ABSTRACT
Several infectious diseases of global importance—e.g., HIV infection and tuberculosis (TB)—require prolonged treatment with combination antimicrobial regimens typically involving high-potency core agents coupled with additional companion drugs that protect against the de novo emergence of mutations conferring resistance to the core agents. Often, the most effective (or least toxic) companion agents are reused in sequential (first-line, second-line, etc.) regimens. We used a multistrain model of Mycobacterium tuberculosis transmission in Southeast Asia to investigate how this practice might facilitate the emergence of extensive drug resistance, i.e., resistance to multiple core agents. We calibrated this model to regional TB and drug resistance data using an approximate Bayesian computational approach. We report the proportion of data-consistent simulations in which the prevalence of pre-extensively drug-resistant (pre-XDR) TB—defined as resistance to both first-line and second-line core agents (rifampin and fluoroquinolones)—exceeds predefined acceptability thresholds (1 to 2 cases per 100,000 population by 2035). The use of pyrazinamide (the most effective companion agent) in both first-line and second-line regimens increased the proportion of simulations in which the prevalence exceeded the pre-XDR acceptability threshold by 7-fold compared to a scenario in which patients with pyrazinamide-resistant TB received an alternative drug. Model parameters related to the emergence and transmission of pyrazinamide-resistant TB and resistance amplification were among those that were the most strongly correlated with the projected pre-XDR prevalence, indicating that pyrazinamide resistance acquired during first-line treatment subsequently promotes amplification to pre-XDR TB under pyrazinamide-containing second-line treatment. These findings suggest that the appropriate use of companion drugs may be critical to preventing the emergence of strains resistant to multiple core agents.
KEYWORDS: pyrazinamide, tuberculosis, antimicrobial combinations, mathematical modeling, multidrug resistance
INTRODUCTION
Antimicrobial resistance has recently been labeled “a problem so serious that it threatens the achievements of modern medicine” (1). Concerns regarding the emergence of drug resistance in the early antimicrobial era, along with the prospect of improving clinical outcomes, led to a shift from monotherapy to combination treatment for infections of global importance, including HIV infection, tuberculosis (TB), and malaria, but the success of combination antimicrobial therapy is increasingly threatened by the rise of multidrug resistance (2–5). Combination regimens often rely on the use of highly effective core drugs that have low toxicity, high microbicidal activity, and/or a high barrier to resistance, supplemented by companion drugs that are typically less active on their own but act to enhance the overall effectiveness of the regimen while also potentially preventing the emergence of resistance to core drugs. For example, in combination therapy against HIV, nucleoside inhibitors often serve as companion agents to prevent resistance to the core drug classes of protease inhibitors, nonnucleoside reverse transcriptase inhibitors, and integrase inhibitors (6). These companion drugs are frequently reused in sequential treatment regimens when alternative companion agents are less effective or more toxic. For instance, due in part to its unique sterilizing activity against Mycobacterium tuberculosis bacilli, pyrazinamide (PZA) is used to augment the effectiveness of several core agents, including rifampin (RIF) in standard first-line TB treatment and fluoroquinolones (FQs) in most second-line regimens (7).
In evaluating the emergence of extensive drug resistance, research and surveillance efforts have historically focused on the role of core agents. However, the recycling of companion drugs in sequential treatment regimens may play a critical and underrecognized role in the emergence of resistance to the core agents. This is the case for PZA, which is a recommended agent in standardized first- and second-line TB treatment regimens (8). If the concomitant use of PZA prevents the emergence of resistance to RIF and FQs (an unproven hypothesis but one that is consistent with the principles of combination drug therapy), PZA resistance may therefore be an important facilitator of the emergence of strains that are resistant to both RIF and FQs, which we define conventionally to be pre-extensively drug resistant (pre-XDR) TB strains. To illustrate this concept, we constructed a dynamic model of M. tuberculosis transmission which incorporates resistance to RIF, PZA, and FQs (Fig. 1). We used this model to generate a large set of simulations consistent with epidemiological data available up to 2013 (Fig. 2). We then evaluated projected levels of pre-XDR TB in 2035, assuming that the concomitant use of PZA protects against de novo resistance to both RIF and FQs. We compared a baseline scenario in which PZA is recycled in first- and second-line regimens to a counterfactual scenario in which PZA is replaced by a hypothetical alternative drug of equal efficacy to demonstrate how the repeated use of companion drugs can facilitate the emergence of extensively drug-resistant strains.
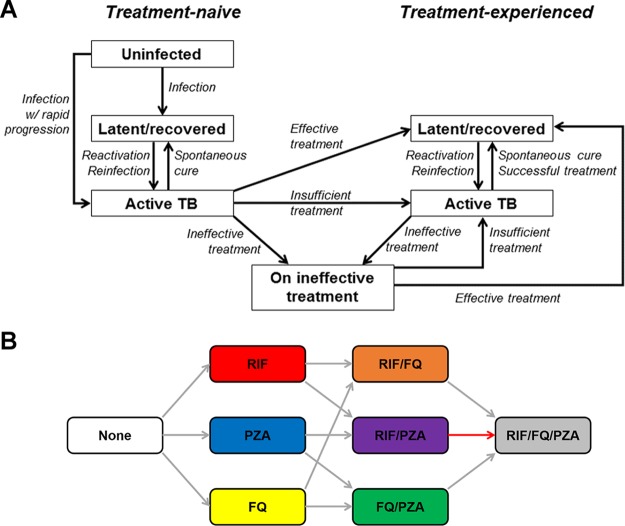
FIG 1.
Model structure diagram. (A) The model features separate compartments for individuals who are uninfected with TB, latently infected with TB, or experiencing active disease. Individuals with TB are further distinguished on the basis of their prior treatment experience. A separate compartment exists for patients who are receiving ineffective treatment; these individuals remain ill with TB and are then initiated on a repeat course of treatment. All five TB compartments (with the exception of the uninfected compartment) are replicated for each of eight drug resistance states for a total of 41 unique compartments. Births and deaths are not shown here for simplicity. (B) Progression between drug resistance states is assumed to result only in increasing resistance. In addition to the transitions shown here, resistance to multiple drugs can be acquired within a single course of treatment. The primary mode of acquiring pre-XDR TB (defined as concomitant resistance to at least RIF and FQ) is highlighted in red and includes acquisition of resistance to PZA, a companion drug that is routinely used in both first- and second-line treatment.
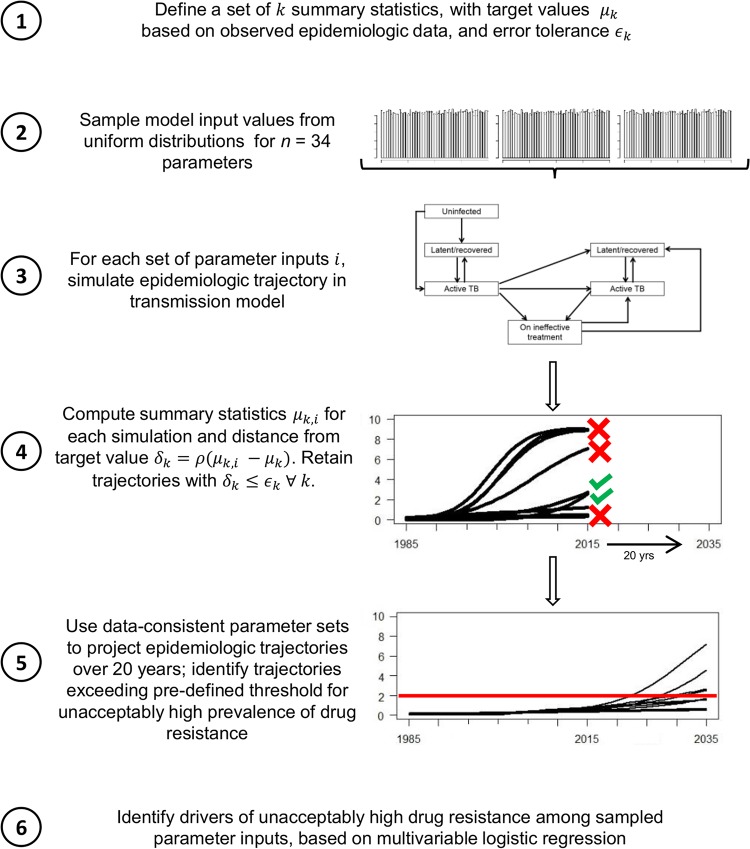
FIG 2.
Experimental approach. Shown here is the step-by-step approach of selecting simulations that are consistent with existing epidemiologic data and projecting outcomes under those simulations, for purposes of elucidating the dynamics between strains with different patterns of resistance to multiple antimicrobial agents. ρ, distance function.
RESULTS
We first attempted to calibrate the model under our baseline assumption that PZA provides protection against de novo resistance to concomitantly administered RIF and FQs, as well as under the alternative assumption that PZA offers no such protection. Attempts to calibrate the model without a protective effect yielded 20-fold fewer simulations that were consistent with existing epidemiologic data (47 versus 1,015 out of 100,000 sampled parameter sets), suggesting that this assumption is probably less consistent with the available data than the assumption that PZA protects against resistance to coadministered drugs. We therefore conducted all subsequent analyses assuming that PZA protects against resistance amplification.
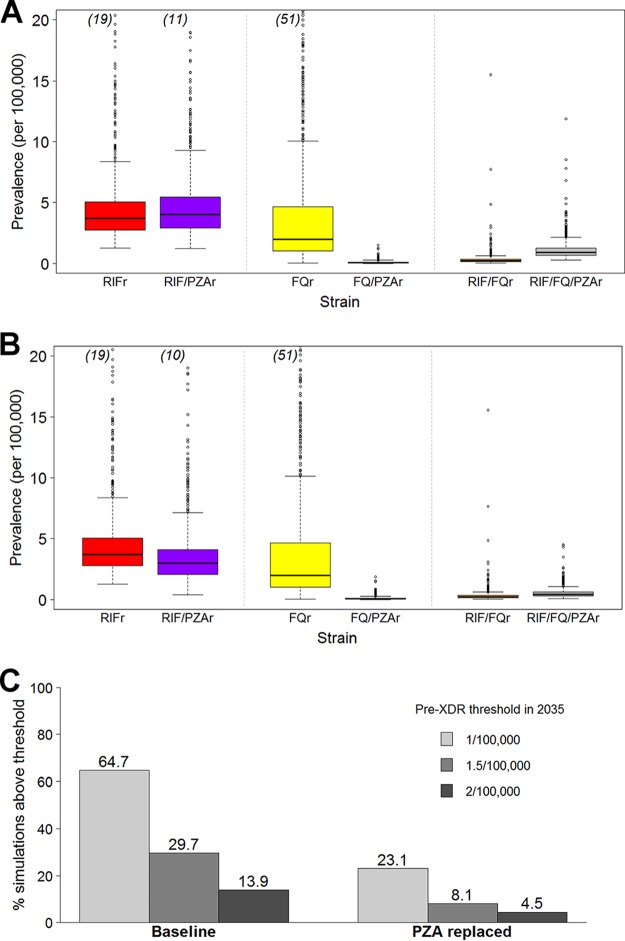
Across the 1,015 simulations consistent with epidemiologic data (assuming a protective effect of PZA on acquired resistance), the median projected prevalence of pre-XDR TB in 2015 was 0.64 per 100,000 population (interquartile range [IQR], 0.51 to 0.79 per 100,000 population). The proportion of RIF-resistant strains in 2035 that harbored additional resistance to PZA was greater in the baseline scenario (median, 51.7%; IQR, 43.7 to 59.5%) than in the alternative scenario, in which PZA was replaced (median, 44.7%; IQR, 36.4 to 51.3%), although the overall TB incidence was similar in both scenarios (median, 205.0 per 100,000 population [IQR, 1,886 to 222.5 per 100,000 population] in the baseline scenario versus 203.7 per 100,000 population [IQR, 187.6 to 221.1 per 100,000 population] in the PZA replacement scenario). There was an even more pronounced difference in the proportion of pre-XDR strains with additional PZA resistance (80.2% [IQR, 72.9 to 85.6%] in the baseline scenario versus 65.8% [IQR, 57.9 to 72.2%] in the PZA replacement scenario) (Fig. 3A and B). Overall, the proportion of simulations in which the pre-XDR prevalence exceeded predefined acceptability thresholds of 1, 1.5, and 2 per 100,000 population in 2035 was 64.7%, 29.7%, and 13.9%, respectively, in the baseline scenario, whereas it was 23.1%, 8.1%, and 4.5%, respectively, in the PZA replacement scenario. This corresponds to relative reductions of 64 to 73% in the proportion of simulations where the prevalence of pre-XDR TB exceeded each acceptability threshold. Similar results were obtained using a stochastic modeling framework: the proportion of simulations in which pre-XDR prevalence exceeded the acceptability thresholds by 2035 decreased from 52.1%, 35.7%, and 24.9%, respectively, in the baseline scenario to 25.1%, 13.7%, and 8.2%, respectively, in the PZA replacement scenario (see Fig. S9 in the supplemental material).
FIG 3.
Reuse of PZA increases the projected prevalence of pre-XDR TB. Projected prevalence of RIF-resistant (RIFr), FQ-resistant (FQr), and pre-XDR (RIF- and FQ-resistant [RIF/FQr] or RIF-, FQ-, and PZA-resistant [RIF/FQ/PZAr]) TB with and without additional resistance to PZA in 2035 under the baseline (A) and PZA replacement (B) scenarios. Box plots show the median, 25th, and 75th percentile values across all data-consistent simulations. Outlier simulations with a projected pre-XDR TB prevalence of greater than 20 per 100,000 population are not shown; the numbers of such outliers, if applicable, are indicated in parentheses at the top of each box plot. (C) Proportion of data-consistent simulations in which the projected pre-XDR TB prevalence in 2035 exceeds three predefined acceptability thresholds. Replacing PZA with an alternative drug of equal efficacy among patients with PZA-resistant TB greatly reduces the proportion of trajectories in which the prevalence exceeds the pre-XDR TB acceptability threshold in 2035.
We used multivariable sensitivity analysis to investigate those parameters that were the most closely associated with the emergence of pre-XDR TB at a prevalence of 1 case per 100,000 population by 2035 (Fig. 4). Five of the 10 most influential parameters involved PZA; these included the probability of cure for RIF- and PZA-resistant TB, the transmission fitness of strains resistant to both RIF and PZA, and the probabilities of acquiring PZA resistance and subsequently developing additional resistance (Fig. 4). Under the PZA replacement scenario, the odds ratios (ORs) associated with the probabilities of acquiring PZA resistance and subsequent resistance amplification were attenuated toward a null effect (i.e., OR = 1). Sensitivity analyses varying the threshold to 1.5 and 2 pre-XDR cases per 100,000 population yielded similar findings, as did alternative analyses using partial rank correlation coefficients (Fig. S6).
FIG 4.
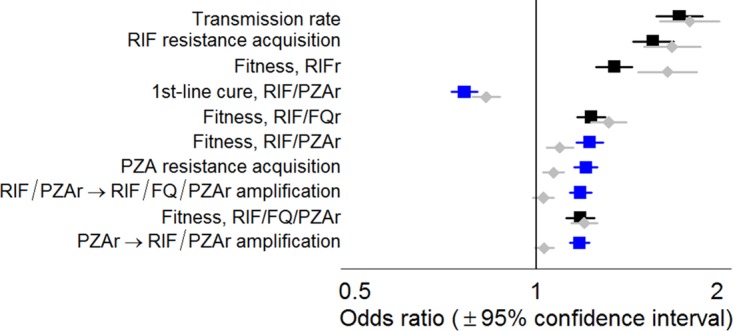
Parameters associated with a high future prevalence of pre-XDR TB. Leading drivers of future pre-XDR TB prevalence, as assessed by logistic regression on the odds of the primary outcome, namely, exceeding a predefined acceptability threshold of 1 case per 100,000 population in 2035, comparing baseline conditions (blue and black squares) to the alternative scenario in which PZA is replaced (gray diamonds). Odds ratios reflect the change in the primary outcome associated with an increase of 1/10 of a standard deviation in the independent variable. Parameters related to strains resistant to PZA only (PZAr) or resistant to both RIF and PZA (RIF/PZAr) are highlighted in blue. As an example of scale, 1/10 of a standard deviation corresponds to absolute changes of 0.5% in the probability of acquiring RIF resistance in a single course of treatment, 6% in the transmission fitness of RIF- and PZA-resistant strains, or 5% in the probability of cure for RIF- and PZA-resistant strains on the first-line regimen.
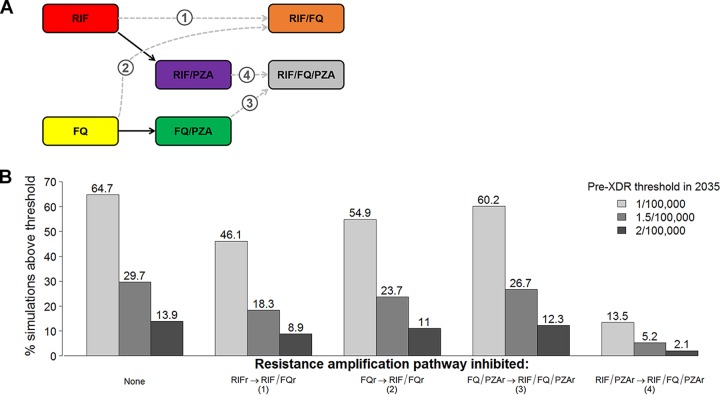
Finally, we evaluated model scenarios in which specific steps in the progression to pre-XDR TB were inhibited, reflecting the potential effect of tailored therapy for patients diagnosed with PZA-resistant TB (Fig. 5). In these analyses, we found that the acquisition of FQ resistance among strains already dually resistant to RIF and PZA was a key step in the development of pre-XDR TB. Blocking this single step in resistance amplification (i.e., allowing pre-XDR TB to emerge only from strains other than RIF- and PZA-resistant strains) reduced the proportion of simulations in which the prevalence exceeded each pre-XDR acceptability threshold by 4- to 7-fold, suggesting that dual RIF and PZA resistance is an important precursor of pre-XDR TB at the population level. In contrast, blocking the emergence of pre-XDR TB from RIF-monoresistant or FQ-monoresistant strains or from FQ- and PZA-resistant strains had a minimal effect on the projected pre-XDR prevalence in 2035.
FIG 5.
Sequential acquisition of resistance and emergence of pre-XDR TB. (A) Pathways from RIF and FQ resistance, with and without additional PZA resistance. We demonstrate that, when PZA prevents the development of resistance to RIF and FQs, the primary pathway to developing pre-XDR TB goes through an intermediate step that includes resistance to both RIF and PZA (RIF/PZAr, arrow 4), rather than directly from RIF or FQ resistance (arrows 1 and 2). (B) Proportion of data-consistent simulations in which the projected pre-XDR TB prevalence in 2035 exceeds various acceptability thresholds, after blocking specific pathways of resistance acquisition. Blocking the progression from combined RIF and PZA resistance to RIF, FQ, and PZA resistance (corresponding to arrow 4 in panel A) greatly reduces the proportion of trajectories in which the prevalence exceeds the pre-XDR TB acceptability threshold in 2035, as shown in the rightmost bars. In contrast, blocking resistance amplification directly from strains that are RIF or FQ monoresistant results in a minimal change from the baseline scenario.
DISCUSSION
This novel population-level modeling framework incorporating resistance to three distinct antimicrobial drugs suggests that, when companion drugs select against de novo resistance mutations in combination regimens, the reuse of these drugs in both first- and second-line treatment may critically facilitate the emergence of strains that are resistant to multiple core agents. Specifically, a scenario projecting the hypothetical effect of perfect susceptibility testing for PZA and replacing PZA with another drug for patients with PZA-resistant TB dramatically reduced the proportion of data-consistent model simulations in which the projected prevalence of pre-XDR TB exceeded predefined acceptability thresholds within 20 years. Simulations in which we assumed that PZA does not apply selection pressure against concomitantly administered core agents were far less likely to match available epidemiologic data. These findings highlight the urgent importance of understanding the potential mechanisms by which PZA (and other companion drugs) enhances combination antimicrobial regimens and of expanding drug susceptibility testing and surveillance for resistance to these agents, rather than focusing such efforts on core drugs alone.
Available evidence from both laboratory and clinical studies supports the sequential acquisition of resistance in TB (9, 10). Our results suggest a similar pattern at the population level and that reusing companion drugs could promote the sequential progression to pre-XDR TB during first- and second-line treatment. Specifically, we found that the prevalence of PZA resistance was greatly increased among RIF-resistant strains and even more so among pre-XDR strains when PZA was used in both first- and second-line TB treatment. Moreover, strains resistant to both RIF and PZA featured as major precursors of pre-XDR. These results suggest that the initial acquisition of RIF or PZA resistance may allow the emergence of resistance to the other agent during first-line treatment, resulting in a large number of RIF- and PZA-resistant strains. These strains are then more likely to develop FQ resistance during second-line therapy that includes both PZA and FQs. These results are highly relevant to the deployment of standardized treatment regimens for multidrug-resistant TB prescribed without prior diagnostic testing for resistance to drugs other than RIF, a practice that may become increasingly common with the scale-up of rapid molecular testing for RIF resistance alone (11–13). In settings where resistance to PZA is common, indiscriminately starting patients on FQ- and PZA-containing standardized second-line regimens (8)—at the very time when the mycobacterial burden, and thus the incidence of spontaneous resistance-conferring mutations, is the highest—may result in the selection of bacilli resistant to other drugs in the regimen, including FQs, before the results of complete drug susceptibility testing (e.g., from culture for M. tuberculosis) are available. If PZA does indeed protect against the development of resistance to FQs during second-line therapy, consistent with our model calibration and the findings of previous empirical studies, routine rapid testing for PZA resistance among patients harboring mycobacterial bacilli with demonstrated RIF resistance would be an important means of preventing the emergence of pre-XDR TB (14, 15). This finding takes on even greater significance in the current drug development climate, as FQs and PZA are considered key agents in the development of many novel regimens for first-line treatment of TB (16, 17).
Overall, our findings highlight the importance of considering not only the interplay between individual antimicrobial drugs but also how these drugs are incorporated into sequential treatment regimens, in order to better control the spread of extensive drug resistance in the long term. Although our model is specific to TB, our insights regarding the importance of recycled companion drugs in facilitating the emergence of multiresistant pathogens may be relevant to other infectious diseases in which resistance to the current arsenal of drugs represents a major public health threat. For example, HIV is a pathogen of major global health significance in which sequential resistance to antiretroviral drugs occurs over the course of treatment (18, 19). As in our study, a previous model of the development of resistance in HIV that explicitly modeled combinations of resistance to three drug classes provided important insights into drug class-specific effects on population-level resistance trajectories (20). Furthermore, by combining a population-level transmission model with policy-relevant outcome thresholds, our study provides useful guidance to decision makers in the setting of sparse empirical data on key parameters related to drug resistance. This approach, which leverages available epidemiologic data and a mechanistic understanding of disease to shed light on future trajectories of drug resistance, can be adapted to other pathogens to inform risk prediction and disease control policies.
This model has several limitations. In seeking to optimize the balance of detail and parsimony, we made several simplifying assumptions, including restricting the model to adult pulmonary TB in an equilibrium population. As our focus was on exploring long-term epidemiologic trajectories rather than clinical outcomes, we chose to exclude forms of TB (i.e., childhood and strictly extrapulmonary disease) that, despite a significant disease burden, do not contribute significantly to transmission. Similarly, we chose the Southeast Asia region, where HIV is not a major driver of the TB epidemic (21), because Southeast Asia currently has higher levels of TB drug resistance. Future adaptations of this model could evaluate different epidemiologic settings, including those in which TB is driven by HIV and those (e.g., the former Soviet Union) with a long history of drug-resistant TB that may reflect high rates of transmission of drug-resistant TB in congregate living settings (e.g., prisons). We limited our model to three key drugs for simplicity, as the addition of additional drugs creates exponentially increasing complexity. As we used a simple acceptance/rejection algorithm to select plausible parameter sets, our results should not be interpreted as probabilistic projections of future TB epidemiology. Rather, our approach allowed us to explore a representative range of data-consistent scenarios—akin to an epidemiologic study selecting a representative sample of the population—and benchmark those scenarios against potentially meaningful decision thresholds. This approach enables us to quantify both the key considerations and the level of uncertainty in such decisions, providing a risk management tool that can inform TB control policies without the need to project the precise future of drug-resistant TB. Our conclusions were unchanged when a stochastic modeling framework that better takes into account rare events in the emergence of drug resistance was used. Finally, in order to simplify our inferences on the acquisition and transmission fitness of drug-resistant strains and the treatment outcomes in patients infected with drug-resistant strains, we kept most other model parameters at fixed values and did not explicitly model changes in transmission fitness over time or potential epistatic effects; our projections may therefore underestimate the true level of uncertainty in future epidemiologic trajectories.
In summary, using a novel, multistrain modeling approach, we evaluated the impact of a companion drug on future trajectories of TB strains resistant to multiple core agents. This approach suggests that, if the companion agent (such as PZA) is used to augment the role of core drugs in both first-line and second-line regimens, the emergence of strains resistant to multiple core drugs may be dramatically hastened. As such, a key research priority should be to collect better data to understand how and to what degree companion drugs enhance the effectiveness of combination regimens (e.g., increase the probability of cure, protect against acquired resistance) and particularly how PZA impacts TB treatment. In the absence of such data, our results support the need for drug susceptibility testing for PZA prior to initiating second-line regimens that include PZA without a sufficient number of additional companion agents. These findings may generalize to infections caused by other microbial pathogens treated with sequential combination regimens, and they highlight an analytic approach that may become increasingly valuable for decision making in the setting of sparse data on resistance to multiple antimicrobial regimens.
MATERIALS AND METHODS
Approach.
Our aim was to understand the population-level dynamics of the emergence of multiple antimicrobial resistance in an infectious pathogen that is treated with combination therapy but for which empirical data on the effects of different resistance patterns are sparse. To achieve this aim, we used a mechanistic simulation of TB transmission and drug resistance to project a range of plausible epidemiologic trajectories, randomly sampling parameter values to reflect inherent uncertainty in key variables related to TB drug resistance (Fig. 1). First, we identified an outcome that could serve as a useful metric for decision making; in our primary analysis, we used the proportion of data-consistent trajectories in which the prevalence of pre-XDR TB exceeds an acceptability threshold of 1 case per 100,000 population at 20 years. We then selected epidemiologic data to which we could calibrate the model. These calibration targets, shown in Table S4 in the supplemental material, included the prevalence and incidence of TB disease from 1990 to 2013 in Southeast Asia (21, 22), which was selected as a target setting because of its high rates of TB and highly drug-resistant TB, as well as the prevalence of resistance against specific drugs for which empirical data were available. Further details of model initialization and calibration are provided in the supplemental material (23–27). For each epidemiologic calibration target, we set a tolerance range based on the degree of uncertainty around available data estimates (Table S4). We then constructed a representative set of scenarios that might be consistent with existing data by randomly sampling parameter sets using an approximate Bayesian process, retaining those sets that resulted in simulated outcomes within our tolerance ranges. We used these data-consistent parameter sets to project epidemiologic trajectories over the ensuing 20 years. These selected parameter sets are therefore not meant to represent the entirety of all possible scenarios, nor are they meant to indicate which scenarios are more likely than others; rather, they are meant as a representative sample that can be useful to inform decision making. This approach is illustrated step by step in Fig. 2.
Mechanistic model structure.
The core structure of our model is similar to that of previous compartmental models of adult pulmonary tuberculosis, assuming a static population size, random mixing, and sequential progression through the stages of TB infection (28–30). As shown in Fig. 1, people are born in the uninfected state and can progress to latent TB infection (an asymptomatic, noninfectious state) and active pulmonary TB disease (symptomatic and infectious). Each compartment of TB infection or disease is subdivided to explicitly track resistance to eight (i.e., 23) possible combinations of the three drugs considered. For any individual being treated for active TB, we assume that the treatment course will be effective, insufficient, or ineffective (defined below), with the probability of each outcome being conditional on both the pathogen's resistance profile and the drug regimen being used (see Table 2).
TABLE 2.
Outcomes upon treatment completion, by resistance profile and treatment regimena
| Final drug resistance profile | Probability (%) of: |
|||
|---|---|---|---|---|
| Cure |
Early relapse after cure |
|||
| First-line agents | Second-line agents | First-line agents | Second-line agents | |
| Drug susceptible | 89–99 | — | 4 | — |
| RIFr | 40–64 | 89–94 | 16 | 4 |
| FQr | 89–99 | — | 4 | — |
| PZAr | 83–90 | — | 8 | — |
| RIFr, FQr | 40–64 | 57–74 | 16 | 12 |
| RIFr, PZAr | 32–59 | 76–86 | 16 | 8 |
| FQr, PZAr | 83–90 | — | 8 | — |
| RIFr, FQr, PZAr | 32–59 | 47–68 | 16 | 12 |
Additional details are provided in Table S1 in the supplemental material. —, not applicable, as the second-line regimen is assumed to be given only to patients with resistance to at least rifampin; RIFr, rifampin resistance; PZAr, pyrazinamide resistance; FQr, fluoroquinolone resistance.
We assume that effective treatment is curative treatment that rapidly renders individuals noninfectious, reflecting the steep decrease in bacillary burden upon treatment initiation (31, 32). We include the possibility that some incomplete treatment courses may nonetheless be effective, reflecting the range of possible interactions between antimicrobial agents and host immune responses. Those patients who do not complete a full course of treatment and are not cured (i.e., patients who receive insufficient treatment) are assumed to remain ill and infectious. Treatment that results in early relapse is also represented in the model as insufficient.
In contrast to insufficient treatment (representing a treatment course that has curative potential but is simply not taken for a sufficient duration of time), ineffective treatment in this model represents a course that does not provide additional curative potential beyond the host's natural immune response. People on ineffective regimens remain infectious in this model, albeit at a reduced level, reflecting regimens that reduce the bacillary burden sufficiently to result in negative sputum smears but do not achieve sterilization and cure. The explicit modeling of ineffective treatment allows us to account for failing treatment regimens, which we assume last for 6 months, on average, reflecting a time point at which treatment effectiveness is commonly assessed (8). Individuals on ineffective regimens are assumed to remain symptomatic and/or test positive on follow-up evaluation (e.g., TB smear or culture), triggering the initiation of a repeat course of treatment. Repeat treatment may in turn be effective (leading to immediate transition to the latent compartment), insufficient (leading to a transition to the active TB compartment), or ineffective (leading to maintenance in the ineffective treatment state), depending on the regimen chosen and the resistance profile of the pathogen.
The model distinguishes patients who are undergoing their first course of TB treatment from those who have previously been treated, incorporating the greater prevalence of drug resistance among treatment-experienced patients. In the baseline scenario, we assume that 5% and 26% of treatment-naive and treatment-experienced patients with RIF-resistant TB, respectively, have access to a standardized second-line treatment regimen, reflecting a combination of access to drug susceptibility diagnostics and presumptive treatment, as estimated in this region (21).
Incorporation of data.
Selected model inputs are shown in Tables 1 and 2 (see Table S3 for more details). Parameters relating to diagnosis and treatment outcomes are based on WHO data and information published in the literature. These data were incorporated in the model using logical assumptions; for instance, with the same regimen, the probability of cure for a patient with TB resistant to two drugs in the regimen cannot be greater than the probability of cure for a patient with TB resistant to just one drug (21, 33–37). We incorporate uncertainty around these baseline outcome probabilities by varying the probability of treatment failure from zero to twice the baseline value for each of the eight strains.
TABLE 1.
Selected input parametersa
| Variable description | Baseline value | Reference(s) |
|---|---|---|
| Protection from reinfection in latent infection state | 0.5 | 41, 42 |
| Proportion progressing rapidly to active TB | 0.15 | 43 |
| Baseline life expectancy (yr) | 70 | 44 |
| TB-specific mortality rate/yr | 0.17 | 45 |
| Probability of endogenous reactivation over the patient's lifetime | 5% | 46 |
| Rate of diagnosis and treatment initiation/yr | 0.69 | 21 |
| Relative infectiousness of patients on ineffective treatment | 0.2 | 47 |
| Rate of spontaneous recovery from active TB/yr | 0.17 | 45 |
| % discontinuing treatment prior to completion | ||
| First-line treatment | 6 | 21 |
| Second-line treatment | 23 | 35 |
| % experiencing early relapse | ||
| Drug-sensitive TB | 4 | 48, 49 |
| RIF-resistant TB | 16 | 50 |
| FQ-resistant TB | 12 | 50 |
| PZA-resistant TB | 8 | 14 |
Additional details are provided in Table S3 in the supplemental material.
Some key parameters that lack reliable empirical estimates include (i) the reduction in transmissibility (transmission fitness) associated with each pattern of drug resistance, (ii) the probability of acquiring new antimicrobial resistance during treatment, and (iii) the effect of each resistance pattern on treatment outcomes for each combination of preexisting drug resistance profile and treatment regimen. For these parameters, we selected values for each simulation from broad and uniform prior distributions, reflecting the inherent uncertainty in the value of these parameters and allowing sufficient coverage of extreme values. Distributions for the probability of acquiring resistance while on each regimen were informed by a published meta-analysis (38), allowing the acquisition of resistance to more than one drug under the assumption of sequential acquisition, with preexisting drug resistance favoring the emergence of further resistance by reducing the number of active drugs.
Baseline and comparison scenarios.
Using these distributions, we randomly sampled 100,000 distinct parameter sets to project trajectories and calibrate the mechanistic model as described above. We initiated simulations from a steady-state condition in the prechemotherapy era, sequentially introducing resistance to RIF, PZA, and FQ. All parameters were varied as described above in the baseline scenario. We also attempted to calibrate the model under the assumption that PZA confers no protection against de novo resistance to RIF or FQs—and, thus, that PZA resistance imposes no additional risk of such mutations—by setting the probability of acquiring resistance to RIF or FQs among individuals with PZA-resistant TB equal to that for patients with PZA-susceptible TB. We conducted all subsequent analyses assuming a protective effect of PZA and compared the baseline scenario to an alternative scenario in which all patients with PZA-resistant TB receive a hypothetical drug of equal efficacy (with regard to its impact on the probability of cure and relapse).
Sensitivity and uncertainty analyses.
For each parameter set considered to be consistent with current epidemiologic data, we compared the proportion of trajectories with levels of pre-XDR TB that exceeded the 20-year prevalence acceptability threshold between the baseline scenario and the alternative scenario in which PZA is replaced by another drug. We then used multivariable logistic regression of standardized input parameter values on the expected probability of exceeding the threshold to identify the parameters (drivers) that are the most strongly correlated with this outcome, varying the acceptability threshold and also considering the partial rank correlation between inputs and pre-XDR prevalence in sensitivity analyses. We conducted additional analyses in which we blocked specific pathways of resistance amplification by setting the corresponding probabilities to zero, reflecting a hypothetical situation in which RIF and/or FQs are replaced by another drug of equal efficacy for patients with PZA-resistant TB. For all scenarios, we expressed uncertainty by providing the proportion of data-consistent simulations in which the prevalence of pre-XDR reached certain acceptability thresholds (rather than point estimates of pre-XDR TB resistance prevalence) and also the median and interquartile ranges of key intermediate outputs (e.g., the proportion of pre-XDR strains with concomitant PZA resistance) across all data-consistent simulations.
In order to assess the potential impact of stochastic events on the emergence (and potential die-out) of drug resistance, we constructed a stochastic adaptation of the model using the stochastic simulation algorithm adaptive tau method of Gillespie and colleagues (39) and replicated the analysis using this stochastic framework.
Software.
The simulation model and all analyses were implemented using the software R (40). All the code necessary to replicate the analyses, tables, and figures presented here is available in an online repository: https://github.com/m-fofana/TB-PZA-model.git.
Supplementary Material
ACKNOWLEDGMENT
We thank Larry Moulton for reviewing the study methods and providing helpful suggestions.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00498-16.
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/drugresistance/documents/surveillancereport/en/ Accessed 29 June 2015. [Google Scholar]
- 2.Barber M. 1965. Drug combinations in antibacterial chemotherapy. Proc R Soc Med 58:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuritzkes DR. 2011. Drug resistance in HIV-1. Curr Opin Virol 1:582–589. doi: 10.1016/j.coviro.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Zumla AI. 2011. Tuberculosis. Lancet 378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 5.Nosten F, Brasseur P. 2002. Combination therapy for malaria: the way forward? Drugs 62:1315–1329. doi: 10.2165/00003495-200262090-00003. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. 2012. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society—USA panel. JAMA 308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 7.Mitchison DA. 2004. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin Respir Crit Care Med 25:307–315. doi: 10.1055/s-2004-829503. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2010. Guidelines for treatment of tuberculosis. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2010/9789241547833/en/ Accessed 29 June 2015. [Google Scholar]
- 9.Lipsitch M, Sousa AO. 2002. Historical intensity of natural selection for resistance to tuberculosis. Genetics 161:1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthys F, Rigouts L, Sizaire V, Vezhnina N, Lecoq M, Golubeva V, Portaels F, Van der Stuyft P, Kimerling M. 2009. Outcomes after chemotherapy with WHO category II regimen in a population with high prevalence of drug resistant tuberculosis. PLoS One 4:e7954. doi: 10.1371/journal.pone.0007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kampen SC, Susanto NH, Simon S, Astiti SD, Chandra R, Burhan E, Farid MN, Chittenden K, Mustikawati DE, Alisjahbana B. 2015. Effects of introducing Xpert MTB/RIF on diagnosis and treatment of drug-resistant tuberculosis patients in Indonesia: a pre-post intervention study. PLoS One 10:e0123536. doi: 10.1371/journal.pone.0123536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyenga I, Roggi A, Sulis G, Diande S, Tamboura D, Tagliani E, Castelli F, Matteelli A. 2015. The impact of Xpert(R) MTB/RIF depends on service coordination: experience in Burkina Faso. Int J Tuberc Lung Dis 19:285–287. doi: 10.5588/ijtld.14.0629. [DOI] [PubMed] [Google Scholar]
- 13.Hossain ST, Isaakidis P, Sagili KD, Islam S, Islam MA, Shewade HD, Kamal SM, Husain A. 2015. The multi-drug resistant tuberculosis diagnosis and treatment cascade in Bangladesh. PLoS One 10:e0129155. doi: 10.1371/journal.pone.0129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, Vernon A, Lienhardt C. 2009. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med 6:e1000150. doi: 10.1371/journal.pmed.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke MF, Becerra MC, Tierney DB, Rich ML, Bonilla C, Bayona J, McLaughlin MM, Mitnick CD. 2015. Counting pyrazinamide in regimens for multidrug-resistant tuberculosis. Ann Am Thorac Soc 12:674–679. doi: 10.1513/AnnalsATS.201411-538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schito M, Migliori GB, Fletcher HA, McNerney R, Centis R, D'Ambrosio L, Bates M, Kibiki G, Kapata N, Corrah T, Bomanji J, Vilaplana C, Johnson D, Mwaba P, Maeurer M, Zumla A. 2015. Perspectives on advances in tuberculosis diagnostics, drugs, and vaccines. Clin Infect Dis 61(Suppl 3):S102–S118. doi: 10.1093/cid/civ609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon YS, Jeong BH, Koh WJ. 2014. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med 20:280–286. doi: 10.1097/MCP.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 18.Nijhuis M, van Maarseveen NM, Boucher CA. 2007. HIV protease resistance and viral fitness. Curr Opin HIV AIDS 2:108–115. doi: 10.1097/COH.0b013e32801682f6. [DOI] [PubMed] [Google Scholar]
- 19.Hamers RL, Schuurman R, Sigaloff KC, Wallis CL, Kityo C, Siwale M, Mandaliya K, Ive P, Botes ME, Wellington M, Osibogun A, Wit FW, van Vugt M, Stevens WS, de Wit TF, PharmAccess African Studies to Evaluate Resistance (PASER) Investigators. 2012. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 12:307–317. doi: 10.1016/S1473-3099(11)70255-9. [DOI] [PubMed] [Google Scholar]
- 20.Smith RJ, Okano JT, Kahn JS, Bodine EN, Blower S. 2010. Evolutionary dynamics of complex networks of HIV drug-resistant strains: the case of San Francisco. Science 327:697–701. doi: 10.1126/science.1180556. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2014. Global tuberculosis control 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ Accessed 29 June 2015. [Google Scholar]
- 22.World Health Organization. 2011. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015: WHO progress report 2011. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2011/mdr_report_2011/en/ Accessed 29 June 2015. [Google Scholar]
- 23.Umubyeyi AN, Rigouts L, Shamputa IC, Fissette K, Elkrim Y, de Rijk PW, Struelens MJ, Portaels F. 2007. Limited fluoroquinolone resistance among Mycobacterium tuberculosis isolates from Rwanda: results of a national survey. J Antimicrob Chemother 59:1031–1033. doi: 10.1093/jac/dkm038. [DOI] [PubMed] [Google Scholar]
- 24.Pierre-Audigier C, Surcouf C, Cadet-Daniel V, Namouchi A, Heng S, Murray A, Guillard B, Gicquel B. 2012. Fluoroquinolone and pyrazinamide resistance in multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 16:221–223, i–ii. doi: 10.5588/ijtld.11.0266. [DOI] [PubMed] [Google Scholar]
- 25.Hannan MM, Desmond EP, Morlock GP, Mazurek GH, Crawford JT. 2001. Pyrazinamide-monoresistant Mycobacterium tuberculosis in the United States. J Clin Microbiol 39:647–650. doi: 10.1128/JCM.39.2.647-650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginsburg AS, Hooper N, Parrish N, Dooley KE, Dorman SE, Booth J, Diener-West M, Merz WG, Bishai WR, Sterling TR. 2003. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis 37:1448–1452. doi: 10.1086/379328. [DOI] [PubMed] [Google Scholar]
- 27.Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, Williams R, Flanagan P, Watt B, Rayner A, Crowe M, Chadwick MV, Middleton AM, Watson JM. 2002. Antibiotic resistant tuberculosis in the United Kingdom: 1993-1999. Thorax 57:477–482. doi: 10.1136/thorax.57.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trauer JM, Denholm JT, McBryde ES. 2014. Construction of a mathematical model for tuberculosis transmission in highly endemic regions of the Asia-Pacific. J Theor Biol 358:74–84. doi: 10.1016/j.jtbi.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha S, Knight GM, Fofana M, Cohen T, White RG, Cobelens F, Dowdy DW. 2014. Drivers and trajectories of resistance to new first-line drug regimens for tuberculosis. Open Forum Infect Dis 1:ofu073. doi: 10.1093/ofid/ofu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills HL, Cohen T, Colijn C. 2013. Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis. Sci Transl Med 5:180ra149. doi: 10.1126/scitranslmed.3005260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joloba ML, Whalen CC, Cave DM, Eisenach KD, Johnson JL, Okwera A, Morrissey A, Bajaksouzian S, Feagin J, Mugerwa R, Ellner J, Jacobs MR. 2000. Determination of drug susceptibility and DNA fingerprint patterns of clinical isolates of Mycobacterium tuberculosis from Kampala, Uganda. East Afr Med J 77:111–115. [PubMed] [Google Scholar]
- 32.Dharmadhikari AS, Mphahlele M, Venter K, Stoltz A, Mathebula R, Masotla T, van der Walt M, Pagano M, Jensen P, Nardell E. 2014. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 18:1019–1025. doi: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee DP, Menzies D, Brassard P. 2012. Clinical outcomes of pyrazinamide-monoresistant Mycobacterium tuberculosis in Quebec. Int J Tuberc Lung Dis 16:604–609. doi: 10.5588/ijtld.11.0376. [DOI] [PubMed] [Google Scholar]
- 34.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. 2009. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 35.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang CY, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O'Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Pena J, Perez-Guzman C, Quelapio MI, Ponce-de-Leon A, et al. 2012. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, Hollm-Delgado MG, Keshavjee S, DeRiemer K, Centis R, D'Ambrosio L, Lange CG, Bauer M, Menzies D, Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. 2013. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 42:156–168. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, Baez J, Kochi A, Dye C, Raviglione MC. 2000. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 38.Lew W, Pai M, Oxlade O, Martin D, Menzies D. 2008. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med 149:123–134. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Gillespie DT, Petzold LR. 2007. Adaptive explicit-implicit tau-leaping method with automatic tau selection. J Chem Phys 126:224101. doi: 10.1063/1.2745299. [DOI] [PubMed] [Google Scholar]
- 40.R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 41.Vynnycky E, Fine PE. 1997. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 119:183–201. doi: 10.1017/S0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland I, Svandova E, Radhakrishna S. 1982. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle 63:255–268. [DOI] [PubMed] [Google Scholar]
- 43.Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. 1995. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med 1:815–821. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 44.United Nations Population Division. 2013. World population prospects: the 2012 revision. United Nations, New York, NY: Accessed 29 June 2015. [Google Scholar]
- 45.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. 2011. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One 6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harries AD, Dye C. 2006. Tuberculosis. Ann Trop Med Parasitol 100:415–431. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]
- 47.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. 1999. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 48.Leung CC, Yew WW, Chan CK, Chang KC, Law WS, Lee SN, Tai LB, Leung EC, Au RK, Huang SS, Tam CM. 2015. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J 45:738–745. doi: 10.1183/09031936.00114214. [DOI] [PubMed] [Google Scholar]
- 49.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, Pappas F, Phillips PP, Nunn AJ, REMoxTB Consortium. 2014. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, Vernon A, Lienhardt C, Burman W. 2009. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med 6:e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.