ABSTRACT
The purpose of this study was to evaluate risk factors for failure of antibiotic treatment within 30 days for uncomplicated skin infections of outpatients treated in a Veterans Affairs hospital. A retrospective chart review of outpatients between January 2006 and July 2015 with an ICD-9 (International Statistical Classification of Diseases and Related Health Problems) code of cellulitis or abscess was included in the analysis. The primary outcome was success versus failure of the antibiotic, with failure defined as another antibiotic prescribed or hospitalization within 30 days for the original indication. A total of 293 patients were included in the final analysis, 24% of whom failed within 30 days. Obesity/overweight (body mass index [BMI] of >25 kg/m2) was identified in 83% of the overall population, with 16% of that population having a BMI greater than 40 kg/m2. An elevated mean BMI of 34.2 kg/m2 (P = 0.0098) was found in the subset of patients who failed oral antibiotics compared to a BMI of 31.32 kg/m2 in patients who were treated successfully. Additionally, the patients who failed had an increased prevalence of heart failure at 16% (P = 0.027). Using multivariate logistic regression, BMI and heart failure were determined to be significant predictors of antibiotic prescription failure. Each 10-kg/m2 unit increase in BMI was associated with a 1.62-fold-greater odds of failure. A diagnosis of heart failure increased the odds of failure by 2.6-fold (range, 1.1- to 5.8-fold). Outpatients with uncomplicated skin infections with an elevated BMI and heart failure were found to have increased odds of failure, defined as hospitalization or additional antibiotics within 30 days.
KEYWORDS: cellulitis, skin and soft tissue infections, antimicrobial, obesity
INTRODUCTION
The incidence of skin and soft tissue infections in outpatients in the United States has increased from 8.6 million in 1997 to 14.2 million in 2005 (1). Hospitalization rates due to poorly managed or untreated skin infections have also continued to rise (1, 2).
Most often, skin and soft tissue infections are treated with oral antibiotics in the outpatient setting, such as emergency rooms or primary care offices. In the literature, the overall treatment failure rate of oral antibiotics ranges from 10% to 21% (3–7). One study found that the mean additional cost associated with antibiotic failure was $1,934 per patient (5).
Knowledge of risk factors for antibiotic prescription failure for skin and soft tissue infections in the outpatient setting is limited. There are two studies that describe risk factors for failure in the emergency room in patients who received oral or intravenous therapy (4, 6). A study that observed patients treated in the emergency room with both oral and intravenous antibiotics for cellulitis found that risk factors associated with treatment failure included fever, chronic leg ulcers or edema, and history of cellulitis or cellulitis around a wound site (4). Additionally, Murray et al. found that in those patients treated with antibiotics in the emergency room, risk factors associated with treatment failure were older age, prior oral antibiotic therapy, and surface area of the infection (6).
Many outpatients with skin and soft tissue infections are treated solely with oral antibiotics, and studies have not identified risks of failure in this subset of patients. As health care costs continue to rise, it is ideal to identify patients at increased risk of failure with oral antibiotics to avoid excessive morbidity and the need for hospital admission.
In this study, the primary objective was to assess the risk factors for failure of oral antibiotic treatment within 30 days for skin and soft tissue infections of outpatients treated in a Veterans Affairs hospital emergency room and primary care offices. We retrospectively evaluated the failure rates of oral antibiotic regimens prescribed for the treatment of cellulitis, abscess, and mixed skin infections in the emergency room and/or primary care offices.
RESULTS
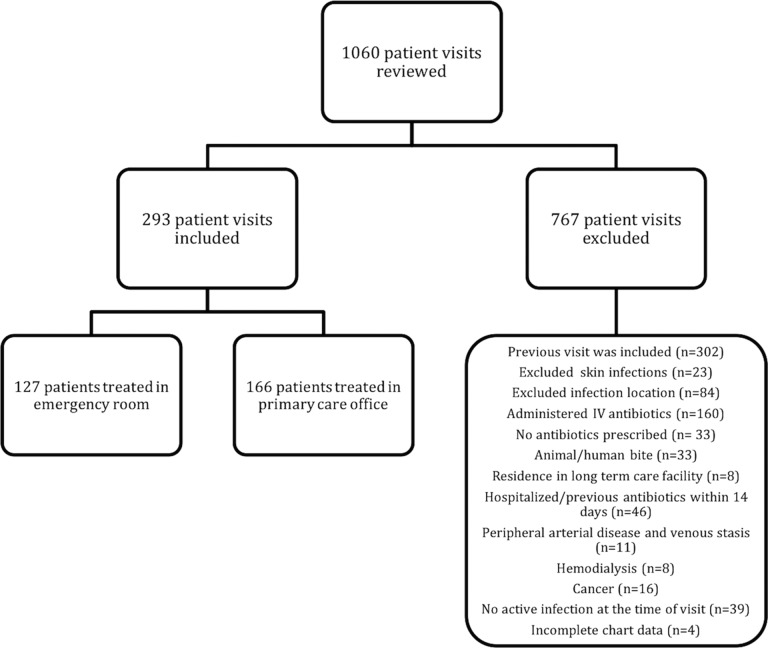
A total of 1,060 patient visits were reviewed using the Veterans Affairs Western New York (VA WNY) Healthcare System electronic medical record (EMR); 293 patients were included and 767 patients were excluded (Fig. 1). There were 127 patients (43%) treated in the emergency department and 166 patients (57%) treated in the primary care offices. Cellulitis was treated in 182 patients (62%), abscesses were treated in 67 patients (23%), and mixed cellulitis and abscess were treated in 44 patients (15%). Of the overall population, 69 patients (24%) failed oral antibiotics as defined by a need for an additional antibiotic or hospitalization within 30 days for the original indication. Of patients treated for abscess, 26.7% failed. Patients with cellulitis alone had a failure rate of 22.5%. Failure was found in 22.7% of patients treated for a mixed infection with abscess that had cellulitis extending beyond what would be expected with abscess alone. Hospitalization for worsening skin infections was required in 14 of the 69 patients who failed oral antibiotic therapy (20.3%).
FIG 1.
Inclusion and exclusion. IV, intravenous.
The majority of patients of the total population were overweight/obese: 83% had a body mass index (BMI) greater than 25 kg/m2. One hundred fifty-nine patients (54%) were overweight (BMI of 25.0 to 29.9 kg/m2), and 84 patients (29%) were obese (BMI of 30 kg/m2 and greater) (8). Forty-eight (16%) patients had a BMI of 40 kg/m2 or greater.
Patients who failed antibiotics and those who succeeded were similar in terms of age, race, height, temperature, serum creatinine, and creatinine clearance (Table 1). Patients who failed treatment were more likely to be obese, with an elevated mean BMI of 34.2 kg/m2 (P = 0.0098). The median (interquartile range) Charlson comorbidity index for those who failed was 1 (0 to 3), compared to 1 (0 to 2) (P = 0.048) for those who succeeded. Additionally, the patients who failed therapy were found to have an increased prevalence of diabetes at 48% (P = 0.026) and of heart failure at 16% (P = 0.027). Diabetics had a higher BMI than did nondiabetics. The mean BMI for diabetics in our cohort was 36 kg/m2, and that in nondiabetics was 29.6 kg/m2 (P < 0.0001).
TABLE 1.
Baseline patient characteristics
| Demographic characteristica | Total cohort (n = 293) | Success (n = 224) | Failure (n = 69) | P value |
|---|---|---|---|---|
| Age (yr), mean ± SD | 60.54 ± 17.20 | 60.79 ± 17.5 | 59.72 ± 6.15 | 0.65 |
| Male, no. (%) | 276 (94.2) | 208 (92.9) | 68 (98.6) | 0.077 |
| Race, no. (%) | 0.95 | |||
| African American | 46 (15.7) | 36 (16.1) | 10 (14.5) | |
| Caucasian | 243 (82.9) | 185 (82.6) | 58 (84.1) | |
| Other | 4 (1.4) | 3 (1.3) | 1 (1.5) | |
| Ht (in.), mean ± SD | 69.82 ± 2.99 | 69.89 ± 3.04 | 69.55 ± 2.83 | 0.40 |
| Wt (kg), mean ± SD | 100.63 ± 26.50 | 98.88 ± 24.57 | 106.3 ± 31.5 | 0.043 |
| BMI (kg/m2), mean ± SD | 32.0 ± 7.89 | 31.32 ± 7.21 | 34.18 ± 9.49 | 0.0098 |
| Temp (°F), mean ± SD | 97.57 ± 0.94 | 97.55 ± 0.94 | 97.63 ± 0.92 | 0.55 |
| Diabetes, no. (%) | 107 (36.5) | 74 (33) | 33 (47.8) | 0.026 |
| Heart failure, no. (%) | 27 (9.2) | 16 (7.1) | 11 (15.9) | 0.027 |
| Mild liver disease, no. (%) | 18 (6.1) | 18 (8) | 0 | 0.15 |
| Charlson score, median (interquartile range) | 1 (0–2) | 1 (0–2) | 1 (0–3) | 0.048 |
| Chronic kidney disease, no. (%) | 26 (8.9) | 19 (8.5) | 7 (10.1) | 0.67 |
| Serum creatinine (mg/dl), mean ± SD | 1.06 ± 0.35 | 1.06 ± 0.35 | 1.08 ± 0.32 | 0.73 |
| Creatinine clearance (ml/min), mean ± SD | 114.05 ± 53.15 | 112.04 ± 51.44 | 120.66 ± 58.34 | 0.25 |
| MRSA colonization (n = 94), no. (%) | 8 (8.5) | 5 (7) | 3 (13) | 0.37 |
| Penicillin allergy, no. (%) | 29 (9.9) | 22 (9.8) | 7 (10.1) | 0.94 |
BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
Cultures were obtained from 45 patients; of those, 20.3% (n = 14) failed and 13.8% (n = 31) experienced success (P = 0.2). Methicillin-resistant Staphylococcus aureus (MRSA) was present in 14 cultures; of those, 9 patients failed and required additional antibiotics. MRSA was present in 10 patients with abscess, 3 patients with mixed infections, and 1 patient with cellulitis.
The types of skin and soft tissue infections and locations, oral antibiotic regimens, and provider locations were similar between the group of patients who failed antibiotics and those who succeeded (Table 2). The most prevalent anatomic area of infection was the lower extremity (58.7%). Trimethoprim-sulfamethoxazole (TMP-SMX) (32.8%) and cephalexin (29.7%) were the most common antibiotics prescribed. Twenty percent of patients received other antibiotic regimens that consisted of dicloxacillin, amoxicillin-clavulanate, doxycycline, and penicillin. There was no statistically significant difference in treatment duration between the two groups, both having an average duration of approximately 9 days.
TABLE 2.
Clinical characteristics of skin infections
| Demographic characteristic | Total cohort (n = 293) | Success (n = 224) | Failure (n = 69) | P value |
|---|---|---|---|---|
| Provider location, no. (%) | 0.98 | |||
| Emergency room | 127 (43.3) | 97 (43.3) | 30 (43.5) | |
| Primary care | 166 (56.7) | 127 (56.7) | 39 (56.5) | |
| Antibiotic prescribed, no. (%) | 0.89 | |||
| Cephalexin | 87 (29.7) | 68 (30.4) | 19 (27.5) | |
| Clindamycin | 45 (15.4) | 35 (15.6) | 10 (14.5) | |
| TMP-SMX | 96 (32.8) | 71 (31.7) | 25 (36.2) | |
| TMP plus cephalexin | 8 (2.7) | 7 (3.1) | 1 (1.4) | |
| Other | 57 (19.4) | 43 (19.2) | 14 (20.3) | |
| Duration of treatment (days), mean ± SD | 9.0 ± 1.92 | 8.94 ± 2.01 | 9.14 ± 1.59 | 0.43 |
| Anatomic area, no. (%) | 0.66 | |||
| Buttocks/groin | 20 (6.8) | 14 (6.2) | 6 (8.7) | |
| Head/neck | 50 (17.1) | 38 (17) | 12 (17.4) | |
| Lower extremity | 172 (58.7) | 129 (57.6) | 43 (62.3) | |
| Trunk/abdomen | 13 (4.4) | 11 (4.9) | 2 (2.9) | |
| Upper extremity | 38 (13) | 32 (14.3) | 6 (8.7) | |
| Incision and drainage, no. (%) | 51 (17.4) | 38 (20) | 13 (18.8) | 0.72 |
| Type of infection, no. (%) | 0.77 | |||
| Abscess | 67 (22.9) | 49 (21.9) | 18 (26.1) | |
| Cellulitis | 182 (62.1) | 141 (62.9) | 41 (59.4) | |
| Mixed | 44 (15) | 34 (15.2) | 10 (14.5) | |
| Culture result (n = 45), no. (%) | 0.012 | |||
| Gram negative | 1 (2.2) | 1 (3.2) | 0 | |
| MRSA | 14 (31.1) | 5 (16.1) | 9 (64.3) | |
| S. aureus | 16 (35.6) | 12 (38.7) | 4 (28.6) | |
| Skin flora | 11 (24.4) | 11 (35.5) | 0 | |
| No growth | 3 (6.7) | 2 (6.4) | 1 (7.1) | |
| Hospitalization within 30 days, no. (%) | 15 (5.1) | 1 (0.5) | 14 (20.3) | <0.0001 |
Doses of the oral antibiotic regimens varied among patients in respect to each of the antibiotics prescribed. Appropriate therapeutic dosing regimens were determined to be the following: cephalexin at 500 mg by mouth every 6 h, clindamycin at 300 mg by mouth every 6 h or 450 mg by mouth every 8 h, and TMP-SMX at 1 double-strength (DS) tablet (800 mg/160 mg, respectively) by mouth every 12 h. In those patients with renal dysfunction, dosing adjustments according to creatinine clearance were considered to be therapeutic per the Lexi-Comp database (Lexi-Comp, Inc., Hudson, OH). Of the 69 patients who failed oral antibiotics, 11 patients (15.9%) were found to have subtherapeutic antibiotic dosing regimens. In those who were treated successfully with oral antibiotics, 33 patients (14.7%) were found to have subtherapeutic dosing regimens.
Significant risk factors for failure were determined using multivariate logistic regression analysis. Charlson comorbidity index (P = 0.99), liver disease (P = 0.99), and diabetes (P = 0.33) were removed, as they were not statistically significant. BMI and heart failure were significant predictors of failure in the final model. The unit odds ratio (OR) for failure with BMI was 1.05 (95% confidence interval [CI] = 1.0 to 1.1, P = 0.0085). Therefore, each 10-kg/m2 unit increase in BMI was associated with a 1.62-fold-higher odds of failure with oral antibiotic treatment for skin and soft tissue infections. Heart failure increased the risk of failure by 2.56 (95% CI = 1.1 to 5.8, P = 0.03). Based on our model, failure of antibiotics in outpatients without heart failure ranged from 13.5% in normal-weight patients with a BMI of 20 kg/m2 to 48.7% in patients with a BMI of 60 kg/m2. In patients with a comorbidity of heart failure, failure of antibiotics was greater; 28.7% would fail with a BMI of 20 kg/m2 and 70.9% were predicted to fail with a BMI of 60 kg/m2 (Table 3). A second logistic regression model examined the interaction between BMI and heart failure; the effect test indicated no interaction (P = 0.48).
TABLE 3.
Predicted failure rates with and without heart failure with various BMIs
| BMI (kg/m2) | Failure rate (%) |
|
|---|---|---|
| No heart failure | Heart failure | |
| 20 | 13.5 | 28.7 |
| 30 | 19.7 | 38.7 |
| 40 | 28.7 | 49.7 |
| 50 | 37.7 | 60.8 |
| 60 | 48.7 | 70.9 |
DISCUSSION
Despite the convenience of treating skin and soft tissue infections with oral antibiotics, the subsets of patients with heart failure and obesity are at increased risk of failing oral therapy, leading to additional antibiotic regimens and hospitalization. The Infectious Diseases Society of America (IDSA) published practice guidelines on the treatment of skin and soft tissue infections in 2014 (9). In these guidelines, oral antibiotic therapy is recommended for patients without systemic inflammatory response syndrome, altered mental status, and hemodynamic instability. This paper provides evidence that patients with perfusion abnormalities are at an increased risk for failure and may not succeed within the limitations of the guidelines. Based on this study, decreasing failure and hospitalization rates may be achieved by focusing on patients with elevated BMI and those with heart failure.
In this study, our failure rate was 24%, which is higher than that in previously published studies with a range of 10% to 21% (3–7). This may be due to the increased number of overweight and obese patients compared to prior studies. Sixteen percent of our patients had a BMI greater than 40 kg/m2; such patients have been excluded from previous studies and thus may not be a true comparison of failure rates to our study population (7).
More than 1.9 billion adults worldwide were considered overweight, with over 600 million categorized as obese, in 2014, and obesity prevalence has more than doubled worldwide since 1980 (10). Obesity has been established as one of the top three leading causes of preventable death in the United States (11). In the United States obesity epidemic, 1 in 3 adults is estimated to be obese. As these numbers continue to rise, treatment of this select population will need to be optimized (12).
It has been found that skin and soft tissue infections in the obese population have increased in incidence and account for some of the most common antibiotic prescriptions (13, 14). In obese patients, the possible proposed mechanism for increased failure may be attributed to pharmacokinetics and pharmacodynamic characteristics of antibiotics that are altered in obese patients (15). Specifically, increased volume of distribution into adipose tissue, increased clearance, and insufficient antibiotic penetration may all result in oral antibiotics not reaching the desired site of action (16).
There is a limited amount of literature on dosing recommendations in the obese population; some studies have drawn the conclusion of increased failure rates in obese patients due to inadequate concentration but have had difficulty accounting for the mechanism of failure. Beta-lactams, such as cephalexin, are known to be hydrophilic drugs with poor penetration into adipose tissue but are generally safe, well-tolerated drugs with few adverse effects. Inadequate tissue concentrations of cefazolin can lead to concentrations below the MIC, which can lead to failure (17–19). In morbidly obese surgical patients, 2-g cefazolin doses resulted in a reduction of surgical site infection rates from 16.5% to 5.6% (19). A study of patients with osteomyelitis concluded that clearance of intravenous clindamycin increases as body weight increases, suggesting that obesity in patients may result in subtherapeutic concentration doses of clindamycin due to increased clearance and a requirement for higher doses (20). This study included 50 patients and 122 plasma concentrations of patients treated with clindamycin. Sixty-eight percent of patients were categorized as overweight, with BMIs ranging from 25 to 30 kg/m2, and 8% were categorized as obese, with BMIs greater than 30 kg/m2 (20). A study suggested that TMP-SMX, a hydrophilic antibiotic, should be dosed on the basis of patients' total body weight due to insufficient concentrations and increased failure in obese patients (17, 21). In a study by Halilovic et al., morbidly obese patients with cellulitis and/or abscess dosed with TMP-SMX as 1 double-strength tablet twice a day were more likely to fail treatment than were nonobese patients (21).
Alon et al. concluded that approximately 8% of infection-associated admissions in heart failure patients were due to skin and soft tissue infections (22). Patients with heart failure often present with chronic edema in the lower extremities, which may predispose patients to cellulitis (23). An additional reason may be due to heart failure patients having reduced cardiac output and peripheral vasoconstriction, leading to reduced volume of distribution and possibly poor penetration of systemic antibiotic into the infection site (24, 25).
The study has several limitations. The retrospective study design relies on the accuracy of the electronic medical record and thus may be subject to selection bias. Because this study was conducted at a single institution and in a veteran population, its external validity may be limited. Patient adherence to medication and nonpharmacologic measures was unable to be assessed. The study was conducted in a predominantly older, male, Caucasian population. It is also conceivable that patients with an abscess and significant cellulitis could have benefited from initial intravenous antibiotic therapy, as this category of patients had cellulitis extending beyond what would have been expected with an abscess alone.
Failure rates of oral antibiotics for skin and soft tissue infections in this largely obese population were elevated. The failure rate of oral antibiotics was 24%. Future directions to help combat the unacceptably high treatment failure rate in the subsets of obese and heart failure patients are needed. Antibiotic doses and duration, as well as route of administration (intravenous versus oral), should be optimized to prevent failure in this vulnerable population.
MATERIALS AND METHODS
Design.
This was a single-center, quality assurance/quality improvement, retrospective cohort of outpatients treated for skin and soft tissue infections in the emergency department or primary care offices at the Veterans Affairs Western New York Healthcare System in Buffalo, NY (WNY VA Healthcare System). Patients' charts were identified through ICD-9 (International Statistical Classification of Diseases and Related Health Problems) diagnosis codes (528.3, 681, and 682) for patients treated for cellulitis and/or abscess skin infection during the period of 1 January 2006 to 1 July 2015.
Study population.
Patients included in the study were those with a diagnosis of skin and soft tissue infection as defined by ICD-9 code. Patients had to have been treated in the emergency department or primary care offices and prescribed an oral antibiotic. Exclusion criteria consisted of any of the following: skin infections (e.g., impetigo, carbuncles, folliculitis, furunculosis, and psoriasis), skin infections at a location that required supplementary management (e.g., bursitis, hand, genital site, mastitis, perirectal site, catheter site, prosthetic site, surgical site, and orbital site), administration of intravenous therapy, any infection caused by human or animal bite, residence in a long-term care facility, hospitalization within the previous 14 days, reception of antibiotic therapy within the previous 14 days, diagnosis of peripheral arterial disease (PAD) and venous stasis, hemodialysis, and immunosuppressant conditions (e.g., immunosuppressive therapy, HIV/AIDS, and active cancer). Patients with mild abscesses treated only with incision and drainage and no oral antibiotics were excluded as well.
Outcome measures.
The primary outcome was the failure rate of oral antibiotic therapy, defined as need for another antibiotic or hospitalization for cellulitis or abscess within 30 days from initial treatment. Baseline demographics included age, gender, race, height, weight, body mass index (BMI), temperature, serum creatinine, penicillin allergy, methicillin-resistant Staphylococcus aureus nasal colonization, and comorbid conditions, including diabetes, heart failure, and chronic kidney disease. Additional data collected included oral antibiotic treatment, including dose and duration, location of provider, type and location of infection, incision and drainage procedure, culture of infection, and additional antibiotic or hospitalization within 30 days of original treatment for skin and soft tissue infection.
Relevant hospital microbiology data.
Methicillin-resistant S. aureus (MRSA) comprises 38 to 50% of all strains of S. aureus at the VA WNY Healthcare System. MRSA susceptibility is 98 to 100% to trimethoprim-sulfamethoxazole (TMP-SMX), 65 to 68% to clindamycin, and 99% to doxycycline. Susceptibility of methicillin-susceptible S. aureus (MSSA) is 100% to cephalexin, 99 to 100% to TMP-SMX, 78 to 88% to clindamycin, and 96% to doxycycline.
Statistics.
Bivariate analysis was used to compare those who had success with treatment with those who failed treatment. To evaluate significant differences with respect to baseline characteristics, the independent Student t test was used for continuous variables and either the chi-square or Fisher's exact test was used for categorical variables. Aggregate significant baseline characteristics (P < 0.05) from the bivariate analysis were built into a multivariate logistic regression analysis to determine predictors of failure. Factors were removed in a backward-elimination fashion until a stable model for predicting failure of the treatment was reached. Results were presented as odds ratios (ORs) with a 95% confidence interval. Statistical analysis was performed utilizing JMP software version 12 (SAS Institute Inc., Cary, NC).
REFERENCES
- 1.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 2.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, Spalding J, Jiang J, Oster G. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 15:1516–1518. doi: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong SL, Kelly KD, Oland RC, Holroyd BR, Rowe BH. 2001. ED management of cellulitis: a review of five urban centers. Am J Emerg Med 19:535–540. doi: 10.1053/ajem.2001.28330. [DOI] [PubMed] [Google Scholar]
- 4.Peterson D, McLeod S, Woolfrey K, McRae A. 2014. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med 21:526–531. doi: 10.1111/acem.12371. [DOI] [PubMed] [Google Scholar]
- 5.Labreche MJ, Lee GC, Attridge RT, Mortensen EM, Koeller J, Du LC, Nyren NR, Trevino LB, Trevino SB, Pena J, Mann MW, Munoz A, Marcos Y, Rocha G, Koretsky S, Esparza S, Finnie M, Dallas SD, Parchman ML, Frei CR. 2013. Treatment failure and costs in patients with methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med 26:508–517. doi: 10.3122/jabfm.2013.05.120247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray H, Stiell I, Wells G. 2005. Treatment failure in emergency department patients with cellulitis. CJEM 7:228–234. doi: 10.1017/S1481803500014342. [DOI] [PubMed] [Google Scholar]
- 7.Miller LG, Daum RS, Creech CB, Young D, Downing MD, Eells SJ, Pettibone S, Hoagland RJ, Chambers HF, DMID 07-0051 Team . 2015. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 372:1093–1103. doi: 10.1056/NEJMoa1403789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2012. Defining adult overweight and obesity. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/obesity/adult/defining.html. [Google Scholar]
- 9.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC, Infectious Diseases Society of America. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2015. Obesity and overweight. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 11.Stein CJ, Colditz GA. 2004. The epidemic of obesity. J Clin Endocrinol Metab 89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2011. The obesity epidemic. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/cdctv/diseaseandconditions/lifestyle/obesity-epidemic.html. [Google Scholar]
- 13.Falagas ME, Kompoti M. 2006. Obesity and infection. Lancet Infect Dis 6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 14.Petersen I, Hayward AC, SACAR Surveillance Subgroup. 2007. Antibacterial prescribing in primary care. J Antimicrob Chemother 60(Suppl 1):i43–47. doi: 10.1093/jac/dkm156. [DOI] [PubMed] [Google Scholar]
- 15.Longo C, Bartlett G, Macgibbon B, Mayo N, Rosenberg E, Nadeau L, Daskalopoulou SS. 2013. The effect of obesity on antibiotic treatment failure: a historical cohort study. Pharmacoepidemiol Drug Saf 22:970–976. doi: 10.1002/pds.3461. [DOI] [PubMed] [Google Scholar]
- 16.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 17.Janson B, Thursky K. 2012. Dosing of antibiotics in obesity. Curr Opin Infect Dis 25:634–649. doi: 10.1097/QCO.0b013e328359a4c1. [DOI] [PubMed] [Google Scholar]
- 18.Wurtz R, Itokazu G, Rodvold K. 1997. Antimicrobial dosing in obese patients. Clin Infect Dis 25:112–118. doi: 10.1086/514505. [DOI] [PubMed] [Google Scholar]
- 19.Forse RA, Karam B, MacLean LD, Christou NV. 1989. Antibiotic prophylaxis for surgery in morbidly obese patients. Surgery 106:750–756. [PubMed] [Google Scholar]
- 20.Bouazza N, Pestre V, Jullien V, Curis E, Urien S, Salmon D, Treluyer JM. 2012. Population pharmacokinetics of clindamycin orally and intravenously administered in patients with osteomyelitis. Br J Clin Pharmacol 74:971–977. doi: 10.1111/j.1365-2125.2012.04292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halilovic J, Heintz BH, Brown J. 2012. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 65:128–134. doi: 10.1016/j.jinf.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Alon D, Stein GY, Korenfeld R, Fuchs S. 2013. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One 8:e72476. doi: 10.1371/journal.pone.0072476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho S, Atwood JE. 2002. Peripheral edema. Am J Med 113:580–586. doi: 10.1016/S0002-9343(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 24.Shammas FV, Dickstein K. 1988. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet 15:94–113. doi: 10.2165/00003088-198815020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. 2009. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]