ABSTRACT
Colistin is often administered by inhalation and/or the parenteral route for the treatment of respiratory infections caused by multidrug-resistant (MDR) Pseudomonas aeruginosa. However, limited pharmacokinetic (PK) and pharmacodynamic (PD) data are available to guide the optimization of dosage regimens of inhaled colistin. In the present study, PK of colistin in epithelial lining fluid (ELF) and plasma was determined following intratracheal delivery of a single dose of colistin solution in neutropenic lung-infected mice. The antimicrobial efficacy of intratracheal delivery of colistin against three P. aeruginosa strains (ATCC 27853, PAO1, and FADDI-PA022; MIC of 1 mg/liter for all strains) was examined in a neutropenic mouse lung infection model. Dose fractionation studies were conducted over 2.64 to 23.8 mg/kg of body weight/day. The inhibitory sigmoid model was employed to determine the PK/PD index that best described the antimicrobial efficacy of pulmonary delivery of colistin. In both ELF and plasma, the ratio of the area under the unbound concentration-time profile to MIC (fAUC/MIC) was the PK/PD index that best described the antimicrobial effect in mouse lung infection (R2 = 0.60 to 0.84 for ELF and 0.64 to 0.83 for plasma). The fAUC/MIC targets required to achieve stasis against the three strains were 684 to 1,050 in ELF and 2.15 to 3.29 in plasma. The histopathological data showed that pulmonary delivery of colistin reduced infection-caused pulmonary inflammation and preserved the integrity of the lung epithelium, although colistin introduced mild pulmonary inflammation in healthy mice. This study showed pulmonary delivery of colistin provides antimicrobial effects against MDR P. aeruginosa lung infections superior to those of parenteral administrations. For the first time, our results provide important preclinical PK/PD information for optimization of inhaled colistin therapy.
KEYWORDS: colistin, respiratory tract infection, intratracheal administration, pharmacokinetics, pharmacodynamics, mouse lung infection model, multidrug-resistant bacteria, Pseudomonas aeruginosa
INTRODUCTION
Respiratory tract infections caused by multidrug-resistant (MDR) bacteria, in particular Pseudomonas aeruginosa, are difficult to treat and often associated with high rates of recurrence, mortality, and morbidity (1–3). For respiratory infections caused by MDR P. aeruginosa, inhalational and intravenous colistin (polymyxin E) is often the only effective option (4, 5).
Colistin is a cationic, multicomponent polypeptide which is usually administered parenterally in its inactive form, colistin methanesulfonate (CMS) (5). Recent pharmacokinetics/pharmacodynamics (PK/PD) and clinical studies demonstrate that intravenous CMS is suboptimal in treating respiratory tract infections caused by P. aeruginosa because of the very low exposure in the lungs and dosing-limiting nephrotoxicity (6–9). In a PK/PD study against P. aeruginosa after parenteral administration in the mouse thigh and lung infection models, the ratio of the area under the unbound concentration-time profile to MIC (fAUC/MIC) required to achieve stasis for treating respiratory infection was approximately 4-fold higher than that required for treating thigh infection (4). Over the last decade, nebulization of colistin has become an alternative in treating MDR Gram-negative respiratory tract infections (10, 11). Inhalation therapy of colistin reduces systemic exposure and thus minimizes potential systemic side effects such as nephrotoxicity (1, 12). PK studies in rats (6–8) and cystic fibrosis patients (9) have clearly demonstrated that nebulization of CMS/colistin resulted in much higher drug exposure in the epithelial lining fluid (ELF) or sputum than by systemic administration. The superior efficacy of nebulized CMS/colistin against lung infections caused by P. aeruginosa and Acinetobacter baumannii has been demonstrated in porcine (13) and mouse (14) lung infection models. However, there is no information on the PK/PD of inhaled colistin in infected animal models that could be used to guide the optimization of dosage regimens of inhaled colistin for the treatment of respiratory tract infections in patients.
The aim of the present study was to identify the most predictive PK/PD index of colistin that best describes its antimicrobial efficacy against P. aeruginosa following pulmonary delivery in a mouse lung infection model and to determine the target values of the PK/PD index to achieve various killing effects. Since cough and bronchospasm have been reported following nebulization of antibiotics in clinical studies (16), the effect of inhaled colistin and its prodrug CMS on the histopathology of the lung tissue was also examined. MDR P. aeruginosa was chosen for this study, because it was listed by the Infectious Disease Society of America as one of the difficult-to-treat-pathogens that required urgent attention (17).
RESULTS
Pharmacokinetics of colistin following intratracheal and intravenous administration.
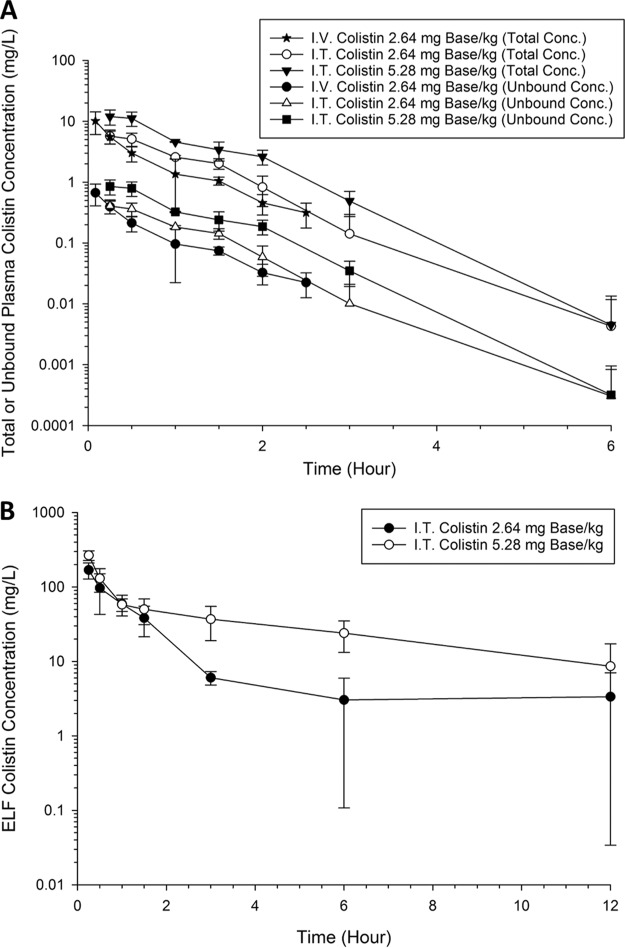
Figure 1A shows the total plasma colistin concentration-time profiles following intratracheal administration of 2.64 and 5.28 mg base/kg of body weight colistin and intravenous administration of 2.64 mg base/kg colistin. The average plasma unbound fraction of 0.084 (4) was employed to generate the corresponding time course profiles for unbound colistin (Fig. 1A). The derived PK parameters for total plasma concentration of colistin are summarized in Table 1. Rapid appearance of colistin in the plasma was observed after intratracheal administration of 2.64 and 5.28 mg base/kg colistin with a time to peak plasma concentration (Tmax) of approximately 30 min (Table 1). The maximum plasma concentration achieved following intratracheal delivery of 2.64 mg base/kg colistin was significantly lower than that after intravenous administration of the same dose (5.72 ± 1.43 versus 10.2 ± 4.11 mg/liter, respectively). Figure 1B shows the ELF colistin concentration as a function of time following intratracheal administration of 2.64 and 5.28 mg/kg colistin. Using urea as an endogenous marker (18), ELF concentration was calculated using an apparent volume of the ELF (VELF) of 0.022 ml and plasma-to-lavage urea dilution factor of 22.1. The derived PK parameters for colistin in ELF are summarized in Table 1.
FIG 1.
(A) Total and unbound plasma concentration versus time of colistin after administration of single intratracheal (I.T.) doses of 2.64 and 5.28 mg base/kg and intravenous (I.V.) administration of 2.64 mg base/kg in neutropenic lung-infected mice. (B) ELF colistin concentration versus time after intratracheal administration of 2.64 and 5.28 mg base/kg colistin in neutropenic lung-infected mice.
TABLE 1.
Pharmacokinetics parameters of colistin in ELF and plasmaa
| Parameter | Value after single dose |
||
|---|---|---|---|
| Intratracheal |
Intravenousc (2.64 mg base/kg) | ||
| 2.64 mg base/kg | 5.28 mg base/kg | ||
| ELF | |||
| Cmax,ELF (mg/liter) | 169b | 266b | ND |
| t1/2,ELF (min) | —d | 252 | ND |
| AUCELF (mg · h/liter) | 181 | 465 | ND |
| Plasma | |||
| Cmax (mg/liter) | 5.72 | 13.1 | ND |
| Tmax (min) | 30 | 30 | NA |
| CL/F (ml/min/kg) | 5.37 | 5.70 | 7.46 |
| V/F (ml/kg) | 181 | 284 | 400 |
| T1/2 (min) | 23.4 | 34.5 | 37.1 |
Shown are pharmacokinetics parameters of colistin in ELF and plasma following intratracheal administration of colistin at 2.64 and 5.28 mg/kg and intravenous administration of colistin at 2.64 mg base/kg (n = 3 or more).
Concentration observed at the first time point.
ND, not determined; NA, not applicable.
—, cannot be reliably estimated.
Relationship between PK/PD index and antibacterial efficacy.
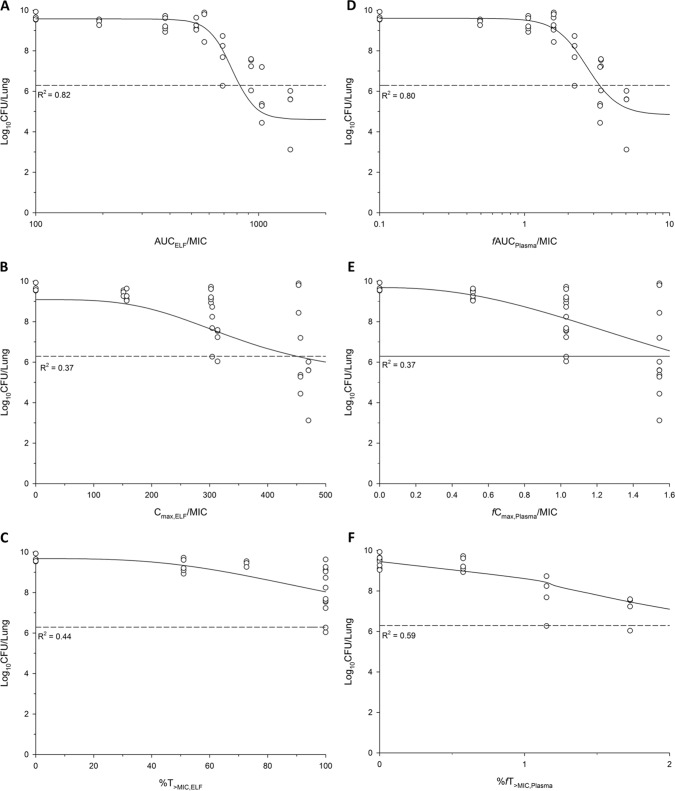
For the untreated growth control group, the bacterial burden increased by 3.50 ± 1.50, 2.92 ± 0.44, and 1.90 ± 1.15 log10/lung over 24 h for ATCC 27853, PAO1, and FADDI-PA022, respectively. For the colistin-treated group, a dose-dependent killing was observed across all three strains. The fAUC/MIC in the ELF and plasma following intratracheal administration of colistin provided the best description of killing effect, with the highest R2 value for all three strains (Fig. 2 and Table 2).
FIG 2.
Sigmoid inhibitory fit for PAO1 between the log10 CFU/lung at 24 h and the PK/PD indices for ELF, namely, AUCELF/MIC (A), Cmax, ELF/MIC (B), and T>MIC, ELF (C), and for plasma, namely, fAUCPlasma/MIC (D), fCmax, Plasma/MIC (E), and fT>MIC, Plasma (F). R2 is the coefficient of determination. The broken line represents the mean bacterial burden in lungs at the start of treatment.
TABLE 2.
PK/PD model parameters for AUC/MIC of colistin in ELF and fAUC/MIC in plasma against three strains of P. aeruginosa after inhalation
| Strain | Value (%) fora: |
||||
|---|---|---|---|---|---|
| Emax (log10 CFU/Lung) | E0 (log10 CFU/Lung) | EI50 | γ | R2 | |
| ELF | |||||
| ATCC 27853 | 4.45 (23.9) | 8.05 (4.20) | 936 (7.10) | 10.0 (83.8) | 0.60 |
| PAO1 | 4.88 (16.9) | 9.56 (2.62) | 846 (8.81) | 5.14 (32.0) | 0.82 |
| FADDI-PA022 | 3.95 (11.0) | 9.20 (2.46) | 672 (5.48) | 7.71 (36.7) | 0.84 |
| Plasma | |||||
| ATCC 27853 | 4.00 (21.2) | 7.99 (4.0) | 2.65 (12.1) | 10.0 (69.6) | 0.64 |
| PAO1 | 4.79 (18.9) | 9.60 (2.9) | 2.70 (12.5) | 4.00 (34.6) | 0.80 |
| FADDI-PA022 | 3.88 (11.7) | 9.16 (2.6) | 2.09 (7.4) | 6.21 (37.1) | 0.83 |
E0 is the effect in the absence of colistin treatment, Emax is the maximal effect, EI50 is the value of fAUC/MIC required to achieve 50% of Emax, and γ is the Hill coefficient. Data in parentheses are the percentages relative to the standard errors.
Targets of fAUC/MIC associated with various magnitudes of antibacterial effect.
Table 3 shows the values of fAUC/MIC in the ELF and plasma to achieve stasis and 1- and 2-log10 reductions in the lung bacterial load. For all three strains, the fAUC/MIC in the ELF and plasma required to achieve stasis were 684 to 1,050 and 2.15 to 3.29, respectively. A 2-log10 reduction was unable to be achieved for all three strains, even at the maximum tolerated dose. Relatively low interstrain variability was observed for intratracheal administration, and FADDI-PA022 was the most susceptible strain, even though all had the same MIC of 1 mg/liter. The fAUC/MIC required to achieve stasis and 1-log10 reduction for FADDI-PA022 were approximately 2-fold lower than the fAUC/MIC of the other two strains (Table 3).
TABLE 3.
Target values of colistin AUC/MIC in ELF and fAUC/MIC in plasmaa
| Strain | Target colistin fAUC/MIC value to achieveb: |
||
|---|---|---|---|
| Stasis | 1-log10 kill | 2-log10 kill | |
| fAUCPlasma/MIC | |||
| ATCC 27853 | 2.99 (2.62–3.27) | ND | ND |
| PAO1 | 3.29 (2.90–3.91) | 4.68 (3.48–5.23) | ND |
| FADDI-PA022 | 2.15 (2.02–2.31) | 2.60 (2.39–2.97) | ND |
| AUCELF/MIC | |||
| ATCC 27853 | 1,050 (931–1,134) | ND | ND |
| PAO1 | 971 (890–1,101) | 1,236 (1,033–1,403) | ND |
| FADDI-PA022 | 684 (653–723) | 796 (751–882) | ND |
Shown are target values of colistin AUC/MIC in ELF and fAUC/MIC in plasma to achieve stasis and 1- and 2-log10 kills against all three strains of P. aeruginosa in the lung infection model.
Values in parentheses are the IQR. ND, not determined.
Histopathological examination of lungs in healthy and infected mice with inhaled colistin.
Table S2 in the supplemental material present the lung histopathological examination and corresponding semiquantitative scores (SQS) following intratracheal administration of CMS and colistin in healthy mice. In the saline-treated control group, no marked injury was observed, with an average SQS of +0.50 (Fig. S1). Inflammation in the lung epithelium was evident for the CMS- and colistin-treated mice, and the severity of inflammation was dose dependent. The most effective inhaled colistin dosage regimen (7.92 mg base/kg thrice daily) resulted in mild to moderate inflammation with infiltration of inflammatory cells (SQS = +2.00). Across all dosage regimens, inhaled CMS resulted in substantially less inflammation than with inhaled colistin (Table S2).
In the infected mice, no significant changes to the lung epithelial cells were identified at 4 h in both the untreated and treated groups (Table S2). However, the histopathological examination revealed that at 26 h postbacterial infection (equivalent to 24 h posttreatment), the infected lungs had necrosis and alveolar inflammation with infiltration of polymorphonuclear cells (Fig. 3). For the intratracheally administered group (2.64 mg base/kg once daily, 5.28 mg base/kg thrice daily, and 7.92 mg base/kg once, twice, and thrice daily), these inflammatory changes in the lung were markedly reduced, and the lung epithelium was mostly preserved (Table S2).
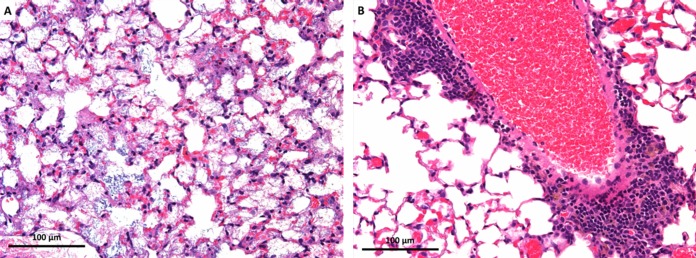
FIG 3.
Representative images of lung sections from histopathological examination of P. aeruginosa-infected mice. (A) Mouse lung from the growth control group showing interstitial necrosis and intra-alveolar flocculus material (severe damage and inflammation; SQS of >+5) (×20 magnification). (B) Mouse lung harvested at 24 h after colistin (2.64 mg/kg thrice daily) with perivascular inflammation (mild damage; SQS of >+1) (×20 magnification). The degree of inflammation and changes may not be consistent across the entire lung tissue, and these images only show significant changes observed.
DISCUSSION
Recent animal and clinical studies indicate that parenteral CMS is suboptimal in treating respiratory tract infections due to limited exposure at the infection site (19). In the mouse lung infection model following subcutaneous administration of colistin, the fAUC/MIC required to achieve stasis for P. aeruginosa was 15.2 to 38.6 (4), which is approximately 10 times higher than those after inhaled colistin (i.e., 2.15 to 3.29) (Table 3). Importantly, nephrotoxicity is a dose-limiting factor, and simply increasing the dose of parenteral CMS is not feasible (20). Over the past few decades, inhaled CMS has become a key practice for the treatment of pulmonary infections in patients with cystic fibrosis (10, 21). However, the majority of the PK/PD studies for CMS/colistin are based on systemic administration (4), which cannot be extrapolated to inhaled CMS. Our study is the first that investigated the PK/PD of pulmonary delivery of colistin in neutropenic lung-infected mice. We are the first to identify that fAUC/MIC in ELF and plasma is the most predictive PK/PD index after inhalation (Fig. 2 and Table 2) and determined the magnitudes of fAUC/MIC in ELF and plasma required to achieve stasis and 1- and 2-log10 killing following pulmonary delivery (Table 3). Although colistin is administered in the form of an inactive product, CMS in clinical practice, colistin was used here because the antimicrobial efficacy of nebulized CMS depends on the exposure of colistin rather than CMS (22). The PK/PD of CMS was not examined in the current study, and CMS was only used in the histology study.
The peak plasma concentration (Cmax) of colistin (5.72 mg/liter) after intratracheal administration was significantly lower than that after intravenous administration (10.2 mg/liter) of the same dose (2.64 mg base/kg). However, at 15 to 30 min posttreatment, plasma concentrations of colistin were similar between intravenous and intratracheal administration (Fig. 1A). Colistin was rapidly absorbed into the systemic circulation with an average bioavailability of approximately 100%, which was significantly higher than the 31 to 69% observed in rats (8, 23). Interestingly, the bioavailability following intratracheal administration of CMS in rats was also 100% (6), which was significantly higher than that observed in humans (7.93% ± 4.26% and 5.37% ± 1.36%, respectively, for 1 and 2 million IU CMS) (9). This low systemic absorption observed in humans highlights the targeting advantage of intratracheal delivery of CMS considering nephrotoxicity and neurotoxicity (9). The significantly higher bioavailability of colistin observed in mice was most likely attributed to the mechanism that was involved in the uptake of the antibiotic by the bronchial epithelium. Diffusion of colistin across the bronchial epithelium was suggested to be driven predominantly by passive diffusion (6). However, recent studies have demonstrated the potential role of specific drug transporters in the absorption of colistin. A number of drug transporters, such as P-glycoprotein (P-gp), organic cation transporters (OCTs), and peptide transporters (PEPTs), have been identified in lung tissue such as airway epithelial cells and endothelium of small blood vessels (8, 25, 26). PEPTs play an important role in transporting endogenous peptides and peptide-like drugs in pulmonary epithelial cells and have two important isomembers, PEPT1 and PEPT2 (26). PEPT2-mediated cellular uptake of colistin has been identified in human embryonic kidney 293 cells (26). Expression of PEPT2 mRNA in mouse lungs was approximately 2-fold higher than that in rat lungs (27), which corresponds well to the higher bioavailability observed in our current mouse study than those reported in rats (8, 23). OCTs are incapable of transporting colistin (25, 26) and were not expressed in rodent lungs (26). Although P-gp is highly expressed in rodent lungs (25), it was unlikely to affect the uptake of colistin, as colistin is not a P-gp substrate (28). Studies are being conducted in our group to characterize the function of PEPT2 in the transport of colistin across the bronchial epithelium.
For PK/PD analysis, it is important to quantify colistin exposure in the ELF, because the plasma colistin exposure may differ from the exposure in infected lungs following intratracheal administration (29). As the total protein concentration in ELF was estimated to be only 6 to 12% of the plasma protein concentration in humans and animals, the protein binding of colistin in ELF was negligible, and the measured total ELF concentration was assumed to be equivalent to the free colistin concentration in ELF (30). As a cationic polypeptide, colistin has the potential to interact with pulmonary surfactants (31) in the ELF and mucin in the sputum (32) via electrostatic interaction. Previously, in an in vitro static time-kill model, the antimicrobial activity of colistin was significantly impaired by pulmonary surfactants (31). However, this inhibitory effect of pulmonary surfactant was found to be reversible at high colistin concentration (>64× MIC). Since the observed ELF concentrations in the current study after inhalation were >64× MIC, the interaction between colistin and pulmonary surfactant was assumed to be negligible. On the contrary, following intravenous administration, colistin exposure in the ELF was minimal, and below 2.21 mg/liter (limit of quantification [LOQ]), the inhibitory effect of pulmonary surfactant is dramatic and should be considered (32).
A significant finding in our PK study was that, following intratracheal delivery (5.28 mg base/kg), the terminal half-life of colistin in ELF (4.2 h) was approximately 7-fold longer than that in plasma (0.57 h), and the colistin concentrations were quantifiable (2.21 mg/liter) for up to 12 h in ELF compared to only 6 h in plasma (Fig. 1 and Table 1). This rate-limiting disposition kinetics suggests that following rapid absorption of colistin into the plasma, a certain portion of the colistin dose was retained in the lung ELF. Colistin is an amphipathic drug with five primary amine groups, and it has the capacity to remain in the lungs due to the electrostatic charge with the negatively charged phospholipids of the cell membrane, such as alveolar macrophages (33, 34) and the alveolar basement membrane (35). The alveolar macrophages and colistin complexes could act like a reservoir of colistin for slow release into the systemic circulation. In addition to the binding of colistin to the alveolar macrophages, a high colistin dose (>5.28 mg/kg) administered could saturate PEPT2 and retard the absorption across the bronchial epithelium (26). Despite the evidence demonstrating the retention of colistin in the lung following intratracheal administration (>5.28 mg/kg), the exact mechanism for colistin retention in the lung is not well known and further studies are warranted.
Similar to the animal PK/PD studies after parenteral administration (4), fAUC/MIC in the ELF and plasma following intratracheal administration of colistin provides the best correlation with the PD data (Fig. 2 and Table 3). The antibacterial effect of colistin on the P. aeruginosa strains following inhalation is much more effective than that by systemic administration (4). For example, to achieve antibacterial stasis for P. aeruginosa ATCC 27853, the plasma fAUC/MIC required following subcutaneous administration (34.1) (4) are 11-fold higher than that required following intratracheal administration (2.99). Both PK/PD breakpoints in ELF and plasma could possibly be used translationally to optimize inhalation doses in clinical settings. However, these values need to be used with the recognition of the design of the animal study. First, the expression of the potential colistin transporter PEPT2 in the lungs in mice is different from that in humans (25), and the colistin plasma unbound fraction in mouse (0.084) is ∼6-fold higher in humans (0.50) (4). Second, direct pulmonary administration of colistin using the MicroSprayer 1A-1B is different from nebulization in humans. The MicroSprayer 1A-1B has droplet diameters of around 10 μm (6), which could be larger than the droplets generated by the nebulization systems in clinics, and the diameters of mouse airways are much smaller than those of humans (6). Similar to the systemic PK/PD study in mice (4), relatively low interstrain variability was observed across all three strains. The fAUC/MIC values required to achieve stasis and 1-log10 killing for all three P. aeruginosa strains were approximately within a 2-fold range (Table 3). For all three strains of P. aeruginosa, it is not possible to achieve a 2-log10 reduction because of the relatively low maximum tolerance dose of colistin aerosols (8.25 mg base/kg) and a cumulative dose of 25 mg base/kg. This moderate net killing following treatment with inhaled colistin was proposed to be due to the presence of multiple purulent plugs obstructing bronchioles, thereby preventing colistin from accessing the distal lung (36). This suggests that the combination of inhaled colistin with intravenous therapy is required for difficult-to-treat lung infections.
We examined the potential lung toxicity of colistin and its prodrug, CMS, following intratracheal administration to healthy mice using histopathological examination (see Table S2 in the supplemental material). Across all dosage regimens, inhaled CMS caused substantially less damage than inhaled colistin. This finding is consistent with the observation in humans in which a significant reduction in pulmonary function was observed in patients who received nebulized colistin compared to those who received nebulized CMS (37). The therapeutic efficacy of inhaled colistin in treating respiratory tract infections is also supported by the histopathological results (Table S2). In the colistin-treated groups (2.64 mg base/kg once daily, 5.28 mg base/kg thrice daily, and 7.92 mg base/kg once, twice, and thrice daily), inflammation with polymorphonuclear cell infiltrate over 24 h was substantially reduced, and lung epithelial cells were not considerably damaged (Fig. 3 and Table S2). Bacterium-induced tissue necrosis, inflammation, and cellular damage are usually associated with lipopolysaccharide (LPS), an outer membrane component of Gram-negative bacteria (38). Inhaled colistin suppresses the LPS activity of Gram-negative organisms in a dose-dependent manner (38), which corresponds well to the histopathological results in the current study (Fig. 3 and Table S2).
In conclusion, our study is the first to identify fAUC/MIC in both ELF and plasma as the most predictive PK/PD index for intratracheal delivery of colistin in a mouse lung infection model against P. aeruginosa, and we determined the fAUC/MIC targets in ELF and plasma for achieving various magnitudes of bacterial kill. The potential advantage of intratracheal administration of colistin is evident for treatment of respiratory tract infections in terms of PK/PD and safety. Importantly, inhaled colistin significantly improved the lung condition by reducing not only the lung bacterial load but also infection-caused lung inflammation. Our study provides key pharmacological information for optimized clinical use of aerosolized colistin against infections caused by P. aeruginosa.
MATERIALS AND METHODS
Chemicals and bacterial strains.
Colistin sulfate (lot 08M1526V; ≥15,000 U/mg) and sodium colistin methanesulfonate (lot 04M1526V; ≥11,500 U/mg) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and solutions were freshly prepared in saline (4, 39, 40). Three strains of P. aeruginosa were examined, ATCC 27853, PAO1, and FADDI-PA022 (MIC of 1 mg/liter for all strains). P. aeruginosa was freshly subcultured from −80°C stock prior to the experiment (4, 39). MICs were determined using the broth microdilution method in cation-adjusted Mueller-Hinton broth (CAMHB) (41).
Animals.
All animal experiments (see Table S1 in the supplemental material) were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee before the experiment was conducted. Pathogen-free, female Swiss mice (8 to 10 weeks old; 25 to 35 g) were obtained from Monash Animal Services (Clayton, Victoria, Australia) and were handled, fed, and housed according to the criteria of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Food and water were available ad libitum.
Neutropenic mouse lung infection model.
A neutropenic mouse lung infection model was employed in the current study (4, 42). In brief, mice were rendered neutropenic by intraperitoneal injection of cyclophosphamide (Baxter Healthcare Pty Ltd., New South Wales, Australia). Bacterial infection was established as described in previous studies (4, 42). Mice were briefly anesthetized via placement into the isoflurane induction chamber. The anesthetized mouse was placed on a Perspex support in a vertical upright position, which allowed the mouse to be temporarily immobilized. A MicroSprayer (model IA-1C; Penn-Century, Philadelphia, PA, USA) (43) was used to deliver 25 μl of bacterial suspension (approximately 106 bacterial cells in early logarithmic phase) directly into the trachea. Mice were maintained in the upright position for approximately 1 to 2 min and then placed onto a warm pad for rapid recovery.
Pharmacokinetics of inhaled colistin in neutropenic infected mice.
Single-dose PK studies (Table S1) were performed in neutropenic lung-infected mice after intratracheal delivery (25 μl) of colistin (2.64 and 5.28 mg base/kg) and intravenous administration (50 μl) of colistin (2.64 mg base/kg). Intratracheal delivery of colistin was performed as described above. Animals (3 or 4 per group) were humanely killed at 15, 30, and 60 min and 2, 4, 6, and 12 h postdose. Terminal blood and bronchoalveolar lavage (BAL) samples were collected from each animal. BAL samples were collected by lavaging the lung with 0.5 ml of 0.9% saline twice per animal, and for each animal the two washings during the BAL sampling were pooled. Samples from each animal were stored separately and analyzed individually. Concentrations of colistin in the plasma and BAL samples from different animals were determined by a validated reversed-phase high-performance liquid chromatography (HPLC) method, with minor modifications (44, 45). Plasma and BAL samples were deproteinized with acetonitrile (1:2 dilution) and centrifuged at 18,210 × g. The calibration range was 0.10 to 10.0 mg/liter for plasma and 0.10 to 6.0 mg/liter for lavage samples. The limit of quantification (LOQ) was 0.10 mg/liter for both plasma and BAL samples. The interday and intraday reproducibility and accuracy of the HPLC assay of plasma and BAL samples were within 12% and 14.7%, respectively.
The apparent volume of the ELF (VELF) was determined using urea as an endogenous dilution marker (18). Urea concentrations in BAL and plasma samples were determined using a QuantiChrom urea assay kit (BioAssay Systems, California, USA). VELF was calculated as ([Urea]BAL/[Urea]Plasma) × VBAL, where [Urea]BAL and [Urea]Plasma are urea concentrations (in micrograms per deciliter) in the plasma and BAL fluid, respectively, and VBAL is the recovered BAL fluid volume.
Colistin concentration in ELF was determined for each animal and was calculated by multiplying the colistin concentration in the BAL fluid by the ratio of the urea concentration in the plasma and BAL fluid: [colistin]ELF = [colistin]BAL × ([Urea]Plasma/[Urea]BAL).
Pharmacokinetic/pharmacodynamic indices for inhaled colistin.
To identify the most predictive PK/PD index for inhaled colistin, neutropenic mice infected with P. aeruginosa were treated with colistin at approximately 2 h postbacterial inoculation (4). The colistin regimens involved intratracheal administration of colistin over a total daily dose range of 2.64 to 23.8 mg base/kg, including once-daily 2.64, 5.28, and 7.92 mg base/kg, twice-daily 5.28 and 7.92 mg base/kg, and thrice-daily 2.64, 5.28, and 7.92 mg base/kg (n = 3 or more animals for each group). Mice were randomly allocated into each group. Lungs from each animal were aseptically collected at 24 h posttreatment and homogenized in 8 ml saline. Viable counting was conducted and the bacterial load was expressed as the log10 CFU per lung (4).
Histopathology of lungs after pulmonary delivery of colistin and its prodrug, CMS.
Histopathological examination was performed after treatment with inhaled colistin in the infected mice and after inhaled colistin or CMS in healthy noninfected mice (n = 3 animals or more for each group) (Table S1). The dosage regimens involved once-, twice-, and thrice-daily administration of colistin at 2.64, 5.28, and 7.92 mg base/kg. For CMS, an equal molar dose was administered using the same regimens as those of colistin doses described above. Mice were euthanized, and lungs were harvested at 24 h and fixed in formalin immediately (7). BAL fluid examination was not performed to avoid any potential effect on the lung epithelial cells. Five layers at 5 μm each were sliced and stained with hematoxylin and eosin for histopathological examination (Australian Phenomics Network Histopathology and Organ Pathology Service, Victoria, Australia).
To quantify the extent of lung damage, a previous rating system was adapted (46) and the following semiquantitative scores (SQS) were assigned: 0, no significant change; +1, mild damage; +2, mild to moderate damage; +3, moderate damage; +4, moderate to severe damage; and +5, severe damage.
Data analysis.
The unbound colistin plasma concentration in the single-dose PK study was calculated using the unbound fraction reported by Cheah et al. (4). The plasma concentration-time profile was subjected to noncompartmental analysis to generate PK parameters (Phoenix WinNonlin software, version 6.3; Pharsight Corporation, Princeton, NJ, USA). The principle of superposition was applied to the single-dose PK concentration-time profile to obtain corresponding concentration-time profiles for multiple dosage regimens (4, 39). Subsequently, the inhibitory sigmoid dose-effect model was employed to calculate the PK/PD indices for plasma and ELF, i.e., the ratio of the area under the unbound concentration-time profile to MIC (fAUC/MIC), the ratio of unbound peak plasma concentration to MIC (fCmax/MIC), and the percentage of time that the unbound plasma concentration exceeded the MIC (%fT>MIC). Various killing magnitudes of the most predictive PK/PD index were determined as previously described (39). Monte Carlo simulation (Crystal Ball Professional V7.2.2 software; n = 10,000) was employed to obtain the interquartile range (IQR) for the target PK/PD index.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from a National Health and Medical Research Council (NHMRC) Project Grant (APP1065046). J.L. is supported by research grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI098771 and R01 AI111965). J.L. is an Australian NHMRC Senior Research Fellow. Y.-W.L. is a recipient of an Australian Postgraduate Award.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. This study utilized the Australian Phenomics Network Histopathology and Organ Pathology Service, University of Melbourne.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02025-16.
REFERENCES
- 1.Geller DE. 2009. Aerosol antibiotics in cystic fibrosis. Respir Care 54:658–670. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed AF, Cars O, Friberg LE. 2014. A pharmacokinetic/pharmacodynamic model developed for the effect of colistin on Pseudomonas aeruginosa in vitro with evaluation of population pharmacokinetic variability on simulated bacterial killing. J Antimicrob Chemother 69:1350–1361. doi: 10.1093/jac/dkt520. [DOI] [PubMed] [Google Scholar]
- 3.Agrafiotis M, Siempos I, Ntaidou T, Falagas M. 2011. Attributable mortality of ventilator-associated pneumonia: a meta-analysis. Int J Tuberc Lung Dis 15:1154–1163. doi: 10.5588/ijtld.10.0498. [DOI] [PubMed] [Google Scholar]
- 4.Cheah S-E, Wang J, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 6.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier J-C, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother 54:3702–3707. doi: 10.1128/AAC.00411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yapa SW, Li J, Porter CJ, Nation RL, Patel K, McIntosh MP. 2013. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother 57:5087–5095. doi: 10.1128/AAC.01127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gontijo AVL, Grégoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob Agents Chemother 58:3950–3956. doi: 10.1128/AAC.02819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yapa SW, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen C, Pressler T, Høiby N. 2008. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros 7:523–530. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Döring G, Conway S, Heijerman H, Hodson M, Høiby N, Smyth A, Touw D, Committee C. 2000. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 16:749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakwan N, Lertpichaluk P, Chokephaibulkit K, Villani P, Regazzi M, Imberti R. 2015. Pulmonary and systemic pharmacokinetics of colistin following a single dose of nebulized colistimethate in mechanically ventilated neonates. Pediatr Infect Dis J 34:961–963. doi: 10.1097/INF.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin M-H, Le Naour G, Marquette C-H. 2010. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 36:1147–1155. doi: 10.1007/s00134-010-1879-4. [DOI] [PubMed] [Google Scholar]
- 14.Chiang S-R, Chuang Y-C, Tang H-J, Chen C-C, Chen C-H, Lee N-Y, Chou C-H, Ko W-C. 2009. Intratracheal colistin sulfate for BALB/c mice with early pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Crit Care Med 37:2590–2595. doi: 10.1097/CCM.0b013e3181a0f8e1. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Velkov T, Rahim NA, Zhou QT, Chan H-K, Li J. 2015. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev 85:65–82. doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 18.Rennard S, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin P, Crystal R. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. [DOI] [PubMed] [Google Scholar]
- 19.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. [DOI] [PubMed] [Google Scholar]
- 20.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48:1724–1728. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 21.Jensen T, Pedersen SS, Garne S, Heilmann C, Hoiby N, Koch C. 1987. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother 19:831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 22.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yapa SW. 2013. Ph.D. thesis. Monash University, Melbourne, Victoria, Australia. [Google Scholar]
- 24.Reference deleted.
- 25.Bosquillon C. 2010. Drug transporters in the lung–do they play a role in the biopharmaceutics of inhaled drugs? J Pharm Sci 99:2240–2255. doi: 10.1002/jps.21995. [DOI] [PubMed] [Google Scholar]
- 26.Lu X, Chan T, Xu C, Zhu L, Zhou QT, Roberts KD, Chan H-K, Li J, Zhou F. 2016. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J Antimicrob Chemother 71:403–412. doi: 10.1093/jac/dkv340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Klaassen C. 2006. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides 27:850–857. doi: 10.1016/j.peptides.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Li J, Nation RL, Nicolazzo JA. 2011. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob Agents Chemother 55:502–507. doi: 10.1128/AAC.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boisson M, Jacobs M, Grégoire N, Gobin P, Marchand S, Couet W, Mimoz O. 2014. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 58:7331–7339. doi: 10.1128/AAC.03510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwameis R, Erdogan-Yildirim Z, Manafi M, Zeitlinger M, Strommer S, Sauermann R. 2013. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother 57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang JX, Blaskovich MA, Pelingon R, Ramu S, Kavanagh A, Elliott AG, Butler MS, Montgomery AB, Cooper MA. 2015. Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother 59:5925–5931. doi: 10.1128/AAC.00808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunin CM, Bugg A. 1971. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J Infect Dis 124:394–400. doi: 10.1093/infdis/124.4.394. [DOI] [PubMed] [Google Scholar]
- 34.Bysani GK, Stokes DC, Fishman M, Shenep JL, Hildner WK, Rufus K, Bradham N, Costlow ME. 1990. Binding of polymyxin B to rat alveolar macrophages. J Infect Dis 162:939–943. doi: 10.1093/infdis/162.4.939. [DOI] [PubMed] [Google Scholar]
- 35.Brody JS, Vaccaro CA, Hill NS, Rounds S. 1984. Binding of charged ferritin to alveolar wall components and charge selectivity of macromolecular transport in permeability pulmonary edema in rats. Circ Res 55:155–167. doi: 10.1161/01.RES.55.2.155. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette C-H, Rouby J-J. 2002. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med 166:1375–1381. doi: 10.1164/rccm.200204-363OC. [DOI] [PubMed] [Google Scholar]
- 37.Westerman EM, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. 2004. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: a pilot study. J Cyst Fibros 3:23–28. doi: 10.1016/j.jcf.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, Yamaguchi K. 2009. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 63:534–542. doi: 10.1093/jac/dkn530. [DOI] [PubMed] [Google Scholar]
- 39.Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 54:1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudhani RV, Turnidge JD, Nation RL, Li J. 2010. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother 65:1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: 23rd information supplement M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 42.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2012. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother 56:2683–2690. doi: 10.1128/AAC.06486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bivas-Benita M, Zwier R, Junginger HE, Borchard G. 2005. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm 61:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother 46:3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 761:167–175. doi: 10.1016/S0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 46.Roberts KD, Azad MA, Wang J, Horne AS, Thompson PE, Nation RL, Velkov T, Li J. 2015. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infect Dis 1:568–575. doi: 10.1021/acsinfecdis.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.