ABSTRACT
Adherence to host surfaces is often mediated by bacterial binding to surface carbohydrates. Although it is widely appreciated that some bacterial species express glycosidases, previous studies have not considered whether bacteria bind to multiple carbohydrates within host glycans as they are modified by bacterial glycosidases. Streptococcus oralis is a leading cause of subacute infective endocarditis. Binding to platelets is a critical step in disease; however, the mechanisms utilized by S. oralis remain largely undefined. Studies revealed that S. oralis, like Streptococcus gordonii and Streptococcus sanguinis, binds platelets via terminal sialic acid. However, unlike those organisms, S. oralis produces a neuraminidase, NanA, which cleaves terminal sialic acid. Further studies revealed that following NanA-dependent removal of terminal sialic acid, S. oralis bound exposed β-1,4-linked galactose. Adherence to both these carbohydrates required Fap1, the S. oralis member of the serine-rich repeat protein (SRRP) family of adhesins. Mutation of a conserved residue required for sialic acid binding by other SRRPs significantly reduced platelet binding, supporting the hypothesis that Fap1 binds this carbohydrate. The mechanism by which Fap1 contributes to β-1,4-linked galactose binding remains to be defined; however, binding may occur via additional domains of unknown function within the nonrepeat region, one of which shares some similarity with a carbohydrate binding module. This study is the first demonstration that an SRRP is required to bind β-1,4-linked galactose and the first time that one of these adhesins has been shown to be required for binding of multiple glycan receptors.
KEYWORDS: endocarditis; neuraminidase; Streptococcus oralis; adherence; β-1,4-linked galactose; host glycans; platelets; serine-rich repeat protein; sialic acid
INTRODUCTION
Infective endocarditis (IE) is an infection (usually bacterial) of the endocardium, most often the surfaces of the valves. Despite improvements in diagnosis, therapeutics, and surgical treatments, there has been no substantive improvement in the survival rate of IE patients over the last 30 years (1–4). Vegetative growths on the endocardium that contain bacteria and host factors lead to the clinical effects of IE, including valvular incompetence and congestive heart failure. Treatment requires several weeks of antibiotics, and approximately 50% of patients undergo surgery (4). The morbidity and mortality due to IE are high, with an in-hospital death rate of 17.7% and a 1-year mortality approaching 40% (2–5).
IE cases can be divided into acute and subacute IE. Acute IE, which is most commonly caused by staphylococci, typically affects previously normal valves and has a severe and sudden onset. Subacute IE requires a previously damaged endocardium and is more subtle in presentation, with infections often progressing for several weeks prior to diagnosis. The precise mechanisms by which subacute IE develops are poorly understood. It is proposed that a preexisting sterile platelet-fibrin nidus on the damaged endocardial surface becomes infected with bacteria, leading to IE (6, 7). It is not clear if bacteria adhere directly to this nidus from the circulation or if bacteria bind to circulating platelets, which then act as “Trojan horses” carrying bacteria to the damaged endothelial surface (7–9). However, it is clear that adherence to platelets is a critical requirement for development of subacute IE. Viridans group streptococci are a common cause of subacute IE that can enter the bloodstream from the oral cavity after dental work and normal brushing activities (4, 10–13). While several species of viridans group streptococci can cause IE, Streptococcus oralis is often identified as the most common cause of viridans group streptococcus-associated IE (11, 14).
Despite the importance of S. oralis in IE, little is known about the adherence mechanisms utilized by this bacterial species. The majority of studies defining mechanisms of streptococcal adherence during IE have focused on S. gordonii. For Streptococcus sanguinis and Streptococcus gordonii, sialic acid residues on glycoprotein Ibα (GPIbα) have been identified as the major receptors on platelets (15–17). Adherence to sialic acid is mediated via serine-rich repeat proteins (SRRPs) that contain a Siglec-like domain (9, 17–20). Mutagenesis of SRRPs in several streptococcal species reduced pathogenesis in a rat model of IE (21–23). These studies demonstrate the essential nature of glycans in bacterium-platelet interactions. The limited studies performed with S. oralis indicate that this organism may also interact with host carbohydrates, including sialic acid (24). Tilley and coworkers recently demonstrated that S. oralis can adhere to and induce aggregation of platelets via interaction with host GPIbα (25). However, the bacterial adhesin and the biochemical nature of this interaction remain unknown. S. oralis has been shown to bind to glycans on several salivary glycoproteins, and neuraminidase treatment reduced binding of some strains to saliva-coated hydroxyapatite and mucin (24–26). Furthermore, agglutination of red blood cells by S. oralis was shown to be inhibited in the presence of some carbohydrates, including galactose and lactose (27).
Here we demonstrate that S. oralis binds to sialic acid on platelets and, following cleavage of terminal sialic acid by S. oralis neuraminidase, to the underlying carbohydrate β-1,4-linked galactose. Given the ability of many bacteria to both bind to and modify host glycans, this novel adherence strategy is likely employed by other bacterial species. Furthermore, our studies provide the first demonstration that an SRRP is required for binding to β-1,4-linked galactose and for adherence to multiple distinct carbohydrate receptors. These data suggest that domains of unknown function in SRRPs may bind additional carbohydrates.
RESULTS
S. oralis binds to sialic acid on platelets.
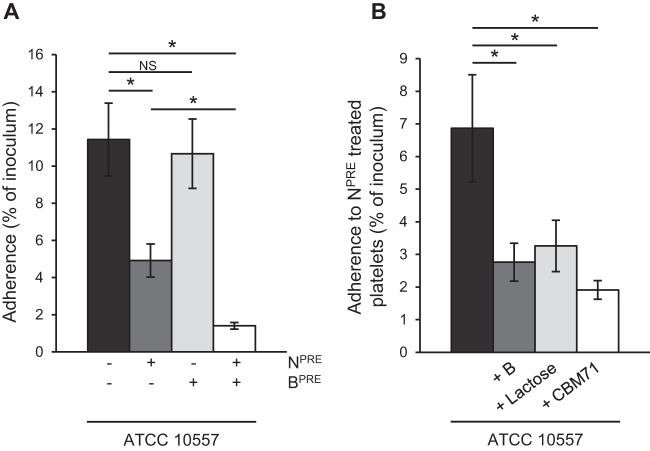
S. gordonii and S. sanguinis, which (like S. oralis) cause subacute infective endocarditis, mediate binding to sialic acid on platelets (15–17). To determine if the S. oralis endocarditis isolate ATCC 10557 binds to sialic acid, we tested its adherence to platelets pretreated with neuraminidase, which removes terminal sialic acid, or with buffer alone. Removal of sialic acid significantly reduced bacterial adherence, indicating that this carbohydrate is a receptor for S. oralis on the platelet surface (Fig. 1). Adherence to platelets was also significantly reduced in the presence of 1 mM free sialic acid, indicating that this carbohydrate can compete with the receptor for binding to the bacterial adhesin (Fig. 1).
FIG 1.
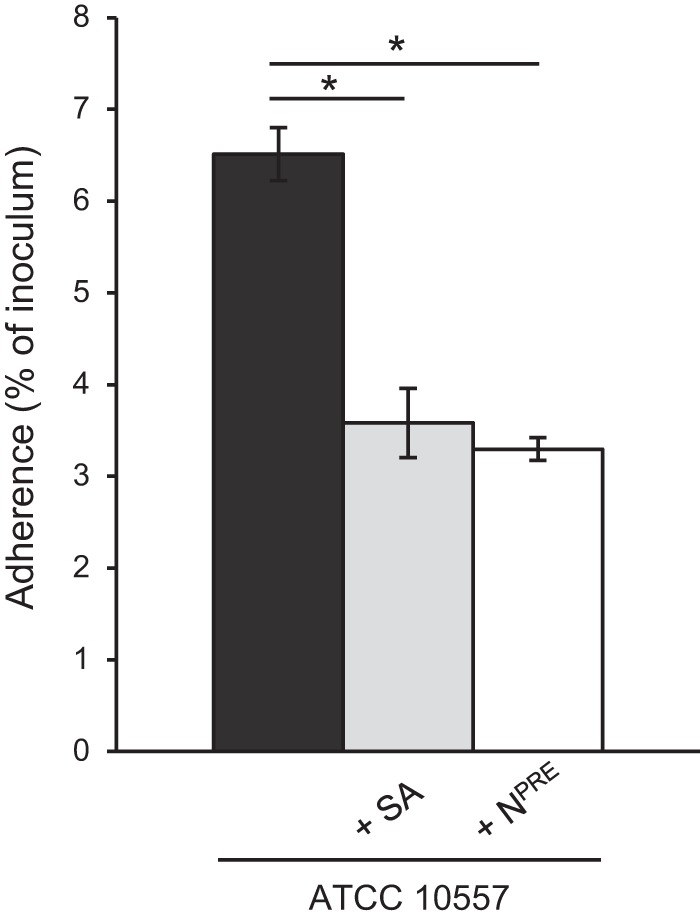
S. oralis binds terminal sialic acid on platelets. The graph shows adherence of S. oralis ATCC 10557 to platelets pretreated with Clostridium perfringens neuraminidase (NPRE; 0.78 μM) or in the presence of sialic acid (SA; 1 mM). Adherence is expressed as a percentage of the inoculum. Values are the means for at least three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.001.
Despite the contribution of sialic acid as a receptor for S. oralis adherence, significant binding was still observed when this receptor was removed, indicating that an additional mechanism(s) exists.
β-1,4-Linked galactose on the surface of platelets acts as a receptor for S. oralis.
Unlike S. sanguinis and S. gordonii, S. oralis produces a neuraminidase that cleaves terminal sialic acid (28). This enzymatic activity both cleaves the sialic acid receptor and exposes underlying carbohydrates (28, 29). Removal of sialic acid reveals a range of glycan structures, whose terminal carbohydrates may act as an additional receptor(s) (27). Previously published data demonstrated that agglutination of red blood cells by S. oralis was inhibited by lactose. Furthermore, it was recently demonstrated that pneumococci bind neuraminidase-exposed β-1,4-linked galactose (30, 31). Together, these data led to the hypothesis that β-1,4-linked galactose may act as a receptor for S. oralis.
Pretreatment of platelets with neuraminidase and the recombinantly expressed active site domain of pneumococcal β-galactosidase BgaA specific for terminal β-1,4-linked galactose (SpBgaA146-990) reduced S. oralis adherence significantly more than treatment with neuraminidase alone did (Fig. 2A). These data indicate that S. oralis can bind both sialic acid and β-1,4-linked galactose. Further supporting the hypothesis that β-1,4-linked galactose is a receptor for S. oralis was the significant reduction in adherence of S. oralis to neuraminidase-treated platelets in the presence of 5 mM lactose and a 250 μM concentration of a recombinantly expressed carbohydrate binding module which specifically binds β-1,4-linked galactose (CBM71) (Fig. 2B) (30). Pretreatment of platelets with SpBgaA146-990 alone had no significant effect on bacterial adherence (Fig. 2A). This enzyme can act only following removal of terminal sialic acid, so these data are consistent with previous studies showing that platelets are highly sialylated and indicate that neuraminidase activity is required to expose β-1,4-linked galactose as a receptor on the platelet surface (Fig. 2A).
FIG 2.
S. oralis binds β-1,4-linked galactose following removal of sialic acid. (A) Adherence of S. oralis ATCC 10557 to platelets was further reduced following pretreatment with Clostridium perfringens neuraminidase (NPRE; 0.78 μM) and the β-1,4-linked galactose-specific β-galactosidase SpBgaA146-990 (BPRE; 0.054 μM). (B) Further data support the binding of ATCC 10557 to β-1,4-linked galactose. Adherence of S. oralis ATCC 10557 to neuraminidase-pretreated platelets was reduced in the presence of SpBgaA146-990 (B; 0.054 μM); CBM71, which is specific for β-1,4-linked galactose (250 μM); or lactose (5 mM). Adherence is expressed as a percentage of the inoculum. Values are the means for three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.03; NS, not significant.
The studies presented here focus on adherence of S. oralis type strain ATCC 10557 to platelets. Adherence to platelets is proposed to be critical to the development of IE; however, adherence to other host factors also likely contributes to development of disease. Binding of ATCC 10557 to endothelial cells was also mediated via adherence to sialic acid and β-1,4-linked galactose (data not shown). Adherence to sialic acid and β-1,4-linked galactose is not limited to ATCC 10557. Adherence of an additional S. oralis strain, 9A2, was significantly reduced by neuraminidase and SpBgaA146-990 (data not shown).
The neuraminidase NanA is required for exposure of β-1,4-linked galactose.
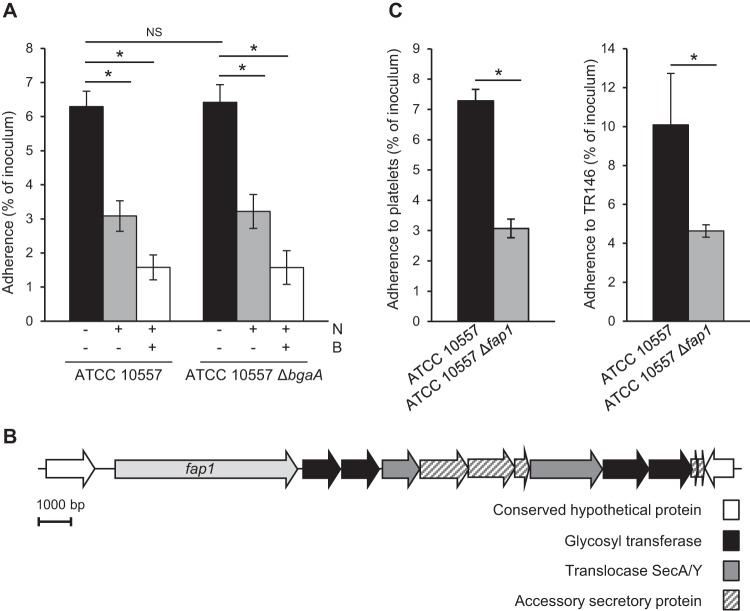
The studies reported thus far demonstrated that S. oralis can bind to terminal sialic acid and that S. oralis can bind to β-1,4-linked galactose following pretreatment of platelets with neuraminidase. In order for this mechanism to be relevant during infective endocarditis, S. oralis neuraminidase must expose β-1,4-linked galactose as a receptor. Addition of SpBgaA146-990 to adherence assays significantly reduced adherence to platelets, demonstrating that S. oralis neuraminidase activity is sufficient to reveal the receptor (Fig. 3). Addition of exogenous neuraminidase with SpBgaA146-990 further reduced adherence. This is likely because the purified neuraminidase used is more effective than that produced by S. oralis at revealing β-1,4-linked galactose.
FIG 3.
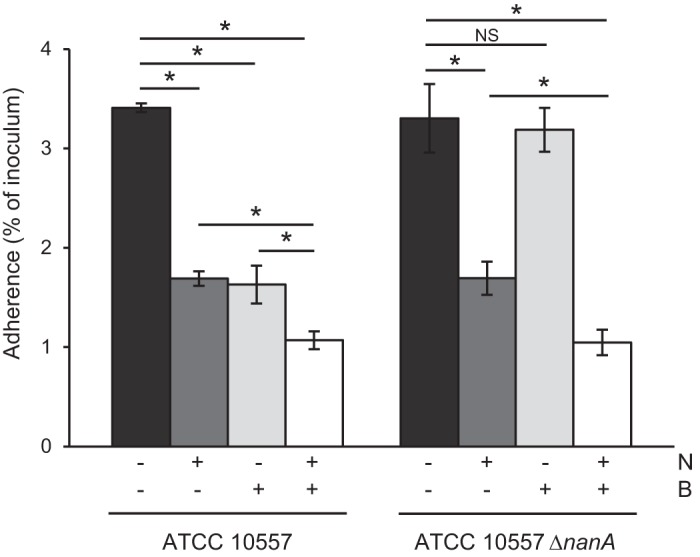
S. oralis reveals β-1,4-linked galactose in a NanA-dependent manner. The graph shows adherence of S. oralis ATCC 10557 and S. oralis ATCC 10557 ΔnanA to platelets in the presence of Clostridium perfringens neuraminidase (N; 0.78 μM), SpBgaA146-990 (B; 0.054 μM), both enzymes, or a PBS control. Adherence is expressed as a percentage of the inoculum. Values are the means for three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.01; NS, not significant.
S. oralis neuraminidase activity is predicted to be encoded by nanA and surface associated in an SrtA-dependent manner (28, 32). Our genome sequence of ATCC 10557 contains a gene predicted to encode a neuraminidase. To determine whether the gene encoding NanA is required to expose β-1,4-linked galactose, a mutant (ATCC 10557 ΔnanA) was generated and tested in adherence assays (Fig. 3). Adherence of the nanA mutant was not significantly different from that of the parent. We expected the mutant to exhibit increased adherence, as this strain cannot remove sialic acid receptors; however, one possible explanation for the observed data is that receptors exposed by cleavage of sialic acid compensate for the partial removal of this receptor. Addition of SpBgaA146-990 did not reduce adherence of the nanA mutant, demonstrating that nanA is required for exposure of β-1,4-linked galactose. These data indicate that S. oralis can bind to terminal sialic acid and that following cleavage of that receptor by bacterial neuraminidase, S. oralis can bind to the underlying β-1,4-linked galactose. Thus, these data strongly suggest that S. oralis binds multiple carbohydrates on the platelet surface.
An ortholog of the S. gordonii serine-rich repeat protein GspB contributes to S. oralis adherence.
ATCC 10557 contains a gene predicted to encode an ortholog of the pneumococcal adhesin BgaA (30). Carbohydrate binding modules (CBMs) within the C-terminal region of BgaA mediate adherence to β-1,4-linked galactose on host cells (30, 31). Despite conservation of these CBMs in S. oralis BgaA, a bgaA mutant was not significantly altered in binding to platelets (Fig. 4A). Furthermore, adherence of this mutant was reduced in the presence of glycosidases, similarly to that of the parental strain. These data demonstrate that there is no role for bgaA in adherence of ATCC 10557 to platelets in this model system. It should be noted that the recombinant SpBgaA146-990 used in this study to cleave terminal β-1,4-linked galactose includes only the enzymatic domain of the protein, not the CBMs (30).
FIG 4.
S. oralis serine-rich repeat protein Fap1, but not the β-galactosidase BgaA, is required for effective binding to platelets. (A) Mutation of bgaA does not significantly alter binding of ATCC 10557 to platelets. (B) Schematic representing the genomic arrangement of the ATCC 10557 fap1 locus. Open reading frames predicted within the fap1 locus are shown by arrows. (C) Fap1 contributes to S. oralis adherence. Adherence levels of ATCC 10557 and ATCC 10557 Δfap1 to platelets and the immortalized oral epithelial cell line TR146 are expressed as percentages of the inoculum. Adherence data are the means for at least three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.03.
Examination of the genome sequence revealed that ATCC 10557, like some S. gordonii and S. sanguinis strains, encodes a member of the SRRP family of adhesins. This is a family of glycosylated surface proteins expressed by many Gram-positive organisms. Many of these proteins are known to play roles in attachment to a variety of host and bacterial surfaces and in biofilm formation (15, 17, 18, 21, 33–50). The S. oralis SRRP is encoded within a typical locus for this protein family, which includes genes predicted to encode proteins for glycosylation and secretion of the SRRP (47) (Fig. 4B). Microscopy suggests that this protein family forms fimbria-like structures on the surfaces of bacteria (33, 34, 51, 52). The SRRP encoded by S. oralis has not previously been studied, but it was annotated in a previous genome sequence as fimbria-associated protein 1 (Fap1), based on very limited sequence similarity to the SRRP from Streptococcus parasanguinis (34, 38, 53). As a result, we use the same nomenclature here despite there being no direct evidence that this protein is associated with fimbriae in S. oralis. To determine if S. oralis Fap1 contributes to adherence to platelets, we generated a fap1 mutant. A significant reduction in adherence of ATCC 10557 Δfap1 compared to that of the parent demonstrated a role for this protein in adherence (Fig. 4C). Although we have successfully complemented other S. oralis mutations, attempts to generate a stable fap1 complement were unsuccessful. This is likely due to the highly repetitive nature of fap1 and likely explains why studies of other SRRPs also lack complementation. However, we confirmed the phenotype with a second, independent fap1 mutant (data not shown). Although the focus of the present study is S. oralis adherence to platelets, it is likely that mechanisms important in that interaction are also important in the oral cavity. This study demonstrated that a fap1 mutant had reduced adherence to oral epithelial cells, but further studies would be required to define the role of Fap1 in the oral cavity (Fig. 4C).
Evidence suggests that S. oralis Fap1 binds both sialic acid and β-1,4-linked galactose.
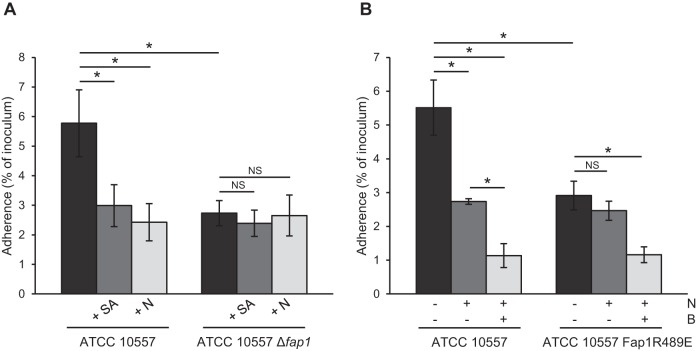
SRRPs contain two glycosylated serine-rich repeat (SRR) regions which comprise the majority of the protein, a cell wall anchor, and one or two nonrepeat regions within the N-terminal region (16, 52). The nonrepeat regions of SRRPs mediate adherence, and diversity in the modular structure of these regions accounts for the different receptors for these proteins, including sialic acid, keratins, and fibrinogen (16, 20, 23, 35, 40, 44, 45, 51, 54–56). BLAST searches identified no significant sequence similarity between the nonrepeat region of S. oralis SRRP and other available sequences. However, HHpred, a server for remote homolog detection using structural information, discovered 24% identity over 107 amino acids with GspB, the SRRP encoded by some S. gordonii strains (15, 57). The region of similarity is within the Siglec domain of GspB, which is required for sialic acid binding. These data suggested that Fap1 may also bind sialic acid. Supporting this hypothesis, neither neuraminidase nor free sialic acid significantly reduced adherence of the fap1 mutant (Fig. 5A). During completion of this study, Bensing et al. identified a threonine-arginine (Thr-Arg) motif conserved in sialoglycan-binding SRRPs that is required for orienting the sialic acid residue (20, 56). This motif is required for efficient glycan binding, platelet binding, and full virulence in the rat model of infective endocarditis (20, 56). These residues are conserved in ATCC 10557 Fap1, and mutagenesis of the arginine residue significantly reduced adherence, further supporting the hypothesis that Fap1 acts as an adhesin binding sialic acid (Fig. 5B). However, adherence was reduced by the presence of SpBgaA146-990, indicating that despite Fap1 being required for binding to β-1,4-linked galactose, the conserved arginine residue does not contribute to binding of this carbohydrate.
FIG 5.
Fap1 is required for binding of ATCC 10557 to sialic acid on platelets, and this interaction requires a conserved residue within the Siglec domain. (A) ATCC 10557 Δfap1 did not show further reduced binding to platelets in the presence of Clostridium perfringens neuraminidase (N; 0.78 μM) or sialic acid (SA; 1 mM). (B) A conserved arginine residue within the nonrepeat region of Fap1 is required for binding of sialic acid but not β-1,4-linked galactose carbohydrates. The graph shows adherence of S. oralis ATCC 10557 and ATCC 10557 Fap1R489E to platelets in the presence of neuraminidase, SpBgaA146-990 (B; 0.054 μM), both enzymes, or a PBS control. Adherence is expressed as a percentage of the inoculum. Values are the means for at least three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.02; NS, not significant.
Thus far, there has been no evidence that SRRPs contribute to binding of multiple glycan receptors. However, as the nonrepeat region of Fap1 is larger than that of GspB, it is possible that this region contains additional receptor binding domains. As a result, the contribution of Fap1 to binding of β-1,4-linked galactose was tested. There was no significant reduction in adherence of the fap1 mutant to neuraminidase-treated platelets in the presence of SpBgaA146-990, lactose, or CBM71 (Fig. 6A). These data indicate that Fap1 is required for binding to both sialic acid and β-1,4-linked galactose. This is the first demonstration that an SRRP is required for binding of β-1,4-linked galactose.
FIG 6.
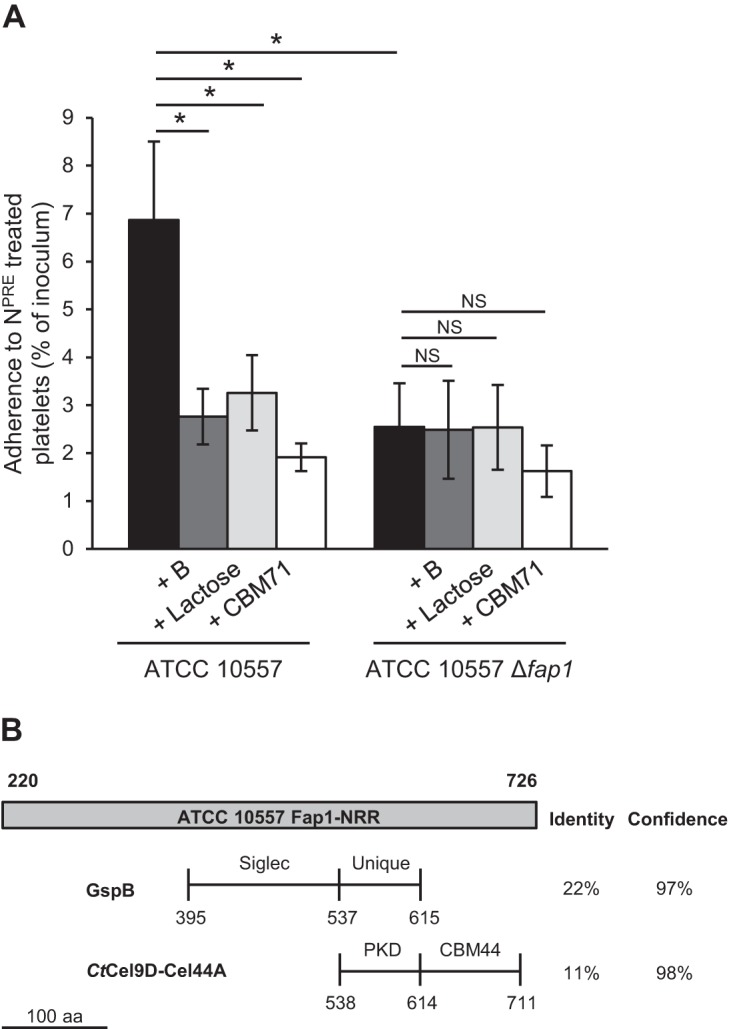
Fap1 is required for binding of ATCC 10557 to β-1,4-linked galactose on platelets. (A) ATCC 10557 Δfap1 did not show further reduced binding to neuraminidase-pretreated (NPRE; 0.78 μM) platelets in the presence of SpBgaA146-990 (B; 0.054 μM), CBM71 (250 μM), or lactose (5 mM). Adherence is expressed as a percentage of the inoculum. Values are the means for three independent experiments, each performed in triplicate, ± SD. Statistical significance was tested by two-tailed Student's t test. *, P ≤ 0.03; NS, not significant. (B) Schematic representing domains predicted by Phyre2 within the nonrepeat region (NRR) of Fap1. Numbers refer to the amino acid positions within the stated protein. The horizontal lines denote the domains identified within GspB (PDB entry 3QC5) and CtCel9D-Cel44A (PDB entry 2C26) as sharing structural similarity with the nonrepeat region of Fap1. The vertical lines indicate the boundaries of the different domains. The percent identity and confidence in the Phyre2 prediction are shown on the right.
During completion of this study, a paper was published characterizing the binding specificity of an SRRP from S. mitis strain SF100 (55). Comparison of the nonrepeat regions of SF100 and Fap1 revealed high levels of amino acid identity (95%). Structural predictions of the nonrepeat region of SF100 indicate the presence of Siglec and unique domains required for binding of sialic acid (55). The study confirmed that this region binds to sialic acid in the context of the sialyl T antigen (Neu5Ac–α-2,3Gal–β-1,3GalNAc). Interestingly, these sialic acid binding domains are flanked by domains that do not resemble any reported structures (Fig. 6B) (55). We identified the same organization in the Fap1 nonrepeat region. Use of the intensive mode of Phyre2 identified a low level of identity (11%) over 174 amino acids between the C-terminal domain of Fap1, encompassing the unique domain and a C-terminal domain of unknown function, and a region of an endoglucanase encoded by Ruminiclostridium thermocellum (58–60). This region of the endoglucanase contains a polycystic kidney disease (PKD) domain found in a wide variety of prokaryotic and eukaryotic proteins and a family 44 CBM with specificity for β-1,4-linked glucose polymers. Despite the high levels of sequence similarity between Fap1 and SF100, Phyre2 did not identify any match between the nonrepeat region of SF100 and the CBM of the endoglucanase (60). These data suggest that there may be an additional carbohydrate binding domain within the nonrepeat region of Fap1. Deletion of the predicted CBM resulted in a strain with an adherence indistinguishable from that of ATCC 10557 Δfap1, which was not further reduced by neuraminidase or SpBgaA146-990 (data not shown). These data indicate that the mutant protein was nonfunctional. Future studies will further address the hypothesis that the nonrepeat region of Fap1 binds a second carbohydrate.
DISCUSSION
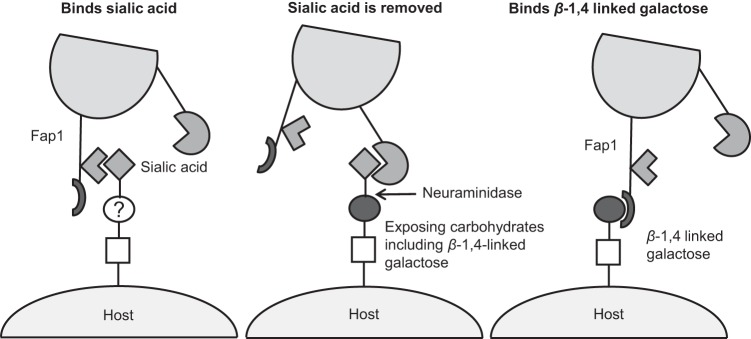
Many bacterial species bind to the host via surface glycans. Furthermore, some bacterial species, including many streptococci, express a range of glycosidases that can modify these host glycans (61). This suggests that some bacterial species bind to receptors that the bacterium can also cleave. This was demonstrated for pneumococci, which can both bind to and cleave β-1,4-linked galactose (30). Here we demonstrate that following initial adherence of S. oralis to sialic acid, the neuraminidase expressed by this bacterium cleaves this carbohydrate receptor and reveals β-1,4-linked galactose, which acts as an additional receptor (Fig. 7). It is not known whether S. oralis can bind other carbohydrate structures revealed by the action of extracellular bacterial glycosidases. Not only can other carbohydrates be revealed by cleavage of sialic acid, but S. oralis also encodes additional glycosidases that can deglycosylate host glycans. These include BgaA, which can cleave β-1,4-linked galactose.
FIG 7.
Schematic of the proposed model of S. oralis adherence to sialic acid and β-1,4-linked galactose.
Both sialic acid and β-1,4-linked galactose are widely distributed within glycan structures on glycoproteins and glycolipids. The wide distribution of these carbohydrates suggests that this adherence mechanism mediates binding to these carbohydrates in a range of contexts and, as a result, is likely important in adherence to not only platelets and endothelial cells but also other host surfaces, including the oral cavity. Previous studies have demonstrated a reduction in binding of other SRRP mutants to salivary proteins and in vitro models of oral surfaces (41, 62–64). The demonstration that a fap1 mutant has reduced adherence for an oral epithelial cell line supports the hypothesis that this protein contributes to S. oralis binding in the oral cavity; however, further studies will be required to determine the precise contribution.
It is not known whether S. oralis binds to carbohydrates decorating a specific glycoprotein(s) on the platelet surface. Sialic acid on GPIbα is the major platelet receptor for SRRPs expressed by S. sanguinis and S. gordonii (15–17). There are other common glycoproteins on the platelet surface, and the basis for the specificity for GPIbα is currently unclear. It has been shown that S. oralis can adhere to and induce aggregation of platelets via interaction with GPIbα, but it is not known if the interaction of S. oralis with this protein requires an SRRP or glycans (25). Furthermore, there is still significant binding of platelets to S. oralis even in the presence of anti-GPIb.
The sialic acid- and β-1,4-linked galactose-containing glycan structures bound by S. oralis remain to be defined. The evidence provided here strongly supports the hypothesis that Fap1 directly binds sialic acid. Given the high level of conservation between the nonrepeat regions of Fap1 and the SF100 SRRP and the specificity of the SF100 nonrepeat region for binding of the sialyl T-antigen structure, it is possible that Fap1 also binds sialic acid in the same context. Interestingly, the most common glycan present on GPIbα is a biantennary O-linked glycan, one branch of which consists of the sialyl T antigen and the other of which contains sialic acid-capped β-1,4-linked galactose (65, 66). Whether or not S. oralis specifically binds GPIbα, this highlights that these carbohydrates can be present on the same glycan structure.
This is the first demonstration that an SRRP is required to bind β-1,4-linked galactose. Furthermore, this is the first report of a requirement for an SRRP to bind two distinct carbohydrate receptors, although it has been suggested that the pneumococcal SRRP PsrP binds both keratin, in a glycan-independent manner, and an as yet undefined glycan structure in saliva (63). While at first glance it may be surprising that BgaA does not mediate binding of S. oralis ATCC 10557 to β-1,4-linked galactose, it has been reported that BgaA does not contribute to adherence of all pneumococcal isolates (30, 31).
We hypothesize that distinct binding sites within Fap1 directly bind sialic acid and β-1,4-linked galactose (Fig. 7). While the evidence strongly suggests that Fap1 binds sialic acid, no direct evidence thus far supports the direct binding of β-1,4-linked galactose by Fap1. However, additional domains of unknown function, one of which shares some structural similarity with a CBM, are present within the nonrepeat region of Fap1. Unfortunately, deletion of this domain resulted in a nonfunctional Fap1 protein. Glycan arrays utilizing the nonrepeat region of SF100 detected no binding to the three β-1,4-linked galactose carbohydrates on the array (Galβ-1,4Glc, Galβ-1,4GlcNAc6S, and Galβ-1,4GlcNAc) (55). This does not rule out Fap1 directly binding to β-1,4-linked galactose. It can be difficult to detect the weak binding of some carbohydrate binding domains, including CBMs, by using glycan arrays. It is the avidity of multiple interactions between CBMs and carbohydrates that allows these modules to mediate binding (30). Furthermore, at present, it is not known if SF100 binds β-1,4-linked galactose, and if so, if the SRRP is required for binding to this carbohydrate. Despite high levels of sequence similarity, no structural similarity with CBMs was identified for the SF100 SRRP. Mutation of the conserved arginine residue previously reported to be required for sialic acid binding does not affect binding to β-1,4-linked galactose. Thus, it is clear that the regions of Fap1 required for binding to the two carbohydrates are distinct. If Fap1 does not directly bind β-1,4-linked galactose, then the most likely explanation for a requirement of Fap1 in binding this carbohydrate is that Fap1 interacts with another protein which is the adhesin or forms an adhesin complex with Fap1 on the bacterial surface. Studies are under way to define the role of Fap1 in binding β-1,4-linked galactose.
At this time, it is unclear whether SRRPs of other related streptococci contribute to binding of β-1,4-linked galactose or other carbohydrates. However, it is highly likely that bacterial species that bind host carbohydrates and express glycosidases, or are present in an environment with these enzymes, have strategies to bind multiple carbohydrates. It has been reported that other viridans group streptococci, including S. gordonii, can bind carbohydrate structures revealed by removal of sialic acid, although the adhesins that bind these carbohydrates remain to be defined (67, 68). Adherence to these underlying carbohydrates is likely not relevant to the binding of S. gordonii to platelets during development of endocarditis, as platelets are highly sialylated and S. gordonii does not produce neuraminidase. However, these mechanisms may play an important role in the complex microbial environment of the oral cavity, where there are many neuraminidase-positive species.
In summary, this study increases our understanding of S. oralis adherence and SRRPs. The data presented here provide strong evidence that S. oralis binds multiple host carbohydrates and that this adherence occurs in a fap1-dependent manner. This is the first report to describe an SRRP required to bind two distinct receptors. Further studies are required to determine whether SRRPs of other related streptococci are required to bind multiple carbohydrates. However, mechanisms of adherence to multiple host carbohydrates are likely employed by other carbohydrate-binding bacterial species that are in an environment containing extracellular carbohydrate-modifying enzymes.
MATERIALS AND METHODS
Bacterial strains, culture media, and chemicals.
The S. oralis strains used in this study are described in Table 1. ATCC 10557 was confirmed as S. oralis by use of a previously published multilocus sequence typing scheme (69). S. oralis was grown on tryptic soy agar plates supplemented with 5% sheep blood. Plates were incubated overnight at 37°C in 5% CO2. Broth cultures were grown statically at 37°C in Todd-Hewitt broth (Becton, Dickinson and Co., Sparks, MD) supplemented with 0.2% (wt/vol) yeast extract (Becton, Dickinson and Co., Sparks, MD) (THY). As appropriate, broth and agar plates were supplemented with 1 μg ml−1 erythromycin, 200 μg ml−1 spectinomycin, 500 μg ml−1 kanamycin, or 200 μg ml−1 streptomycin (Fisher Scientific, Fair Lawn, NJ). C medium with 5% yeast extract (pH 8; C+Y) was used for S. oralis transformations (70).
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Genotype, phenotype, and/or characteristica | Source or reference |
|---|---|---|
| Strains | ||
| Streptococcus oralis strains | ||
| ATCC 10557 | Endocarditis isolate | 78 |
| ATCC 10557 ΔbgaA | ΔbgaA::aad9 Spcr | This study |
| ATCC 10557 ΔnanA | ΔnanA::erm Ermr | This study |
| ATCC 10557 Δfap1 | Δfap1::aad9 Spcr | This study |
| ATCC 10557 Smr | Lys → Thr mutation in RpsL [rpsL(K56T)] conferring Smr | This study |
| ATCC 10557 Fap1 Janus | Δfap1::kan/rpsL+ rpsL(K56T) Kanr Sms | This study |
| ATCC 10557 Fap1R489E | Arg → Glu mutation in Fap1 [fap1(R489E)]; rpsL(K56T) Smr | This study |
| ATCC 10557 Fap1Δ622-708 Janus | Δfap1::kan/rpsL+ rpsL(K56T) Kanr Sms | This study |
| ATCC 10557 Fap1Δ622-708 | Deletion of nucleotides encoding amino acid residues 622 to 708 in Fap1; rpsL(K56T) Smr | This study |
| 9A2 | Human oral cavity isolate | 69 |
| Escherichia coli strain | ||
| Stellar | Cloning host | Clontech |
| Plasmids | ||
| pEASY-T1ermB | pEASY-T1 containing ermB cassette; Ampr Kanr | This study |
| pDrive | Cloning vector; Ampr Kmr | Qiagen |
| pDriveΔbgaA | pDriveΔbgaA::aad9 Spcr Ampr Kanr | This study |
| pDriveΔnanA | pDriveΔnanA::erm Ermr Ampr Kanr | This study |
| pDriveΔfap1 | pDriveΔfap1::aad9 Spcr Ampr Kanr | This study |
| pGEX-5X-3 | Expression vector; Ampr | GE Biosciences |
| pGEX-5X-3 Fap1NRR | pGEX-5X-3 encoding Fap1 amino acids 220 to 726; Ampr | This study |
| pGEX-5X-3 Fap1NRR Janus | pGEX-5X-3-Fap1NRR with Janus cassette; Ampr Kanr | This study |
| pGEX-5X-3 Fap1NRRR489E | pGEX-5X-3-Fap1NRR with Arg → Glu mutation in Fap1 [fap1(R489E)]; Ampr | This study |
| pJET1.2/Blunt | Cloning vector; Ampr | Thermo Fisher Scientific |
| pJET Fap1C | pJET1.2/Blunt containing Fap1 sequence corresponding to amino acids 500 to 860 | This study |
| pJET Fap1C Janus | pJET Fap1C with Janus cassette; Ampr Kanr | This study |
| pJET Fap1Δ622-708 | pJET Fap1C with in-frame deletion of fap1 region encoding amino acids 622 to 708 | This study |
Smr, streptomycin resistant; Sms, streptomycin sensitive; Kanr, kanamycin resistant; Ampr, ampicillin resistant; Spcr, spectinomycin resistant; Ermr, erythromycin resistant.
Escherichia coli strains were grown at 37°C and 200 rpm in Luria-Bertani (LB) broth or statically on LB agar plates. As appropriate, the medium was supplemented with 200 μg ml−1 erythromycin, 100 μg ml−1 ampicillin, 50 μg ml−1 kanamycin, or 50 μg ml−1 spectinomycin (Fisher Scientific, Fair Lawn, NJ).
Unless otherwise specified, all chemicals, substrates, and enzymes were purchased from Sigma-Aldrich (St. Louis, MO).
Generation of S. oralis mutants.
Insertion-deletion mutants of ATCC 10557 were generated using allelic exchange methods. All genomic DNAs were prepared as previously described, with the addition of 100 U ml−1 mutanolysin to the resuspension buffer (71). Fragments of approximately 500 bp from the 5′ and 3′ regions of the target gene were amplified from S. oralis genomic DNA by use of appropriate primer pairs (1/2 and 3/4, respectively) (Table 2). The spectinomycin resistance cassette, amplified by using primers S.F and S.R, and flanking fragments were cloned into EcoRI-digested pDrive (Qiagen, Germantown, MD) by use of an In-Fusion EcoDry HD cloning kit (Clontech, Mountain View, CA) per the manufacturer's protocol and then transformed into E. coli Stellar (Clontech). Plasmid constructs were confirmed by PCR with the M13F and T7 promoter primers followed by sequencing. Due to recombination of the spectinomycin resistance cassette at an unexpected site within the nanA locus, the spectinomycin resistance cassette was exchanged for an erythromycin resistance cassette. The ermB cassette was amplified from pEASY-T1ermB (primers E.F and E.R) and was ligated into an inverse PCR product of the original construct amplified using primers 2 and 3.
TABLE 2.
Primers used in this study
| Target or group | Name | Sequence (5′-3′) | Location (accession no.) |
|---|---|---|---|
| nanA | N.1 | TCGGATCCAGAATTCAGTCGGTGCAGCATCGGTAGa | 506101–506121 (FR720602) |
| N.2 | CACGAACGAAAATCGGTTTGAACTCCATGTGAACTGb | 506596–506575 (FR720602) | |
| N.3 | ATAAACCCTTGCATACACCGGTCCATTATCAACAGb | 508977–508998 (FR720602) | |
| N.4 | CTTGTCGACGAATTCCTTTTCTCTTCTTGCCAAATGCa | 509436–509415 (FR720602) | |
| N.5 | GGTGAGGTCAAGATGATTACG | 505512–505532 (FR720602) | |
| N.6 | ATACCGACTGCTCCACCTTG | 509829–509810 (FR720602) | |
| bgaA | B.1 | TCGGATCCAGAATTCCAGATGTTCGAGGTCAATACa | 664999–665019 (FR720602) |
| B.2 | CACGAACGAAAATCGCCAATGGCCTTTTTCCATb | 665731–665714 (FR720602) | |
| B.3 | ATAAACCCTTGCATAGAAGTGCAGAAGGTCAAGCAGb | 672597–672617 (FR720602) | |
| B.4 | CTTGTCGACGAATTCTTATACTCTGTCGCATGTACa | 673259–673240 (FR720602) | |
| B.5 | TCTTGCCAAATGACCAACTC | 664767–664786 (FR720602) | |
| B.6 | GTTGTTGTATCACCTTCATG | 673397–673378 (FR720602) | |
| fap1 | F.1 | TCGGATCCAGAATTCAGAAACAGACCGTGTAACTCa | 1541056–1541037 (FR720602) |
| F.2 | CACGAACGAAAATCGGAGCTGACTCACTTGCTTGGb | 1540428–1540447 (FR720602) | |
| F.3 | ATAAACCCTTGCATACAGCTGTGACAGGTATCGGTCb | 1535264–1535244 (FR720602) | |
| F.4 | CTTGTCGACGAATTCGGTACAAGGCTAGGTCTTCGCa | 1534668–1534688 (FR720602) | |
| F.5 | AAGTTACCTCGGCTACCGGC | 1541474–1541455 (FR720602) | |
| F.6 | ATCTAAGTTTGATCTAACGATGA | 1534633–1534655 (FR720602) | |
| Fap1R489E | NRR.F | TCGAAGGTCGTGGGATCCGCTCGGGAGACAGTGAAAGAATCc | 1540431–1540409 (FR720602) |
| NRR.R | CACGATGCGGCCGCTCGAGATTACTTACTGAAATTGACGc | 1538911–1538930 (FR720602) | |
| NRRI.F | AACCCGTGATTGAAACGGTG | 1539663–1539682 (FR720602) | |
| NRRI.R | TTAGCGGAAATACTACTGACG | 1539590–1539570 (FR720602) | |
| R489E.F | CTTCCTGATCATAGGTGAAAACATACTCGGTCCACAAACTATTTACTGCTTGAd | 1539597–1539649 (FR720602) | |
| R489E.R | TCAAGCAGTAAATAGTTTGTGGACCGAGTATGTTTTCACCTATGATCAGGAAGd | 1539649–1539597 (FR720602) | |
| R489E.5 | ACTGCTAGTTCAAGTGACTC | 1540536–1540517 (FR720602) | |
| R489E.6 | ACTAGCACTTGCTGAAGCAGAC | 1538884–1538905 (FR720602) | |
| Fap1Δ622-708 | Δ622-708.R | TGACTGAAGCGGATTGGCTCG | 4777–4797 (KX792093) |
| Δ622-708I.F | GCGCACAGTAAAGCTCACGTC | 4058–4079 (KX792093) | |
| Δ622-708I.R | GATGTGTTTAATGGACAAATTAC | 4341–4363 (KX792093) | |
| Δ622-708.5 | TCTAGCGAAGAATGAGATAG | 1540162–1540143 (FR720602) | |
| aad9 | S.F | CGATTTTCGTTCGTGAATAC | 5418–5399 (KM009065) |
| S.R | TATGCAAGGGTTTATTGTTTTC | 4265–4286 (KM009065) | |
| erm | E.F | CTCGAGCGGCCGCCAGTGe | 264–282 (EU233623) |
| E.R | AACGGCCGCCAGTGTGCTGe | 319–336 (EU233623) | |
| Janus | J.F | CCGTTTGATTTTTAATGGATAATG | 7–30 (AY334019) |
| J.R | GGGCCCCTTTCCTTATGCTT | 247511–247527 (AE005672) | |
| pDrive | T7 promoter | GTAATACGACTCACTATAG | 239–258 (DQ996013) |
| M13F | GTAAAACGACGGCCAGT | 431–447 (DQ996013) |
Underlining indicates nucleotides introduced to allow In-Fusion cloning into the pDrive vector (Qiagen).
Underlining indicates nucleotides introduced to allow In-Fusion cloning with aad9 (spectinomycin cassette).
Underlining indicates nucleotides introduced to allow In-Fusion cloning into the pGEX-5X-3 vector (GE Biosciences).
Bold letters indicate nucleotide changes to alter Arg 489 to Glu in Fap1.
These primers were used to amplify the erm cassette from pEASY-T1ermB.
S. oralis cells were grown to an optical density at 600 nm (OD600) of 0.12 ± 0.02 diluted in fresh medium (1:20), and competence was induced by the addition of competence-stimulating peptide (2 μg ml−1) and CaCl2 (1 mM) (72). Approximately 100 ng of plasmid was added, and the culture was incubated at 37°C for 2 h. Transformants were selected on TS agar plates supplemented with the appropriate antibiotic. The resulting colonies were screened for the targeted deletion by PCR using primers 5 and 6 and were shown not to have any generalized growth defect during growth on rich medium. Mutagenesis of bgaA and nanA was also confirmed by enzymatic activity assays as previously reported (73, 74). In cases where phenotypes were observed, flanking regions were sequenced to ensure that no spurious changes were introduced.
ATCC 10557 Fap1R489E and ATCC 10557 Fap1Δ622-708 were generated using the two-step Janus cassette system (75). For ATCC 10557 Fap1R489E, a gene fragment encoding the Fap1 nonrepeat region (amino acid residues 220 to 726; primers NRR.F and NRR.R) was cloned into pGEX-5X-3 (GE Healthcare, Chicago, IL) by use of In-Fusion to generate pGEX-5X-3 Fap1NRR. For ATCC 10557 Fap1Δ622-708, a gene fragment encoding amino acid residues 500 to 860 (primers NRRI.R and Δ622-708.R) was cloned into the pJET1.2/Blunt PCR cloning vector (Thermo Fisher Scientific, Waltham, MA) to create pJETFap1C. The constructs for generation of the Janus intermediates were made by blunt-end ligation of the Janus cassette (primers J.F and J.R) into inverse PCR products from pGEX-5X-3 Fap1NRR (primers NRRI.F and NRRI.R) and pJETFap1C (primers Δ622-708I.F and Δ622-708I.R). Following confirmation of the constructs (pGEX-5X-3 Fap1NRRJanus and pJET Fap1C Janus), these plasmids were transformed into ATCC 10557 Smr. Transformants were selected on kanamycin and confirmed by PCR with primers flanking the construct (R489E.5/R489E.6 and Δ622-708.5/F.6). The second round of transformation replaced the Janus cassette with a fragment of DNA containing the desired mutation. For generation of ATCC 10557 Fap1R489E, this was plasmid pGEX-5X-3 Fap1NRRR489E, in which the codon for Glu 489 was changed to a codon for Arg (primers R489E.F and R489E.R) by use of a QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). In the case of ATCC 10557 Fap1Δ622-708, plasmid pJETFap1-Δ622-708 was generated by self-ligation of an inverse PCR product from pJETFap1C (primers Δ622-708I.F and Δ622-708I.R), resulting in an in-frame deletion in the predicted CBM (amino acids 622 to 708). Final mutants were selected on streptomycin and confirmed by sequencing.
Adherence assays.
Assays to determine adherence to human platelets were adapted from the work of Sullam et al. (76). In brief, blood from healthy donors was collected in 1:5 (vol/vol) acid citrate dextrose solution (56 mM trisodium citrate dehydrate, 65 mM citric acid, 100 mM dextrose). As the blood was drawn from healthy donors and no identifiers were collected, the Institutional Review Board at Nationwide Children's Hospital deemed this study to not involve human subjects. Pooled platelet-rich plasma (PRP) was collected by centrifugation of blood samples at 200 × g for 10 min at room temperature and diluted 1:4 (vol/vol) in platelet wash buffer (9.3 mM trisodium citrate dehydrate, 5.3 mM citric acid, 17.3 mM dextrose, 145.5 mM NaCl, pH 6.5). Platelets were collected by centrifugation at 750 × g for 10 min and washed twice in platelet wash buffer. Platelets were suspended in platelet wash buffer at 1 × 107 platelets ml−1 and fixed with 1% paraformaldehyde for 10 min at room temperature. Following three washes in phosphate-buffered saline (PBS), platelets were resuspended in PBS. To test bacterial adherence to platelets, each well of a flat-bottomed 96-well microtiter plate was coated with 1 × 106 fixed platelets for 60 min at 37°C, and unbound platelets were removed by three PBS washes. Control wells were coated with 1% (wt/vol) bovine serum albumin (BSA) in PBS. All wells were blocked with 1% BSA in PBS for 60 min at 37°C prior to adherence assays.
Where appropriate, wells were pretreated for 30 min with 50 μl of PBS containing 0.78 μM purified Clostridium perfringens sialidase, a 0.054 μM concentration of the recombinantly expressed SpBgaA146-990, or both. Control wells were incubated with PBS alone, and all wells were washed three times with PBS prior to addition of bacteria. Where stated, glycosidases, sialic acid, lactose, or CBM71 was mixed with bacterial cells at the specified concentration, and adherence was assessed in the same manner. SpBgaA146-990 and CBM71 were recombinantly expressed and purified as previously described (30).
Bacteria were grown to an OD600 of 0.6 ± 0.05, and approximately 2 × 105 bacteria in PBS were allowed to adhere at 37°C for 60 min. Nonadherent bacteria were removed by three washes in PBS, and adherent bacteria were lifted with 0.25% trypsin-1 mM EDTA and enumerated by serial dilution. All adherence assays were performed in triplicate on at least three independent occasions. Bacterial adherence was calculated as the percentage of the inoculum adherent to the platelets. Data are presented as means ± standard deviations (SD). The preparation of platelets from pooled blood from different donors and multiple platelet preparations resulted in some differences in adherence of wild-type bacteria between sets of experiments; however, the trends were maintained. Statistical significance was determined using two-tailed Student's t test, and data points with P values of ≤0.05 were considered significant.
Human umbilical vein endothelial cells (HUVECs) were grown in 24-well plates in EBM-2 medium supplemented with EGM-2 SingleQuats (Lonza, Walkersville, MD). Adherence to HUVECs was assessed in the same way as that described above, except that pretreatment with enzymes was conducted in 200-μl aliquots of antibiotic-free medium and 5 × 105 bacteria were added per well.
The buccal keratinizing squamous cell line TR146 was grown in 24-well plates in F-12 medium with 10% fetal bovine serum (vol/vol), 2 mM glutamic acid, and 1% penicillin-streptomycin solution (77). Adherence to TR146 cells was assessed as described for other cells, except that 1 × 106 bacteria were added per well.
Accession number(s).
As there were small sequence differences between our strain and the published genome sequences, the nucleotide sequences of the nanA, bgaA, and fap1 regions were submitted to GenBank under accession numbers KX792091, KX792092, and KX792093, respectively.
ACKNOWLEDGMENTS
We thank Steve Goodman of Nationwide Children's Hospital and David Beighton, formerly of Kings College London, for providing strains.
We thank the Infectious Disease Consortium at Nationwide Children's Hospital for a trainee grant to A.K.S.
REFERENCES
- 1.Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, Briancon S, Casalta JP, Danchin N, Delahaye F, Etienne J, Le Moing V, Leport C, Mainardi JL, Ruimy R, Vandenesch F, Association pour l'Etude et la Prevention de l'Endocardite Infectieuse (AEPEI) Study Group . 2002. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 2.Nissen H, Nielsen PF, Frederiksen M, Helleberg C, Nielsen JS. 1992. Native valve infective endocarditis in the general population: a 10-year survey of the clinical picture during the 1980s. Eur Heart J 13:872–877. [DOI] [PubMed] [Google Scholar]
- 3.Benn M, Hagelskjaer LH, Tvede M. 1997. Infective endocarditis, 1984 through 1993: a clinical and microbiological survey. J Intern Med 242:15–22. doi: 10.1046/j.1365-2796.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH, International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators . 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabell CH, Abrutyn E. 2002. Progress toward a global understanding of infective endocarditis. Early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin North Am 16:255–272. [DOI] [PubMed] [Google Scholar]
- 6.Karchmer AW. 2001. Infective endocarditis, p 1723–1748. In Zipes DP, Libby P, Bonow RO, Braunwald E (ed), Braunwald's heart disease: a textbook of cardiovascular medicine, 6th ed Elsevier Saunders, Philadelphia, PA. [Google Scholar]
- 7.Werdan K, Dietz S, Loffler B, Niemann S, Bushnaq H, Silber RE, Peters G, Muller-Werdan U. 2014. Mechanisms of infective endocarditis: pathogen-host interaction and risk states. Nat Rev Cardiol 11:35–50. doi: 10.1038/nrcardio.2013.174. [DOI] [PubMed] [Google Scholar]
- 8.Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, Chia JS. 2012. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis 205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Bensing BA, Thamadilok S, Yu H, Lau K, Chen X, Ruhl S, Sullam PM, Varki A. 2014. Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood. PLoS Pathog 10:e1004540. doi: 10.1371/journal.ppat.1004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. 2008. Bacteremia associated with toothbrushing and dental extraction. Circulation 117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naveen Kumar V, van der Linden M, Menon T, Nitsche-Schmitz DP. 2014. Viridans and bovis group streptococci that cause infective endocarditis in two regions with contrasting epidemiology. Int J Med Microbiol 304:262–268. doi: 10.1016/j.ijmm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, Moustafa SE, Hoskin TL, Mandrekar JN, Wilson WR, Baddour LM. 2005. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA 293:3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 13.Mylonakis E, Calderwood SB. 2001. Infective endocarditis in adults. N Engl J Med 345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 14.Westling K, Julander I, Ljungman P, Vondracek M, Wretlind B, Jalal S. 2008. Identification of species of viridans group streptococci in clinical blood culture isolates by sequence analysis of the RNase P RNA gene, rnpB. J Infect 56:204–210. doi: 10.1016/j.jinf.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Bensing BA, Lopez JA, Sullam PM. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect Immun 72:6528–6537. doi: 10.1128/IAI.72.11.6528-6537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takamatsu D, Bensing BA, Cheng H, Jarvis GA, Siboo IR, Lopez JA, Griffiss JM, Sullam PM. 2005. Binding of the Streptococcus gordonii surface glycoproteins Gspβ and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibα. Mol Microbiol 58:380–392. doi: 10.1111/j.1365-2958.2005.04830.x. [DOI] [PubMed] [Google Scholar]
- 17.Plummer C, Wu H, Kerrigan SW, Meade G, Cox D, Ian Douglas CW. 2005. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br J Haematol 129:101–109. doi: 10.1111/j.1365-2141.2005.05421.x. [DOI] [PubMed] [Google Scholar]
- 18.Bensing BA, Sullam PM. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol Microbiol 44:1081–1094. doi: 10.1046/j.1365-2958.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Konishi K, Cisar JO, Yoshikawa M. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect Immun 70:1209–1218. doi: 10.1128/IAI.70.3.1209-1218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyburn TM, Bensing BA, Xiong YQ, Melancon BJ, Tomasiak TM, Ward NJ, Yankovskaya V, Oliver KM, Cecchini G, Sulikowski GA, Tyska MJ, Sullam PM, Iverson TM. 2011. A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog 7:e1002112. doi: 10.1371/journal.ppat.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong YQ, Bensing BA, Bayer AS, Chambers HF, Sullam PM. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb Pathog 45:297–301. doi: 10.1016/j.micpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Y, Takashima E, Shimazu K, Yagishita H, Aoba T, Konishi K. 2006. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect Immun 74:740–743. doi: 10.1128/IAI.74.1.740-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo HS, Xiong YQ, Sullam PM. 2013. Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis. PLoS One 8:e64204. doi: 10.1371/journal.pone.0064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu SD, Cisar JO, Sandberg AL, Kilian M. 1994. Adhesive properties of viridans streptococcal species. Microb Ecol Health Dis 7:125–137. [Google Scholar]
- 25.Tilley DO, Arman M, Smolenski A, Cox D, O'Donnell JS, Douglas CW, Watson SP, Kerrigan SW. 2013. Glycoprotein Ibα and FcγRIIa play key roles in platelet activation by the colonizing bacterium, Streptococcus oralis. J Thromb Haemost 11:941–950. doi: 10.1111/jth.12175. [DOI] [PubMed] [Google Scholar]
- 26.Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ. 1992. Adherence of oral streptococci to salivary glycoproteins. Infect Immun 60:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray PA, Levine MJ, Tabak LA, Reddy MS. 1982. Specificity of salivary-bacterial interactions. II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAcα2, 3Galβ1, 3GalNAc sequence. Biochem Biophys Res Commun 106:390–396. [DOI] [PubMed] [Google Scholar]
- 28.Beighton D, Whiley RA. 1990. Sialidase activity of the “Streptococcus milleri group” and other viridans group streptococci. J Clin Microbiol 28:1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byers HL, Tarelli E, Homer KA, Beighton D. 2000. Isolation and characterisation of sialidase from a strain of Streptococcus oralis. J Med Microbiol 49:235–244. doi: 10.1099/0022-1317-49-3-235. [DOI] [PubMed] [Google Scholar]
- 30.Singh AK, Pluvinage B, Higgins MA, Dalia AB, Woodiga SA, Flynn M, Lloyd AR, Weiser JN, Stubbs KA, Boraston AB, King SJ. 2014. Unravelling the multiple functions of the architecturally intricate Streptococcus pneumoniae beta-galactosidase, BgaA. PLoS Pathog 10:e1004364. doi: 10.1371/journal.ppat.1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limoli DH, Sladek JA, Fuller LA, Singh AK, King SJ. 2011. BgaA acts as an adhesin to mediate attachment of some pneumococcal strains to human epithelial cells. Microbiology 157:2369–2381. doi: 10.1099/mic.0.045609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichmann P, Nuhn M, Denapaite D, Bruckner R, Henrich B, Maurer P, Rieger M, Klages S, Reinhard R, Hakenbeck R. 2011. Genome of Streptococcus oralis strain Uo5. J Bacteriol 193:2888–2889. doi: 10.1128/JB.00321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handley PS, Correia FF, Russell K, Rosan B, DiRienzo JM. 2005. Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol Immunol 20:131–140. doi: 10.1111/j.1399-302X.2004.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Mintz KP, Ladha M, Fives-Taylor PM. 1998. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol 28:487–500. doi: 10.1046/j.1365-2958.1998.00805.x. [DOI] [PubMed] [Google Scholar]
- 35.Samen U, Eikmanns BJ, Reinscheid DJ, Borges F. 2007. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect Immun 75:5405–5414. doi: 10.1128/IAI.00717-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 37.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. 2009. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis 199:1479–1487. doi: 10.1086/598217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Fives-Taylor PM. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol Microbiol 34:1070–1081. doi: 10.1046/j.1365-2958.1999.01670.x. [DOI] [PubMed] [Google Scholar]
- 39.Mistou MY, Dramsi S, Brega S, Poyart C, Trieu-Cuot P. 2009. Molecular dissection of the secA2 locus of group B streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J Bacteriol 191:4195–4206. doi: 10.1128/JB.01673-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. 2009. The Streptococcus pneumoniae adhesin PsrP binds to keratin 10 on lung cells. Mol Microbiol 73:663–679. doi: 10.1111/j.1365-2958.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. 2006. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect Immun 74:1933–1940. doi: 10.1128/IAI.74.3.1933-1940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siboo IR, Chambers HF, Sullam PM. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect Immun 73:2273–2280. doi: 10.1128/IAI.73.4.2273-2280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. 2010. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog 6:e1001044. doi: 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo HS, Mu R, Kim BJ, Doran KS, Sullam PM. 2012. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS Pathog 8:e1002947. doi: 10.1371/journal.ppat.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YH, Jiang YL, Zhang J, Wang L, Bai XH, Zhang SJ, Ren YM, Li N, Zhang YH, Zhang Z, Gong Q, Mei Y, Xue T, Zhang JR, Chen Y, Zhou CZ. 2014. Structural insights into SraP-mediated Staphylococcus aureus adhesion to host cells. PLoS Pathog 10:e1004169. doi: 10.1371/journal.ppat.1004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheen TR, Jimenez A, Wang NY, Banerjee A, van Sorge NM, Doran KS. 2011. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J Bacteriol 193:6834–6842. doi: 10.1128/JB.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou MX, Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155:317–327. doi: 10.1099/mic.0.025221-0. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, Morimoto Y, Kuroda M, Cui L, Takahashi M, Ankai A, Baba S, Fukui S, Lee JC, Hiramatsu K. 2005. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol 187:7292–7308. doi: 10.1128/JB.187.21.7292-7308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Froeliger EH, Fives-Taylor P. 2001. Streptococcus parasanguis fimbria-associated adhesin Fap1 is required for biofilm formation. Infect Immun 69:2512–2519. doi: 10.1128/IAI.69.4.2512-2519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim AR, Ahn KB, Kim HY, Seo HS, Yun CH, Han SH. 2016. Serine-rich repeat adhesin gordonii surface protein B is important for Streptococcus gordonii biofilm formation. J Endod 42:1767–1772. doi: 10.1016/j.joen.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Ramboarina S, Garnett JA, Zhou M, Li Y, Peng Z, Taylor JD, Lee WC, Bodey A, Murray JW, Alguel Y, Bergeron J, Bardiaux B, Sawyer E, Isaacson R, Tagliaferri C, Cota E, Nilges M, Simpson P, Ruiz T, Wu H, Matthews S. 2010. Structural insights into serine-rich fimbriae from Gram-positive bacteria. J Biol Chem 285:32446–32457. doi: 10.1074/jbc.M110.128165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lizcano A, Sanchez CJ, Orihuela CJ. 2012. A role for glycosylated serine-rich repeat proteins in Gram-positive bacterial pathogenesis. Mol Oral Microbiol 27:257–269. doi: 10.1111/j.2041-1014.2012.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilian M, Riley DR, Jensen A, Bruggemann H, Tettelin H. 2014. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio 5:e01490-14. doi: 10.1128/mBio.01490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stephenson AE, Wu H, Novak J, Tomana M, Mintz K, Fives-Taylor P. 2002. The Fap1 fimbrial adhesin is a glycoprotein: antibodies specific for the glycan moiety block the adhesion of Streptococcus parasanguis in an in vitro tooth model. Mol Microbiol 43:147–157. doi: 10.1046/j.1365-2958.2002.02725.x. [DOI] [PubMed] [Google Scholar]
- 55.Bensing BA, Khedri Z, Deng L, Yu H, Prakobphol A, Fisher SJ, Chen X, Iverson TM, Varki A, Sullam PM. 2016. Novel aspects of sialoglycan recognition by the Siglec-like domains of streptococcal SRR glycoproteins. Glycobiology 26:1221–1233. doi: 10.1093/glycob/cww042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bensing BA, Loukachevitch LV, McCulloch KM, Yu H, Vann KR, Wawrzak Z, Anderson S, Chen X, Sullam PM, Iverson TM. 2016. Structural basis for sialoglycan binding by the Streptococcus sanguinis SrpA adhesin. J Biol Chem 291:7230–7240. doi: 10.1074/jbc.M115.701425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soding J, Biegert A, Lupas AN. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soding J. 2005. Protein homology detection by HMM-HMM comparison. Bioinformatics 21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 59.Najmudin S, Guerreiro CI, Carvalho AL, Prates JA, Correia MA, Alves VD, Ferreira LM, Romao MJ, Gilbert HJ, Bolam DN, Fontes CM. 2006. Xyloglucan is recognized by carbohydrate-binding modules that interact with beta-glucan chains. J Biol Chem 281:8815–8828. doi: 10.1074/jbc.M510559200. [DOI] [PubMed] [Google Scholar]
- 60.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King SJ. 2010. Pneumococcal modification of host sugars: a major contributor to colonization of the human airway? Mol Oral Microbiol 25:15–24. doi: 10.1111/j.2041-1014.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 62.Fachon-Kalweit S, Elder BL, Fives-Taylor P. 1985. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun 48:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thamadilok S, Roche-Hakansson H, Hakansson AP, Ruhl S. 2016. Absence of capsule reveals glycan-mediated binding and recognition of salivary mucin MUC7 by Streptococcus pneumoniae. Mol Oral Microbiol 31:175–188. doi: 10.1111/omi.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plummer C, Douglas CW. 2006. Relationship between the ability of oral streptococci to interact with platelet glycoprotein Ibα and with the salivary low-molecular-weight mucin, MG2. FEMS Immunol Med Microbiol 48:390–399. doi: 10.1111/j.1574-695X.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji T, Tsunehisa S, Watanabe Y, Yamamoto K, Tohyama H, Osawa T. 1983. The carbohydrate moiety of human platelet glycocalicin. J Biol Chem 258:6335–6339. [PubMed] [Google Scholar]
- 66.Korrel SA, Clemetson KJ, Van Halbeek H, Kamerling JP, Sixma JJ, Vliegenthart JF. 1984. Structural studies on the O-linked carbohydrate chains of human platelet glycocalicin. Eur J Biochem 140:571–576. doi: 10.1111/j.1432-1033.1984.tb08140.x. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi Y, Ruhl S, Yoon JW, Sandberg AL, Cisar JO. 2002. Adhesion of viridans group streptococci to sialic acid-, galactose- and N-acetylgalactosamine-containing receptors. Oral Microbiol Immunol 17:257–262. doi: 10.1034/j.1399-302X.2002.170409.x. [DOI] [PubMed] [Google Scholar]
- 68.Ruhl S, Sandberg AL, Cisar JO. 2004. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J Dent Res 83:505–510. doi: 10.1177/154405910408300614. [DOI] [PubMed] [Google Scholar]
- 69.Do T, Jolley KA, Maiden MC, Gilbert SC, Clark D, Wade WG, Beighton D. 2009. Population structure of Streptococcus oralis. Microbiology 155:2593–2602. doi: 10.1099/mic.0.027284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lacks S, Hotchkiss RD. 1960. A study of the genetic material determining an enzyme in pneumococcus. Biochim Biophys Acta 39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 71.Whatmore AM, Barcus VA, Dowson CG. 1999. Genetic diversity of the streptococcal competence (com) gene locus. J Bacteriol 181:3144–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida Y, Yang J, Peaker PE, Kato H, Bush CA, Cisar JO. 2008. Molecular and antigenic characterization of a Streptococcus oralis coaggregation receptor polysaccharide by carbohydrate engineering in Streptococcus gordonii. J Biol Chem 283:12654–12664. doi: 10.1074/jbc.M801412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King SJ, Hippe KR, Weiser JN. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol 59:961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- 74.King SJ, Whatmore AM, Dowson CG. 2005. NanA, a neuraminidase from Streptococcus pneumoniae, shows high levels of sequence diversity, at least in part through recombination with Streptococcus oralis. J Bacteriol 187:5376–5386. doi: 10.1128/JB.187.15.5376-5386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol 67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullam PM, Bayer AS, Foss WM, Cheung AL. 1996. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect Immun 64:4915–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rupniak HT, Rowlatt C, Lane EB, Steele JG, Trejdosiewicz LK, Laskiewicz B, Povey S, Hill BT. 1985. Characteristics of four new human cell lines derived from squamous cell carcinomas of the head and neck. J Natl Cancer Inst 75:621–635. [PubMed] [Google Scholar]
- 78.Washburn MR, White JC, Niven CF Jr. 1946. Streptococcus S.B.E.: immunological characteristics. J Bacteriol 51:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]