Abstract
Eukaryotic cells can die from physical trauma, resulting in necrosis. Alternately, they can die via programmed cell death upon stimulation of specific signalling pathways. Here we discuss the utility of four cell death pathways in innate immune defence against bacterial and viral infection: apoptosis, necroptosis, pyroptosis and NETosis. We describe the interactions that interweave different programmed cell death pathways, which create complex signalling networks that cross-guard each other in the evolutionary arms race with pathogens. Finally, we describe how the resulting cell corpses — apoptotic bodies, pore-induced intracellular traps (PITs) and neutrophil extracellular traps (NETs) — promote clearance of infection.
Introduction
Cell death can be either programmed or accidental. Programmed cell death results in either lytic or non-lytic morphology, depending upon the signalling pathway. For example, apoptosis1,2 is a non-lytic and typically immunologically silent form of cell death. On the other hand, programmed lytic cell death is highly inflammatory, and includes necroptosis3–5, pyroptosis6, and the rapid release of so-called neutrophil extracellular traps (NETs) in a process known as NETosis7. The elimination of host immune cells by programmed cell death can be thought to benefit an infecting pathogen8. However, programmed cell death is increasingly understood to benefit the host, for example, by eliminating the intracellular niche of certain pathogens9. Furthermore, the resulting cellular corpses coordinate an appropriate innate immune response to promote the resolution of infection. However, since our understanding of innate immune defences is mainly based on work with pathogens that evade these defences, it is often difficult to recognize how programmed cell death functions in response to infections. Indeed, many discoveries of cell death functions are based on experiments with pathogens that have been genetically modified to remove their normal host evasion strategies.
Significant crosstalk exists between different cell death pathways, and as a result, innate immune signalling pathways are well guarded against pathogen attack. The ‘guard hypothesis’ was first developed in plant immunity, where studies have shown that innate immune sensors monitor for perturbation of other innate immune signalling pathways10. For example, in Arabidopsis thaliana, pathogens often attempt to inhibit key proteins that are involved in innate immune signalling. When pathogens attack one such protein, RPM1-interacting protein 4 (RIN4), the perturbation is detected by two different nucleotide oligomerization domain (NOD)-like receptors (NLRs), which activate programmed cell death — a phenomenon known as the hypersensitive response in plants — to combat the pathogen11,12. This guard mechanism is echoed in mammalian programmed cell death pathways, which function to guard each other as well as other innate immune signalling pathways.
In this Review, we discuss how the host utilizes programmed cell death to fight infections with a focus on in vivo studies. We also discuss how difficult, but not impossible, it can be for pathogens to evade programmed cell death because of the guard functions built into the signalling pathways. We discuss the role of pyroptosis, necroptosis and apoptosis during infection, and how their signalling pathways are functionally interwoven to make them guard each other. Finally, we discuss the fate of dead cell corpses such as apoptotic bodies, NETs, and pore-induced intracellular traps (PITs), and how their physical properties are beneficial to fight infection.
Inflammasomes trigger pyroptosis
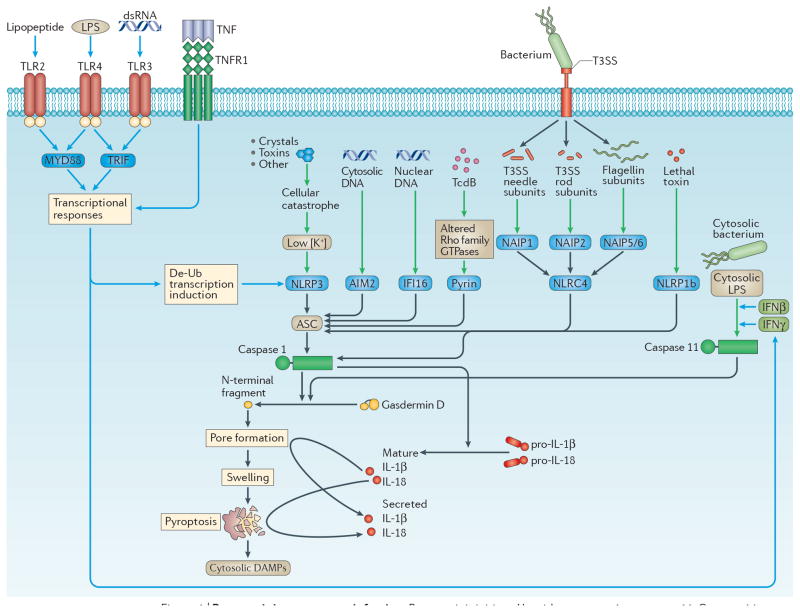
Inflammasomes are cytosolic sensors that activate caspase 113. Once activated, caspase 1 cleaves pro-interleukin-1β (IL-1β) and pro-IL-18 into their mature forms, and cleaves gasdermin D (encoded by Gsdmd) to induce pore opening and pyroptosis. (Figure 1). Inflammasomes are cytoplasmic platforms composed of either NLR, AIM2-like receptor (ALR), or tripartite motif-containing (TRIM) proteins that detect contamination of the cytosol by DNA, bacterial flagellin, the type 3 secretion system (T3SS) needle and rod subunits, toxins and cellular perturbations. Mouse caspase-11 — which is duplicated in humans as caspase-4 and caspase-5 — also induces pyroptosis14, but does not cleave pro-IL-1β or pro-IL-18 on its own. Caspase-11 directly detects lipopolysaccharide (LPS) when it enters the cytosol15–17, and thus protects against infection by cytosol-invasive Gram-negative bacteria such as Burkholderia thailandensis18,19, but can be evaded by pathogens such as Francisella novicida15 and Shigella flexneri20. Caspases-11, -4 and -5 are often called the non-canonical inflammasome14. The role of inflammasomes in pyroptosis has been reviewed in detail6 and is summarized in Fig. 1.
Figure 1. Pyroptosis.
Pyroptosis is initiated by either caspases 1 or 11. Caspase 1 is activated by one of several inflammasomes, NOD-, LRR- and pyrin domain-containing 3 (NLRP3), AIM2, interferon-γ (IFNγ)-inducible protein 16 (IFI16), pyrin, NOD-, LRR- and CARD-containing 4 (NLRC4) and NLRP1b6. NLRP3 responds to numerous agonists, which may converge upon low cellular potassium concentration, although this remains controversial. NLRP3 will not respond to these agonists unless it also receives a priming stimulus via various Toll-like receptors (TLRs) or from tumour necrosis factor (TNF), which trigger post-translational priming (probably de-ubiquitination), as well as boosting NLRP3 sensitivity by transcriptional induction162. AIM2 detects cytosolic DNA83,84, and IFI16, which lacks a clear mouse homolog166, also detects viral nucleic acids50. Pyrin detects modulation of Rho-family GTPases by bacterial toxins167. NLRC4 is activated by one of three bacterial flagellin or type III secretion rod or needle proteins that signal via an upstream NLR in the NAIP family9,36,168,169. NLRP1b detects the protease activity of the anthrax lethal toxin170,171. By contrast, caspase 11 itself is the sensor for cytosolic lipopolysaccharide (LPS)15–17. Similar to NLRP3, caspase 11 requires priming, but priming is by either type I interferon or interferon-γ (IFNγ). Either caspases 1 or 11 independently cleaves gasdermin D, from which the released N-terminal fragment associates with the cell membrane and oligomerizes to form the pyroptotic pore26–29. The cell then swells, resulting in membrane rupture that is called pyroptosis. In addition, caspase 1 will cleave pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to their mature forms (caspase 11 cannot do this directly)14. Mature IL-1β and IL-18 can escape through the gasdermin D pore, or be released later by membrane rupture26,28. Red and blue lines represent initiating and priming events, respectively.
Recently, two research groups independently discovered that caspases-1 and -11 cleave gasdermin D and this cleavage is required to trigger pyroptosis21,22. This finding was later confirmed by a third research group23. Interestingly, cleavage of gasdermin D was observed a few years earlier, but its importance remained uninvestigated24. Gasdermin D is a member of the greater gasdermin-domain protein family, consisting of 6 members in humans and 10 in mice25. The function of other gasdermin family members remains to be further elucidated, and while many probably induce pyroptosis21, it remains unclear how they are activated.
Gasdermins are bipartite proteins whose N- and C-terminal domains are connected by a linker, and have several proposed roles in immune-related diseases25. Caspases-1 or -11 cleave this linker of gasdermin D, releasing the N-terminal domain21,22, which then associates with the inner leaflet of the plasma membrane and oligomerizes to form pores. These pores range in size from 10–33 nm26–29, just large enough to allow passage of mature IL-1β (which is 4.5 nm)26,28; this could explain how IL-1β can be released from cells that do not lyse, which might occur, hypothetically, if a very low number gasdermin D pores form. Small, but detectable, amounts of IL-1β may escape through these pores, while small amounts of sodium and water enter. The resulting mild cell swelling should be counteracted by regulatory volume decrease mechanisms, preventing membrane rupture30. Later, the cell could remove the pores by normal membrane repair processes31. Conversely, if more gasdermin D pores form, significant swelling should overwhelm the regulatory volume decrease, resulting in membrane rupture that we call pyroptosis, and the release of any remaining processed IL-1β from the cytosol. The N-terminal domain of gasdermin D has affinity not only for the inner leaflet of the plasma membrane, but also for other phospholipids and cardiolipin26–28,32, which suggests that it may also target organelles. In this regard, permeabilization of the endoplasmic reticulum may explain the calcium flux that occurs during pyroptosis33,34.
Although numerous pathogens trigger pyroptosis in vitro, the role of pyroptosis in vivo is much less studied. Mice deficient in caspase 1 have an increased susceptibility to a variety of pathogens (Table S1). The importance of pyroptosis in vivo is primarily inferred from comparing the resistance to infection of Casp1−/− mice with Il1b−/−Il18−/− mice. This comparison remains to be performed for many pathogens. The recently generated Gsdmd−/− mice are defective for both pyroptosis and release of IL-1β and IL-18 (at least in vitro)21–23, so a genetic approach to inhibit pyroptosis while maintaining cytokine secretion remains elusive. Finally, it is important to remember that defence against infection may require IL-1β, IL-18 and pyroptosis in conjunction35.
Pyroptosis defends against intracellular bacteria
The most direct evidence for pyroptosis in clearing infections comes from the investigation of bacterial strains engineered to activate the NLR family, CARD-containing 4 (NLRC4) inflammasome and induce pyroptosis in vivo. Normally, Salmonella enterica subsp. enterica serovar Typhimurium and Listeria monocytogenes efficiently evade inflammasomes during the systemic phase of infection in mice9,36–39. By contrast, strains engineered to persistently express flagellin are detected by NLRC4 and are rapidly cleared. The observation that mice deficient in IL-1β and IL-18 could still clear these flagellin-engineered bacteria suggests that pyroptosis is the primary mechanism for bacterial clearance9,36–38. Similarly, caspase 11 provides in vivo defence against S. Typhimurium strains (sifA mutants) that have lost the ability to maintain the integrity of the Salmonella-containing vacuole and thus aberrantly enter the cytosol18. After pyroptosis in vitro, bacteria are damaged40, perhaps by gasdermin D pores28 and are more susceptible to secondary insults such as hydrogen peroxide or antibiotics40. However, the physiologic relevance of this observation remains unclear, since pyroptosis and gasdermin D are insufficient to kill intracellular bacteria in vivo9. Instead, after pyroptosis in mice, bacteria are transferred to neutrophils, where they are killed by reactive oxygen species (ROS)9,40.
Pyroptosis also protects against non-engineered, opportunistic microorganisms in vivo, including Chromobacterium violaceum, B. thailandensis, Burkholderia pseudomallei and F. novicida18,19,39,41,42. However, additional supporting data is needed to confirm this protection43. Amongst these bacteria, B. thailandensis and C. violaceum have the strongest phenotypes of all tested pathogens in inflammasome-deficient mice19,39 (Table S1)44. As few as 100 colony forming units (CFUs) of C. violaceum or B. thailandensis are lethal to inflammasome-deficient mice, whereas wild-type mice survive challenges by 1,000,000 to 20,000,000 CFU. The strength of these phenotypes leads us to propose the Red Pawn Hypothesis, a corollary to the Red Queen Hypothesis. We propose that despite their evasion by host-adapted pathogens, inflammasomes may be maintained over evolutionary time due to their critical role in defence against pathogens that are co-evolving with a non-mammalian host that lacks inflammasome defences. Thus, some ubiquitous environmental bacteria have specific virulence traits capable of manipulating a generic eukaryotic cell, making them potentially lethal pathogens. However, they are unprepared for the mammalian innate immune system, including inflammasome-driven responses (Box 1 and further discussion in reference 44).
Box 1. Hypothesis to explain inflammasome importance despite evasion by pathogens.
In Lewis Carol’s Through the Looking Glass, Alice finds herself in a race with the Red Queen, in which “it takes all the running you can do, to keep in the same place.” This inspired the evolutionary hypothesis that constant change is required to survive within a constantly changing environmental niche alongside constantly changing competitors. But in the end, each organism tends to maintain its place within the niche. This concept applies to the host-pathogen interaction: as hosts (Alice) evolve new immune responses, pathogens (the Red Queen) evolve virulence factors to overcome them. For example, numerous pathogens evade inflammasome detection156. Thus, many successful pathogens appear to be perpetually one step ahead in the evolutionary equilibrium between inflammasome detection and evasion. On the other hand, aberrant inflammasome activation can be lethal15,16,34, which might select for loss of these sensors over time. One must ask, then, why would Alice run with inflammasomes that fail to fight the Red Queen?
Interestingly, there are two pathogens which inflammasomes decisively control in vivo, Burkholderia thailandensis19 and Chromobacterium violaceum39 (Table S1)44. Both employ significant virulence traits that make them lethal to inflammasome-deficient mice and immunocompromised people. But in normal animals, inflammasomes render them harmless. B. thailandensis and C. violaceum are ubiquitous soil/mud bacteria whose natural host remains unknown; perhaps it is a lower eukaryote that lacks inflammasomes, such as insects or nematodes.
This leads us to propose a corollary to the Red Queen Hypothesis. We propose that C. violaceum and B. thailandensis are not running an evolutionary race alongside humans because they are not natural human pathogens. Instead, they run a completely separate race altogether, alongside a non-human host. They may occasionally lose their way and stumble clumsily onto the human racecourse, where they find themselves brushed aside by inflammasomes. Hence, the mammalian innate immune response is perpetually winning the evolutionary race against environmental pathogens that may be dangerous, but that lack sophisticated immune evasion strategies.
This could explain why inflammasomes are maintained over evolutionary time despite the prevalence of evasive pathogens. Inflammasomes may act as a barrier to prevent opportunistic infection. Such a barrier may not be perfect since B. thailandensis has two close relatives that have advanced on the pathway to mammalian pathogenesis – environmental bacteria that have evolved into Red Queens. Burkholderia pseudomallei is broadly pathogenic to mammals and causes melioidosis157. Further along the adaptation course is B. mallei, which is the highly virulent equine-adapted cause of glanders158. Further caveats to the this hypothesis are described in ref 44.
Our hypothesis thus predicts that certain innate immune sensors provide a near-permanent victory for the host in defence against a large reservoir of potentially deadly environmental pathogens, while host-adapted pathogens must evade these sensors.
Additionally, inflammasomes provide defence against microorganisms that have adapted to mammalian hosts. For example, although S. Typhimurium efficiently evades inflammasomes during systemic infection, they are detected in the gut45. Intestinal epithelial cell NLRC4 triggers epithelial cell shedding during infection. Whether this process is a precursor stage during pyroptosis, or is fully independent of pyroptosis requires further study45. Caspase-11 appears to play a similar, parallel role in epithelial shedding to eliminate the low frequency but rapidly replicating sub-population of S. Typhimurium that aberrantly enter the cytosol46.
Pyroptosis during viral infection
Inflammasomes also induce pyroptosis in vitro following the detection of viruses, such as murine cytomegalovirus (MCMV) via the double-stranded DNA-detecting cytosolic sensor AIM2. Parallel in vivo studies show that Aim2−/− mice are susceptible to MCMV infection47. However, whether this susceptibility arises from pyroptosis, IL-1β or IL-18 remains to be explored. Conversely, a detrimental effect of pyroptosis has been described during HIV infection. Interferon-γ (IFNγ)-inducible protein 16 (IFI16) — which is an AIM2-like receptor that detects viral nucleic acids — induces pyroptosis in CD4+ T cells abortively infected by HIV48. This is proposed as the major pathway by which HIV depletes CD4+ T cells in vivo49,50. Conversely, IFI16 has also been suggested to play a beneficial role in the defence against HIV infection, although pyroptosis was not examined as a possible mechanism51.
RIPK3 triggers necroptosis
Necroptosis, like pyroptosis, is a form of programmed lytic cell death. Necroptosis can be triggered by a variety of intricate pathways (discussed below) and perhaps the simplest pathway is through Z-DNA binding protein 1 (ZBP1; also known as DAI and DLM-1).
Induction of necroptosis by ZBP1
ZBP1 binds either Z-DNA or Z-RNA via two Z-DNA binding motifs, and can respond to MCMV (a DNA virus) or Influenza (a RNA virus)52–54. Unlike the normal B-form of DNA, Z-form DNA results from tosional strain that may occur during rapid DNA synthesis that could occur during viral infection. Once activated, its receptor-interacting protein (RIP) homotypic interaction motif (RHIM) binds the RHIM of RIP kinase 3 (RIPK3)52. This interaction stimulates RIPK3 kinase activity, auto-phosphorylation and oligomerization55–57 and RIPK3 phosphorylates the necroptosis effector mixed lineage kinase domain-like (MLKL)58 (Figure 2). Following phosphorylation by RIPK3, the MLKL pseudokinase undergoes a conformational switch59, licensing interaction with the inner leaflet of the plasma membrane where MLKL oligomerizes to form the necroptotic pore60–62, which is analogous to gasdermin D for pyroptosis. Additional steps may also regulate MLKL63. After pore formation, the terminal events in necroptosis are similar to those in pyroptosis; osmotic pressure from ion and water influx results in rupture of the plasma membrane. In our hands pyroptosis and necroptosis (both at 2h post treatment) are morphologically identical in primary macrophages40, with the exception of slightly different nuclear morphology (I.J. and E.A.M, unpublished observation). Other research laboratories visualize differences in RAW264.7 macrophages in settings where pyroptosis is fast (2h) while necroptosis is slow (4h)32. The morphologies in comparison deserve further study. Necroptosis can be investigated in vivo by using Ripk3−/− or Mlkl−/− mice; however, RIPK3 has been suggested to have non-necroptotic functions during influenza infection64 and during inflammatory disease models65. Therefore, before attributing a phenotype to necroptosis, it is important to verify that phenotypes observed in Ripk3−/− mice are also seen in Mlkl−/− mice.
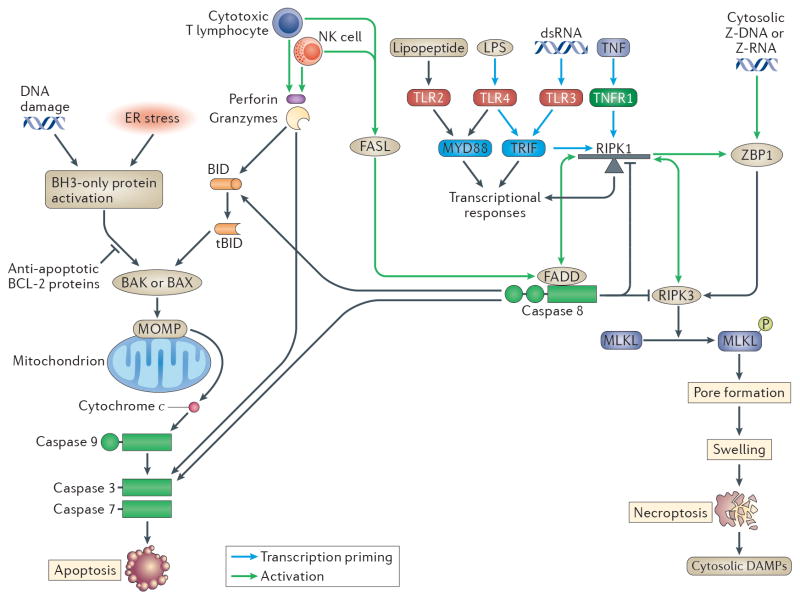
Figure 2. Apoptosis and Necroptosis.
(Left) Apoptosis can be triggered via intrinsic or extrinsic pathways, including by natural killer (NK) cells and cytotoxic T lymphocyte (CTL) granzymes. These stimulate the cleavage of BID to tBID, which causes BAX and/or BAK to trigger mitochondrial outer-membrane permeabilization (MOMP), resulting in release of cytochrome C (CytC), which binds to apoptotic protease activating factor 1 (APAF1). APAF1 then oligomerizes into the apoptosome, the platform for caspase 9 activation. Either caspases 8 or 9 will cleave the effector caspases 3 and 7 into their active forms, which leads to apoptosis2. (Right) Necroptosis can be triggered by DNA-sensing via ZBP152, which activates receptor-interacting protein kinase 3 (RIPK3) followed by phosphorylation of MLKL. Phosphorylated MLKL binds to the inner leaflet of the plasma membrane and forms the necroptotic pore60–62. (Center) In the more complex necroptotic pathway, tumour necrosis factor receptor 1 (TNFR1), Toll-like receptor 3 (TLR3)–TRIF, or TLR4–TRIF signal via RIPK1 to activate NF-κb (but RIPK1 is not required for the TRIF-type I IFN response97,98). RIPK1 can be thought of as a finely balanced teeter-totter with two guards: caspase 8 on one side balanced by a duo of RIPK3 and ZBP1 on the other. When TNFR1–TLR3–TLR4 signalling proceed normally, RIPK1 is “balanced” and functions as a scaffolding protein that becomes poly-ubiquitinated, and serving as a platform for signalling complex assembly5 driving transcriptional responses. When signalling is inhibited, for example by inhibition of cIAP1/2 pharmacologic inhibitors (with SMAC mimetics), by inhibiting TAK1, by inhibition of protein synthesis, or by blocking RIPK1 ubiquitination 3, or potentially by virulence factors, RIPK1 becomes “unbalanced”. RIPK1 then recruits FADD (a DD-DED adaptor) via DD-DD interactions, and FADD recruits caspase 8 via DED-DED interaction thereby triggering extrinsic apoptosis172–174. Successful caspase 8 activation provides negative feedback to prevent necroptosis by cleaving RIPK1 and RIPK3 in the kinase domains175,176. However, inhibition of caspase-8 pharmacologically or by viral virulence factors permits continued RIPK1 kinase activity, and the teeter-totter swings the other direction. RIPK1 recruits RIPK3 via RHIM-RHIM interaction, and phosphorylates it, triggering RIPK3 kinase activity and necroptosis177–179. Thus, RIPK1, RIPK3, and caspase 8 act as a cross guard system for TNFR1, TLR3, and TLR4. TLRs such as TLR2 that do not signal through TRIF76,100,108,114, unless longer time points are examined75 where TLR2-driven TNF could require caspase 8 to cause paracrine TNFR1 stimulation.
Necroptosis defends against viral infections
The clearest phenotypes for necroptosis during in vivo infection highlight an antiviral role. MCMV encodes a viral inhibitor of RIP activation (vIRA), whose RHIM mimic inhibits RIPK3 signalling, and thereby prevents necroptosis52. A RHIM domain mutant virus is attenuated, but this attenuation is reversed in Ripk3−/− and Zbp1−/− mice52. Similarly, human-adapted herpes simplex virus 1 (HSV-1) and HSV-2 express two protein homologs, ICP6 and ICP10, that inhibit human RIPK366,67. However, there appears to be some degree of species tropism; human HSV-1 ICP6 interacts with mouse RIPK3, presumably attempting to inhibit it, but due to the species mismatch, RIPK3 is activated, which triggers necroptosis to prevent viral replication66–68. Accordingly, Ripk3−/− mice are susceptible to HSV-1 infection69. This idea does not hold true for MCMV, which inhibits necroptosis in both human and mouse cells66.
The antiviral role for necroptosis is further supported by the observation that Ripk3−/− mice are susceptible to vaccinia virus infection56. The RIPK3 pathway also defends against a mouse adapted influenza infection. Zbp1−/− and Ripk3−/− mice have increased mortality during infection influenza A PR854,64. However, the susceptibility of Ripk3−/− mice to influenza virus was not replicated in another study70 and a third study shows the reverse, where Zbp1−/− mice have higher viral titers but reduced tissue damage and mortality53, suggesting a simultaneously beneficial role in defense paired with a detrimental role in immunopathology.
Finally, Ripk3−/− mice have normal resistance to mouse hepatitis virus71. Many other mouse viral infection models remain to be examined in Ripk3−/− and Mlkl−/− mice. All reported lethality phenotypes of Ripk3−/− and related necroptosis pathway mice are summarized in Table S1.
Necroptosis during bacterial infections
To date, in vivo data does not support an important role for necroptosis against bacterial infections. The most prominent report proposed that reduced necroptosis leads to increased susceptibility to S. Typhimurium infection in mice lacking Ifnar1 (which encodes interferon α/β-receptor subunit 1)72. However, this study did not compare other competing hypotheses, most importantly the hyper-responsiveness of Ifnar1−/− mice to IFNγ (which combats intracellular bacteria such as S. Typhimurium) due to increased expression of the IFNγ receptor73. Wild-type and Ripk3−/− mice showed similar susceptibility to S. Typhimurium infection72, demonstrating that necroptosis plays little role in IFNAR1-sufficient mice. Similarly, Ripk3−/− mice and wild-type mice are comparably sensitive to Yersinia pseudotuberculosis74 and Citrobacter rodentium infections75. Ripk3−/− mice may have a subtle (but statistically insignificant) susceptibility to an LPS engineered Yersinia pestis strain that cannot evade TLR476 (Table S1). Interestingly, Mlkl−/− mice are susceptible to Staphylococcus aureus skin infection77, but paradoxically Ripk3−/− mice are resistant to both skin and lung infection77,78. Inhibitor treatment suggests that necroptosis may be pathologic during Serratia marcescens infection79, but this needs further study using knockout mice. Specific inhibition of RIPK3 by bacteria has not yet been described. Many more bacterial infection models remain to be examined in Ripk3−/− and/or Mlkl−/− mice, and it seems likely that some will establish a role for necroptosis in defence against specific bacteria.
Apoptosis during infections
Apoptosis is programmed cell death characterized by cytoplasmic shrinking, cell rounding, chromatin condensation, DNA fragmentation and membrane blebbing1,2. Apoptosis can be initiated by cell extrinsic pathways (which are mediated by death receptors) or cell intrinsic (mitochondrial) pathways, both of which culminate in the activation of the effector caspases 3, 6 and 7 (Figure 2). Apoptosis can also be initiated by cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells that deliver granzymes, which activate apoptotic caspases. Upon completion of apoptosis, cellular contents are encapsulated within membrane bound apoptotic bodies. If these bodies are not cleared, they will undergo secondary necrosis (rupture), releasing cytosolic damage-associated molecular patterns (DAMPs) to the extracellular space80. The role of apoptosis against infection is perhaps best appreciated by inference, since numerous viruses and bacteria encode apoptosis inhibitors that are essential for virulence (reviewed in 81,82). This again raises the conundrums posed in the Red Pawn Hypothesis, if most viruses evade apoptosis, are there pathogens that apoptosis effectively counters, providing fully penetrant innate immunity (Box 1). Here, we focus on some of the recent advances in apoptosis that are relevant to infection.
Guarding cell intrinsic apoptosis with interferons
Intrinsic apoptosis is often driven by cell intrinsic stresses such as DNA damage and growth factor withdrawal, which leads to mitochondrial outer-membrane permeabilization (MOMP) 83,84. Although its structure remains to be fully elucidated, the MOMP pore appears to be formed by BAX and BAK83,84. MOMP leads to release of mitochondrial contents, including cytochrome c, into the cytosol where it binds to the NLR protein apoptotic protease activating factor 1 (APAF1), which oligomerizes into a hub to form the apoptosome — an activating platform for the initiator caspase 9. Activated caspase 9 in turn cleaves and activates the effector caspases 3 and 72. Since apoptosis destroys the replicative niche of intracellular pathogens, particularly viruses, those that prevent apoptosis will replicate better as the infected cell lives longer.
Historically, apoptosis is considered immunologically silent owing to the collective efforts of caspases. For example, apoptotic DAMPs that can induce a robust type I interferon-mediated proinflammatory response are dampened by caspases85,86. During viral infection of Casp9−/− or Casp3−/−Casp7−/− cells, MOMP is initiated, but apoptosis fails to proceed. MOMP eventually releases mitochondrial DNA (mtDNA) into the cytosol, where it is detected by the DNA sensor cGAS, resulting in a type I IFN response85,86. Thus, pathogens that only block caspases will encounter a cGAS-driven type I IFN response that effectively guards events downstream of MOMP. This interferon response promotes resistance to viral infections including encephalomyocarditis virus (EMCV) and vesicular stomatitis virus (VSV). By contrast, blockade upstream of MOMP — which can be studied in Bax−/−Bak−/− mice — does not trigger cGAS because MOMP is not initiated and mitochondria retain their DNA85,86.
Apoptosis/necroptosis guard innate immune signalling
Cell extrinsic apoptosis is typically initiated by extracellular stress signals that engage a subset of TNF-superfamily of receptors called death receptors2. Prototypical extrinsic stimuli include FasL, TNF and TNF-related apoptosis-inducing ligand (TRAIL). Following death receptor engagement, caspase 8 (human caspase 10) are recruited and activated via the death inducing signalling complex (DISC). In certain cell types, caspase 8 can also cleave BID into tBID, initiating MOMP in a positive feedback loop that leads to caspase 9, another initiator caspase, thus ensuring a strong initiation of. Thus, caspase 8 activation connects extrinsic and intrinsic apoptosis (Figure 2).
Additionally, caspase 8 is ensconced within the TNF receptor 1 (TNFR1), TLR3, and TLR4 signalling pathways3–5,87. Caspase 8 interacts with RIPK1, which is a key scaffolding protein within the signalling cascade3–5,87. Thus, caspase 8 is often interpreted as a node that regulates the divergence of signalling towards either transcriptional responses or apoptosis. For example, it is frequently stated that caspase 8 regulates TNF-dependent transcriptional responses and TNF-driven apoptosis3–5,87. In this section, we will present this pathway from the perspective of a pathogen.
TNFR1, TLR3, and TLR4 are important innate innate immune sensors — which respond to TNF, double-stranded RNA and LPS, respectively — that drive potent transcriptional responses (but do not trigger cell death under normal circumstances) 3. Hence, these sensors are attractive targets for pathogens to inhibit, particularly since all three use RIPK1 as a scaffolding node to assemble signalling complexes (Figure 2). However, when signalling at or below RIPK1 is pharmacologically inhibited, this causes an alteration in the flow of signals through the pathway, which triggers a counter response that activates caspase 83,5. In other words, if pathogens target these signalling pathways they would be met by a caspase 8 tripwire that triggers apoptosis. If pathogens possessed virulence strategies to simultaneously target RIPK1 and caspase 8, they would encounter a second tripwire in RIPK3, which also interacts with RIPK1. As the first caspase 8 tripwire fails to function, signalling build-up at RIPK1 results in the activation of RIPK3, triggering this second guard pathway that drives the response to infection to necroptosis rather than apoptosis (Figure 2). Thus, caspase 8, RIPK1 and RIPK3 essentially function as a triangular guard system.
This cross-guarded system becomes activated during embryogenesis in Casp8−/− mice, causing embryonic lethality4. In line with the cross-guard model, Casp8−/− lethality is rescued by simultaneous deletion of Ripk388,89. Thus, caspase-8 is guarded by RIPK1-RIPK3 driven necroptosis.
Further, recent evidence suggests that RIPK3 is guarded by caspase 8, essentially reverse guarding the guard. Although Ripk3−/− mice are viable and healthy, mice engineered to carry some (but a not all) point mutations within the Ripk3 kinase enzymatic site suffer from neonatal lethality as a caspase 8 guard program becomes activated, triggering lethal apoptosis during development90,91. In this guard system, the RIPK3 protein must be present and abnormal in order to trigger caspase 8, whereas simple absence of RIPK3 does not trip this guard pathway (as it does when caspase 8 is absent. This could explain instances where stimuli cause necroptosis, but Mlkl−/− cells still die whereas Ripk3−/− cells live; in Mlkl−/− cells signalling events may accumulate at RIPK3, triggering the guard function of caspase 8, but this does not happen if Ripk3 is deleted53.
Finally, RIPK1 is guarded by both caspase 8 and RIPK3. Deletion of Ripk1 can only be rescued by simultaneous deletion of both Casp8 and Ripk392–94, illustrating redundant guarding. On the necroptosis side, there are two pathways that guard RIPK1. The first is direct, where RIPK3 activates autonomously in response to deleted or abnormal RIPK1. The second is upstream, when RIPK1 is absent or its RHIM domain is mutated, this triggers activation of ZBP1, which normally docks on RIPK1 to hold it in check95,96. Thus, Ripk1RHIM mice can be rescued by crossing with either Mlkl−/−, Ripk3−/− or Zbp1−/− mice95,96. ZBP1 then activates RIPK3. These essentially double guard RIPK1.
To add to the complexity, the caspase 8 guard function is built into the RIPK1 scaffold function; TNFR1–TLR3–TLR4 signalling fails to drive transcription in Casp8−/− cells97,98. It seems that RIPK1 fails to function when it is unable to interact with its guard caspase 8. The same may be true for Ripk3−/− cells but only in specific cell types99. In contrast, TLR2 signalling, which does not use TRIF–RIPK1, remains intact76,100 (although one study did see defects in TLR2 signaling75, perhaps due to autocrine TNF signalling; also see Figure 2 legend and Box 2).
Box 2. Compound defects in Casp8−/− mice.
Investigators generated mice lacking caspase 8 to study cell extrinsic apoptosis in vivo, but these mice turned out not to be viable4. A caspase 8 deletion induces necroptosis and destruction of the yolk sac vasculature, and this circulatory failure leads to embryonic death. However, introduction of Ripk3−/− complements the lethality, and Casp8−/− Ripk3−/− mice are viable since cells in these mice cannot undergo necroptosis88,89. Comparing Casp8−/− Ripk3−/− mice to Ripk3−/− controls allows study of caspase 8 genetic deficiency in vivo. However, these mice must be interpreted with great caution since the Casp8−/− mutation eliminates multiple signalling pathways: i) it eliminates extrinsic apoptosis driven by TNF, Fas, and TNF-related apoptosis-inducing ligand (TRAIL), which could be beneficial during infection88,89,159; ii)) it alters homeostasis in adaptive immune cells, resulting in a lymphoproliferative disorder that is apparent at 2 months of age88,89; iii) it significantly impedes or fully abrogates transcriptional responses via tumour necrosis factor receptor (TNFR), Toll-like receptor 3 (TLR3) and TLR4 due to loss of the caspase 8–RIPK1 scaffolding platform97,98; iv) its effect on TNF, TLR3 and TLR4 also prevent these receptors from post-translational priming of the NLRP3 inflammasome75,76,100,107–109. Fadd−/− Ripk3−/− mice are similarly affected71,74,75,160. This makes it difficult to assign a precise caspase 8 function to phenotypes observed by comparing Casp8−/− Ripk3−/− to Ripk3−/− mice. In order to do this, one could start by comparing various Ripk3−/− background mice. For example, the resistance of Casp8−/− Ripk3−/− mice to endotoxic shock88 may be similar to Tlr4−/− Ripk3−/− mice but not Nlrp3−/− Ripk3−/− mice. This would begin to ascribe the mechanism to the effect of Casp8 deletion upon TLR4 signalling and not to its influence upon NLRP3.
One way to try to study cell extrinsic apoptosis in vivo is to compare Casp8−/−Ripk3−/− mice to Ripk3−/− control mice. However, given the compound effects of deleting Casp8 (elaborated in Box 2), it is not surprising that Casp8−/−Ripk3−/− mice are highly susceptible to multiple infectious challenges. Casp8−/−Ripk3−/− mice have more rapid dissemination and/or lethality of fully virulent Y. pestis or Y. pseudotuberculosis74,76. These mice also have higher stool burdens during C. rodentium infection75. Furthermore, they succumb to infections by Y. pestis strains engineered to enhance TLR4 detection 76, perhaps due to defective TLR4 signalling. Defective TLR4 signalling would also explain the resistance of Casp8−/− background mice to endotoxic shock88. Fadd−/−Mlkl−/− mice (which are similar to Casp8−/−Ripk3−/− mice, see Box 2) have increased mortality to influenza A virus infection64, perhaps due to defects in TLR3 signalling.
In summary, multiple innate immune defence pathways intersect at the caspase 8–RIPK1–RIPK3 triangle. It seems likely that the intricate cross wiring of these pathways plays an important role in preventing pathogens from inhibiting the function of TLR3, TLR4, TNFR1, extrinsic apoptosis and necroptosis.
Neutrophil NETosis makes NETs
Whereas necroptosis and apoptosis are seen in many cell types, another form of cell death is uniquely observed in neutrophils — NETosis, which primarily functions to extrude NETs101 (Figure 3). NETosis is triggered by various microbial and sterile activators — a process that was recently shown to differentiate between microbes based on their size102 — or by ligation of specific receptors including complement, antibody, cytokine, and TLRs103. During NETosis, ROS-dependent release of elastase and myeloperoxidase from neutrophil granules to the nucleus (though not under all conditions104) promotes histone degradation and chromatin decondensation105. The effects of ROS and proteases on the nuclear material leads to extrusion of a meshwork of chromatin fibers dotted with granules containing anti-microbial molecules, which traps and kills extracellular bacteria and fungi.
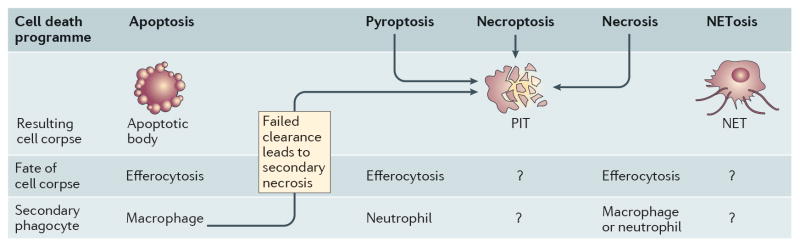
Figure 3. Apoptotic bodies, neutrophil extracellular traps and pore-induced intracellular traps.
The fate of the cell corpse is dependent on the cell death pathway. Extrinsic or intrinsic apoptosis leads to the formation of apoptotic bodies that are engulfed by a secondary phagocyte, a process that is referred to as efferocytosis. In the absence of efferocytosis, apoptotic bodies can undergo secondary necrosis. Lytic death of cells infected with an intracellular pathogen, including pyroptosis, leads the formation of a pore-induced intracellular trap (PIT), which traps the pathogen within the cell corpse. The PIT also presents ligands that are recognized by neutrophils (and potentially macrophages), which efferocytose the PITs and associated bacteria, ultimately killing the pathogen. Efferocytosis of pathogen-containing apoptotic bodies also eliminates the pathogen. Necroptosis and necrosis also result in PITs in vitro, although the physiologic importance in vivo needs further study. Following detection of extracellular pathogens, neutrophils extrude a meshwork of chromatin dotted with granules loaded with antimicrobial molecules. These neutrophil extracellular traps (NETs) trap and kill extracellular pathogens.
NETosis is accompanied by ruptures in the plasma membrane and neutrophil lysis101. However, a mounting body of evidence demonstrates that neutrophils can release NETs while maintaining an intact plasma membrane103; this is paradoxically referred to as ‘vital NETosis’, in contrast to the typical ‘suicidal NETosis’. After vital NETosis, neutrophils continue to function in chemotaxis, phagocytosis and bacterial killing106. Whether a neutrophil undergoes suicidal or vital NETosis appears to be dependent on the NET-inducing stimulus. It is unknown how NETs are extruded from neutrophils without lysing the cell.
Whether NETosis has antimicrobial functions that are independent of NET extrusion remains unknown, but one could imagine that pathogens within neutrophils during NETosis could be killed by the release of granule contents into the nucleus or cytosol (see REF 7 for a comprehensive review on NET signalling mechanisms). How NETs combat infection is described further below in our discussion of consequences of cell death.
Cross-talk between cell death pathways
There are many cross-pathway signalling events between apoptosis, necroptosis and pyroptosis (Figure 4). Most prominent amongst these are NLR family, pyrin domain-containing 3 (NLRP3) interactions with the difference cell death pathways. Although the precise signal detected by NLRP3 is uncertain, NLRP3 can be considered as the “sensor for cellular catastrophe” (Box 3), activating when normal cellular physiology breaks down.
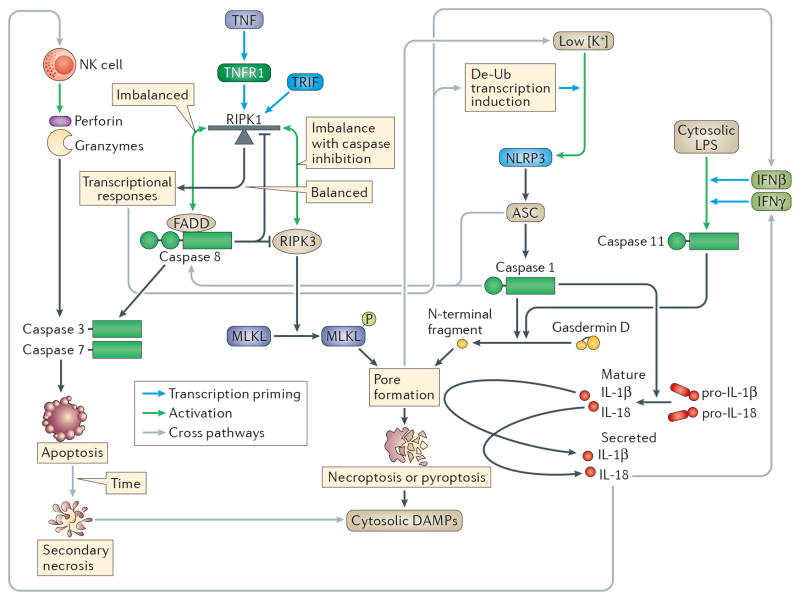
Figure 4. Interactions between apoptotic, necroptotic and pyroptotic pathways.
Cell death pathways can interact with each other, with the caveat that once a cell death pathway runs to completion, the interaction ends. The exception to this is the lysis of apoptotic bodies by secondary necrosis, which releases cytosolic contents, effectively converting apoptosis to lytic cell death. Signalling pathways running through RIPK1 will fail if Casp8 is deleted, affecting priming of NOD-, LRR- and pyrin domain-containing 3 (NLRP3) and caspase 1175,76,100,107–109. Caspase 1 driven IL-18 can have two interactions with other pathways, first by inducing interferon-γ (IFNγ) production to prime caspase 1119, and second by stimulating NK cytotoxic activity39. The terminal events occurring after the MLKL or gasdermin D pores open are catastrophic, resulting in loss of cellular potassium, which is the trigger for NLRP3 activation163,164. In the window between potassium loss and membrane rupture, NLRP3 activity will trigger caspase 1-dependent processing of interleukin-1β (IL-1β) and IL-18 processing. Finally, ASC and caspase 1 can activate caspase 8, triggering apoptosis22,23,117,118. Purple lines are interacting pathways.
Box 3. As the sensor of cellular catastrophe, NLRP3 detects the pyroptotic and necroptotic pores.
Although NLRP3 is the most extensively investigated inflammasome, it remains the most enigmatic. NLRP3 is activated in response to numerous insults, including pore-forming toxins, ionophores and phagocytosis of indigestible particulates. As such, NLRP3 detects catastrophic events, including the inability to maintain a cytosolic potassium concentration161 via an unknown biochemical mechanism. Besides loss of cytosolic potassium, other mechanisms have also been proposed to explain NLRP3 activation, including ER stress and mitochondrial dysfunction162.
NLRP3 is also activated downstream of lipopolysaccharide (LPS) detection by caspase 1114. When caspase 11 cleaves gasdermin D, it polymerizes into the pyroptotic pore. The resulting loss of cytosolic potassium triggers NLRP3 and consequent interleukin-1β (IL-1β) and IL-18 processing163,164 (Fig. 3). Thus, NLRP3 has a very short activation window before cellular swelling causes rupture of the plasma membrane. IL-1β secretion downstream of cytosolic LPS is thus much lower than other direct inflammasome agonists. To date there is no in vivo evidence supporting physiologic relevance of NLRP3 activation downstream of caspase 11. In fact, caspase 11-dependent defence against Burkholderia thailandensis does not require NLRP319, which argues against an in vivo role, at least in this model system.
Similarly, NLRP3 activation downstream of necroptosis53,100,110 may be caused by the same mechanism. It is likely that potassium loss after opening of the MLKL pore is detected by NLRP3. This pathway may promote IL-1β secretion in vivo during influenza virus and vesicular stomatitis virus infection165, but more work is needed to determine whether MLKL is the primary pathway whereby NLRP3 is activated in vivo, or whether NLRP3 is actually responding to some other catastrophic event during infection in vivo.
Role of caspase 8 upstream of inflammasome signalling
Caspase 8 is a component of the TNFR1–TLR3–TLR4 signalling scaffold. Therefore, loss of caspase 8 prevents these receptors from providing both post-translational and transcriptional priming of inflammasome components. For example, pro-IL-1β and NLRP3 transcriptional induction is compromised in Casp8-deficient cells75,76,100,107–109, although this may not hold true for all cell types110. Furthermore, NLRP3 requires TNFR1 or TLR signalling to provide post-translational priming111–113. Thus, Casp8−/− cells fail to prime NLRP3 after TNFR1, TLR3, or TLR4 stimulation, but can prime NLRP3 within 10 minutes of TLR2 stimulation76,100,108,114. However, when longer time points (4 hours) are examined, TLR2-driven TNF may require caspase 8 to cause paracrine stimulation75. In contrast, Casp8-deficiency does not prevent activation of inflammasomes that do not need priming, such as NLRC475,107. The in vivo relevance of these interactions remains to be assessed.
Caspase-8 downstream of inflammasomes ensures death
Caspase 8 can also function downstream of inflammasomes, which makes caspase 8 interactions more complicated. Caspase 8 may guard pyroptosis, and thereby ensure activation of cell death in a hypothetical situation where a pathogen attempt to inhibit pyroptosis. ASC is a small adaptor between most inflammasomes and caspase 1; the ASC pyrin domain (PYD) interacts with the PYD of inflammasomes, while its casapse activation and recruitment domain (CARD) interacts with the CARD of caspase 113. Upon activation by inflammasomes, ASC polymerizes to provide a large surface area of activating CARD domains115,116. In the absence of caspase 1, cell death still occurs after ASC polymerization, but under slower kinetics and with apoptotic morphology. Here, caspase 8 is proposed to interact with ASC via an unusual heterotypic death domain fold interaction between the ASC CARD and the caspase 8 death effector domain117–119 (Figure 4). This pathway may be most relevant when a pathogen directly inhibits caspase 1 without preventing ASC polymerization120. This situation is mimicked by comparing Asc−/− to Casp1−/− mice, which reveal an in vivo effect of cytokine responses via this pathway during a F. novicida infection117,121, but this has no effect on the F. novicida bacterial load117,121.
In addition, caspase 8 may be directly activated by caspase 1 in settings where gasdermin D is absent. Although cell death was fully ablated in Gsdmd−/− cells in one study21, a slower form of cell death was observed in another study22 that was later suggested to be apoptosis associated with caspase 8 activation23. By contrast, this pathway to apoptosis was not observed after activation of caspase 1122 (Figure 4).
Some researchers describe this pathway as “suppression of apoptosis by pyroptosis” 23, or as a “balance between pyroptosis and apoptosis” that depends on the quantity of agonist118. However, we prefer the interpretation that this is a versatile pathway that can “switch from pyroptosis to apoptosis” in the hypothetical case where a pathogen inhibits pyroptosis, thus ensuring cell death117. Apoptosis could thus be considered as a backup guard pathway for pyroptosis, however the in vivo relevance in controlling pathogen burdens remains to be elucidated.
Finally, caspase 8 has been proposed to substitute for caspase 1 as an pro-IL-1β and pro-IL-18 cleaving enzyme121–125, although this function warrants further study.
In summary, caspase 8 has been proposed to function upstream of inflammasomes, but also downstream of inflammasomes, which creates a quite confusing picture when one attempts to integrate the multiple functions into a single model. In vivo validation of these interactions will hopefully reveal their physiological importance.
Caspase 1 driven IL-18 primes caspase 11
Mouse caspase 11 activation is strictly dependent upon priming; in vitro this is commonly accomplished by studying cells previously primed by type I IFNs (via TLR3 or TLR4 driven type I IFN secretion)6, or by TLR2 stimulation16. The TLR3 pathway has been used in vivo to enable caspase 11 priming during LPS shock models15,16. Surprisingly, these pathways were insufficient to prime caspase 11 during infection with the cytosol-invasive bacterium B. thailandensis in vivo19. Instead, NLRC4-driven caspase 1 activation was required to drive IL-18 production, which in turn stimulated IFNγ within the first day of infection. IFNγ was absolutely essential for caspase 11 priming in this model, while endogenous TLR agonists and type I IFN were insufficient. The IL-18 requirement could be bypassed only under delayed kinetics after 3 days as alternate mediators eventually promoted IFNΓ production19. Thus, in the context of intracellular bacterial infection in vivo, caspase 1 acts upstream of caspase 11 priming, which was surprising as NLRP3 and caspase 1 are commonly thought to function downstream of caspase 11 (Box 3).
IL-18 drives NK cytotoxicity vs intracellular bacteria
NK-derived IFNΓ is well established to combat intracellular bacterial infection. However, whereas NK cytotoxicity is critical in combating viruses such as MCMV, HSV-1, influenza virus, and cancer, initial in vitro studies suggesting that bacterially infected cells are killed by NK cells have been uniformly disproven by subsequent in vivo studies126,127. Collectively, this data led to the conclusion that cytokines rather than cytotoxicity is the primary mode of NK cell- mediated protection against intracellular pathogens. However, our group recently showed that NK lysis of infected hepatocytes is crucial for controlling infection by an intracellular bacterial pathogen39. In this model, infected phagocytes detected C. violaceum via NLRC4, which lead to production of IL-18 and activation of liver NK cells. Activated NK cells then directly lysed infected hepatocytes in a perforin-dependent manner. NK cytotoxicity also controlled the inflammasome-evasive L. monocytogenes infection, but only when mice were treated with exogenous IL-1839. This example highlights the importance of direct cytotoxicity against bacteria and crosstalk between two different cell death modalities — inflammasomes and apoptosis — to efficiently fight infection. Similarly, NK cytotoxicity initiated by inflammasome detection of S. Typhimurium in a colitis model was recently shown to promote inflammation128 and Prf1−/− mice were also susceptible to C. rodentium during the innate phase of infection129. Interestingly, human, but not mouse, NK cells have additional means to directly kill bacteria, since granulysin delivered upon NK degranulation is directly toxic for both bacteria and parasites130,131.
How programmed cell death clears infection
Recent studies have examined the fate of pathogens after apoptosis, necroptosis and pyroptosis, and how these cell death mechanisms promote the resolution of infection. Apoptosis converts cells into apoptotic bodies, which release a series of find-me and eat-me signals that promote efferocytosis (phagocytosis of dead cells). How the necroptotic and pyroptotic cells are cleared, is currently under thorough investigation. These mechanisms remove the niche for intracellular viruses and bacteria, but pathogens frequently survive host cell death. Finally, NETosis generates structures that are specifically microbicidal. In this final section we discuss what happens to pathogens after programmed cell death (Figure 3).
Efferocytosis of apoptotic bodies
The resolution phase of apoptosis is another example of how apoptotic caspases maintain immunological silence. Failure to clear apoptotic debris can contribute to inflammatory or autoimmune disease{Poon:2014ik}. During normal development and homeostasis, apoptotic bodies are efferocytosed by a secondary phagocyte, thereby preventing secondary necrosis and maintaining immunological quiescence80. Efferocytosis is currently an active area of research in the context of infection.
Until recently, apoptosis was often considered intrinsically bactericidal. For example, apoptosis was thought to kill Mycobacteria spp. in vitro132 and in vivo133. However, recent studies suggest that Mycobacteria tuberculosis and Mycobacterium mariunum survive apoptosis, but are subsequently killed after efferocytosis by a secondary macrophage134,135. This efferocytic phagosome matures, acidifies and kills the bacteria via ROS. Notably, pathogens that resist apoptosis, such as Mycobacterium, also interfere with efferocytosis, which increases their survival136. L. monocytogenes, on the other hand, exploits efferocytosis receptors in order to spread from cell to cell137. The Listeriolysin O toxin damages the plasma membrane, exposing phosphatidyl serine, which is recognized by TIM4. As L. monocytogenes propels itself with actin comets, TIM4 on the neighbouring cell promotes efferocytosis of the protruding membrane, enabling L. monocytogenes to enter the next cell137.
Virus-containing apoptotic bodies can also be efferocytosed; for example, influenza A virus induces apoptosis of infected epithelial cells, and neighbouring macrophages efferocytose apoptotic bodies containing viral progeny, which reduces viral titers138. On the other hand, some viruses use mimicry to induce apoptosis in order to spread to neighbouring cells139.
NETs trap extracellular microbes
In addition to killing pathogens after phagocytosis, neutrophils can target extracellular pathogens using NETs101. NETs play two distinct anti-microbial roles by trapping and killing pathogens. Extensive intravital and confocal microscopy of neutrophils in infected tissue show in real time how bacterial and fungal pathogens can be trapped in NETs103,105,140. Moreover, additional pathogens are trapped in the extracellular chromatin in vitro. In fact, systemic DNase treatment in a mouse model of a S. aureus skin infection, which induces NET formation, resulted in the escape of the bacteria from the site of inoculation and increased vascular bacterial loads106.
The extent to which NETs are directly capable of killing bacterial and fungal pathogens remains controversial, since not all bacterial pathogens trapped in NETs are killed and there are no known specific inhibitors that unlink NET release from NET antimicrobial activity. For example, in vitro DNase treatment of Streptococcus pneumonia and Candida albicans trapped in NETs results in the release of viable bacteria141. This lack of microbial killing may stem from the loss of optimal protease activity of key bactericidal enzymes, such as elastase, under in vitro conditions142. Moreover, many research groups have clearly demonstrated anti-microbial activity of NETs against a variety of microbes in vitro140, and trapping is obviously beneficial to the host103,143. For example, intravital microscopy of Escherichia coli trapped in NETs within liver sinusoids demonstrate that disruption of NETs via platelet depletion or DNase treatment prevents trapping and promotes dissemination143. The use of vital DNA-intercalating dyes demonstrate that bacteria trapped within NETs are killed101,144. A thorough discussion of the controversies regarding the composition and antimicrobial properties of NETs was recently published145.
Many pathogens directly disrupt NETs — for example group B Streptococcus146, Vibrio cholera147, Yersinia enterocolitica148 and Neisseria gonorrhoeae149 — by expressing nucleases that degrade the NET-associated chromatin, which enables them to escape from NETs and may result in systemic dissemination and sepsis. Other pathogens, such as Haemophilus influenza150, Pseudomonas aeruginosa151 and S. pneumoniae152, evade NETs by incorporating NET-associated DNA into their biofilms, which promotes their replication in vivo. Hence, trapping of some microorganisms by NETs in the absence of bacterial killing could be advantageous to the pathogen. Finally, some pathogens evade killing by NETs by sidestepping the specific antimicrobial activity of NET components by expressing polysaccharide capsules153,154. A full overview of exotoxins that disrupt NETs and other evasion mechanisms are reviewed elsewhere155.
Pore-induced intracellular traps
Recent evidence from our group establishes the fate of the pyroptotic cell corpse and its role in orchestrating a downstream innate immune response. Similar to apoptosis, pyroptosis does not kill bacteria9,40. We initially assumed that pyroptosis released intracellular bacteria to the extracellular space9. However, in a follow-up study we show that viable bacteria remain trapped within the cellular debris of pyroptotic macrophages40. This trapping appears to be mediated by the pyroptotic cell membrane, which remains largely intact despite containing discreet ruptures, encompassing collapsed organelles and cellular structures. We call this structure the pore-induced intracellular trap (PIT) 35,40 (Figure 3), which is conceptually parallel to NETs. Both PITs and NETs trap the pathogen and prevent microbial dissemination. In contrast to NETs, which act against extracellular microbes, PITs are trap intracellular microorganisms. In addition to pyroptosis, necroptosis and necrosis also result in formation of a PIT that is efferocytosed by neutrophils and macrophages40, although the in vivo relevance of necroptotic and necrotic PITs in the context of infection remains to be elucidated.
PITs coordinate innate immune responses to combat intracellular bacteria; PIT formation leads to complement activation, release of IL-1β and IL-18, and eicosanoid production. These mediators recruit neutrophils, which utilize phagocytic scavenger and complement receptors to efferocytose the PIT and entrapped bacteria35,40, which is conceptually parallel to efferocytosis of bacteria trapped within apoptotic bodies134. Ultimately, this secondary phagocyte kills the bacteria via ROS40.
We therefore propose that PITs are highly effective immunologically important structures with two key functions; PITs immobilize the pathogen to prevent dissemination and promote efferocytosis by a secondary phagocyte that kills the pathogen.
Concluding remarks
Much work remains to be done to fully understand how pyroptosis, necroptosis, apoptosis and NETosis defend against infection in vivo. Still, genetic tools to study programmed cell death in vivo remain confounded by effects upon other aspects of host defence. For example, Gsdmd deletion prevents pyroptosis, but also prevents IL-1β and IL-18 secretion; similarly, Casp8 deletion prevents cell extrinsic apoptosis, but also may trigger necroptosis, cause a lymphoproliferative disorder, and inhibit innate immune receptor signalling (Box 2). Despite such difficulties, our understanding of programmed cell death has significantly advanced in recent years, revealing the utility of programmed cell death during infection both to destroy intracellular niches, and to coordinate an appropriate innate immune response thereafter. Finally, if a programmed cell death modality is exquisitely effective against a specific virulence strategy, this exerts strong selective pressure for those pathogens to evade the response. Therefore, when interpreting the results of experimental infections it is important to keep in mind the competing possibilities that the host may be benefiting, the pathogen may be evading, or that host defence and pathogenic evasion may both be partially effective. In this regard, identification of new infectious agents that fail to evade specific cell death pathways can aid our understanding of the true function of programmed cell death during infection.
Supplementary Material
Online summary.
Pyroptosis is programmed lytic cell death caused by caspase-1/4/5/11 cleavage of GSDMD. Recent work suggests that pyroptosis defends against infection by vacuolar or cytosol-invasive bacteria.
Necroptosis is programmed lytic cell death caused by RIPK3 activation of MLKL. Recent work suggests that necroptosis defends against viral infection.
Apoptosis can be activated by extrinsic and intrinsic signals. It is a critical host defence mechanism, and pathogens have evolved to both evade and co-opt apoptosis.
Apoptosis and necroptosis guard each other to make it difficult for pathogens to inhibit these programmed cell death mechanisms. Despite this, pathogens such as MCMV seem to inhibit both pathways simultaneously.
The signaling cascades leading to apoptosis, necroptosis, and pyroptosis have numerous interactions, creating a complex web of programmed cell death mechanisms to defend against infection. In this regard, physiologic relevance for apoptosis and necroptotic cross guarding is established, but most other interactions remain to be fully explored.
Neutrophil extracellular traps (NETs) and pore-induced intracellular traps (PITs) are mechanism to physically restrain bacteria in the extracellular or intracellular space, respectively. These cellular corpses can be considered as structures that exist in parallel to apoptotic bodies (the corpse of an apoptotic cell).
Acknowledgments
Edward A. Miao’s lab is supported by NIH grants AI097518 and AI119073, and the Yang Biomedical Scholars Award.
Glossary
- Apoptosis
A common form of cell death, which is also known as intrinsic or programmed cell death. Many physiological and developmental stimuli cause apoptosis, and this mechanism is frequently used to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation
- Pyroptosis
Programmed lytic cell death via caspase-1/4/5/11 cleavage of GSDMD, and recently expanded to include lytic cell death caused by other gasdermin family proteins
- Necroptosis
Programmed lytic cell death via RIPK3 activation of MLKL
- Apoptosis
Programmed non-lytic cell death in which initiator caspases-2/8/9/10 activate executioner caspases-3/6/7
- NETosis
Programmed neutrophil death that results in formation of NETs
- Apoptotic bodies
Apoptotic bodies form after apoptosis
- NETs
Neutrophil extracellular traps that form after NETosis
- pore-induced intracellular traps (PITs)
Structures that form after pyroptosis and necroptosis to retain organelles and bacteria
Biographies
Dr. Jorgensen received her PhD from Duke University where she studied Chlamydia pathogenesis, and did post-doctoral research with Dr. Miao at the University of North Carolina at Chapel Hill studying how pyroptosis clears intracellular bacteria. She is now a researcher at Oslo University Hospital at Rikshospitalet in Oslo, Norway.
Dr. Rayamajhi received her PhD from the University of Colorado and National Jewish Health, then did post doctoral research with Dr. Miao at the University of North Carolina at Chapel Hill studying apoptotic and inflammatory caspase responses to bacterial infection. She is now a research scientist at Camargo Pharmaceuticals.
Dr. Miao received MD and PhD degrees from the University of Washington, and is now an Associate Professor at the University of North Carolina at Chapel Hill. His laboratory studies the mechanisms whereby the innate immune system differentiates pathogenic from non-pathogenic microbes with a focus on intracellular bacteria. His work considers the concepts within microbial pathogenesis to understand innate immune surveillance.
Footnotes
Highlighted References
| 21,22 | Discovery of GSDMD as the effector of pyroptosis cleaved by caspase-1 and -11 |
| 26,28 | GSDMD forms a pore in membranes. |
| 39 | In vivo importance of NK cytotoxicity as a parallel effector mechanism to pyroptosis in clearing intracellular niches. |
| 19 | In vivo importance of caspase-11 in defense against cytosol-invasive bacteria |
| 9 | First demonstration that pyroptosis is an innate immune effector mechanism in vivo. |
| 52 | ZBP1 detects MCMV to trigger necroptosis, but is counteracted by MCMV vIRA. |
| 69 | Ripk3−/− mice are susceptible to HSV-1 infection |
| 56 | RIPK3 critical for necroptosis and Ripk3−/− mice susceptible to vaccinia infection. |
| 60–62 | MLKL permeabilizes membranes. |
| 85,86 | cGAS as guard for MOMP / caspase-9, triggering a type I IFN response when apoptosis fails to complete. |
| 88,89 | The embryonic lethality of Casp8−/− mice is complemented by crossing with Ripk3−/−, demonstrating that necroptosis guards caspase-8. |
| 100 | Description of the effect of Casp8−/− mutation upon TLR3 signalling and NLRP3 priming. |
| 134 | Efferocytosis as an effector mechanism against M. tuberculosis. |
| 101 | Discovery of neutrophil extracellular traps (NETs). |
| 106 | Evidence of vital NETosis, wherein neutrophils form NETs but survive. |
| 40 | Pore-induced intracellular traps (PITs) as a mechanism to hold intracellular bacteria in place after lytic cell death |
| 55–57 | RIPK3 activation drives necroptosis. |
Competing interests statement
The authors declare no competing interests.
References
- 1.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 3.Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. CMLS, Cell Mol Life Sci. 2016;73:2137–2152. doi: 10.1007/s00018-016-2189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillon CP, Tummers B, Baran K, Green DR. Developmental checkpoints guarded by regulated necrosis. CMLS, Cell Mol Life Sci. 2016;73:2125–2136. doi: 10.1007/s00018-016-2188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondelinger Y, Darding M, Bertrand MJM, Walczak H. Poly-ubiquitination in TNFR1-mediated necroptosis. CMLS, Cell Mol Life Sci. 2016;73:2165–2176. doi: 10.1007/s00018-016-2191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remijsen Q, et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dangl JL, Jones JDG. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 11.Mackey D, Holt BF, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 12.Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112:379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 13.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 14.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 15.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 18.Aachoui Y, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aachoui Y, et al. Canonical Inflammasomes Drive IFN-γ to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host Microbe. 2015;18:320–332. doi: 10.1016/j.chom.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, et al. The Shigella OspC3 Effector Inhibits Caspase-4, Antagonizes Inflammatory Cell Death, and Promotes Epithelial Infection. Cell Host Microbe. 2013;13:570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 22.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 23.He WT, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agard NJ, Maltby D, Wells JA. Inflammatory Stimuli Regulate Caspase Substrate Profiles. Mol Cell Proteomics. 2010;9:880–893. doi: 10.1074/mcp.M900528-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeki N, Sasaki H. In: Endothelium and Epithelium. Carrasco J, Mota M, editors. 2012. pp. 193–211. [Google Scholar]
- 26.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 27.Aglietti RA, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sborgi L, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 31.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsbaken T, Fink SL, Hartigh den AB, Loomis WP, Cookson BT. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J Immunol. 2011;187:2748–2754. doi: 10.4049/jimmunol.1100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorgensen I, Lopez JP, Laufer SA, Miao EA. IL-1β, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol. 2016 doi: 10.1002/eji.201646647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer JD, et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc Natl Acad Sci USA. 2011;108:12419–12424. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren SE, et al. Generation of a Listeria vaccine strain by enhanced caspase-1 activation. Eur J Immunol. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltez VI, et al. Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium. Immunity. 2015;43:987–997. doi: 10.1016/j.immuni.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213:2113–2128. doi: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maltez VI, Miao EA. Reassessing the Evolutionary Importance of Inflammasomes. J Immunol. 2016;196:956–962. doi: 10.4049/jimmunol.1502060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellin ME, et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16:237–248. doi: 10.1016/j.chom.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Knodler LA, et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe. 2014;16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rathinam VAK, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monroe KM, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakobsen MR, et al. From the Cover: IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci USA. 2013;110:E4571–80. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuriakose T, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Science Immunology. 2016;1:aag2045–aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thapa RJ, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 56.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 59.Murphy JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Dondelinger Y, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanzer MC, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J. 2015;471:255–265. doi: 10.1042/BJ20150678. [DOI] [PubMed] [Google Scholar]
- 64.Nogusa S, et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newton K, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo H, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu X, et al. Herpes Simplex Virus 1 (HSV-1) and HSV-2 Mediate Species-Specific Modulations of Programmed Necrosis through the Viral Ribonucleotide Reductase Large Subunit R1. J Virol. 2016;90:1088–1095. doi: 10.1128/JVI.02446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigue-Gervais IG, et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe. 2014;15:23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Lu JV, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci USA. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson N, et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rayamajhi M, Humann J, Penheiter K, Andreasen K, Lenz LL. Induction of IFN-αβ enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J Exp Med. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Philip NH, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc Natl Acad Sci USA. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weng D, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kitur K, et al. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitur K, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González-Juarbe N, et al. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog. 2015;11:e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Robinson KS, Aw R. The Commonalities in Bacterial Effector Inhibition of Apoptosis. Trends Microbiol. 2016;24:665–680. doi: 10.1016/j.tim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Zheng JH, Viacava Follis A, Kriwacki RW, Moldoveanu T. Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J. 2016;283:2690–2700. doi: 10.1111/febs.13527. [DOI] [PubMed] [Google Scholar]
- 84.Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122:2801–2808. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.White MJ, et al. Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silke J, Hartland EL. Masters, marionettes and modulators: intersection of pathogen virulence factors and mammalian death receptor signaling. Curr Opin Immunol. 2013;25:436–440. doi: 10.1016/j.coi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 91.Mandal P, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rickard JA, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 93.Dillon CP, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaiser WJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin J, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newton K, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 97.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 98.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 99.Moriwaki K, et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang S, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 102.Branzk N, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 104.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 105.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Man SM, et al. Salmonella Infection Induces Recruitment of Caspase-8 to the Inflammasome To Modulate IL-1β Production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganesan S, et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allam R, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 111.López-Castejón G, et al. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J Biol Chem. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Juliana C, et al. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Lawlor KE, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cai X, et al. Prion-like Polymerization Underlies Signal Transduction in Antiviral Immune Defense and Inflammasome Activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu A, et al. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pierini R, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sagulenko V, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vajjhala PR, et al. The Inflammasome Adaptor ASC Induces Procaspase-8 Death Effector Domain Filaments. J Biol Chem. 2015;290:29217–29230. doi: 10.1074/jbc.M115.687731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pierini R, et al. ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J Immunol. 2013;191:3847–3857. doi: 10.4049/jimmunol.1203326. [DOI] [PubMed] [Google Scholar]
- 122.Maelfait J, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bossaller L, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Antonopoulos C, et al. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J Biol Chem. 2015;290:20167–20184. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vince JE, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 126.Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 127.Laochumroonvorapong P, et al. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Müller AA, et al. An NK Cell Perforin Response Elicited via IL-18 Controls Mucosal Inflammation Kinetics during Salmonella Gut Infection. PLoS Pathog. 2016;12:e1005723–30. doi: 10.1371/journal.ppat.1005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Solaymani-Mohammadi S, et al. Lack of the programmed death-1 receptor renders host susceptible to enteric microbial infection through impairing the production of the mucosal natural killer cell effector molecules. J Leukoc Biol. 2016;99:475–482. doi: 10.1189/jlb.4A0115-003RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walch M, et al. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi: 10.1016/j.cell.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dotiwala F, et al. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat Med. 2016;22:210–216. doi: 10.1038/nm.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 133.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin CJ, et al. Efferocytosis Is an Innate Antibacterial Mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang CT, et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hartman ML, Kornfeld H. Interactions between naïve and infected macrophages reduce Mycobacterium tuberculosis viability. PLoS ONE. 2011;6:e27972. doi: 10.1371/journal.pone.0027972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Czuczman MA, et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature. 2014;509:230–234. doi: 10.1038/nature13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amara A, Mercer J. Viral apoptotic mimicry. Nat Rev Microbiol. 2015;13:461–469. doi: 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 141.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 142.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 143.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular Neutrophil Extracellular Traps Capture Bacteria from the Bloodstream during Sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 144.Lauth X, et al. M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun. 2009;1:202–214. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nauseef WM, Kubes P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016;18:1349–1357. doi: 10.1111/cmi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beiter K, et al. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 147.Seper A, et al. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9:e1003614. doi: 10.1371/journal.ppat.1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Möllerherm H, et al. Yersinia enterocolitica-mediated degradation of neutrophil extracellular traps (NETs) FEMS Microbiology Letters. 2015;362:fnv192. doi: 10.1093/femsle/fnv192. [DOI] [PubMed] [Google Scholar]
- 149.Juneau RA, Stevens JS, Apicella MA, Criss AK. A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J Infect Dis. 2015;212:316–324. doi: 10.1093/infdis/jiv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hong W, Juneau RA, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1:215–224. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fazli M, et al. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ Microbiol. 2014;16:1961–1981. doi: 10.1111/1462-2920.12448. [DOI] [PubMed] [Google Scholar]
- 152.Reid SD, et al. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199:786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- 153.Wartha F, et al. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 154.Cole JN, et al. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. mBio. 2010;1:e00191-10–e00191-17. doi: 10.1128/mBio.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.von Köckritz-Blickwede M, Blodkamp S, Nizet V. Interaction of Bacterial Exotoxins with Neutrophil Extracellular Traps: Impact for the Infected Host. Front Microbiol. 2016;7:402. doi: 10.3389/fmicb.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stewart MK, Cookson BT. Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses. 2016;14:346–359. doi: 10.1038/nrmicro.2016.50. [DOI] [PubMed] [Google Scholar]
- 157.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 158.Khan I, et al. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis. 2013;60:204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 159.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muñoz-Planillo R, et al. K(+) Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wen H, Miao EA, Ting JPY. Mechanisms of NOD-like Receptor-Associated Inflammasome Activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rühl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 164.Schmid-Burgk JL, et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 165.Wang X, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15:1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 166.Brunette RL, et al. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu H, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 168.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 169.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levinsohn JL, et al. Anthrax lethal factor cleavage of nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chavarría-Smith J, Vance RE. Direct Proteolytic Cleavage of NLRP1B Is Necessary and Sufficient for Inflammasome Activation by Anthrax Lethal Factor. PLoS Pathog. 2013;9:e1003452. doi: 10.1371/journal.ppat.1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruckdeschel K, et al. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- 173.Han KJ, et al. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem. 2004;279:15652–15661. doi: 10.1074/jbc.M311629200. [DOI] [PubMed] [Google Scholar]
- 174.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 175.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & Development. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cellular Signalling. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 177.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaiser WJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.