65% of CD11c cells. Nondepleted, CD19-depleted, CD11c-depleted, and CD11b-depleted cells were plated at 5 × 106/well in 24-well plates and cultured with pLAg and 0, 40, 200, 1,000, or 5,000 nM CpG 1826 for 72 h. IFN-γ, IL-10, IL-13, and IL-17 levels were measured in the culture supernatant using a multiplex mouse cytokine kit (R&D Systems).
Statistical analyses.
Statistical analyses were conducted using the Student t test for comparison between two groups. One-way analysis of variance (ANOVA), with Dunnet's test or Dunn's test (according to the parametric or nonparametric distribution of data) correcting for multiple comparisons, was used to establish statistical differences from the control group when comparing among three or more groups and the control group. For parasite burden analysis, data were log transformed prior to the t test. P values of ≤0.05 were considered significant.
ACKNOWLEDGMENTS
This work was supported by Fogarty International Center (NIH) (grant number D43 TW006589), National Institute of Allergy and Infectious Diseases (R01AI093775), and the 2011 Gorgas Memorial Institute Research Award to D.R.-P. A.K.E. was supported in part through training grant T32 AI07404.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Liliana Valderrama and Alexandra Cossio for coordination of the studies of human leishmaniasis patients; Wilson Cortez, María del Mar Castro, Javier Martínez, and Jimena Jojoa for patient evaluation and recruitment; Maryori Vidarte for PBMC processing and cryopreservation in Biobank; and Beatriz Parra and Melissa Pelaez of the Universidad del Valle for their collaboration in the conduct of Luminex assays.
REFERENCES
- 1.Bañuls AL, Hide M, Prugnolle F. 2007. Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol 64:1–109. doi: 10.1016/S0065-308X(06)64001-3. [DOI] [PubMed] [Google Scholar]
- 2.Lipoldová M, Demant P. 2006. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet 7:294–305. doi: 10.1038/nrg1832. [DOI] [PubMed] [Google Scholar]
- 3.Varela MR, Munoz DL, Robledo SM, Kolli BK, Dutta S, Chang KP, Muskus C. 2009. Leishmania (Viannia) panamensis: an in vitro assay using the expression of GFP for screening of antileishmanial drug. Exp Parasitol 122:134–139. doi: 10.1016/j.exppara.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis 193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 5.Berman JD. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Bour-Jordan H. 2012. Current and future immunomodulation strategies to restore tolerance in autoimmune diseases. Cold Spring Harb Perspect Biol 4(11):pii=a007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilboa E, McNamara J II, Pastor F. 2013. Use of oligonucleotide aptamer ligands to modulate the function of immune receptors. Clin Cancer Res 19:1054–1062. doi: 10.1158/1078-0432.CCR-12-2067. [DOI] [PubMed] [Google Scholar]
- 8.Convit J, Ulrich M, Zerpa O, Borges R, Aranzazu N, Valera M, Villarroel H, Zapata Z, Tomedes I. 2003. Immunotherapy of American cutaneous leishmaniasis in Venezuela during the period 1990-99. Trans R Soc Trop Med Hyg 97:469–472. doi: 10.1016/S0035-9203(03)90093-9. [DOI] [PubMed] [Google Scholar]
- 9.Arevalo I, Ward B, Miller R, Meng TC, Najar E, Alvarez E, Matlashewski G, Llanos-Cuentas A. 2001. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis 33:1847–1851. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 10.Badaro R, Lobo I, Munos A, Netto EM, Modabber F, Campos-Neto A, Coler RN, Reed SG. 2006. Immunotherapy for drug-refractory mucosal leishmaniasis. J Infect Dis 194:1151–1159. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 11.Datta N, Mukherjee S, Das L, Das PK. 2003. Targeting of immunostimulatory DNA cures experimental visceral leishmaniasis through nitric oxide up-regulation and T cell activation. Eur J Immunol 33:1508–1518. doi: 10.1002/eji.200323671. [DOI] [PubMed] [Google Scholar]
- 12.Khan MA, Maruno M, Khaskhely NM, Ramzi ST, Hosokawa A, Uezato H, Landires EA, Hashiguchi Y, Nonaka S. 2002. Inhibition of intracellular proliferation of Leishmania parasites in vitro and suppression of skin lesion development in BALB/c mice by a novel lipid A analog (ONO-4007). Am J Trop Med Hyg 67:184–190. [DOI] [PubMed] [Google Scholar]
- 13.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, Carvalho EM. 2001. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg 65:87–89. [DOI] [PubMed] [Google Scholar]
- 14.Raman VS, Bhatia A, Picone A, Whittle J, Bailor HR, O'Donnell J, Pattabhi S, Guderian JA, Mohamath R, Duthie MS, Reed SG. 2010. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol 185:1701–1710. doi: 10.4049/jimmunol.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wagner H, Heeg K. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol 160:3627–3630. [PubMed] [Google Scholar]
- 16.Castilho TM, Goldsmith-Pestana K, Lozano C, Valderrama L, Saravia NG, McMahon-Pratt D. 2010. Murine model of chronic L. (Viannia) panamensis infection: role of IL-13 in disease. Eur J Immunol 40:2816–2829. doi: 10.1002/eji.201040384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrlich A, Castilho TM, Goldsmith-Pestana K, Chae WJ, Bothwell AL, Sparwasser T, McMahon-Pratt D. 2014. The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection. J Immunol 193:2961–2970. doi: 10.4049/jimmunol.1400728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Pinto D, Navas A, Blanco VM, Ramirez L, Garcerant D, Cruz A, Craft N, Saravia NG. 2012. Regulatory T cells in the pathogenesis and healing of chronic human dermal leishmaniasis caused by Leishmania (Viannia) species. PLoS Negl Trop Dis 6:e1627. doi: 10.1371/journal.pntd.0001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer J, Krieg AM. 2009. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev 61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Miyake K, Onji M. 2013. Endocytosis-free DNA sensing by cell surface TLR9 in neutrophils: rapid defense with autoimmune risks. Eur J Immunol 43:2006–2009. doi: 10.1002/eji.201343882. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy EJ, Parker AE, O'Neill LA. 2010. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 23.Tuon FF, Amato VS, Bacha HA, Almusawi T, Duarte MI, Amato Neto V. 2008. Toll-like receptors and leishmaniasis. Infect Immun 76:866–872. doi: 10.1128/IAI.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmakar S, Bhaumik SK, Paul J, De T. 2012. TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog 8:e1002646. doi: 10.1371/journal.ppat.1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Pine SO, Cowgill KD, Reed SG, Piazza FM. 2010. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine 28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 26.von Stebut E, Belkaid Y, Nguyen B, Wilson M, Sacks DL, Udey MC. 2002. Skin-derived macrophages from Leishmania major-susceptible mice exhibit interleukin-12- and interferon-gamma-independent nitric oxide production and parasite killing after treatment with immunostimulatory DNA. J Invest Dermatol 119:621–628. doi: 10.1046/j.1523-1747.2002.01850.x. [DOI] [PubMed] [Google Scholar]
- 27.Cabrera M, Blackwell JM, Castes M, Trujillo D, Convit J, Shaw MA. 2000. Immunotherapy with live BCG plus heat killed Leishmania induces a T helper 1-like response in American cutaneous leishmaniasis patients. Parasite Immunol 22:73–79. doi: 10.1046/j.1365-3024.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- 28.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. 2009. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med 10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 30.Pourgholaminejad A, Jamali A, Samadi-Foroushani M, Amari A, Mirzaei R, Ansaripour B, Khansari N, Aghasadeghi MR, Baban B, Hadjati J. 2011. Reduced efficacy of multiple doses of CpG-matured dendritic cell tumor vaccine in an experimental model. Cell Immunol 271:360–364. doi: 10.1016/j.cellimm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Waibler Z, Anzaghe M, Konur A, Akira S, Muller W, Kalinke U. 2008. Excessive CpG 1668 stimulation triggers IL-10 production by cDC that inhibits IFN-alpha responses by pDC. Eur J Immunol 38:3127–3137. doi: 10.1002/eji.200838184. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JD, Kell SA, Kozy HM, Lum JA, Sweetwood R, Chu M, Cunningham CR, Salamon H, Lloyd CM, Coffman RL, Hessel EM. 2014. A limited CpG-containing oligodeoxynucleotide therapy regimen induces sustained suppression of allergic airway inflammation in mice. Thorax 69:565–573. doi: 10.1136/thoraxjnl-2013-204605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin L, Shelite TR, Gong B, Mendell NL, Soong L, Fang R, Walker DH. 2012. Systemic treatment with CpG-B after sublethal rickettsial infection induces mouse death through indoleamine 2,3-dioxygenase (IDO). PLoS One 7:e34062. doi: 10.1371/journal.pone.0034062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, Puccetti P, Romani L, Grohmann U. 2009. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol 183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 35.Broide DH. 2009. Immunomodulation of allergic disease. Annu Rev Med 60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, Mahmood U, Weissleder R, Carulli J, Benoist C, Mathis D. 2007. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross talk. J Exp Med 204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, Carvalho LP, Machado P, Carvalho EM, Gollob KJ. 2006. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol 63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 38.Bittar RC, Nogueira RS, Vieira-Goncalves R, Pinho-Ribeiro V, Mattos MS, Oliveira-Neto MP, Coutinho SG, Da-Cruz AM. 2007. T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz 102:625–630. doi: 10.1590/S0074-02762007005000069. [DOI] [PubMed] [Google Scholar]
- 39.Gomes-Silva A, de Cassia Bittar R, Dos Santos Nogueira R, Amato VS, da Silva Mattos M, Oliveira-Neto MP, Coutinho SG, Da-Cruz AM. 2007. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol 149:440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faria DR, Gollob KJ, Barbosa J Jr, Schriefer A, Machado PR, Lessa H, Carvalho LP, Romano-Silva MA, de Jesus AR, Carvalho EM, Dutra WO. 2005. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun 73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. 2005. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN type 1 signaling. J Immunol 175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 42.Barnard R, Gurevich KG. 2005. In vitro bioassay as a predictor of in vivo response. Theor Biol Med Model 2:3. doi: 10.1186/1742-4682-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W, Huang L, Mendez S. 2010. A live Leishmania major vaccine containing CpG motifs induces the de novo generation of Th17 cells in C57BL/6 mice. Eur J Immunol 40:2517–2527. doi: 10.1002/eji.201040484. [DOI] [PubMed] [Google Scholar]
- 44.Wu W, Weigand L, Belkaid Y, Mendez S. 2006. Immunomodulatory effects associated with a live vaccine against Leishmania major containing CpG oligodeoxynucleotides. Eur J Immunol 36:3238–3247. doi: 10.1002/eji.200636472. [DOI] [PubMed] [Google Scholar]
- 45.Pan American Health Organization. 2012. Leishmaniasis en las Americas recomendaciones para el tratamiento. Pan American Health Organization, Washington, DC. [Google Scholar]
- 46.Blum J, Lockwood DN, Visser L, Harms G, Bailey MS, Caumes E, Clerinx J, van Thiel PP, Morizot G, Hatz C, Buffet P. 2012. Local or systemic treatment for New World cutaneous leishmaniasis? Re-evaluating the evidence for the risk of mucosal leishmaniasis. Int Health 4:153–163. [DOI] [PubMed] [Google Scholar]
- 47.Baban B, Chandler PR, Johnson BA III, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. 2011. Physiologic control of IDO competence in splenic dendritic cells. J Immunol 187:2329–2335. doi: 10.4049/jimmunol.1100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorn A, Ludwig RJ, Bock A, Thaci D, Hardt K, Bereiter-Hahn J, Kaufmann R, Bernd A, Kippenberger S. 2007. Oligonucleotides suppress IL-8 in skin keratinocytes in vitro and offer anti-inflammatory properties in vivo. J Invest Dermatol 127:846–854. doi: 10.1038/sj.jid.5700620. [DOI] [PubMed] [Google Scholar]
- 49.Inoue J, Aramaki Y. 2007. Suppression of skin lesions by transdermal application of CpG-oligodeoxynucleotides in NC/Nga mice, a model of human atopic dermatitis. J Immunol 178:584–591. doi: 10.4049/jimmunol.178.1.584. [DOI] [PubMed] [Google Scholar]
- 50.Croft SL, Neal RA, Pendergast W, Chan JH. 1987. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol 36:2633–2636. doi: 10.1016/0006-2952(87)90543-0. [DOI] [PubMed] [Google Scholar]
- 51.Yardley V, Croft SL, De Doncker S, Dujardin JC, Koirala S, Rijal S, Miranda C, Llanos-Cuentas A, Chappuis F. 2005. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am J Trop Med Hyg 73:272–275. [PubMed] [Google Scholar]
- 52.Weinkopff T, Mariotto A, Simon G, Hauyon-La Torre Y, Auderset F, Schuster S, Zangger H, Fasel N, Barral A, Tacchini-Cottier F. 2013. Role of Toll-like receptor 9 signaling in experimental Leishmania braziliensis infection. Infect Immun 81:1575–1584. doi: 10.1128/IAI.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbi J, Pardoll D, Pan F. 2014. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev 259:115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. 2014. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev 259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. 2011. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med 17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao Y, Cao X. 2014. The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review. J Autoimmun 55:10–23. doi: 10.1016/j.jaut.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Hajoui O, Janani R, Tulic M, Joubert P, Ronis T, Hamid Q, Zheng H, Mazer BD. 2004. Synthesis of IL-13 by human B lymphocytes: regulation and role in IgE production. J Allergy Clin Immunol 114:657–663. doi: 10.1016/j.jaci.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 58.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, Flynn JL, Porcelli SA, Jacobs WR Jr, Chan J. 2013. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog 9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Pinto D, Saravia NG, McMahon-Pratt D. 2014. CD4 T cell activation by B cells in human Leishmania (Viannia) infection. BMC Infect Dis 14:108. doi: 10.1186/1471-2334-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. 2010. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol 40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 61.Szili D, Banko Z, Toth EA, Nagy G, Rojkovich B, Gati T, Simon M, Herincs Z, Sarmay G. 2014. TGFbeta activated kinase 1 (TAK1) at the crossroad of B cell receptor and Toll-like receptor 9 signaling pathways in human B cells. PLoS One 9:e96381. doi: 10.1371/journal.pone.0096381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley LM, Harbertson J, Biederman E, Zhang Y, Bradley SM, Linton PJ. 2002. Availability of antigen-presenting cells can determine the extent of CD4 effector expansion and priming for secretion of Th2 cytokines in vivo. Eur J Immunol 32:2338–2346. doi:. [DOI] [PubMed] [Google Scholar]
- 63.Schriefer A, Wilson ME, Carvalho EM. 2008. Recent developments leading toward a paradigm switch in the diagnostic and therapeutic approach to human leishmaniasis. Curr Opin Infect Dis 21:483–488. doi: 10.1097/QCO.0b013e32830d0ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ives A, Masina S, Castiglioni P, Prevel F, Revaz-Breton M, Hartley MA, Launois P, Fasel N, Ronet C. 2014. MyD88 and TLR9 dependent immune responses mediate resistance to Leishmania guyanensis infections, irrespective of Leishmania RNA virus burden. PLoS One 9:e96766. doi: 10.1371/journal.pone.0096766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salhi A, Rodrigues V Jr, Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, Rafati S, Chevillard C, Prata A, Alcais A, Argiro L, Dessein A. 2008. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol 180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 66.Ciorba MA, Bettonville EE, McDonald KG, Metz R, Prendergast GC, Newberry RD, Stenson WF. 2010. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol 184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, Orabona C, De Luca A, Boon L, Romani L, Grohmann U, Puccetti P. 2013. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun 4:1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- 68.Ramírez C, Diaz-Toro Y, Tellez J, Castilho TM, Rojas R, Ettinger NA, Tikhonova I, Alexander ND, Valderrama L, Hager J, Wilson ME, Lin A, Zhao H, Saravia NG, McMahon-Pratt D. 2012. Human macrophage response to L. (Viannia) panamensis: microarray evidence for an early inflammatory response. PLoS Negl Trop Dis 6:e1866. doi: 10.1371/journal.pntd.0001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMahon-Pratt D, Alexander J. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev 201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 70.Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. 2011. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs 20:361–372. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 71.Gupta GK, Agrawal DK. 2010. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs 24:225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Ushach I, Zlotnik A. 2016. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100:481–489. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, Proudfoot AE, Tacchini-Cottier F. 2010. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog 6:e1000755. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja SK, Ahuja SS. 1999. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J Immunol 163:5519–5525. [PubMed] [Google Scholar]
- 75.Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. 2004. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect Immun 72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. 2006. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med 203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gómez MA, Navas A, Marquez R, Rojas LJ, Vargas DA, Blanco VM, Koren R, Zilberstein D, Saravia NG. 2014. Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: impact on intracellular parasite survival. J Antimicrob Chemother 69:139–149. doi: 10.1093/jac/dkt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ritter U, Moll H. 2000. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur J Immunol 30:3111–3120. doi:. [DOI] [PubMed] [Google Scholar]
- 79.Brandonisio O, Panaro MA, Fumarola I, Sisto M, Leogrande D, Acquafredda A, Spinelli R, Mitolo V. 2002. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1 alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin Exp Med 2:125–129. doi: 10.1007/s102380200017. [DOI] [PubMed] [Google Scholar]
- 80.Ramasawmy R, Menezes E, Magalhaes A, Oliveira J, Castellucci L, Almeida R, Rosa ME, Guimaraes LH, Lessa M, Noronha E, Wilson ME, Jamieson SE, Kalil J, Blackwell JM, Carvalho EM, de Jesus AR. 2010. The −2518bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infect Genet Evol 10:607–613. doi: 10.1016/j.meegid.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navas A, Vargas DA, Freudzon M, McMahon-Pratt D, Saravia NG, Gomez MA. 2014. Chronicity of dermal leishmaniasis caused by Leishmania panamensis is associated with parasite-mediated induction of chemokine gene expression. Infect Immun 82:2872–2880. doi: 10.1128/IAI.01133-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. 2010. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol 184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 83.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med 201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med 191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. 2006. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med 203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ronet C, Voigt H, Himmelrich H, Doucey MA, Hauyon-La Torre Y, Revaz-Breton M, Tacchini-Cottier F, Bron C, Louis J, Launois P. 2008. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J Immunol 180:4825–4835. [DOI] [PubMed] [Google Scholar]
- 87.Guarin N, Palma GI, Pirmez C, Valderrama L, Tovar R, Saravia NG. 2006. Comparative immunohistological analysis of the Montenegro skin test reaction in asymptomatic infection and in acute and chronic cutaneous leishmaniasis. Biomedica 26(Suppl 1):S38–S48. [PubMed] [Google Scholar]
- 88.Morlacchi S, Soldani C, Viola A, Sarukhan A. 2011. Self-antigen presentation by mouse B cells results in regulatory T-cell induction rather than anergy or clonal deletion. Blood 118:984–991. doi: 10.1182/blood-2011-02-336115. [DOI] [PubMed] [Google Scholar]
- 89.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. 2004. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+ CD25+ regulatory T cells. J Immunol 173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 90.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. 2012. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carneiro G, Aguiar MG, Fernandes AP, Ferreira LA. 2012. Drug delivery systems for the topical treatment of cutaneous leishmaniasis. Expert Opin Drug Deliv 9:1083–1097. doi: 10.1517/17425247.2012.701204. [DOI] [PubMed] [Google Scholar]
- 92.Hirobe S, Okada N, Nakagawa S. 2013. Transcutaneous vaccines–current and emerging strategies. Expert Opin Drug Deliv 10:485–498. doi: 10.1517/17425247.2013.760542. [DOI] [PubMed] [Google Scholar]
- 93.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 94.Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. 2003. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun 71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]