ABSTRACT
Exposure to blood-stage malaria infection is often persistent, leading to generation of CD4 effector and effector memory T cells that contribute to protection. We showed previously that chronic exposure to blood-stage Plasmodium chabaudi offers the best protection from parasitemia and pathology in reinfection cases, correlating with an increase in Th1 cells. Although much is known about the features of resting or exhausted memory T cells (Tmem), little is known about the functional capacities of chronically stimulated but protective T cells. To determine the functional capacity of CD4 T cells generated by chronic infection upon reexposure to parasite, we compared their responses to known features of classical Tmem. The numbers of cytokine-producing T cells increased following infection in the polyclonal populations, suggesting an increase in pathogen-specific T cells. Malaria antigen-specific B5 T cell receptor (TCR) transgenic (Tg) T cells from chronic infection proliferated on reinfection and were highly sensitive to TCR stimulation without costimulation, as shown for Tmem in acute stimulations. However, B5 Tmem did not accumulate more than naive B5 T cells in vivo or in vitro and became apoptotic. Failure to accumulate was partly the result of chronic stimulation, since eliminating persistent parasites before reinfection slightly increased the accumulation of B5 Tg T cells upon reinfection. The levels of specific gamma interferon-positive, interleukin-10-positive T cells, which protect animals from pathology, increased after malaria infection. These data demonstrate that although chronic infection generates a protective T cell population with increased TCR sensitivity and cytokine production, they do not reexpand upon reexposure due to increased apoptosis.
KEYWORDS: T cells, immune memory, malaria, mouse
INTRODUCTION
Blood-stage malaria infection, like other chronic infections, generates effector memory T cells (Tem) in mice and humans (1, 2). Children living in areas with high malaria transmission demonstrate a decrease in the incidence of malaria disease as they grow older (3). This decrease in malarial incidence is associated with an increased number of gamma interferon (IFN-γ)-producing effector/effector memory CD4 T cells upon parasite exposure (1, 2). However, the levels of malaria-responsive T cells and malaria antigen-specific antibody titers decay over time (4–6), as does clinical immunity. These data support the conclusion that there are disease-protective memory B and T cells in individuals repeatedly infected with malaria that decay in the absence of exposure. However, the effector mechanisms by which these immune cells contribute to protection from repeated parasitemia and malaria disease are poorly understood.
Chronic infection with Plasmodium chabaudi maintains a protective immune response against reinfection that we are just beginning to understand. Reinfection before the parasite is cleared naturally or using antimalarial drugs leads to lower secondary parasitemia than if the parasite is already cleared before reinfection (7). Our previous work suggests that in the chronic phase of this infection, there is a T cell population expressing markers of the memory T cell (Tmem) phenotype (CD44hi IL-7Rαhi) (2). However, the T cell population in the memory phase of this infection also contains a mixture of effector T cells (Teff; IL-7Rα−) and effector memory T cells (Tem; CD44hi IL-7Rαhi CD62Llo), with a small fraction of central memory T cells (Tcm; CD44hi IL-7Rαhi CD62Lhi) (2). In addition, we showed that there is an increase in a Th1 Teff/Tem population (CD44hi CD62Llo IFN-γ+ TNF+ IL-2−) during chronic infection compared to treated infections that corresponds with increased protection from P. chabaudi infection during chronic infection. The predominance of effector/effector memory T cells over central memory T cells could explain the decay of immunity in malaria, since both Teff and Tem are reported to be short-lived (8). An increase in Tem has also been documented in several other chronic infections (9). On the other hand, we recently defined the pathway of generation of Tem in P. chabaudi infection and showed that both Tem and Tcm are made before the peak of infection, from CD62Lhi early Teff (10). We also showed that the ratio of Tcm to Tem is determined before T cell proliferation, suggesting that there is still much to learn about the generation of Tem and their role in chronic infection. However, our previous work showed that curing long-term chronic infection, although it decreases protection, does not change the expression of CD62L on Tmem (2). This result suggests that there are other important functional features besides expression of CD62L, or the ratio of Tem to Tcm, required for protection. This view is consistent with the work of Hikono et al. (11), which showed that the protective capacity of T cells correlates with several other surface markers of activation besides CD62L. Together, these data suggest that the functional features of T cells in the memory phase are more important than their surface phenotype. For example, the increased protection in chronically infected animals is due to an increase in Th1 cells and not in Tem overall. Other functional features of Tmem in chronic infection are less well understood.
The most salient feature of the memory T cell population is an increase in precursor frequency of pathogen-specific cells, the result of clonal expansion. This increase leads to increased secondary responses due to faster T cell expansion and more cytokine production overall on restimulation. Another general feature of Tmem is heightened intrinsic T cell receptor (TCR) sensitivity, including a reduced requirement for costimulation (12). Although there is a significant amount of literature documenting the functional features of memory T cells formed in response to acute stimuli, little is known about the mechanisms of immunity that are dependent on reexposure (13, 14). Furthermore, it is not clear which features are most important to the overall memory effect or to protection, especially in chronic infection. Although memory T cells are reported to respond faster than naive cells to generate an improved immune response to reinfection (15, 16), the secondary T cell response to persistent infections has not been fully studied.
In order to understand the functional features of the T cell population in chronic P. chabaudi, we investigated the responsiveness of antigen-experienced (CD44hi) malaria antigen-specific CD4 T cells 2 months postinfection (p.i.), in the presence or absence of persistent infection. In this study, we test the functional attributes of memory T cells that develop in malaria infection by comparing the responses of naive and memory T cells to reinfection with P. chabaudi. In short, we show that the polyclonal memory T cell response to reinfection is enhanced during chronic P. chabaudi infection. However, although merozoite surface protein 1 (MSP-1)-specific B5 TCR transgenic (Tg) memory T cells are more sensitive to suboptimal stimulation and able to proliferate, they do not accumulate more than specific naive T cells and appear to be apoptotic. Furthermore, the responsiveness of the memory T cells 2 months after infection does not improve substantially with the clearance of antigen with the antimalarial drug chloroquine (CQ), suggesting that the failure of these cells to accumulate is not primarily due to exposure to chronic infection. Importantly, the interleukin-10 (IL-10) response of malaria antigen-specific Th1 cells is maintained, suggesting a potential mechanism for the reduction of pathology provided by T cells generated in this infection.
RESULTS
The polyclonal malaria-antigen-responsive CD4 T cell number is increased postinfection.
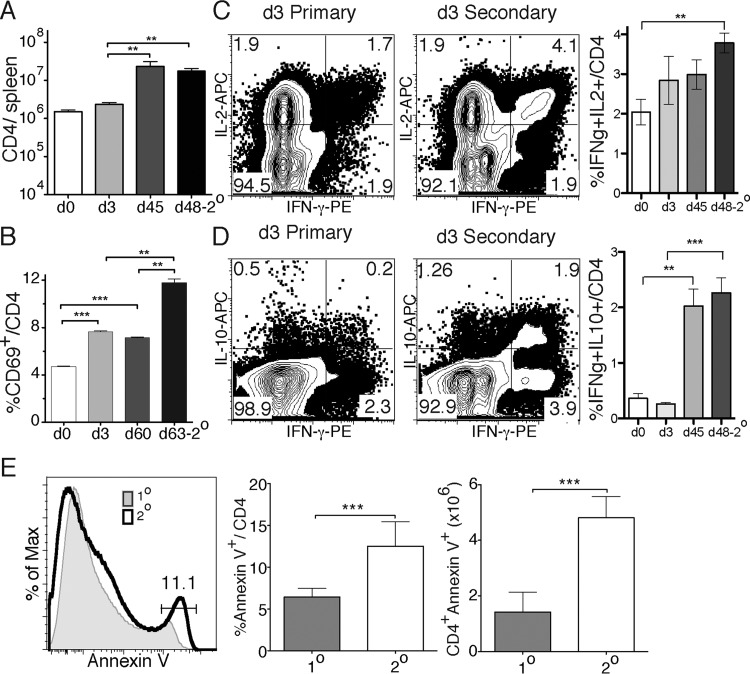
CD4+ memory T cells are known to respond faster to a secondary than a primary challenge (17), and an increase in the precursor frequency of pathogen-specific T cells in the polyclonal T cell population is likely the most important contributor to this effect. Memory T cells are frequently defined in the literature as antigen-experienced T cells that reexpand in response to secondary stimulation. Despite the reexpansion assay being a standard assay of memory cell function, the reexpansion of CD4 T cells has not been clearly linked to protection, especially from chronic infections, where effector memory T cells predominate. To examine the relationship between expansion and protection in the context of malaria infection, we first tested whether P. chabaudi infection generates an increase in malaria parasite-specific T cell frequency after infection. BALB/c mice were first infected with P. chabaudi-parasitized red blood cells and reinfected 45 or 60 days later during the chronic phase of infection. For each study, controls consisted of age-matched animals that were either naive mice or mice that had been infected only once 3 days prior to isolation of their splenocytes for analysis. Three days after the secondary challenge (i.e., day 48 of the secondary infection [d48-2°] or d63-2°), we measured the fraction of endogenous CD4 T cells that responded to reinfection by expressing an early activation marker (CD69) or by producing cytokines (IL-2, IFN-γ, and IL-10).
Over the course of the infection, there was an increase in the number of CD4 T cells up to d45 of the primary infection (Fig. 1A). A 3-day infection does not lead to an increase in CD4 T cells in the spleen after either the first (1°; day 3) or second (d48-2°) infection. To determine the fraction of T cells activated in the first and second infections, we used CD69 to mark recently activated CD4 T cells. There was a significant increase in the fraction of CD69+ T cells both day 0 and day 3, and even up to day 60, after primary infection. We also observed an increase in CD69+ CD4 T cells from day 60 postinfection to day 3 postreinfection (d63-2°, Fig. 1B). The increase in the proportion of CD4 T cells that are CD69+ on day 3 of the second infection compared to day 3 of the first infection suggests that the T cell population after the primary infection includes more malaria antigen-responsive T cells than the naive population due to clonal expansion occurring during the first infection. In addition, naive T cells are likely to be activated in the second infection, as in the first. We also observed a higher proportion of IFN-γ+ multi-cytokine-producing CD4 T cells (IL-2+ IFN-γ+ and IL-10+ IFN-γ+) by day 3 of the second infection (d48-2°) than after the first infection (Fig. 1C and D), suggesting a faster cytokine response. Increases in IFN-γ+ IL-2+ T cells occur in a manner similar to that for the CD69+ T cells, although the differences before the secondary response were not statistically significant (Fig. 1C). The fraction of IL-10+ IFN-γ+ T cells was strongly increased by day 45 of infection (Fig. 1D). IL-10 expression by IFN-γ+ CD4 T cells has been shown previously to start on day 15 after infection with P. chabaudi (18).
FIG 1.
More polyclonal CD4 T cells are activated quickly after a second infection than after the first infection. Endogenous CD4 T cells from the spleens of P. chabaudi-infected age-matched BALB/c mice were analyzed after primary or secondary infection. (A) Total CD4 T cell numbers recovered on day 0, 3, or 45 after primary infection or day 48, 3 days after a second infection (d48-2°). (B) Percentages of CD69+ CD4 T cells on day 0, 3, or 60 of a primary infection or day 63, 3 days after a second infection (d63-2°). (C and D) Contour plots and summary graphs showing cytokine profiles of IFN-γ and either IL-2 (C) or IL-10 (D) by intracellular cytokine staining of CD4+ T cells from day 3 after primary or day 3 after secondary infection (day 48). (E) Histogram and summary graphs showing annexin V staining of CD4 T cells at day 5 p.i. of a first (1°) or second (2°) infection (day 50). The data represent three independent experiments with five mice per group, and error bars represent the standard errors of the mean (SEM). The plots are contour plots with outliers of a representative mouse from each group. **, P < 0.01; ***, P < 0.005 (Student t test).
In order to test whether CD4 T cells are sensitive to apoptosis in P. chabaudi infection, as previously described (19), we measured the apoptotic marker annexin V (exposed phosphatidylserine) 5 days after the first or second challenge. We observed a significant increase in annexin V-positive CD4 T cells after the second infection (d60-2°) compared to the level after the first infection (Fig. 1E). Taken together, these data suggest that there is an increase in malaria-responsive T cells in the spleen after a P. chabaudi infection that express markers of activation or cytokines in the second infection. However, the T cells present after P. chabaudi infection also show an increased propensity for apoptosis.
Malaria-specific memory T cells do not expand as well as naive T cells in response to reinfection.
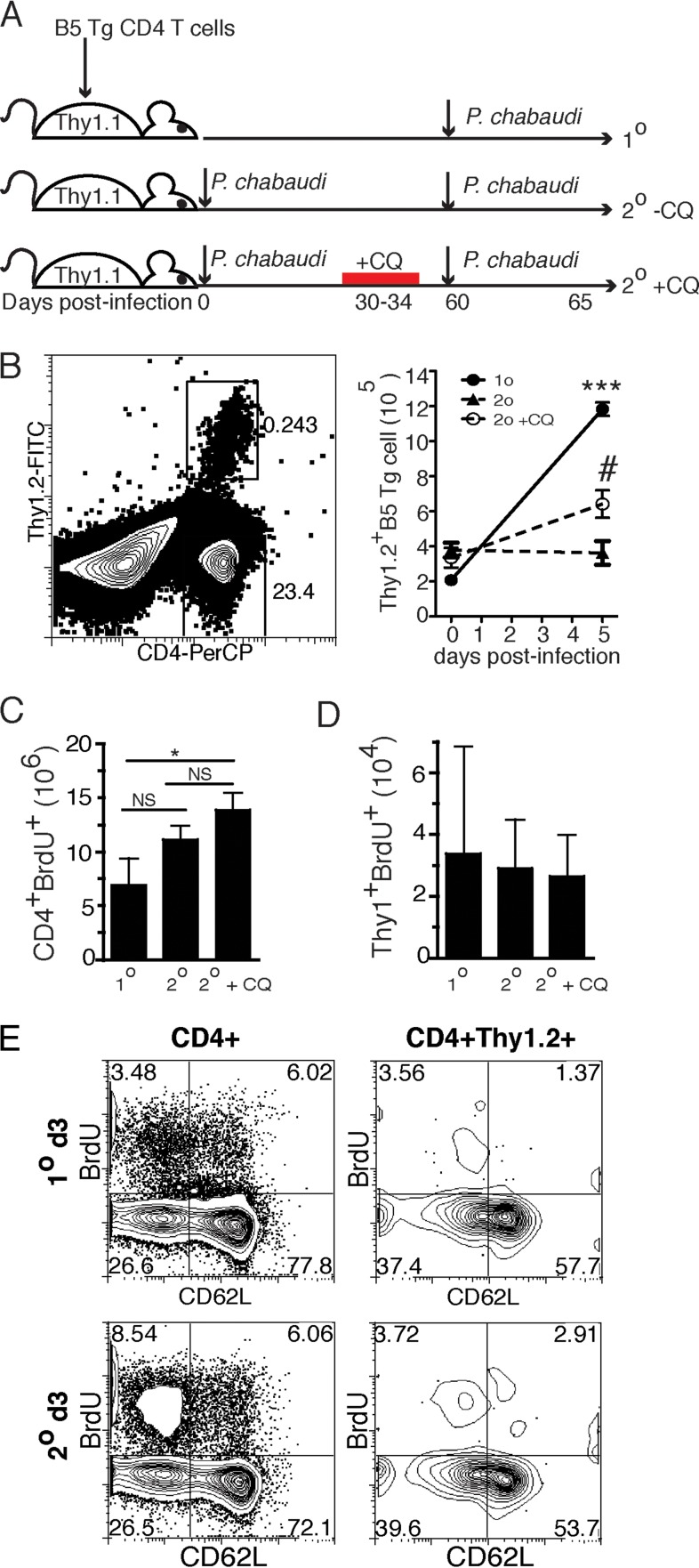
To test whether malaria antigen-specific T cells respond to the second infection with different kinetics than the first, we adoptively transferred P. chabaudi merozoite surface protein 1 (MSP-1)-specific B5 T cell receptor (TCR) transgenic (Tg) T cells before infection. Purified CD4+ B5 TCR Tg T cells (2 × 106) were transferred into age-matched Thy1.1 congenic mice. The transfer of B5 TCR Tg T cells does not affect parasitemia, as previously reported (20). Animals were then infected either the next day and again 2 months later (2°), or only once at 2 months after T cell transfer (1°), as shown in Fig. 2A. This was done to directly compare primary and secondary cell numbers by flow cytometry of the samples on the same day. Unexpectedly, when analyzed on day 5 after each infection, B5 TCR Tg T cells did not expand more after the second infection compared to the ∼6-fold expansion in response to the primary infection (Fig. 2B). One potential explanation for this consistent observation is that the persistence of parasites in the chronic infection, documented to last up to 3 months (20), inhibits the proliferation of T cells, as recently reported in human P. falciparum infection (21).
FIG 2.
MSP-1-specific memory T cells do not expand more than naive T cells. The experimental design is schematically represented in panel A. The primary-infection group was Thy1.1 recipients of adoptively transferred B5 TCR Tg T cells (2 × 106) infected with P. chabaudi one time, 60 days after T cell transfer. In the secondary-infection groups, recipients were infected the same day as the adoptive transfer and again 60 days later. Recipients in the secondary +CQ group were also treated with the antimalarial drug chloroquine between days 30 and 34 p.i. (B) Representative cytometry plot and graph of number of recovered CD4+ Thy1.2+ B5 T cells per spleen on days 0 and 5 p.i. for the 1° group and days 0 and 5 after secondary infection for the 2° and 2° +CQ groups. Data are representative of six independent experiments with five mice per group. (C and D) On day 60 posttransfer, all mice were given BrdU for the first 3 days of infection and then analyzed. Graphs indicate proliferating (BrdU+) CD4 T cells (C) or B5 TCR Tg (Thy1.2+) cells (D). (E) Contour plots showing BrdU staining and CD62L expression on CD4+ and B5 TCR Tg (Thy1.2+ CD4+) T cells. Error bars represent the SEM. #, P < 0.05 compared to −CQ; ***, P < 0.005 compared to 1°; *, P < 0.05; NS, not significant (Student t test).
To test whether chronic parasitemia was indeed inhibiting T cell proliferation, we treated P. chabaudi-infected mice with chloroquine from days 30 to 34 postinfection (p.i.). Chloroquine has been previously shown to eliminate chronic P. chabaudi parasitemia (22). We have also shown that chloroquine treatment at this time point reduces protection from parasitemia and decreases polyclonal secondary B and T cell responses to this infection (7, 23, 24). In this experiment, chronic exposure of T cells to parasite antigens was eliminated for approximately 24 days before reinfection. This treatment significantly increased the number of malaria antigen-specific B5 TCR Tg T cells recovered 5 days after the second infection compared to untreated (2°+CQ versus 2°−CQ). However, treatment did not cause the T cells to expand as much as we observed in response to the first exposure (1°, Fig. 2B). To directly measure T cell proliferation, we monitored bromodeoxyuridine (BrdU) incorporation into the DNA of dividing polyclonal or MSP-1-specific B5 TCR Tg cells over the first 3 days p.i. in the context of chronic or treated infection. We observed a small increase in the early proliferation of total CD4 T cells after secondary challenge compared to primary challenge, which became significant in the chloroquine-treated mice (Fig. 2C). This observation supports the increased responsiveness of the polyclonal population seen above, at least in treated infection. There were no detectable changes in division of B5 TCR Tg T cells among groups in this short period of exposure to parasite and BrdU (Fig. 2D), but BrdU staining was clearly detectable in both CD4 and B5 TCR Tg T cells (Fig. 2E). BrdU staining is validated by the increased division in the activated (CD62Llo) population. These data suggest that MSP-1-specific T cells present after primary infection do not expand in numbers as much as they did when they were naive.
Deficient secondary activation is partially due to chronic infection.
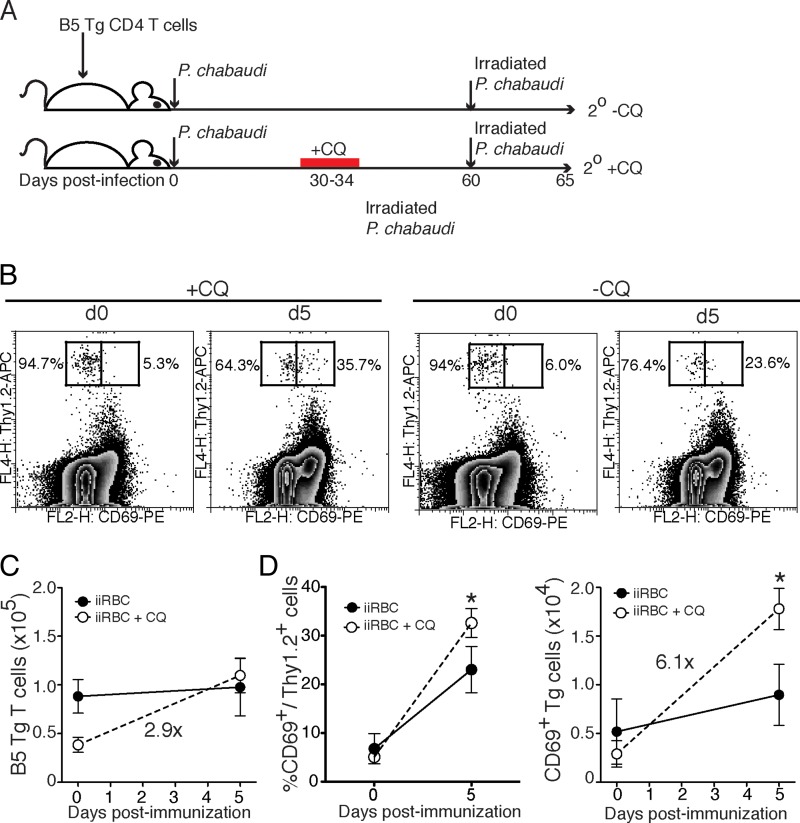
A caveat to the previous experiment is that secondary infection during the chronic infection (−CQ) leads to a secondary parasitemia that is a log less than in animals treated with chloroquine to clear chronic infection before rechallenge (+CQ), as shown previously (7). Parasite load is likely to affect the degree of proliferation. Therefore, we tested whether the reduced proliferation of T cells seen in the chronically infected (−CQ) group compared to the chloroquine-treated (+CQ) animals would be similar in the presence of equal parasite antigen. In order to test this possibility, we repeated the adoptive-transfer strategy used in the previous experiment, replacing the secondary challenge with equal doses of irradiated (nonproliferating) blood-stage parasites (109 irradiated infected red blood cells [iiRBCs]), as shown in Fig. 3A. This approach allowed us to make a direct comparison of the proliferative abilities of resting (+CQ) and chronically stimulated (−CQ) T cells in response to equal amounts of antigen. It is not known whether irradiated P. chabaudi trophozoites are protective, and we did not test this here. Interestingly, we observed that resting B5 TCR Tg T cells (+CQ) were more highly activated by iiRBCs, as measured by CD69, than chronically stimulated memory T cells (−CQ, Fig. 3B). Strikingly, the number of resting (+CQ) B5 memory T cells increased by 2.9-fold by day 5 versus day 0 after iiRBC challenge (day 60 after primary infection), whereas the chronically stimulated cells did not increase in number at all (Fig. 3C). The numbers of chronically stimulated B5 T cells were higher to start with in this experiment. Therefore, to test expansion, we compared the fold increases. The percentage of resting (+CQ) memory T cells expressing CD69 increased significantly from day 0 to day 5 postchallenge, and the number increased 6.1-fold (Fig. 3D). These data suggest that reducing the chronic stimulation in the memory phase of P. chabaudi infection restores some responsiveness, even under conditions of equivalent challenge dose.
FIG 3.
Rested memory T cells expand more than chronically stimulated memory cells in response to equivalent irradiated parasite challenge in vivo. As shown in panel A, B5 TCR Tg T cells (2 × 106) were transferred into Thy1.1 congenic mice, which were then infected with P. chabaudi. Chloroquine was administered to the 2° +CQ group on days 30 to 34 p.i. On day 60 p.i., all animals were given irradiated infected red blood cells (iiRBCs, 109), and splenocytes were analyzed both before and 5 days after challenge. (B) Contour plots with outliers of concatenated data from five animals to identify CD69+ B5 TCR Tg cells. The percentages of B5 TCR Tg cells that are CD69+ or CD69− are shown on the plots. (C and D) Summary graphs of the total numbers of B5 TCR transgenic cells (C) and the percentages or numbers of CD69+ Thy1.2+ and CD69+ CD4+ T cells (D). Numbers on the graph represent average fold increases between groups. Error bars represent the SEM. *, P < 0.05 (Student t test).
Malaria antigen-specific T cells proliferate and die.
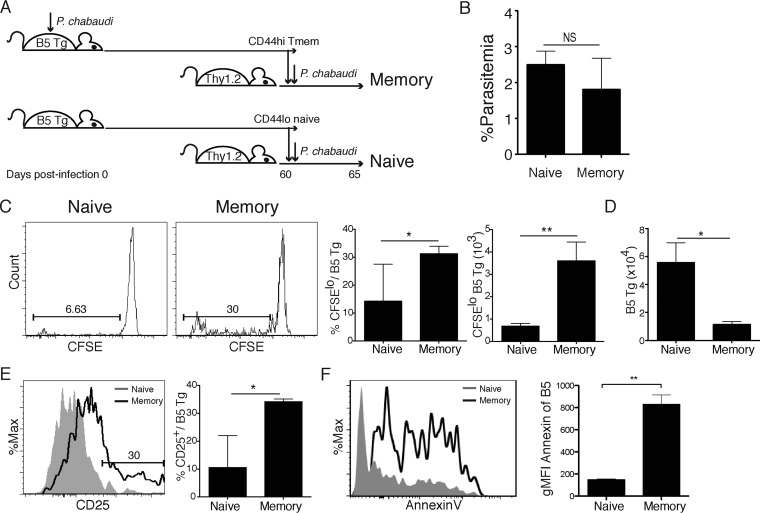
Another potential caveat in the design of the experiments shown in Fig. 2 is that the naive T cells acquired their ability to expand during the long incubation in vivo. This possibility arises due to reports that naive T cells can attain a pseudo-memory T cell phenotype over time in a naive recipient and reexpand like memory T cells (25). Therefore, we also tested proliferation of naive and memory T cells that were generated in different donor animals and then transferred into naive animals the day before challenge. The experimental design is shown schematically in Fig. 4A. First, we sorted CD4+ memory T cells (CD44hi CD25−) from P. chabaudi-infected (day 60 p.i.) B5 TCR Tg mice and naive T cells (CD44lo CD25−) from age-matched, uninfected B5 TCR Tg mice. Next, we transferred the sorted cells (2 × 105, CFSE+) into congenic (Thy1.1) animals, which were then infected with P. chabaudi on the same day as the adoptive transfer. Five days later, we measured the parasitemia of recipient animals, splenic T cell proliferation, activation (CD25+), and apoptosis (annexin V+). There was no significant difference in parasitemia between the groups on day 5 p.i. (Fig. 4B). This finding supports our previous data showing that transfer of B5 TCR Tg T cells into Thy1 animals does not affect parasitemia (20). Surprisingly, we observed higher proportions and numbers of proliferating (CFSElo) B5 memory T cells on day 5 p.i. than naive T cells (Fig. 4C). Total B5 T cell numbers (Thy1.2+ CD4+) on day 5 p.i. were significantly higher in animals receiving naive T cells than in animals receiving memory T cells (Fig. 4D). This was striking given that memory T cells showed greater proliferation, and both groups received equal numbers of transferred cells on day 0. Memory T cells were significantly more activated than naive T cells after 5 days of infection (CD25hi, Fig. 4E).
FIG 4.
Memory T cells proliferate more than naive cells but do not accumulate in vivo. Naive (CD44lo CD25−) and day 60 p.i. memory (CD44hi CD25−) B5 Tg CD4 T cells were sorted from spleens of B5 TCR Tg mice and labeled with CFSE. T cells were transferred (106) into congenic Thy1.1 hosts that were infected with P. chabaudi the following day. Splenocytes were analyzed on day 5 p.i. The experimental design is shown schematically in panel A. (B) Graph showing the percent parasitemia (%iiRBC/RBC) on day 5 p.i. (C) Histogram and summary graphs of the percentages and numbers of proliferating (CFSElo) Thy1.2+ B5 Tg T cells in the spleen on day 5 p.i. (D) Summary graph of numbers of recovered Thy1.2+ B5 Tg T cells on day 5 p.i. (E) Histogram overlay and summary graph of CD25+ B5 Tg T cells recovered day 5 p.i. (F) Histogram overlay and summary graph of annexin V+ B5 Tg T cells isolated on day 5 p.i. Data are representative of three independent experiments with three to five mice per group. Error bars represent the SEM. *, P < 0.05; **, P < 0.01 (Student t test).
To better understand why memory cells proliferated but did not expand, we measured early apoptosis. Memory T cells were significantly more apoptotic (annexin V+, Fig. 4F), suggesting activation-induced cell death of reactivated memory T cells. Therefore, chronically stimulated memory T cells responding to malaria infection sense antigen and divide but do not expand numerically as the naive T cells. Taken together, these findings led us to conclude that the memory T cells proliferate significantly more than naive T cells but fail to accumulate in their second encounter with parasite, potentially due to increased activation-induced cell death.
Malaria antigen-specific T cells are more sensitive to TCR stimulation after infection than naive T cells with the same TCR.
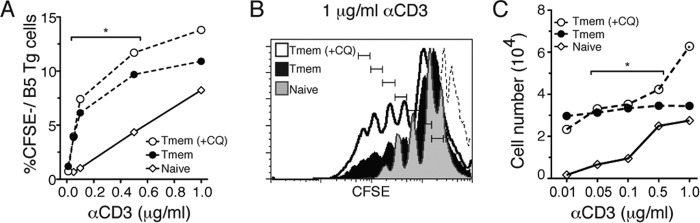
The functional avidity of the TCR increases for both effector and memory T cells compared to naive cells (26). In order to test memory T cells generated in P. chabaudi infection for improvement in sensitivity of TCR signaling in memory cells compared to naive bearing the same TCR, we stimulated B5 TCR transgenic T cells in vitro using proliferation as a readout. To do this, we sorted and carboxyfluorescein succinimidyl ester (CFSE) labeled memory T cells (Tmem, CD44hi CD25−) from chronic malaria-infected B5 TCR Tg mice on day 60 p.i. (−CQ) and also from infected and subsequently chloroquine-treated mice (Tmem +CQ). The Tmem groups were compared to naive (CD44lo CD25−) CD4 T cells from uninfected B5 TCR Tg mice. Equal numbers of cells per well (106/ml) were stimulated with increasing concentrations of plate-bound anti-CD3, and proliferation was measured by flow cytometry at 5 days poststimulation. Memory T cells are more costimulation independent than effector cells (12). Therefore, in order to test for memory T cell behavior, we did not include anti-CD28 in the stimulation conditions. Memory T cells from both chloroquine-treated and untreated mice proliferated in response to even the lowest doses of TCR stimulation (anti-CD3, 0.01 μg/ml), while naive B5 TCR Tg T cells did not proliferate until exposed to anti-CD3 at 0.5 μg/ml (Fig. 5A). These data indicate that the memory T cells generated in P. chabaudi infection do have enhanced functional TCR avidity. Upon comparing the average proliferative response of 0.05 to 0.5 μM anti-CD3 for naive and memory T cells, both the +CQ (P = 0.044) and the −CQ (P = 0.046) groups exhibited significantly more proliferation than did naive cells using a one-sided t test. At the highest concentration of anti-CD3 (1 μg/ml), Tmem from chloroquine-treated mice proliferated slightly more than chronically stimulated Tmem and naive T cells (Fig. 5B), a finding consistent with the previous results. However, the difference between Tmem from chloroquine-treated and untreated mice was not statistically significant. Most naive T cells died by day 3 of culture, especially at the lower stimulation levels (Fig. 5C). The number of resting memory T cells collected increased with increasing stimulation and proliferation. Tmem wells have significantly more cells alive at day 5 than naive (Fig. 5C). Intriguingly, chronically stimulated memory T cells (−CQ) survived with even the lowest levels of stimulation but did not accumulate. On the other hand, resting memory T cells (+CQ) accumulated slightly at the highest (1 μg/ml) stimulation, an observation consistent with the in vivo results. Taken together, these results suggest that even though malaria antigen-specific memory T cells do not expand in vivo as naive T cells do, they are still highly sensitive to restimulation, and they did not require costimulation to survive or proliferate, even at low concentrations of anti-CD3. These data also support a T cell intrinsic basis for the slight increase in responsiveness seen in rested compared to chronically stimulated memory T cells in vivo.
FIG 5.
Memory T cells generated in P. chabaudi infections have high intrinsic TCR sensitivity. Naive (CD44lo CD25−) or memory (CD44hi CD25−, day 60 p.i., +CQ or −CQ, on days 30 to 34) B5 TCR Tg CD4 T cells were sorted, CFSE labeled, and stimulated in vitro with plate-bound anti-CD3 (αCD3) for 5 days before analysis. (A) Percentages of recovered proliferating (CFSElo) T cells. (B) Histogram of proliferating cells, as measured by CFSE dilution after 5 days of stimulation with anti-CD3 at 1 μg/ml. Results for unstimulated, CFSEhi T cells are indicated by the dotted line; each division is marked. (C) T cells collected per sample after culture. Data are representative of two independent experiments, with one well per condition. The average numbers of Tmem (middle three concentrations) were significantly different from the average numbers of naive T cells, as determined by Student t test (Tmem [+/−CQ] > naive; *, P < 0.05).
IL-10-producing Th1 cells are maintained beyond acute infection.
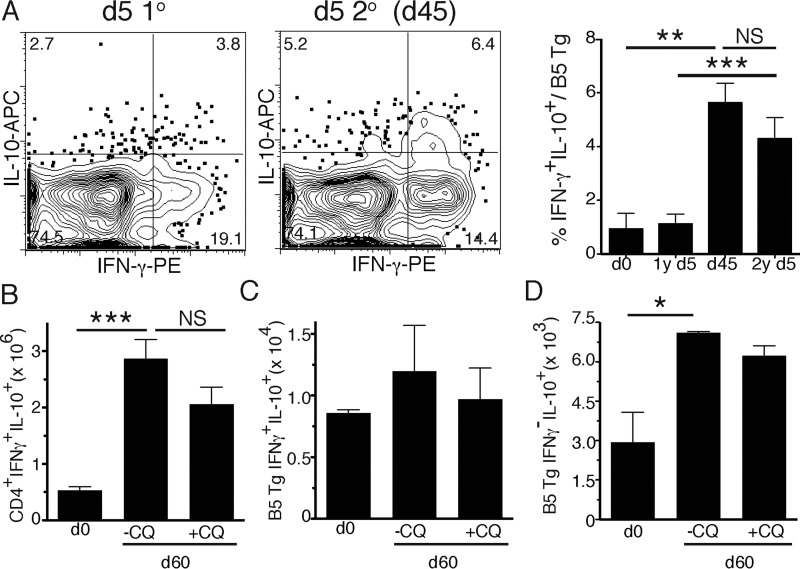
The primary function of CD4 T cells in fighting infection is to produce cytokines. In this malaria infection, IFN-γ and IL-10 are required to reduce parasitemia and pathology, respectively (18). Therefore, we investigated the ability of chronically stimulated memory T cells to produce these cytokines on restimulation. Previously uninfected or infected adoptive recipients of naive B5 TCR Tg T cells were reinfected on day 45 or 60 (as shown in Fig. 2A), and we tested for intracellular IFN-γ and IL-10 at 5 days after primary (day 5) or secondary infection (Fig. 6A). There were higher proportions of IFN-γ+ IL-10+ B5 TCR Tg T cells on day 45, as seen in the polyclonal population (Fig. 1). These double-cytokine-producing cells were maintained up to day 45, but did not increase on day 5 after rechallenge with parasite. This observation suggests that this persistent infection maintains production of regulatory cytokines by T cells. Therefore, we tested the role of chronic infection in maintenance of CD4 T cell cytokines by treating the animals with chloroquine (days 30 to 34 p.i.) and measuring IL-10 production 30 days later on day 60 p.i. We observed a trend toward decreased numbers of CD4+ IFN-γ+ IL-10+ T cells (Fig. 6B), but not malaria antigen-specific B5 T cells (Fig. 6C), in chloroquine-treated (+CQ) animals compared to untreated animals. However, this trend does not reach significance, suggesting that IL-10 production in IFN-γ+ CD4 T cells is programmed earlier than day 30. The total numbers of B5 TCR Tg T cells producing IL-10, but not IFN-γ, were significantly increased in chronically infected (−CQ) mice at day 60 of infection (Fig. 6D). These data suggest that IL-10 production increases over the course of infection and is promoted by chronic infection.
FIG 6.
IL-10-producing Th1 cells remain increased after infection. Thy1.1 recipients of adoptively transferred CD4 T cells (2 × 106) from B5 TCR Tg mice were infected with 105 P. chabaudi organisms (1°), and some mice were treated with the antimalarial drug chloroquine (CQ) on days 30 to 34 p.i. The second infection (2°) was administered on day 45 or 60 (as indicated), and the spleens were collected after 5 days (as for Fig. 2A). IFN-γ and IL-10 secretion was determined by intracellular cytokine staining on day 5 p.i. of the primary or secondary infection. (A) Plots show the results for representative mice in each group, while the summary graph shows the percentages of B5 Tg cells that are IFN-γ+ IL-10+. (B and C) Graphs show the total numbers of (B) CD4+ or (C) B5 TCR Tg T cells that are IFN-γ+ IL-10+. (D) Numbers of single positive IL-10+ IFN-γ− B5 T cells at 60 days p.i. The data represent five mice per group from two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005; NS, not significant (Student t test).
DISCUSSION
Immunity to malaria is not strong enough to prevent heterologous reinfection, and the nature of T cell memory to malaria is poorly understood (27). In other systems, some enhanced features of memory T cells, such as increased accumulation in response to second infection, have been identified. In order to determine whether memory T cells generated in malaria infection acquire these improved features compared to naive T cells, we tested the responses of T cells generated by a primary malaria infection in response to a secondary infection. The assays that we applied were designed to test for the functional attributes of memory T cells described in previous literature. From work in model systems, the memory T cell population is expected to include an increased antigen-specific precursor frequency after infection, to maintain the ability to expand and produce cytokines faster, and to lack a requirement for costimulation in reinfection, as well as show a higher intrinsic TCR sensitivity than naive T cells (12, 17, 26, 28–30). Our results show that the polyclonal T cell response to P. chabaudi has some of the general features previously described for T cell immunity. The specificity of the polyclonal T cell response to parasite antigens is suggested primarily by the increased fraction of CD4+ T cells that are primed by parasites on reinfection compared to a first infection. There is also an increased fraction of fast-responding cytokine producers in the polyclonal response compared to the first infection. The increase in polyclonal activation that we observed in our studies is likely due to an increased precursor frequency within the memory T cell pool. In addition, the total T cell numbers increased up to day 45 postinfection. This finding is similar to an increase observed in studies of CD8 Tem-inducing vaccination, which expands the T cell pool and the spleen size (31). We also determined that MSP-1-specific T cells from the memory phase of P. chabaudi have increased TCR sensitivity, demonstrate a reduced need for costimulation, and can proliferate in response to low doses of TCR stimulation even in the presence of chronic subpatent parasitemia in most animals at 2 months p.i. (as documented previously [7]).
Although there were some similarities, not all predicted memory T cell functions were observed in B5 malaria epitope-specific TCR Tg T cells. For example, whereas MSP-1-specific memory T cells are able to divide more than naive TCR Tg T cells on reinfection, they do not accumulate more either in vivo or in vitro. This results in a lower number of memory T cells than naive T cells 5 days after reinfection, even though Tmem clearly divide more than naive T cells, even in the context of an equivalent infection. Our data suggest that this is due to a higher rate of apoptosis in restimulated T cells in the memory phase, which is suggestive of increased activation-induced cell death. Interestingly, CD8 memory T cells generated in response to P. berghei ANKA expand less than Tmem generated in response to Listeria (32).
Since Tcm are expected to respond to restimulation by expansion, secondary proliferation has often been used as a measurement of the memory capacity of T cells (33–36). We demonstrated previously in both polyclonal (23) and transgenic (2) CD4 T cells that Tem make up more than 70% of the memory cells in P. chabaudi, with effector T cells around 10% in the memory phase of chronic infection. The current work focused on T cells in the spleen, as previous work has suggested that there is no T cell activation in lymph nodes in P. chabaudi. We have also shown previously that memory T cells found in lymph nodes at later time points mostly have an effector memory phenotype, similar to that observed in the spleen (2). Tem may not be expected to expand as efficiently as Tcm. Furthermore, Tem are predicted to be activated and prone to die upon activation, as seen here, like effector cells (35, 37). Although the lack of reexpansion could be due to continued effector T cell activation, and this has been correlated with a large fraction of Tem among memory cells, we have not defined the T cell subsets within the responding cells in the current work. Furthermore, in previous work, we showed that there is no detectable increase in the fraction of memory that is Tcm upon chloroquine treatment 1 month after infection (2), suggesting that the ratio of phenotypic Tcm to Tem does not explain the small difference between ±CQ seen here in proliferation or sensitivity. Intriguingly, we have recently shown in another report that only stopping the infection after 3 days (with mefloquine) can increase the fraction of Tcm produced in response to P. chabaudi infection (38), a system more suited for further inquiry.
Interestingly, CD4 memory T cells do not always reexpand more than naive, even in acute model antigen systems. Jenkins and coworkers showed recently that antigen-experienced CD4 T cells divide but expand less than their naive counterparts on challenge with ovalbumin (OVA) and that successful reexpansion can be restored after 2 weeks in a naive recipient (33). Interestingly, inflammation, specifically IFN-γ, rather than antigen, was shown to be responsible for the delay of acquisition of this memory trait in CD8 T cells (39), and both IFN-γ and low autocrine IL-2 have this effect on CD4 memory cells (34). In our system, reexpansion was not restored after 1 month in a treated mouse, suggesting that inflammation may be slow to be restored to homeostasis. These would be interesting targets to explore in this system as well. Indeed, extended expression of type I IFN or IFN-γ has recently been shown to inhibit germinal center production in chronic P. berghei and P. yoelii infections (40, 41).
Studies of the T cell response to chronic infection have primarily focused on defects termed exhaustion, which reduce proliferation and cytokine production of memory T cells. This exhausted phenotype has been reported for CD8 T cells in chronic infections, such as lymphocytic choriomeningitis virus (LCMV) infections (42, 43). Exhaustion has been implicated in P. falciparum, P. yoelii, and P. chabaudi malaria (44–46). However, there is no indication of functional unresponsiveness in CD4 T cells in malaria (2, 45, 46). Actually, CD4 T cell exhaustion has not been well documented in any situation. Instead, there is a concerted change in the cytokine profile of chronically stimulated T cells in LCMV and malaria away from Th1 or Tfh commitment (40, 41, 47–49), suggesting that the effect of chronic infection on CD4 T cells is different than that on CD8 cells. On the other hand, PD-1 is expressed on human T cells in P. falciparum infections (44), and T cell proliferation is reduced in areas of continuous exposure to P. falciparum compared to those with successful control programs (21). The reduction in T cell proliferation in chronically exposed children supports the finding here that this is a factor in the reduced accumulation of T cells upon reinfection. Although there is no known function for CD8 T cells in protection, blocking PD-1 and LAG3 does lead to complete clearance of P. chabaudi after day 30 of infection (45), and PD-1 knockout (KO) CD8 T cells play a role in this, implying a role for CD8 exhaustion in this infection (50). In our studies, Teff quickly become PD-1int (2), a finding in agreement with studies in Mycobacterium tuberculosis showing that PD-1int Teff are proliferative, are short-lived, and produce cytokines (51), suggesting that PD-1 expression at the peak of infection does not define unresponsive CD4 T cells. In summary, the effect of chronic infection is not the primary factor responsible for reducing T cell expansion in response to reinfection, and the mechanism responsible for the slight, but significant, improvement of secondary proliferation upon elimination of the chronic phase with drug treatment is unclear.
We have previously shown that chronic infection protects animals from high parasitemia (7) and that T cells from chronic infection protect better than those from treated infection (2). Enhanced protection is due to the presence of persistently activated Th1 cells within the Teff, Tem, and resident memory T cell populations (2, 13, 52). Therefore, in contrast to expectations of T cell inhibition by chronic infection, the data in chronic infections suggest that previously activated cells, even if they do not reexpand, are protective. The best example of this is that persistent Leishmania skin infection protects from new lesions and visceral dissemination. To date, leishmanization remains the most successful vaccination protocol for this disease due to the persistence of the parasite and activated T cells in the healed lesion (13, 53). Enhanced protection cannot be explained by exhaustion; therefore, antigen-specific T cells that survive due to continuous exposure can be helpful, as suggested by Zinkernagel and Hengartner (54).
The memory T cells generated by P. chabaudi proliferated in response to suboptimal stimulation with anti-CD3. This is consistent with the lower activation threshold and costimulation independence expected of memory T cells. This increased sensitivity for memory T cells has been attributed to more extensive lipid rafts with prephosphorylated proteins (28, 29). Chronically activated memory T cells also have increased basal phosphorylation of TCR signaling chains (30). Phosphatidylinositol 3-kinase and Akt, which signal downstream of costimulation and cytokines, regulate the apoptosis of expanding effector or effector memory CD8 T cells (55). Therefore, it will be interesting to test the contribution of these molecules to the poor secondary expansion seen here, since they may provide a mechanism for the regulation of proliferation and accumulation by activation-induced cell death.
Cytokine production by CD4 T cells is critical for protection against malaria (20, 56–58), and the balance of IFN-γ, tumor necrosis factor (TNF), and IL-21 with the regulatory cytokines IL-10 and transforming growth factor β (TGF-β) in response to parasite antigens is associated with protection against malaria disease in naturally infected people (58, 59). IL-10, in particular, plays an important role in protection from pathology in both mice and human malaria by reducing the production of pathogenic inflammatory cytokines (58, 60). Mice deficient in IL-10 succumb to P. chabaudi infection, and the survival rate is decreased further by inhibition of the regulatory cytokine TGF-β or reduced by neutralization of TNF (61). IL-10 has been shown to increase throughout chronic infection (18). The finding that IL-10 remains elevated into the memory phase and is dramatically higher upon secondary infection than upon primary infection represents an important mechanism of protection from pathology by malaria-specific T cells in the memory phase of chronic infection. Similarly in humans, children exposed to two or more malaria infections showed an increase in IFN-γ- and IL-10-coproducing CD4 T cells (59). However, the difference in IL-10 presented here does not explain the protection to parasitemia elicited by chronically infected animals compared to chloroquine-treated animals, which we believe is controlled by an increase in Th1 cells (2).
Overall, our data suggest that whereas chronic infection generates a memory T cell population that remains highly sensitive to restimulation, similar to classical memory T cells (12), these cells do not accumulate significantly in response to reinfection, potentially due to higher rates of apoptosis. Given that we have shown that chronically stimulated T cells protect in P. chabaudi infection (2), we propose that reexpansion upon reinfection is not essential for CD4 T cells to be protective. Instead, it is possible that they are primarily required to maintain an elevated malaria-specific precursor frequency and a high level of sensitivity to antigen and to produce a well-balanced cytokine response that promotes parasite killing while limiting immunopathology.
MATERIALS AND METHODS
Mice and parasites.
Female BALB/c mice were maintained in the breeding facilities of the MRC National Institute of Medical Research or purchased from The Jackson Laboratory. Thy1.1 BALB/c congenic mice (N15 BALB/c) were kindly provided by David Tough (Jenner Institute, Compton, United Kingdom) or purchased from Jackson Laboratories (Bar Harbor, ME) and further backcrossed four generations to BALB/c (MRC) or BALB/cJ mice for adoptive transfers. B5 TCR Tg mice (kindly provided by Jean Langhorne, NIMR, London, United Kingdom) were generated as described previously (20). The B5 TCR recognizes MSP-1 (1157 to 1171, ISVLKSRLLKRKKYI/I-Ed); B5 TCR Tg mice were typed using the primers Vα2 (GAACGTTCCAGATTCCATGG and ATGGACAAGATCCTGACAGCATCG) and Vβ8.1 (CAGAGACCCTCAGGCGGCTGCTCAGG and ATGGGCTCCAGGCTGTTCTTTGTGGTTTTGATTC). Mice, 6 to 8 weeks of age, were infected with 105 P. chabaudi chabaudi (AS)-infected erythrocytes (iRBC, intraperitoneally [i.p.]) for all experiments. Figure 3 used irradiated P. chabaudi parasites (iiRBCs), which were gamma irradiated on ice in a 137Cs source for 30,000 rads, and 108 nonproliferating parasites were given i.p. to each animal. To eliminate chronic infections in some studies, mice were treated three times with 50 mg/kg chloroquine (Sigma, Dorset, United Kingdom) in saline (Sigma) on alternate days between days 30 and 34 p.i. P. chabaudi (AS) is sensitive to chloroquine at a low parasite density, as on day 30 p.i. (7). Animals were maintained in sterile caging in a specific-pathogen-free animal facility with ad libitum access to irradiated food and water. All experiments were carried out in accordance with the protocols approved by the National Institute of Medical Research Institutional Ethical Review Panel and The University of Texas Medical Branch Institutional Animal Care and Use Committee.
Flow cytometry.
Single-cell suspensions of spleens were made in Hanks balanced salt solution, incubated in red blood cell lysis buffer (Sigma), and stained in phosphate-buffered saline (PBS), 2% fetal bovine serum (FBS; PAA Laboratories, Somerset, United Kingdom), and 0.01% sodium azide (staining buffer) with anti-CD16/32 (24G2) supernatant, followed by combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)-, biotin-, or allophycocyanin (APC)-conjugated antibodies (Cambridge Biosciences, Oxford, United Kingdom). For the second-step reagents, streptavidin-PerCP (BD Biosciences) and streptavidin-PE (Invitrogen) were used. For intracellular cytokine staining, the cells were stimulated for 5 h in complete Iscove medium (cIMDM; Sigma), 10% FBS, 2 mM l-glutamine, 0.5 mM sodium pyruvate, 100 U of penicillin, 100 mg of streptomycin, and 50 mM β2-mercaptoethanol (Gibco/Invitrogen) with phorbol myristate acetate (50 ng/ml), ionomycin (500 ng/ml), and brefeldin A (10 mg/ml; all from Sigma) for the last 2 h. Cells were fixed in 2% paraformaldehyde for 20 min and resuspended in staining buffer overnight. Cells were then permeabilized in Perm/Wash buffer (BD Biosciences) for 25 min and washed twice; they were then incubated for 40 min with anti-IFN-γ-PE (XMG1.2), IL-2-APC (JES6-5H4), or IL-10-APC (JES5-16E3; all from BD Biosciences). The cells were washed three times in Perm/Wash solution and resuspended in staining buffer. The cells were then collected and compensated on a FACSCalibur, using CellQuest software (BD Biosciences), or on a nine-color CyAn ADP (Dako, Beckman Coulter), using Summit software (Cytomation) or BDFortessa (BD Biosciences), and analyzed in FlowJo (Tree Star, Ashland, OR). Further compensation was performed in FlowJo using single stained splenocytes.
Cell sorting and in vitro culture.
For in vitro stimulation and adoptive transfer of naive and memory T cells, B5 Tg CD4+ cells were purified by positive selection using Miltenyi magnetic CD4-microbeads to >95% purity (Miltenyi, Germany) or by negative selection with the EasySep biotin selection kit (STEMCELL Technologies, Vancouver, Canada) using biotinylated anti-CD8a (55-6.7), B220 (RA3-6B2), CD11b (MI/70), CD11c (N418), F4/80 (BM8), and Ter119 (eBioscience). Enriched T cells were then stained with anti-CD4-FITC, CD44-APC-Cy7, and CD25-PE for naive (CD44lo CD25−) or memory (CD44hi CD25−) cell sorts. Cells were sorted on a FACSAria I using FACSDiva software (BD Biosciences). Sort purity was consistently >99%. The cells were washed three times in calcium- and magnesium-free PBS and then labeled for 10 min at 37°C with 1 mM CFSE (carboxyfluorescein succinimidyl ester; Sigma) or Cell Trace Violet (CTV; Invitrogen) and transferred i.p. For in vitro cultures, the cells were labeled with CFSE, plated at 106 cells/ml, and stimulated with the stated concentrations of plate-bound anti-CD3 (OKT3).
Adoptive transfers.
Splenic CD4+ T cells were purified by magnetically activated cell sorting (MACS) positive selection (95% purity; Miltenyi). Equal numbers of B5 TCR Tg CD4+ T cells (1 × 106 to 2 × 106) were transferred into congenic Thy1.1 BALB/c mice on the same day for all groups. Recipient mice were infected once at 60 days posttransfer for the primary (1°) group. The secondary (2°) group was infected the day after adoptive transfer and again 60 days later. To minimize variation in flow cytometry analyses, infections were staggered so that flow analyses for all groups occurred on the same day. When memory (CD44hi CD25− from day 60 p.i.) and naive (CD44lo CD25− from uninfected cells) B5 TCR Tg CD4+ T cells (MACS purified, sorted) were transferred into uninfected Thy1.1 congenic recipients, the same numbers of each were transferred into their respective recipients.
Statistics.
Statistics were performed in Prism (GraphPad, La Jolla, CA). All groups in an experiment were compared by analysis of variance and individually by using an unpaired Student t test, with P < 0.05 considered significant.
ACKNOWLEDGMENTS
We sincerely appreciate the contributions of Jean Langhorne (Francis Crick Institute, London, United Kingdom) for general supervision, reagents, and resources for this work from its inception. We appreciate the help of Margarita Ramirez and the technical contributions of Mark Griffin of the UTMB Flow Cytometry & Cell Sorting Core Facility. We also thank Peter Melby, Christine Arcari, and Linsey Yeager for critical feedback on the manuscript.
R.S. and M.M.O. conceptualized and performed experiments, analyzed data, and wrote the manuscript.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. However, the work was made possible by the Medical Research Council, United Kingdom; the University of Texas Medical Branch, Galveston, TX; and NIH NIAID grants R01AI089953 and T327536-15 (M.M.O.). The funders had no role in study design, data collection or interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Chelimo K, Embury PB, Sumba PO, Vulule J, Ofulla AV, Long C, Kazura JW, Moormann AM. 2011. Age-related differences in naturally acquired T cell memory to Plasmodium falciparum merozoite surface protein 1. PLoS One 6:e24852. doi: 10.1371/journal.pone.0024852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens R, Langhorne J. 2010. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog 6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird JK. 1998. Age-dependent characteristics of protection v. susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol 92:367–390. doi: 10.1080/00034989859366. [DOI] [PubMed] [Google Scholar]
- 4.Achtman AH, Bull PC, Stephens R, Langhorne J. 2005. Longevity of the immune response and memory to blood-stage malaria infection. Curr Top Microbiol Immunol 297:71–102. [DOI] [PubMed] [Google Scholar]
- 5.Bouchaud O, Cot M, Kony S, Durand R, Schiemann R, Ralaimazava P, Coulaud JP, Le Bras J, Deloron P. 2005. Do African immigrants living in France have long-term malarial immunity? Am J Trop Med Hyg 72:21–25. [PubMed] [Google Scholar]
- 6.Deloron P, Chougnet C. 1992. Is immunity to malaria really short-lived? Parasitol Today 8:375–378. doi: 10.1016/0169-4758(92)90174-Z. [DOI] [PubMed] [Google Scholar]
- 7.Achtman AH, Stephens R, Cadman ET, Harrison V, Langhorne J. 2007. Malaria-specific antibody responses and parasite persistence after infection of mice with Plasmodium chabaudi chabaudi. Parasite Immunol 29:435–444. doi: 10.1111/j.1365-3024.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 8.Macallan DC, Wallace D, Zhang Y, De Lara C, Worth AT, Ghattas H, Griffin GE, Beverley PC, Tough DF. 2004. Rapid turnover of effector-memory CD4+ T cells in healthy humans. J Exp Med 200:255–260. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DL, Tarleton RL. 2005. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J Immunol 174:1594–1601. doi: 10.4049/jimmunol.174.3.1594. [DOI] [PubMed] [Google Scholar]
- 10.Opata MM, Stephens R. 2013. Early decision: effector and effector memory T cell differentiation in chronic infection. Curr Immunol Rev 9:190–206. doi: 10.2174/1573395509666131126231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hikono H, Kohlmeier J, Takamura S, Wittmer S, Roberts A, Woodland D. 2007. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med 204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft M, Bradley LM, Swain SL. 1994. Naive versus memory CD4 T cell response to antigen: memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types, including resting B cells. J Immunol 152:2675–2685. [PubMed] [Google Scholar]
- 13.Peters NC, Pagan AJ, Lawyer PG, Hand TW, Henrique Roma E, Stamper LW, Romano A, Sacks DL. 2014. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+ CD4+ effector T cells that are required for protection against reinfection. PLoS Pathog 10:e1004538. doi: 10.1371/journal.ppat.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A 101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol 1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 16.Swain SL, Hu H, Huston G. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science 286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 17.Rogers PR, Dubey C, Swain SL. 2000. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol 164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 18.Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O'Garra A, Langhorne J. 2012. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmby H, Jonsson G, Troye-Blomberg M. 2000. Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect Immun 68:1485–1490. doi: 10.1128/IAI.68.3.1485-1490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens R, Albano F, Quin S, Pascal B, Harrison V, Stockinger B, Kioussis D, Weltzien H-U, Langhorne J. 2005. Malaria-specific transgenic CD4+ T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 106:1676–1684. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 21.Bediako Y, Ngoi JM, Nyangweso G, Wambua J, Opiyo M, Nduati EW, Bejon P, Marsh K, Ndungu FM. 2016. The effect of declining exposure on T cell-mediated immunity to Plasmodium falciparum: an epidemiological “natural experiment.” BMC Med 14:143. doi: 10.1186/s12916-016-0683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt P, Cravo PV, Donleavy P, Carlton JM, Walliker D. 2004. Chloroquine resistance in Plasmodium chabaudi: are chloroquine-resistance transporter (crt) and multidrug resistance (mdr1) orthologues involved? Mol Biochem Parasitol 133:27–35. doi: 10.1016/j.molbiopara.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Stephens R, Seddon B, Langhorne J. 2011. Homeostatic proliferation and IL-7Rα expression do not correlate with enhanced T cell proliferation and protection in chronic mouse malaria. PLoS One 6:e26686. doi: 10.1371/journal.pone.0026686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens R, Ndungu FM, Langhorne J. 2009. Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cell. Parasite Immunol 31:20–31. doi: 10.1111/j.1365-3024.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Ahmed R. 2000. Cutting edge: naive T cells masquerading as memory cells. J Immunol 165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 26.Slifka MK, Whitton JL. 2001. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat Immunol 2:711–717. doi: 10.1038/35096027. [DOI] [PubMed] [Google Scholar]
- 27.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat Immunol 9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 28.Farber DL. 2009. Biochemical signaling pathways for memory T cell recall. Semin Immunol 21:84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kersh EN, Fitzpatrick DR, Murali-Krishna K, Shires J, Speck SH, Boss JM, Ahmed R. 2006. Rapid demethylation of the IFN-γ gene occurs in memory but not naive CD8 T cells. J Immunol 176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadzadeh M, Hussain SF, Farber DL. 1999. Effector CD4 T cells are biochemically distinct from the memory subset: evidence for long-term persistence of effectors in vivo. J Immunol 163:3053–3063. [PubMed] [Google Scholar]
- 31.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. 2009. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 32.Miyakoda M, Kimura D, Honma K, Kimura K, Yuda M, Yui K. 2012. Development of memory CD8+ T cells and their recall responses during blood-stage infection with Plasmodium berghei ANKA. J Immunol 189:4396–4404. doi: 10.4049/jimmunol.1200781. [DOI] [PubMed] [Google Scholar]
- 33.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. 2000. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol 164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 34.MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P. 2008. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci U S A 105:14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. 2005. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med 201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts A, Ely K, Woodland D. 2005. Differential contributions of central and effector memory T cells to recall responses. J Exp Med 202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi A, Hanson MGV, Norell HR, Havelka AM, Kono K, Malmberg KJ, Kiessling RVR. 2005. Preferential cell death of CD8+ effector memory (CCR7-CD45RA−) T cells by hydrogen peroxide-induced oxidative stress. J Immunol 174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 38.Opata MM, Carpio VH, Ibitokou SA, Dillon BE, Obiero JM, Stephens R. 2015. Early effector cells survive the contraction phase in malaria infection and generate both central and effector memory T cells. J Immunol 194:5346–5354. doi: 10.4049/jimmunol.1403216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med 11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 40.Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, Hansen DS. 2016. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep 14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Zander RA, Guthmiller JJ, Graham AC, Pope RL, Burke BE, Carr DJ, Butler NS. 2016. Type I interferons induce T regulatory 1 responses and restrict humoral immunity during experimental malaria. PLoS Pathog 12:e1005945. doi: 10.1371/journal.ppat.1005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barber D, Wherry E, Masopust D, Zhu B, Allison J, Sharpe A, Freeman G, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 44.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. 2013. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2012. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horne-Debets JM, Karunarathne DS, Faleiro RJ, Poh CM, Renia L, Wykes MN. 2016. Mice lacking programmed cell death-1 show a role for CD8+ T cells in long-term immunity against blood-stage malaria. Sci Rep 6:26210. doi: 10.1038/srep26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. 2015. Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep 13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. 2014. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpio VH, Opata MM, Montanez ME, Banerjee PP, Dent AL, Stephens R. 2015. IFN-γ and IL-21 double-producing T cells are Bcl6-independent and survive into the memory phase in Plasmodium chabaudi infection. PLoS One 10:e0144654. doi: 10.1371/journal.pone.0144654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horne-Debets JM, Faleiro R, Karunarathne DS, Liu XQ, Lineburg KE, Poh CM, Grotenbreg GM, Hill GR, MacDonald KP, Good MF, Renia L, Ahmed R, Sharpe AH, Wykes MN. 2013. PD-1-dependent exhaustion of CD8+ T cells drives chronic malaria. Cell Rep 5:1204–1213. doi: 10.1016/j.celrep.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. 2010. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. 2015. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 212:1405–1414. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okwor I, Mou Z, Dong L, Uzonna JE. 2012. Protective immunity and vaccination against cutaneous leishmaniasis. Front Immunol 3:128. doi: 10.3389/fimmu.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zinkernagel RM, Hengartner H. 2006. Protective ‘immunity’ by preexistent neutralizing antibody titers and preactivated T cells but not by so-called ‘immunological memory.’ Immunol Rev 211:310–319. doi: 10.1111/j.0105-2896.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 55.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. 2010. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A 107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meding SJ, Langhorne J. 1991. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol 21:1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- 57.Stevenson MM, Tam MF, Wolf SF, Sher A. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol 155:2545–2556. [PubMed] [Google Scholar]
- 58.May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. 2000. Plasma interleukin-10:tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J Infect Dis 182:1570–1573. doi: 10.1086/315857. [DOI] [PubMed] [Google Scholar]
- 59.Jagannathan P, Nankya F, Stoyanov C, Eccles-James I, Sikyomu E, Naluwu K, Wamala S, Nalubega M, Briggs J, Bowen K, Bigira V, Kapisi J, Kamya MR, Dorsey G, Feeney ME. 2014. IFN-γ responses to pre-erythrocytic and blood-stage malaria antigens exhibit differential associations with past exposure and subsequent protection. J Infect Dis 211:1987–1996. doi: 10.1093/infdis/jiu814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C, Corraliza I, Langhorne J. 1999. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67:4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Sanni LA, Omer F, Riley E, Langhorne J. 2003. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun 71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]