ABSTRACT
Gepotidacin (formerly GSK2140944) is a novel, first-in-class, triazaacenaphthylene antibacterial that inhibits bacterial DNA gyrase and topoisomerase IV via a unique mechanism and has demonstrated in vitro activity against Neisseria gonorrhoeae, including drug-resistant strains, and also targets pathogens associated with other conventional and biothreat infections. Broth microdilution was used to evaluate the MIC and minimum bactericidal concentration (MBC) activity of gepotidacin and comparators against 25 N. gonorrhoeae strains (including five ciprofloxacin-nonsusceptible strains). Gepotidacin activity was also evaluated against three N. gonorrhoeae strains (including a ciprofloxacin-nonsusceptible strain) for resistance development, against three N. gonorrhoeae strains (including two tetracycline- and azithromycin-nonsusceptible strains) using time-kill kinetics and checkerboard methods, and against two N. gonorrhoeae strains for the investigation of postantibiotic (PAE) and subinhibitory (PAE-SME) effects. The MIC50 and MIC90 for gepotidacin against the 25 N. gonorrhoeae isolates tested were 0.12 and 0.25 μg/ml, respectively. The MBC50 and MBC90 for gepotidacin were 0.25 and 0.5 μg/ml, respectively. Gepotidacin was bactericidal, and single-step resistance selection studies did not recover any mutants, indicating a low rate of spontaneous single-step resistance. For combinations of gepotidacin and comparators tested using checkerboard methods, there were no instances where antagonism occurred and only one instance of synergy (with moxifloxacin; fractional inhibitory concentration, 0.375). This was not confirmed by in vitro time-kill studies. The PAE for gepotidacin against the wild-type strain ranged from 0.5 to >2.5 h, and the PAE-SME was >2.5 h. These in vitro data indicate that further study of gepotidacin is warranted for potential use in treating infections caused by N. gonorrhoeae.
KEYWORDS: Neisseria gonorrhoeae, minimum bactericidal activity, postantibiotic effect
INTRODUCTION
Neisseria gonorrhoeae causes a range of sexually transmitted diseases and is the second most reported notifiable infectious disease in the United States (1). N. gonorrhoeae is known to facilitate the acquisition of HIV infection (2). The incidence of infection caused by N. gonorrhoeae in the United States is currently estimated at 820,000 cases per year, with an estimated yearly health care cost of $162 million (1).
N. gonorrhoeae has proven to be very adept at developing resistance to antimicrobials used to treat gonococcal disease. After penicillin resistance became prevalent, fluoroquinolones became a mainstay of treatment until resistance emerged in this class in the early to mid-2000s (3), leaving only cephalosporins as a treatment option in the United States. However, declining cefixime susceptibility was found to correlate with treatment failures (4) and, in 2012, oral cephalosporins were no longer recommended for treatment in the United States (5). In addition, it has been found that azithromycin resistance has coevolved with cephalosporin resistance, hence compromising combination therapy with these two classes of antibiotics (6). Faced with an already limited and potentially complete lack of treatment options in the future, there is an urgent need to research and develop new antimicrobial agents to treat gonococcal infections.
Gepotidacin (formerly GSK2140944) is a novel, first-in-class, triazaacenaphthylene antibacterial that selectively inhibits bacterial DNA gyrase and topoisomerase IV by a unique mechanism, one that is not utilized by any currently approved human therapeutic agent. Structural data with a type IIA topoisomerase enzyme, DNA gyrase, has revealed the novel binding mode of the triazaacenaphthylene class and has distinguished it from the binding mode of the quinolone antibacterials (7). As a consequence of its novel mode of action, gepotidacin is active in vitro against target pathogens carrying resistance determinants to established antibacterials, including fluoroquinolones (8). Gepotidacin has demonstrated in vitro activity against key pathogens, including drug-resistant strains, associated with a range of conventional and biothreat infections (8).
In the present study, in vitro methods were used to evaluate the MIC/MBC activity of gepotidacin and comparator agents against N. gonorrhoeae. Gepotidacin in vitro activity was also evaluated for resistance development and using time-kill kinetics, for broth microdilution checkerboard methods for synergy testing, and for postantibiotic effects.
RESULTS
Quality control.
The recommended method for N. gonorrhoeae susceptibility testing is an agar dilution method (9). Quality control criteria for gepotidacin against N. gonorrhoeae by agar dilution have been proposed at 0.25 to 1 μg/ml (10, 11). In the present study, the MIC values for gepotidacin for the quality control strain (N. gonorrhoeae ATCC 49226) were 0.5 μg/ml when tested on agar for the resistance development analysis and 0.12 to 0.25 μg/ml when tested in fastidious broth (FB). Quality control results for ceftriaxone were all within published Clinical and Laboratory Standards Institute (CLSI) ranges (11).
MIC/MBC studies.
The MIC50/90 for gepotidacin against 25 N. gonorrhoeae isolates selected for this study was 0.12/0.25 μg/ml (Table 1). The highest gepotidacin MIC value was 0.25 μg/ml. The ceftriaxone MIC50 and MIC90 for the N. gonorrhoeae isolates were 0.002 and 0.008 μg/ml. The MBC50/90 for gepotidacin against 25 N. gonorrhoeae isolates selected for this study was 0.25/0.5 μg/ml. The highest gepotidacin MBC value was 1 μg/ml. The ceftriaxone MBC50/90 for the N. gonorrhoeae isolates was 0.002/0.008 μg/ml. Gepotidacin was bactericidal when tested against N. gonorrhoeae, with a total of 100% (25/25) of isolates exhibiting an MBC/MIC ratio of ≤4. For 80% (20/25) of the isolates tested, the MBC/MIC ratio was ≤2. Ceftriaxone was bactericidal against N. gonorrhoeae, with a total of 100% (25/25) of the N. gonorrhoeae isolates tested exhibiting an MBC/MIC ratio of ≤2 (Table 1).
TABLE 1.
Summary of MIC50/90 values, MBC50/90 values, and MBC/MIC ratios for gepotidacin and ceftriaxone against 25 N. gonorrhoeae strains
| MIC or MBC/MIC ratio | Gepotidacin | Ceftriaxone |
|---|---|---|
| MIC (μg/ml) | ||
| MIC50 | 0.12 | 0.002 |
| MIC90 | 0.25 | 0.008 |
| MBC50 | 0.25 | 0.002 |
| MBC90 | 0.5 | 0.008 |
| MBC/MIC ratioa | ||
| MBC50/MIC50 | 2 | 1 |
| MBC90/MIC90 | 2 | 1 |
All isolates exhibited an MBC/MIC ratio of ≤4.
Frequency of single-step resistance development.
The MIC values for gepotidacin against the three N. gonorrhoeae strains tested ranged from 0.25 to 0.5 μg/ml (Table 2). The ciprofloxacin MIC value for N. gonorrhoeae isolate 12 was 16 μg/ml, with a corresponding gepotidacin MIC value of 0.25 μg/ml (Table 2). This isolate was determined to have the following genotype: GyrA (S91F, D95A) and ParC (S87R) mutations. The ciprofloxacin MIC value for N. gonorrhoeae isolates MS11 and ATCC 49226 was 0.004 μg/ml. The MIC values for the three N. gonorrhoeae strains tested ranged from 0.004 to 0.015 μg/ml for ceftriaxone, 0.25 to 2 μg/ml for azithromycin, and 0.12 to 1 μg/ml for tetracycline (data not shown). No mutants were observed for gepotidacin, ciprofloxacin, ceftriaxone, azithromycin, or tetracycline against any of the three strains tested. These results show a spontaneous single-step mutation rate among these N. gonorrhoeae strains to be less than 1.25 × 10−9 for gepotidacin (Table 2).
TABLE 2.
Summary of MIC values and mutation rates for ciprofloxacin and gepotidacin for three N. gonorrhoeae strains
| Isolate | Ciprofloxacin agar MIC (μg/ml) | Ciprofloxacin mutation frequency | Gepotidacin baseline agar MIC (μg/ml) | Gepotidacin mutation frequency |
|---|---|---|---|---|
| 12 | 16a | <1.25 × 10−9 | 0.25 | <1.25 × 10−9 |
| MS11 | 0.004 | <9.09 × 10−10 | 0.5 | <9.09 × 10−10 |
| ATCC 49266 | 0.004 | <9.09 × 10−10 | 0.5 | <9.09 × 10−10 |
Isolate 12 was determined to have the following genotype: GyrA S91F and D95A mutations and a ParC S87R mutation.
Time-kill studies.
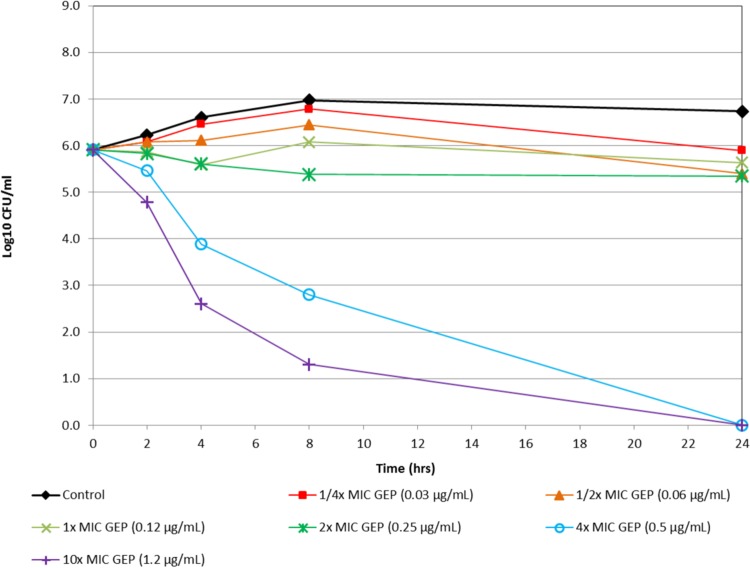
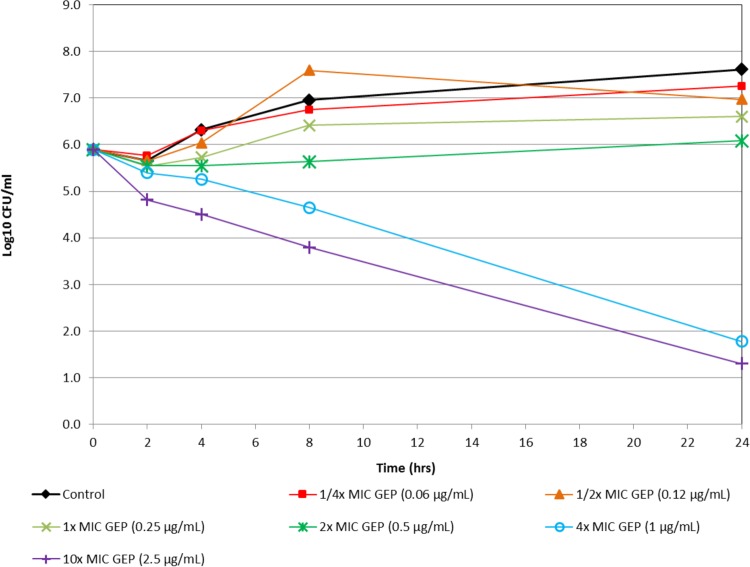
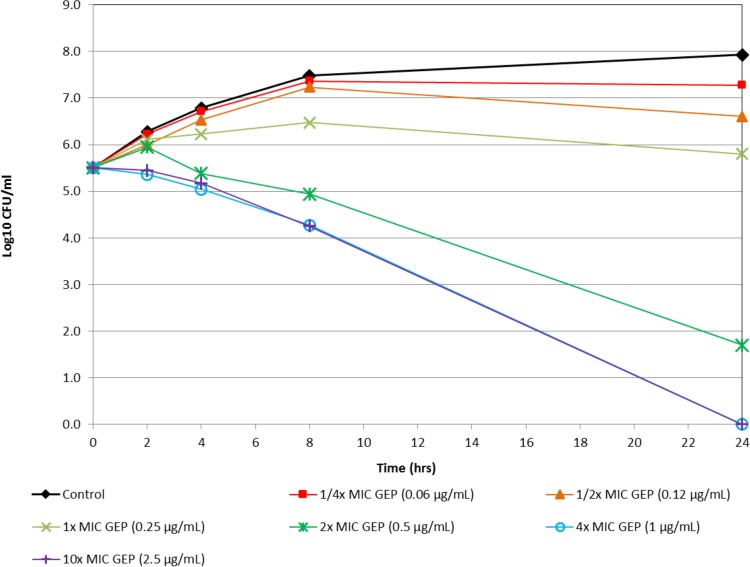
Figure 1 shows the in vitro time-kill activity of gepotidacin against N. gonorrhoeae isolate 12584, which was nonsusceptible to both azithromycin and tetracycline. Against this isolate, gepotidacin was bactericidal at 4× MIC at the 8-h time point. At 10× MIC, gepotidacin was bactericidal at the 4-h time point. Figure 2 shows the in vitro time-kill activity of gepotidacin against a second isolate of N. gonorrhoeae (12588), which was also nonsusceptible to both azithromycin and tetracycline. Gepotidacin was bactericidal against this isolate at the 24-h time point at both 4× and 10× MICs. No regrowth for gepotidacin was seen for either isolate. Figure 3 shows the in vitro time-kill activity of gepotidacin against a wild-type reference isolate of N. gonorrhoeae, ATCC 49226. Gepotidacin was bactericidal against this isolate at 24 h at 2×, 4×, and 10× MICs. No regrowth for gepotidacin was noted. No colonies grew on the gepotidacin 4× MIC agar screen plates inoculated from the 24-h time point time-kill curve tubes containing either 2×, 4×, or 10× MIC gepotidacin concentrations for any of the 3 N. gonorrhoeae isolates tested.
FIG 1.
Time-kill curve for gepotidacin against N. gonorrhoeae (isolate 12584).
FIG 2.
Time-kill curve for gepotidacin against isolate N. gonorrhoeae (isolate 12588).
FIG 3.
Time-kill curve for gepotidacin against isolate N. gonorrhoeae ATCC 49226.
Determination of interactions (checkerboard) evaluation.
For all of the combinations of gepotidacin and comparators tested in vitro against N. gonorrhoeae, there were no occurrences of antagonism, and there was only one occurrence of synergy (data not shown). For the remaining combinations, indifference was noted for 80.0% of in vitro checkerboard results, and 13.3% of results could not be determined due to off-scale MIC values (data not shown). There was one occurrence of synergy (fractional inhibitory concentration [FIC] index, 0.375) for gepotidacin tested in combination with moxifloxacin against N. gonorrhoeae strain 12588). To further confirm this synergy, an in vitro time-kill study was performed with various concentrations of gepotidacin and moxifloxacin. This in vitro time-kill study did not confirm the potential synergy noted in the in vitro checkerboard testing.
Determination of postantibiotic and subinhibitory effects.
Against the wild-type reference strain (ATCC 49226), the PAE-SME value for gepotidacin was >2.5 h at 0.25× MIC and 0.5× MIC. The PAE-SME values for ceftriaxone were 0.5 h at 0.25× MIC and 0.1 h at 0.5× MIC (Tables 3 and 4).
TABLE 3.
PAE results for time-kill curves for gepotidacin and comparator agents after 1 h of exposure at 1×, 5×, and 10× baseline MIC values
| N. gonorrhoeae isolate | Gepotidacin |
Ceftriaxone |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline MIC (μg/ml) | PAE (h) at various MICs |
Baseline MIC (μg/ml) | PAE (h) at various MICs |
|||||
| 1× | 5× | 10× | 1× | 5× | 10× | |||
| ATCC 49226 | 0.25 | 0.5 | 1.0 | >2.5 | 0.004 | 0.3 | 0.0 | 0.0 |
| 12584 | 0.12 | 0.7 | 0.7 | 0.7 | 0.004 | 0.7 | 0.2 | 0.0 |
TABLE 4.
PAE-SME results observed for time-kill curves for gepotidacin and comparator agents after 1 h of exposure at 5× MIC and addition of 0.25× MIC or 0.5× MIC of the antimicrobial agent
| N. gonorrhoeae isolate | Gepotidacin |
Ceftriaxone |
||||
|---|---|---|---|---|---|---|
| 5× MIC (μg/ml) | PAE-SME (h) at: |
5× MIC (μg/ml) | PAE-SME (h) at: |
|||
| 5× MIC + 0.25× MIC | 5× MIC + 0.5× MIC | 5× MIC + 0.25× MIC | 5× + 0.5× MIC | |||
| ATCC 49226 | 1.25 | >2.5 | >2.5 | 0.02 | 0.5 | 0.1 |
| 12584 | 0.625 | 1.2 | 2.7 | 0.02 | 1.3 | 0.0 |
Against the tetracycline- and azithromycin-nonsusceptible strain (isolate 12584), the PAE-SME values for gepotidacin were 1.2 h at 0.25× MIC and 2.7 h at 0.5× MIC. The PAE-SME values for ceftriaxone were 1.3 h at 0.25× MIC and 0.0 h at 0.5× MIC (Tables 3 and 4).
DISCUSSION
In the present study, gepotidacin was shown to be active (MIC50/90 value of 0.12/0.25 μg/ml) and bactericidal against N. gonorrhoeae (MBC/MIC ratios of ≤4 against 100% of isolates tested), including strains nonsusceptible to penicillin, ciprofloxacin, and tetracycline. In vitro resistance selection studies indicated a low rate of mutational resistance with gepotidacin. Gepotidacin also demonstrated bactericidal activity in time-kill curves against N. gonorrhoeae, including isolates that were nonsusceptible to azithromycin and tetracycline. In vitro checkerboard experiments showed no occurrences of antagonism when testing gepotidacin in combination with a variety of currently used antimicrobial agents against N. gonorrhoeae. The PAE for gepotidacin that occurred when testing N. gonorrhoeae ranged from short to modest, with an extended PAE-SME observed for the two isolates tested.
Gepotidacin is a member of the new triazaacenaphthylene class of novel bacterial topoisomerase inhibitors (NBTIs) and inhibits both bacterial gyrase and topoisomerase IV. Gepotidacin and other mechanistically related (but structurally different) NBTIs have a mode of action which differs from fluoroquinolones in that they bind to the DNA-protein complex at a location that is away from the cleavage site and do not form a cleavage complex (12, 13). Since gepotidacin and NBTIs bind at a different location than fluoroquinolones, they have demonstrated in vitro activity against strains resistant to other antibacterial classes, including fluoroquinolones (7, 8).
Resistance frequencies for NBTIs have been previously reported for other organisms and were shown to be in the range of 10−8 to 10−10 (13–15). In our study, spontaneous mutations to gepotidacin in N. gonorrhoeae were not obtained for the strains tested; however, the experimental approach used to select for resistance was a method that would most likely identify single-step and not multistep mutations. Although in our study, with our methodology, the spontaneous single-step mutation rate for ciprofloxacin was low (10−9), others have reported rates of ∼10−8 or that ciprofloxacin resistance was readily selected (16, 17). Further studies are needed to evaluate the relative development of resistance potential for gepotidacin compared to fluoroquinolones in the treatment of infections caused by N. gonorrhoeae.
These in vitro data demonstrating the attributes of gepotidacin, including potency, bactericidal activity, postantibiotic effect, and single-step resistance development, indicate that further study is warranted for potential use in treating infections caused by N. gonorrhoeae. Rapidly developing antimicrobial resistance in N. gonorrhoeae to all current treatment options for gonococcal infections means new therapies are urgently needed (5).
MATERIALS AND METHODS
For all liquid in vitro assays described in the present study, testing was performed using FB (18).
In vitro broth microdilution methods were used to evaluate the MIC/MBC activity of gepotidacin and ceftriaxone (comparator agent) against 25 strains of N. gonorrhoeae (including five ciprofloxacin-nonsusceptible, five penicillin-nonsusceptible, five penicillin- and ciprofloxacin-nonsusceptible, and five azithromycin- or tetracycline-nonsusceptible strains) (9, 19). Quality control was tested daily for the broth microdilution method and inoculum density was monitored by colony counts for each test run. ATCC quality control strains were selected as appropriate for the conditions being tested and included N. gonorrhoeae ATCC 49226 (11). A drug was considered to exhibit bactericidal activity against a particular isolate when the MBC/MIC ratio was ≤4.
For frequency of resistance development studies, three strains were tested by reference agar dilution MIC methods against gepotidacin plus the control agents, ciprofloxacin, ceftriaxone, azithromycin, and tetracycline (9). N. gonorrhoeae MS11 (ATCC BAA-1833) was provided by Ann Jerse, Uniformed Services University of the Health Sciences (Bethesda, MD). Based on the baseline agar dilution MIC results for gepotidacin, individual agar plates were prepared with 4× and 8× MIC values in duplicate for each of the three strains for both 0.1- and 1.0-ml inoculum volumes. Agar plates were inoculated and incubated at 35°C in 5% CO2 atmosphere for 24 and 48 h. Visible colonies of N. gonorrhoeae were quantitated and compared to the starting inoculum concentration to determine the mutation rate (i.e., calculated as the number of mutants/inoculum concentration).
For time-kill kinetics, gepotidacin and comparator agents were tested in FB at concentrations of 0.25×, 0.5×, 1×, 2×, 4×, and 10× MICs for each isolate and sampled at time zero hour (T0), T2, T4, T8 and T24 (20). Time-kill curves were performed in duplicate. Bactericidal activity was defined as a ≥3-log reduction in bacterial counts (log10 CFU/ml). Three strains were used in these studies; including ATCC 49226 and fluoroquinolone-, tetracycline-, and azithromycin-nonsusceptible isolates.
Checkerboard synergy testing was performed by broth microdilution, as previously described (21). Gepotidacin was tested alone and in combination with moxifloxacin, levofloxacin, azithromycin, tetracycline, and ceftriaxone. The total fractional inhibitory concentration index (ΣFIC) was scored as follows: synergy, ≤0.5; indifference, >0.5 to ≤4.0; and antagonism, >4.0.
Gepotidacin was tested against isolates using time-kill methods to the determine postantibiotic effect (PAE) and the PAE-sub-MIC effect (SME) (22–24). In addition to gepotidacin, ceftriaxone was tested as a comparator. The organisms were exposed for 1 h to gepotidacin or the comparison agent at 1×, 5×, and 10× MICs as determined from broth microdilution testing for each agent. For the PAE-SME, only an initial 5× exposure was used; followed by 0.25× or 0.5× MIC reexposure. Antibacterials were removed by centrifugation at 3,000 × g for 3 min and resuspension of the cells in prewarmed media, followed by a 1:1,000 dilution in additional prewarmed media. Colony counts were performed at T0 (preantimicrobial exposure) and T1 (after antimicrobial exposure). Colony counts were taken immediately after antimicrobial removal and at each subsequent hour until visible turbidity was observed or up to 9 h.
ACKNOWLEDGMENTS
This study was supported by GlaxoSmithKline (GSK; Collegeville, PA) and has been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract HHSO100201300011C.
JMI Laboratories, Inc., also contracted to perform services in 2016 for Achaogen, Actelion, Allecra, Allergan, Ampliphi, API, Astellas, AstraZeneca, Basilea, Bayer, BD, Biomodels, Cardeas, CEM-102 Pharma, Cempra, Cidara, Cormedix, CSA Biotech, Cubist, Debiopharm, Dipexium, Duke, Durata, Entasis, Fortress, Fox Chase Chemical, Medpace, Melinta, Merck, Micurx, Motif, N8 Medical, Nabriva, Nexcida, Novartis, Paratek, Pfizer, Polyphor, Rempex, Scynexis, Shionogi, Spero Therapeutics, Symbal Therapeutics, Synolgoic, TGV Therapeutics, The Medicines Company, Theravance, ThermoFisher, Venatorx, Wockhardt, and Zavante. Some JMI employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, Cempra, and Theravance. There are no speakers' bureaus or stock options to declare.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2014. 2013 sexually transmitted disease surveillance. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2007. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 56:332–336. [PubMed] [Google Scholar]
- 4.Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, Siebert H, Towns L, Melano RG, Low DE. 2013. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 309:163–170. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 6.Allen VG, Seah C, Martin I, Melano RG. 2014. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother 58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 8.Biedenbach DJ, Bouchillon SK, Hackel M, Miller LA, Scangarella-Oman NE, Jakielaszek C, Sahm DF. 2016. In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother 60:1918–1923. doi: 10.1128/AAC.02820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Jones RN, Fedler KA, Scangarella-Oman NE, Ross JE, Flamm RK. 2016. Multicenter investigation of gepotidacin (GSK2140944) agar dilution quality control determinations for Neisseria gonorrhoeae ATCC 49226. Antimicrob Agents Chemother 60:4404–4406. doi: 10.1128/AAC.00527-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2016. M100-S26. Performance standards for antimicrobial susceptibility testing: 26th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Black MT, Stachyra T, Platel D, Girard AM, Claudon M, Bruneau JM, Miossec C. 2008. Mechanism of action of the antibiotic NXL101, a novel nonfluoroquinolone inhibitor of bacterial type II topoisomerases. Antimicrob Agents Chemother 52:3339–3349. doi: 10.1128/AAC.00496-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri SD, Kutschke A, McCormack K, Alm RA. 2015. Insights into the mechanism of inhibition of novel bacterial topoisomerase inhibitors from characterization of resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother 59:5278–5287. doi: 10.1128/AAC.00571-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayar AS, Dougherty TJ, Reck F, Thresher J, Gao N, Shapiro AB, Ehmann DE. 2015. Target-based resistance in Pseudomonas aeruginosa and Escherichia coli to NBTI 5463, a novel bacterial type II topoisomerase inhibitor. Antimicrob Agents Chemother 59:331–337. doi: 10.1128/AAC.04077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty TJ, Nayar A, Newman JV, Hopkins S, Stone GG, Johnstone M, Shapiro AB, Cronin M, Reck F, Ehmann DE. 2014. NBTI 5463 is a novel bacterial type II topoisomerase inhibitor with activity against gram-negative bacteria and in vivo efficacy. Antimicrob Agents Chemother 58:2657–2664. doi: 10.1128/AAC.02778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz J, Jurado A, Garcia-Mendez E, Marco F, Aguilar L, Jimenez de Anta MT, Vila J. 2001. Frequency of selection of fluoroquinolone-resistant mutants of Neisseria gonorrhoeae exposed to gemifloxacin and four other quinolones. J Antimicrob Chemother 48:545–548. doi: 10.1093/jac/48.4.545. [DOI] [PubMed] [Google Scholar]
- 17.Jeverica S, Golparian D, Hanzelka B, Fowlie AJ, Maticic M, Unemo M. 2014. High in vitro activity of a novel dual bacterial topoisomerase inhibitor of the ATPase activities of GyrB and ParE (VT12-008911) against Neisseria gonorrhoeae isolates with various high-level antimicrobial resistance and multidrug resistance. J Antimicrob Chemother 69:1866–1872. doi: 10.1093/jac/dku073. [DOI] [PubMed] [Google Scholar]
- 18.Takei M, Yamaguchi Y, Fukuda H, Yasuda M, Deguchi T. 2005. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J Clin Microbiol 43:4321–4327. doi: 10.1128/JCM.43.9.4321-4327.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 1999. M26-A: methods for determining bactericidal activity of antimicrobial agents; approved guideline. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Leber AL. (ed). 2016. Antimicrobial susceptibility testing, p. 5.16.1–5.16.21. Clinical microbiology procedures handbook, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 21.Leber AL. (ed). 2016. Antimicrobial susceptibility testing, p. 5.14.2.1–5.14.2.12. Clinical microbiology procedures handbook, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 22.Odenholt-Tornqvist I. 1993. Studies on the postantibiotic effect and the postantibiotic sub-MIC effect of meropenem. J Antimicrob Chemother 31:881–892. doi: 10.1093/jac/31.6.881. [DOI] [PubMed] [Google Scholar]
- 23.Odenholt-Tornqvist I, Lowdin E, Cars O. 1992. Postantibiotic sub-MIC effects of vancomycin, roxithromycin, sparfloxacin, and amikacin. Antimicrob Agents Chemother 36:1852–1858. doi: 10.1128/AAC.36.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangler SK, Lin G, Jacobs MR, Appelbaum PC. 1998. Postantibiotic effect and postantibiotic sub-MIC effect of levofloxacin compared to those of ofloxacin, ciprofloxacin, erythromycin, azithromycin, and clarithromycin against 20 pneumococci. Antimicrob Agents Chemother 42:1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]