ABSTRACT
The clinical and economic impacts of bloodstream infections (BSI) due to multidrug-resistant (MDR) Gram-negative bacteria are incompletely understood. From 2009 to 2015, all adult inpatients with Gram-negative BSI at our institution were prospectively enrolled. MDR status was defined as resistance to ≥3 antibiotic classes. Clinical outcomes and inpatient costs associated with the MDR phenotype were identified. Among 891 unique patients with Gram-negative BSI, 292 (33%) were infected with MDR bacteria. In an adjusted analysis, only history of Gram-negative infection was associated with MDR BSI versus non-MDR BSI (odds ratio, 1.60; 95% confidence interval [CI], 1.19 to 2.16; P = 0.002). Patients with MDR BSI had increased BSI recurrence (1.7% [5/292] versus 0.2% [1/599]; P = 0.02) and longer hospital stay (median, 10.0 versus 8.0 days; P = 0.0005). Unadjusted rates of in-hospital mortality did not significantly differ between MDR (26.4% [77/292]) and non-MDR (21.7% [130/599]) groups (P = 0.12). Unadjusted mean costs were 1.62 times higher in MDR than in non-MDR BSI ($59,266 versus $36,452; P = 0.003). This finding persisted after adjustment for patient factors and appropriate empirical antibiotic therapy (means ratio, 1.18; 95% CI, 1.03 to 1.36; P = 0.01). Adjusted analysis of patient subpopulations revealed that the increased cost of MDR BSI occurred primarily among patients with hospital-acquired infections (MDR means ratio, 1.41; 95% CI, 1.10 to 1.82; P = 0.008). MDR Gram-negative BSI are associated with recurrent BSI, longer hospital stays, and increased mean inpatient costs. MDR BSI in patients with hospital-acquired infections primarily account for the increased cost.
KEYWORDS: Gram negative, bloodstream infection, cost
INTRODUCTION
Antibiotic resistance has been associated with multiple poor outcomes, including increased mortality, length of hospital stay, and health care costs. For example, it has been estimated that by 2050 resistance will be responsible for 300 million deaths and drain up to $100 trillion from the world's gross domestic product (GDP) (1). Gram-negative bacteria are particular drivers of this trend toward increased antibiotic resistance.
The clinical and economic effects of antibiotic resistance can be challenging to study across different bacteria due to variability in intrinsic and acquired antibiotic resistance phenotypes. In order to harmonize definitions used to classify bacterial antibiotic resistance, a group of international experts were brought together through the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) to create a standardized international terminology to describe bacterial antibiotic resistance profiles (2). One such bacterial antibiotic resistance profile that emerged from this work was the multidrug-resistant (MDR) phenotype. A bacterium is defined as MDR if “the isolate is nonsusceptible to at least one agent in greater than or equal to three antimicrobial categories.” These “antimicrobial categories” differ for each bacterial species (2).
Using this defined antibiotic resistance phenotype, the aim of this study was to better understand the clinical and economic impacts of antibiotic resistance in a large cohort of prospectively enrolled patients with Gram-negative bloodstream infections (BSI). In this study, we describe the demographic differences between patients with MDR versus non-MDR BSI, factors associated with acquisition of MDR BSI, factors associated with increased in-hospital mortality and poor clinical outcome, and the impact of MDR BSI on in-hospital mortality and inpatient costs in a large, prospectively enrolled cohort of patients with Gram-negative BSI.
RESULTS
Patient demographics.
A total of 891 patients with monomicrobial MDR BSI at Duke University Medical Center were included in the current analysis (Table 1). Of these 891 patients, 292 patients (33%) had BSI due to MDR pathogens. Compared to patients with BSI due to non-MDR bacteria, patients with MDR BSI were significantly more likely to have a history of transplant (19% versus 13%; P = 0.02), a history of a prior Gram-negative infection (46% versus 33%; P = 0.0003), and hospital-acquired infection (35% versus 28%; P = 0.05).
TABLE 1.
Baseline characteristics for patients with bloodstream infections caused by MDR and non-MDR Gram-negative bacteria
| Clinical parameter | Valuea |
P valueb | ||
|---|---|---|---|---|
| Overall (n = 891) | Non-MDR (n = 599) | MDR (n = 292) | ||
| Age | ||||
| Mean (SD) | 61.9 (15.5) | 61.8 (16.2) | 62.3 (13.8) | 0.63 |
| Median (IQR) | 64.0 (53.0–72.0) | 64.0 (52.0–73.0) | 63.0 (55.0–72.0) | |
| Male gender | 485 (54.4) | 331 (55.3) | 154 (52.7) | 0.48 |
| Race | ||||
| White | 603 (67.7) | 419 (69.9) | 184 (63.0) | 0.11 |
| Black | 247 (27.7) | 155 (25.9) | 92 (31.5) | |
| Other | 41 (4.6) | 25 (4.2) | 16 (5.5) | |
| Diabetes mellitus | 313 (35.1) | 198 (33.1) | 115 (39.4) | 0.06 |
| Rheumatoid arthritis | 22 (2.5) | 16 (2.7) | 6 (2.1) | 0.58 |
| Corticosteroids in the last 30 days | 238 (26.7) | 151 (25.2) | 87 (29.8) | 0.15 |
| History of transplant | 136 (15.3) | 80 (13.4) | 56 (19.2) | 0.02 |
| HIV positive | 18 (2.0) | 12 (2.0) | 6 (2.1) | 0.96 |
| History of neoplasm | 352 (39.5) | 232 (38.7) | 120 (41.1) | 0.50 |
| History of Gram-negative infection | 334 (37.5) | 200 (33.4) | 134 (45.9) | 0.0003 |
| Surgery in the 30 days prior to bacteremia | 204 (22.9) | 133 (22.2) | 71 (24.3) | 0.48 |
| Route of infection | ||||
| Hospital acquired | 269 (30.2) | 168 (28.0) | 101 (34.6) | 0.05 |
| Nonnosocomial health care associated | 472 (53.0) | 320 (53.4) | 152 (52.1) | |
| Non-health care associated | 150 (16.8) | 111 (18.5) | 39 (13.4) | |
| APACHE II acute physiology score, median (IQR) | 7.0 (4.0–10.0) | 7.0 (4.0–10.0) | 7.0 (4.0–10.0) | 0.84 |
| APACHE II chronic health score | ||||
| 0 | 241 (27.0) | 159 (26.5) | 82 (28.1) | 0.63 |
| 5 | 650 (73.0) | 440 (73.5) | 210 (71.9) | |
| Initial source of bacteremia | 298 (33.4) | 197 (32.9) | 101 (34.6) | 0.17 |
| Urine/pyelonephritis | ||||
| Line | 90 (10.1) | 60 (10.0) | 30 (10.2) | |
| Pneumonia | 84 (9.4) | 61 (10.2) | 23 (7.9) | |
| Biliary tract | 83 (9.3) | 53 (8.8) | 30 (10.3) | |
| Abscess | 44 (4.9) | 24 (4.0) | 20 (6.8) | |
| Wound | 29 (3.2) | 21 (3.5) | 8 (2.7) | |
| Cellulitis | 13 (1.5) | 13 (2.2) | 0 (0.0) | |
| Other | 99 (11.1) | 67 (11.2) | 32 (11.0) | |
| No known source identified | 151 (16.9) | 103 (17.2) | 48 (16.4) | |
Values are number (percent) unless otherwise indicated.
Boldface indicates significance.
The most commonly isolated Gram-negative bacteria were Escherichia coli (330/891 [37%]), Klebsiella pneumoniae (166/891 [19%]), and Pseudomonas aeruginosa (119/891 [13%]) (Table 2). The MDR phenotype was most common in E. coli (165/330 [50%]) and Citrobacter freundii (4/9 [44%]). Additional bacterial antibiotic resistance patterns are described in Table S1 in the supplemental material.
TABLE 2.
Gram-negative bacterial species causing bloodstream infections (n = 891) at Duke University Medical Center from 2009 to 2015
| Organism | No. (%)a of isolates |
||
|---|---|---|---|
| Non-MDR | MDR | Total | |
| Escherichia coli | 165 (50) | 165 (50) | 330 (37) |
| Klebsiella pneumoniae | 127 (77) | 39 (23) | 166 (19) |
| Pseudomonas aeruginosa | 86 (72) | 33 (28) | 119 (13) |
| Enterobacter species | 62 (70) | 27 (30) | 89 (10) |
| Serratia marcescens | 51 (94) | 3 (6) | 54 (6) |
| Proteus mirabilis | 28 (88) | 4 (13) | 32 (4) |
| Klebsiella oxytoca | 17 (77) | 5 (23) | 22 (2) |
| Acinetobacter species | 14 (78) | 4 (22) | 18 (2) |
| Salmonella species | 9 (82) | 2 (18) | 11 (1) |
| Morganella morganii | 8 (89) | 1 (11) | 9 (1) |
| Citrobacter freundii | 5 (56) | 4 (44) | 9 (1) |
| Other species | 28 (85) | 5 (15) | 33 (4) |
For non-MDR and MDR, the percentage of the particular species that is non-MDR or MDR is indicated. For the totals, the percentage of the particular species relative to all Gram-negative bloodstream infection isolates is indicated.
Risk factor analysis.
A multivariable logistic regression analysis of clinical factors associated with acquisition of MDR versus non-MDR BSI revealed that a history of any Gram-negative infection (odds ratio [OR], 1.60; 95% confidence interval [CI], 1.19 to 2.16; P = 0.002) was the only factor associated with higher odds of developing MDR BSI (Table 3).
TABLE 3.
Multivariable logistic regression analysis of clinical factors affecting acquisition of bloodstream infections caused by MDR versus non-MDR Gram-negative bacteria
| Parameter | Odds ratio | 95% confidence interval | P valuea |
|---|---|---|---|
| Age | 1.00 | 0.99–1.01 | 0.45 |
| Race, blackb | 1.38 | 0.99–1.94 | 0.06 |
| Race, otherb | 1.53 | 0.78–2.98 | 0.21 |
| Female gender | 1.06 | 0.79–1.41 | 0.72 |
| APACHE II acute physiology score | 1.00 | 0.97–1.03 | 0.91 |
| APACHE II chronic health score | 0.94 | 0.87–1.01 | 0.07 |
| Diabetes mellitus | 1.29 | 0.95–1.74 | 0.11 |
| Rheumatoid arthritis | 0.87 | 0.33–2.33 | 0.79 |
| Corticosteroid use in last 30 days | 1.06 | 0.71–1.59 | 0.78 |
| History of transplant | 1.50 | 0.92–2.46 | 0.10 |
| HIV positive | 1.14 | 0.40–3.21 | 0.81 |
| History of neoplasm | 1.30 | 0.95–1.78 | 0.11 |
| History of Gram-negative infection | 1.60 | 1.19–2.16 | 0.002 |
| Surgery in 30 days prior to bacteremia | 1.02 | 0.70–1.47 | 0.93 |
| Hospital-acquired vs community-acquired infectionc | 1.38 | 0.99–1.92 | 0.06 |
Boldface indicates significance.
The reference group for the race parameter is white patients.
Community acquired includes both nonnosocomial health care-associated and non-health care-associated bloodstream infections.
Clinical outcome analysis.
In the unadjusted analysis, patients with MDR BSI were more likely than patients with non-MDR BSI to have recurrent BSI (5/292 [1.7%] versus 1/599 [0.2%]; P = 0.02) and to have longer hospital stays (median days, 10.0 [interquartile range {IQR}, 5.5 to 23.0] versus 8.0 [4.0 to 17.0]; P = 0.0005). Differences in unadjusted in-hospital mortality (77/292 [26.4%] versus 130/599 [21.7%]; P = 0.12) and poor clinical outcome (168/292 [57.5%] versus 317/599 [52.9%]; P = 0.19) between patients with MDR and non-MDR BSI did not achieve statistical significance. Regression analyses of clinical factors associated with length of hospital stay and poor clinical outcome are described in Tables S2 and S3 in the supplemental material, respectively. In these adjusted analyses, the MDR phenotype was associated with increased length of hospital stay (mean ratio, 1.20; 95% CI, 1.06 to 1.35; P = 0.003) but not poor clinical outcome (OR, 1.21; 95% CI, 0.87 to 1.69; P = 0.26). In a multivariable logistic regression analysis of in-hospital mortality, age (OR, 1.03; 95% CI, 1.02 to 1.04; P < 0.0001), higher acute physiology and chronic health evaluation (II) (APACHE II) acute physiology score (OR, 1.23; 95% CI, 1.18 to 1.28; P < 0.0001), higher APACHE II chronic health score (OR, 1.26; 95% CI, 1.14 to 1.40; P < 0.0001), and hospital-acquired BSI (OR, 5.37; 95% CI, 3.54 to 8.15; P < 0.0001) were associated with increased mortality. The MDR phenotype was not associated with in-hospital mortality (OR, 1.28; 95% CI, 0.87 to 1.89; P = 0.22) (Table 4).
TABLE 4.
Multivariable logistic regression of in-hospital mortality among patients with hospital-acquired bloodstream infections caused by Gram-negative bacteria at Duke University Medical Center from 2009 to 2015
| Clinical variable | Odds ratio | 95% confidence interval | P valuea |
|---|---|---|---|
| MDR | 1.28 | 0.87–1.89 | 0.22 |
| Age | 1.03 | 1.02–1.04 | <0.0001 |
| Race, blackb | 0.89 | 0.57–1.40 | 0.63 |
| Race, otherb | 0.99 | 0.42–2.34 | 0.99 |
| Female gender | 0.91 | 0.62–1.33 | 0.64 |
| APACHE II acute physiology score | 1.23 | 1.18–1.28 | <0.0001 |
| APACHE II chronic health score | 1.26 | 1.14–1.40 | <0.0001 |
| Diabetes mellitus | 1.07 | 0.72–1.58 | 0.74 |
| Rheumatoid arthritis | 1.11 | 0.33–3.75 | 0.87 |
| Corticosteroid use in last 30 days | 1.15 | 0.70–1.89 | 0.59 |
| History of transplant | 0.78 | 0.42–1.44 | 0.42 |
| HIV positive | 0.73 | 0.15–3.56 | 0.69 |
| History of neoplasm | 0.91 | 0.61–1.35 | 0.63 |
| History of Gram-negative infection | 0.87 | 0.59–1.29 | 0.50 |
| Surgery in 30 days prior to bacteremia | 0.63 | 0.40–1.01 | 0.06 |
| Hospital-acquired vs community-acquired infectionc | 5.37 | 3.54–8.15 | <0.0001 |
Boldface indicates significance.
The reference group for the race parameter is white patients.
Community acquired includes both nonnosocomial health care-associated and non-health care-associated bloodstream infections.
Health care cost analysis in full cohort.
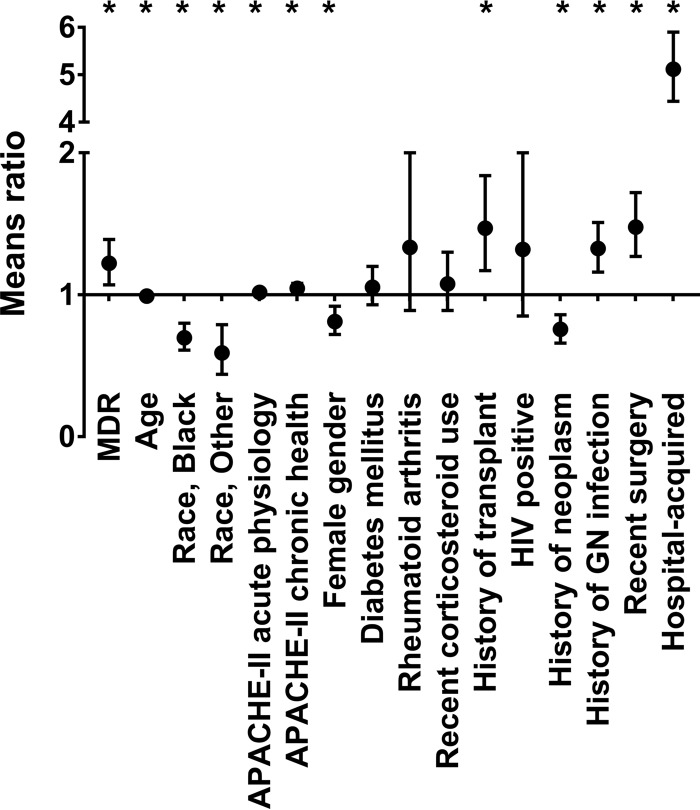
The mean inpatient cost for an episode of Gram-negative BSI was $43,929 (standard deviation [SD], $92,344), and the median cost was $12,939 (IQR, $6,205 to $38,278). The unadjusted mean inpatient cost was significantly higher for patients with MDR BSI than for patients with non-MDR BSI ($59,266 versus $36,452; P = 0.003). Using a generalized linear model (GLM), the relative increase in inpatient costs among patients with MDR Gram-negative BSI was 63% higher (mean ratio of MDR versus non-MDR 1.63; 95% CI, 1.37 to 1.94; P < 0.0001) than that for patients with non-MDR Gram-negative BSI. We also examined the relationship between MDR BSI and inpatient costs using a generalized linear model adjusted for patient characteristics. Inpatient costs for patients with MDR BSI were significantly higher than those for patients with non-MDR BSI (mean cost ratio for MDR versus non-MDR, 1.22; 95% CI, 1.07 to 1.39; P = 0.003). Decreased age (means ratio, 0.99; 95% CI, 0.99 to 1.00; P < 0.0001), higher APACHE II acute physiology score (means ratio, 1.02; 95% CI, 1.00 to 1.03; P = 0.01), higher APACHE II chronic physiology score (means ratio, 1.05; 95% CI, 1.01 to 1.08; P = 0.004), history of transplant (means ratio, 1.47; 95% CI, 1.17 to 1.84; P = 0.0008), history of Gram-negative infection (means ratio, 1.33; 95% CI, 1.16 to 1.51; P < 0.0001), surgery in past 30 days (means ratio, 1.48; 95% CI, 1.27 to 1.72; P < 0.0001), and hospital-acquired BSI (means ratio, 5.12; 95% CI, 4.44 to 5.90; P < 0.0001) were also associated with higher costs (Fig. 1). Female gender (means ratio, 0.83; 95% CI, 0.73 to 0.93; P = 0.002), black race (means ratio, 0.71; 95% CI, 0.61 to 0.81; P < 0.0001), and history of neoplasm (means ratio, 0.76; 95% CI, 0.67 to 0.86; P < 0.0001) were associated with lower costs. MDR BSI continued to be associated with higher inpatient costs than non-MDR BSI (means ratio, 1.18; 95% CI, 1.03 to 1.36; P = 0.01) after adjustment for appropriate empirical antibiotic therapy (see Table S4 in the supplemental material). However, when we adjusted the model for two other covariates, length of hospital stay and in-hospital mortality, the association between MDR BSI and increased inpatient cost was no longer statistically significant (means ratio, 1.07; 95% CI, 0.97 to 1.17; P = 0.17) (see Table S5 in the supplemental material).
FIG 1.
Factors associated with Gram-negative-bacterium bloodstream infection inpatient costs in a multivariable logistic regression model. A generalized linear model with gamma distribution and log link was adjusted for all listed patient/bacterial characteristics. In this model, a mean ratio of inpatient costs for MDR versus non-MDR of greater than 1 indicates higher inpatient costs. The mean ratio and 95% confidence intervals for each parameter are plotted. *, parameter that is statistically significant (P < 0.05). GN, Gram negative.
Health care cost analysis stratified by route of infection.
In patients with hospital-acquired BSI (n = 269), mean inpatient costs were higher in patients with MDR BSI ($136,945 versus $89,197; P = 0.02). In addition, the MDR phenotype was associated with increased inpatient costs after adjustment for patient characteristics (means ratio, 1.41; 95% CI, 1.10 to 1.82; P = 0.008) (Table 5). When we adjusted the model for two additional covariates, length of hospital stay and in-hospital mortality, the association between MDR BSI and increased inpatient cost was no longer statistically significant (means ratio, 1.09; 95% CI, 0.92 to 1.29; P = 0.32) (see Table S6 in the supplemental material).
TABLE 5.
Relative impact of MDR bacteria and other factors on inpatient costs among patients with hospital-acquired bloodstream infections caused by Gram-negative bacteria at Duke University Medical Center from 2009 to 2015
| Clinical variable | Mean ratio | 95% confidence interval | P valuea |
|---|---|---|---|
| MDR | 1.41 | 1.10–1.82 | 0.008 |
| Age | 0.99 | 0.98–1.00 | 0.008 |
| Race, blackb | 0.88 | 0.67–1.16 | 0.37 |
| Race, otherb | 0.75 | 0.48–1.18 | 0.22 |
| APACHE II acute physiology score | 0.99 | 0.96–1.01 | 0.32 |
| APACHE II chronic health score | 1.07 | 1.01–1.13 | 0.03 |
| Female gender | 0.63 | 0.49–0.80 | 0.0001 |
| Diabetes mellitus | 1.22 | 0.96–1.55 | 0.10 |
| Rheumatoid arthritis | 0.41 | 0.16–1.02 | 0.05 |
| Corticosteroid use in last 30 days | 0.94 | 0.67–1.31 | 0.71 |
| History of transplant | 1.81 | 1.21–2.71 | 0.004 |
| HIV positive | 2.48 | 0.41–15.12 | 0.32 |
| History of neoplasm | 0.62 | 0.48–0.79 | 0.0002 |
| History of Gram-negative infection | 1.59 | 1.23–2.04 | 0.0003 |
| Surgery in 30 days prior to bacteremia | 1.32 | 1.04–1.69 | 0.02 |
Boldface indicates significance.
The reference group for the race parameter is white patients.
In the 622 patients with community-acquired BSI, both mean (MDR, $18,190 [SD, $28,094]; non-MDR, $15,893 [SD $25,314]) and median (MDR, $9,282 [IQR, $5,523 to $17,587]; non-MDR, $7,895 [IQR $4,558 to $14,421]) inpatient costs were numerically higher in patients with MDR BSI than in those with non-MDR BSI, though this did not reach statistical significance (P = 0.31). In addition, the MDR phenotype was not significantly associated with higher inpatient costs in patients with community-acquired BSI (means ratio, 1.07; 95% CI, 0.92 to 1.25; P = 0.40) after adjustment for patient characteristics (see Table S7 in the supplemental material). Similarly, the MDR phenotype was not significantly associated with higher inpatient costs in patients with community-acquired BSI when length of stay and mortality were added as covariates (see Table S8 in the supplemental material).
Health care cost analysis by bacterial species.
We also examined the influence of MDR BSI on inpatient costs for individual bacterial groups, including E. coli, K. pneumoniae, and Enterobacter (both E. aerogenes and E. cloacae). For all three bacterial groups, the mean inpatient costs were numerically higher in patients with MDR BSI than in those with non-MDR BSI (Table 6). Modeling each genus separately, adjusted analyses revealed that MDR BSI were associated with increased inpatient costs relative to non-MDR BSI for E. coli (means ratio, 1.43; 95% CI, 1.20 to 1.71; P < 0.0001), K. pneumoniae (means ratio, 1.62; 95% CI, 1.05 to 2.51; P = 0.03), and Enterobacter (means ratio, 2.02; 95% CI, 1.29 to 3.15; P = 0.002) (see Tables S9 to S11 in the supplemental material).
TABLE 6.
Descriptive statistics of inpatient costs by infectious pathogen and MDR status
| Bacterial group | MDR | n | Cost |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 25th percentile | 75th percentile | |||
| Escherichia coli | No | 165 | $14,776 | $24,416 | $7,503 | $4,301 | $13,447 |
| Yes | 165 | $18,917 | $29,394 | $9,527 | $5,915 | $19,648 | |
| Klebsiella pneumoniae | No | 127 | $28,877 | $48,116 | $11,183 | $5,955 | $29,452 |
| Yes | 39 | $115,868 | $163,881 | $46,934 | $12,470 | $153,881 | |
| Enterobacter aerogenes + Enterobacter cloacae | No | 58 | $42,717 | $60,877 | $19,544 | $6,693 | $43,621 |
| Yes | 26 | $108,163 | $89,579 | $97,543 | $19,278 | $166,265 | |
DISCUSSION
In this study, we used a large cohort of inpatients with Gram-negative BSI from 2009 to 2015 at a single U.S. tertiary medical center to investigate the clinical and economic ramifications of the MDR phenotype (i.e., resistance to ≥3 antibiotic classes) (2). While multiple studies have evaluated the clinical and economic influences of particular antibiotic resistance phenotypes on Gram-negative infections (3–27), this study is unique for several reasons: (i) it used among the largest cohorts of patients with Gram-negative BSI studied to evaluate the influence of antibiotic resistance on health care costs, (ii) all patients in this study were prospectively enrolled, (iii) all Gram-negative species that could be defined as MDR were evaluated, and (iv) the antibiotic resistance phenotype that we selected for our primary analysis, the MDR phenotype, is more commonly encountered in the clinical setting than other phenotypes of interest (e.g., extended-spectrum β-lactamase [ESBL]-producing or carbapenem-resistant Enterobacteriaceae). In total, we believe that this study provides a comprehensive picture of the detrimental effects of antibiotic resistance on clinical and economic outcomes in hospitalized patients with Gram-negative BSI.
We found that the MDR phenotype was associated with increased inpatient costs in patients with Gram-negative BSI. This is consistent with an earlier study in Singapore that similarly noted an increased cost of care in patients with MDR Gram-negative BSI (17). Interestingly, the increased cost of MDR BSI was noted even after we adjusted for appropriate empirical antibiotic therapy. The increased costs associated with MDR BSI may stem from factors that are not modifiable by early appropriate antibiotic therapy, such as higher costs of agents needed to treat MDR BSI, greater likelihood for procedures such as line placement for intravenous (i.v.) antibiotics, and complications from these procedures. We also noted that patients with MDR BSI had increased length of hospital stay. In an adjusted analysis, the increased cost of inpatient care for MDR BSI was accounted for by the longer hospital stay among patients with MDR BSI. Again, this finding is consistent with past work which has demonstrated an association between antibiotic resistance, length of hospital stay, and increased costs (17, 25–27). However, to our knowledge, this is the first study to identify particular patient subpopulations that are driving the increased cost of care associated with drug-resistant infections. We found that MDR BSI in patients with hospital-acquired infections were associated with increased cost of inpatient care.
The reasons behind these subpopulation-specific findings are not entirely clear, though they likely are related to the types of patients who contract hospital-acquired BSI. For example, of the patients in this cohort with hospital-acquired BSI, 78% had an APACHE II chronic health score of 5, indicating the presence of severe chronic organ dysfunction or immunosuppression. Immunosuppression due to metastatic cancer or chemotherapy was particularly common in this patient cohort. The deleterious effects of the MDR phenotype may be magnified in this population, as individuals who are more chronically ill or are immunosuppressed are likely at increased risk of therapy complications from the i.v. antibiotics or procedures that are often needed to treat MDR pathogens. Therapy complications will increase inpatient costs.
The MDR phenotype was also associated with recurrent BSI, defined in this study as culture-confirmed Gram-negative BSI during the same hospitalization after microbiological resolution of the initial Gram-negative BSI. To our knowledge, this is the first study to demonstrate an association between antibiotic resistance and recurrent Gram-negative BSI in a controlled patient population. Such a finding has been suggested, however, by patients with BSI caused by ESBL-producing or carbapenem-resistant Enterobacteriaceae, who have rates of recurrent bacteremia far exceeding that noted in this and other studies (15 to 29%) (29, 30). Multiple additional studies have evaluated risk factors for recurrent Gram-negative BSI (31–36), though antibiotic resistance patterns were not considered. The MDR phenotype was not associated with either in-hospital mortality or poor clinical outcome. This is consistent with the literature, as multiple large studies have demonstrated antibiotic resistance to have either a detrimental or neutral effect on clinical outcomes (37–40). In this study, the assessment of how antibiotic resistance influences clinical outcomes is likely complicated by heterogeneity in bacterial species, antibiotic resistance profiles, and patient clinical factors.
This study has several limitations. First, inpatient costs represented costs incurred during the entire hospitalization rather than the cost incurred after the first positive blood culture. For the hospital-acquired BSI, this makes it more difficult to assess the true cost of BSI, since the inpatient costs from hospital days before the BSI will be included in the total cost. Second, the data come from a single U.S. medical center. However, given that this is one of the largest cohorts of prospectively enrolled patients with Gram-negative BSI over a significant time period (2009 to 2015), we believe that these data make a significant contribution to our understanding of how antibiotic resistance affects clinical and economic outcomes. Finally, in this study we account only for inpatient costs. This may underestimate the true cost of MDR BSI, which are more likely than non-MDR BSI to require expensive outpatient therapy such as i.v. antibiotics.
In summary, we used a large cohort of prospectively enrolled inpatients with Gram-negative BSI from 2009 to 2015 to show several important findings. First, MDR BSI, relative to non-MDR BSI, were associated with increased mean inpatient costs ($59,266 versus $36,452), and this was significant even after adjustment for patient demographics, medical comorbidities, and treatment factors. Second, the increased cost of MDR BSI stemmed primarily from increased length of hospital stay. Third, patients with hospital-acquired infections were the primary drivers of the increased inpatient costs associated with the MDR phenotype. Finally, MDR BSI were associated with recurrent BSI during the same hospital stay. This study highlights the detrimental effects of antibiotic resistance and the need for novel therapeutics to treat these pathogens.
MATERIALS AND METHODS
Clinical data.
The Duke Bloodstream Infection Biorepository (BSIB) has prospectively ascertained all eligible adult inpatients at Duke University Medical Center (Durham, NC, USA) with monomicrobial Gram-negative-bacterium BSI since 1 January 2009. Patients or their representatives provide written informed consent. Clinical data are collected prospectively within 36 h of positive blood culture. Detailed clinical data, including patient characteristics, treatment patterns, and in-hospital outcomes, are collected on a standardized case report form and entered into an electronic database (Access, Microsoft). Patient sera, DNA, and the bloodstream bacterial isolates are also collected. In this study, only patients with Enterobacteriaceae, Pseudomonas aeruginosa, or Acinetobacter species BSI were included. Other Gram-negative species (e.g., anaerobes such as Bacteroides species) are not routinely tested for antimicrobial susceptibility, and a specific set of antibiotic classes on which to base the MDR definition has not been defined (2). The study had full approval from the Duke University Institutional Review Board.
Definitions.
Four primary endpoints were defined: (i) cure, no evidence of recurrent Gram-negative BSI within the index hospital admission; (ii) recurrence, clinical and microbiological resolution of the initial episode of infection after treatment but culture-confirmed Gram-negative BSI (same species and antibiogram) documented within the index hospital admission; (iii) attributable mortality, persistent signs or symptoms of infection, positive blood cultures, or a persistent focus of infection at the time of death in the absence of another explanation for death; and (iv) death due to a defined underlying disease other than Gram-negative BSI, defined according to a review of clinical events by one of the investigators (J.T.T.). In this study, we assessed in-hospital mortality, which consisted of either attributable mortality or death due to underlying disease other than Gram-negative BSI. Our endpoint, poor clinical outcome, is a composite endpoint of in-hospital mortality or any of the following complications: septic shock (41), acute kidney injury (42), acute lung injury/acute respiratory distress syndrome (43), disseminated intravascular coagulation (DIC) (44), or shock liver (45). The route of infection refers to whether the BSI was hospital acquired or community acquired. Hospital-acquired infection was defined as an infection beginning ≥48 h after hospital admission (46). Community-acquired BSI was defined as an infection beginning <48 h after hospital admission. Community-acquired BSI was further subdivided into (i) nonnosocomial health care-associated BSI and (ii) non-health care-associated BSI. The nonnosocomial health care-associated BSI definition was modified from that of Friedman et al. (46) and defined as a BSI beginning prior to 48 h after hospital admission in patients that meet one or more of the following criteria: hospitalized in the past 90 days, resident of a nursing home or long-term-care facility, actively receiving home intravenous therapy, received wound care or specialized nursing care in the previous 30 days, received hemodialysis in the past 30 days, immunosuppressed (e.g., presence of metastatic cancer, history of solid organ or hematological transplant, chemotherapy in last 30 days, or currently on immunosuppressive medication for any reason), or had surgery in the last 180 days. Non-health care-associated BSI is any community-acquired BSI not meeting criteria for nonnosocomial health care-associated BSI. The source of infection refers to the primary focus of the BSI (e.g., urine/pyelonephritis, line, etc.) and was determined by review of the medical record by one of the investigators (J.T.T.). The APACHE II score (47) was calculated at the time of initial positive blood cultures. Appropriate empirical antibiotic therapy was achieved when a patient received an antibiotic to which the BSI bacterial isolate was susceptible within 24 h of the index positive blood culture.
Antibiotic susceptibility data.
MIC data were obtained for all bacterial isolates by the Duke Clinical Microbiology Laboratory as part of routine clinical care. MIC data were generated via the broth microdilution technique with the MicroScan WalkAway system (Siemens Healthcare Diagnostics). Definitions of antibiotic susceptibilities (i.e., susceptible, intermediate, and resistant) were defined according to the most recent Clinical and Laboratory Standards Institute (CLSI) guidelines. A bacterium was considered to be MDR if it exhibited resistance to at least one antibiotic in at least three relevant antimicrobial categories (2).
Health care costs.
Inpatient hospital costs are based on patient-level data from the cost accounting systems used by Duke University Medical Center, which use activity-based costing to assign costs based on labor and supply relative value units (RVUs) for every service provided to hospitalized patients. Cost estimates include fixed direct costs and variable direct costs for all medical services received, inclusive of supplies, laboratory tests, drugs, and dietary needs, as well as associated salary and fringe benefits for all levels of personnel (excluding physicians). Overhead costs and depreciation are also included. Physician fees for procedures related to the Gram-negative BSI were estimated using the Medicare physician fee schedule. All costs were adjusted to 2015 dollars using the medical care component of the consumer price index and represented the entire inpatient stay.
Statistical analyses.
In descriptive analyses, continuous variables were reported as means, standard deviations (SD), medians, and 25th and 75th percentiles (i.e., interquartile range [IQR]) and compared using t tests in unadjusted analyses. Dichotomous variables are reported as counts and percentages and were compared using two-tailed Fisher exact tests or Pearson chi-square tests when appropriate. For multivariable analyses, we used generalized linear models (GLMs) to compare inpatient days, mortality rates, poor clinical outcomes, and inpatient costs between groups. The variables included in all the models were MDR status, age, gender, race, APACHE II acute physiology score, APACHE II chronic health score, diabetes mellitus, rheumatoid arthritis, corticosteroid use in the last 30 days, solid organ or hematopoietic transplant, HIV, neoplasm, history of Gram-negative infections, surgery in the 30 days prior to bacteremia, source of BSI, and route of infection. The models were specified with gamma distributions and log links to compare costs, negative binomial distributions and log links to compare inpatient days, and binomial distributions and logit links to compare mortality rates and poor outcomes (equivalent to logistic regression).
Supplementary Material
ACKNOWLEDGMENTS
J.T.T., Y.L., F.R., S.D.R., and V.G.F. received funding through an Investigator-Initiated Research Grant from Merck & Co. Additionally, J.T.T. received funding from the U.S. FDA (5R18-FD005292-02), S.A.M. received funding from an NIH T32 grant (5T32-AI052080-12), and V.G.F. received funding from an NIH K24 grant (K24-AI093969).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01709-16.
REFERENCES
- 1.The Review on Antimicrobial Resistance. 11 December 2014, posting date. Antimicrobial resistance: tackling a crisis for the future health and wealth of nations. https://amr-review.org/sites/default/files/AMR Accessed 3 June 2016.
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Apisarnthanarak A, Kiratisin P, Saifon P, Kitphati R, Dejsirilert S, Mundy LM. 2007. Clinical and molecular epidemiology of community-onset, extended-spectrum beta-lactamase-producing Escherichia coli infections in Thailand: a case-case-control study. Am J Infect Control 35:606–612. doi: 10.1016/j.ajic.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Carmeli Y, Troillet N, Karchmer AW, Samore MH. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med 159:1127–1132. doi: 10.1001/archinte.159.10.1127. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL, Lefrak SN, Lyman J, Smith RL, Chong TW, McElearney ST, Schulman AR, Hughes MG, Raymond DP, Pruett TL, Sawyer RG. 2007. Cost of Gram-negative resistance. Crit Care Med 35:89–95. doi: 10.1097/01.CCM.0000251496.61520.75. [DOI] [PubMed] [Google Scholar]
- 6.Gasink LB, Fishman NO, Weiner MG, Nachamkin I, Bilker WB, Lautenbach E. 2006. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med 119:526.e19–526.e25. [DOI] [PubMed] [Google Scholar]
- 7.Hu B, Ye H, Xu Y, Ni Y, Hu Y, Yu Y, Huang Z, Ma L. 2010. Clinical and economic outcomes associated with community-acquired intra-abdominal infections caused by extended spectrum beta-lactamase (ESBL) producing bacteria in China. Curr Med Res Opin 26:1443–1449. doi: 10.1185/03007991003769068. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 9.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, Kim M. 2009. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol 30:1186–1192. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, Kim M. 2010. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect Control Hosp Epidemiol 31:47–53. doi: 10.1086/649021. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. 2006. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol 27:893–900. doi: 10.1086/507274. [DOI] [PubMed] [Google Scholar]
- 12.Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC. 2007. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 28:713–719. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP. 2006. Impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol 27:1226–1232. doi: 10.1086/507962. [DOI] [PubMed] [Google Scholar]
- 14.Leistner R, Gurntke S, Sakellariou C, Denkel LA, Bloch A, Gastmeier P, Schwab F. 2014. Bloodstream infection due to extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: an analysis of the disease burden in a large cohort. Infection 42:991–997. doi: 10.1007/s15010-014-0670-9. [DOI] [PubMed] [Google Scholar]
- 15.MacVane SH, Tuttle LO, Nicolau DP. 2014. Impact of extended-spectrum beta-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med 9:232–238. doi: 10.1002/jhm.2157. [DOI] [PubMed] [Google Scholar]
- 16.Neidell MJ, Cohen B, Furuya Y, Hill J, Jeon CY, Glied S, Larson EL. 2012. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis 55:807–815. doi: 10.1093/cid/cis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng E, Earnest A, Lye DC, Ling ML, Ding Y, Hsu LY. 2012. The excess financial burden of multidrug resistance in severe Gram-negative infections in Singaporean hospitals. Ann Acad Med Singapore 41:189–193. [PubMed] [Google Scholar]
- 18.Riu M, Chiarello P, Terradas R, Sala M, Garcia-Alzorriz E, Castells X, Grau S, Cots F. 2016. Cost attributable to nosocomial bacteremia. Analysis according to microorganism and antimicrobial sensitivity in a university hospital in Barcelona. PLoS One 11:e0153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y. 2006. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 50:1257–1262. doi: 10.1128/AAC.50.4.1257-1262.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewardson A, Fankhauser C, De Angelis G, Rohner P, Safran E, Schrenzel J, Pittet D, Harbarth S. 2013. Burden of bloodstream infection caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae determined using multistate modeling at a Swiss university hospital and a nationwide predictive model. Infect Control Hosp Epidemiol 34:133–143. doi: 10.1086/669086. [DOI] [PubMed] [Google Scholar]
- 21.Tansarli GS, Karageorgopoulos DE, Kapaskelis A, Falagas ME. 2013. Impact of antimicrobial multidrug resistance on inpatient care cost: an evaluation of the evidence. Expert Rev Anti Infect Ther 11:321–331. doi: 10.1586/eri.13.4. [DOI] [PubMed] [Google Scholar]
- 22.Tumbarello M, Spanu T, Di Bidino R, Marchetti M, Ruggeri M, Trecarichi EM, De Pascale G, Proli EM, Cauda R, Cicchetti A, Fadda G. 2010. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother 54:4085–4091. doi: 10.1128/AAC.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson SJ, Knipe CJ, Zieger MJ, Gabehart KM, Goodman JE, Volk HM, Sood R. 2004. Direct costs of multidrug-resistant Acinetobacter baumannii in the burn unit of a public teaching hospital. Am J Infect Control 32:342–344. doi: 10.1016/j.ajic.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Young LS, Sabel AL, Price CS. 2007. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol 28:1247–1254. doi: 10.1086/521660. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove SE, Kaye KS, Eliopoulous GM, Carmeli Y. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med 162:185–190. doi: 10.1001/archinte.162.2.185. [DOI] [PubMed] [Google Scholar]
- 26.de Kraker ME, Davey PG, Grundmann H, BURDEN Study Group. 2011. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. 2010. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant Gram-negative bacteria. Antimicrob Agents Chemother 54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Lee CH, Su LH, Chen FJ, Tang YF, Chien CC, Liu JW. 2015. Clinical and microbiologic characteristics of adult patients with recurrent bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Clin Microbiol Infect 21:1105 e1101–e1108. [DOI] [PubMed] [Google Scholar]
- 30.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, Silveira FP, Abdel-Massih R, Bogdanovich T, Humar A, Perlin DS, Kreiswirth BN, Hong Nguyen M. 2013. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Hasan MN, Eckel-Passow JE, Baddour LM. 2010. Recurrent Gram-negative bloodstream infection: a 10-year population-based cohort study. J Infect 61:28–33. doi: 10.1016/j.jinf.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen US, Knudsen JD, Ostergaard C, Gradel KO, Frimodt-Moller N, Schonheyder HC. 2010. Recurrent bacteraemia: a 10-year regional population-based study of clinical and microbiological risk factors. J Infect 60:191–199. doi: 10.1016/j.jinf.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Jensen US, Knudsen JD, Wehberg S, Gregson DB, Laupland KB. 2011. Risk factors for recurrence and death after bacteraemia: a population-based study. Clin Microbiol Infect 17:1148–1154. doi: 10.1111/j.1469-0691.2011.03587.x. [DOI] [PubMed] [Google Scholar]
- 34.Wendt C, Messer SA, Hollis RJ, Pfaller MA, Wenzel RP, Herwaldt LA. 1999. Recurrent Gram-negative bacteremia: incidence and clinical patterns. Clin Infect Dis 28:611–617. doi: 10.1086/515152. [DOI] [PubMed] [Google Scholar]
- 35.Marschall J, Doherty J, Warren DK. 2010. The epidemiology of recurrent Gram-negative bacteremia in a tertiary-care hospital. Diagn Microbiol Infect Dis 66:456–459. doi: 10.1016/j.diagmicrobio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Lopez Dupla M, Martinez JA, Vidal F, Almela M, Lopez J, Marco F, Soriano A, Richart C, Mensa J. 2005. Clinical characterization of breakthrough bacteraemia: a survey of 392 episodes. J Intern Med 258:172–180. doi: 10.1111/j.1365-2796.2005.01513.x. [DOI] [PubMed] [Google Scholar]
- 37.Blot S, Vandewoude K, De Bacquer D, Colardyn F. 2002. Nosocomial bacteremia caused by antibiotic-resistant Gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis 34:1600–1606. doi: 10.1086/340616. [DOI] [PubMed] [Google Scholar]
- 38.Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, Agodi A, Frank U, Mertens K, Schumacher M, Wolkewitz M. 2011. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 39.Lye DC, Earnest A, Ling ML, Lee TE, Yong HC, Fisher DA, Krishnan P, Hsu LY. 2012. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect 18:502–508. doi: 10.1111/j.1469-0691.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 40.Giske CG, Monnet DL, Cars O, Carmeli Y. 2008. Clinical and economic impact of common multidrug-resistant Gram-negative bacilli. Antimicrob Agents Chemother 52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, Dellinger RP. 2015. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 43:3–12. doi: 10.1097/CCM.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 42.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragaller M, Richter T. 2010. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock 3:43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi M, Toh CH, Thachil J, Watson HG. 2009. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 45.Seeto RK, Fenn B, Rockey DC. 2000. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med 109:109–113. doi: 10.1016/S0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 46.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 47.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.