ABSTRACT
We report a case of infective endocarditis (IE) caused by ceftaroline-resistant, daptomycin-tolerant, and heterogeneous vancomycin-intermediate methicillin-resistant S. aureus (MRSA). Resistance to ceftaroline emerged in the absence of drug exposure, and the E447K substitution in the active site of PBP2a previously associated with ceftaroline resistance was identified. Additionally, we present evidence of patient-to-patient transmission of the strain within the same unit. This case illustrates the difficulties in treating MRSA IE in the setting of a multidrug-resistant phenotype.
KEYWORDS: endocarditis, Staphylococcus aureus, ceftaroline
INTRODUCTION
Infective endocarditis (IE) due to methicillin-resistant Staphylococcus aureus (MRSA) is a life-threatening infection for which vancomycin (VAN) has long been considered the drug of choice. However, its effectiveness has been questioned due to the emergence of S. aureus strains with reduced susceptibility to VAN (VAN-intermediate S. aureus [VISA] or heterogeneous resistance [hVISA]). Daptomycin (DAP) is a lipopeptide antibiotic with reliable in vitro bactericidal activity that is frequently used to treat invasive MRSA infections, including IE. Unfortunately, the emergence of DAP nonsusceptibility during therapy threatens its efficacy against severe MRSA infections, especially in the setting of decreased susceptibility to vancomycin (1). Among other alternatives, ceftaroline (CPT), a β-lactam with high affinity for penicillin-binding protein 2a (PBP2a), has been successfully used as salvage therapy for recalcitrant MRSA bacteremia and IE and has emerged as an interesting drug to manage these infections (2). However, recent reports of CPT-resistant MRSA isolates also threaten the clinical utility of this drug (3).
CASE PRESENTATION
The patient is a 36-year-old man without significant past medical history who was admitted to the burn unit after a motor vehicle accident resulting in burns of 28% of body surface. On day 46 of admission, the patient developed fever (102°F), and blood cultures yielded MRSA (VAN MIC, 1 μg/ml). The patient was started on VAN (1 g every 8 h), and a transesophageal echocardiogram revealed a 0.8- by 0.5-cm vegetation on the aortic valve. On day 7 of VAN therapy (trough levels between 15 and 20 μg/ml), blood cultures remained positive, and the patient developed an acute myocardial infarction attributed to an embolic occlusion of the coronary artery due to septic emboli. VAN was stopped, and therapy was switched to DAP (8 mg/kg body weight daily) plus CPT (600 mg every 8 h). The MICs of the isolate recovered from the bloodstream before the change of therapy were 2, 1, and 4 μg/ml for VAN, DAP, and CPT, respectively (Table 1). The patient rapidly improved after starting the new regimen, blood cultures cleared after 24 h, and he completed 6 weeks of combination therapy without recurrence of the bacteremia. Seven days after the onset of bacteremia in the patient described above, a 60-year-old man who had been admitted to the same unit with burns encompassing 55% of the body surface was diagnosed with ventilator-associated pneumonia (VAP). The organism recovered from bronchoalveolar lavage (BAL) fluid was an MRSA isolate that was also found to have a CPT MIC above the established clinical breakpoint (6 μg/ml) (Table 1). The patient was treated with VAN monotherapy with clinical improvement.
TABLE 1.
Susceptibility profiles of isolates from both patients
| Antimicrobial(s) | MIC (μg/ml) for isolatea: |
Interpretation (CLSI breakpoint MIC [μg/ml])b | |
|---|---|---|---|
| IE | VAP | ||
| Vancomycin | 2* | 2* | S (≤2) |
| Daptomycin | 1* | 1* | S (≤1) |
| Ceftaroline | 4* | 6* | R (≤1) |
| Clindamycin | >4 | >4 | R |
| Erythromycin | >4 | >4 | R |
| Gentamicin | >8 | >8 | R |
| Levofloxacin | >4 | >4 | R |
| Oxacillin | >2 | >2 | R |
| Linezolid | 1 | 1 | S |
| Rifampin | ≤1 | ≤1 | S |
| Tetracycline | ≤1 | ≤1 | S |
| TMP-SMXc | ≤0.5/9.5 | ≤0.5/9.5 | S |
IE, infective endocarditis; VAP, ventilator-associated pneumonia; *, MIC was determined with the Etest.
S, susceptible; R, resistant.
TMP-SMX, trimethoprim-sulfamethoxazole.
CHALLENGE QUESTION
What is the rationale for using the combination of DAP plus CPT in the treatment of recalcitrant infections caused by MRSA?
A. Development of reduced susceptibility to DAP is associated with an increase in susceptibility to β-lactams that target penicillin-binding protein 1 (PBP1).
B. CPT has intrinsic activity against MRSA.
C. β-Lactams seem to increase the binding of DAP to the cell membrane target.
D. The increased susceptibility to β-lactams has also been identified in hVISA and VISA MRSA strains that exhibit similar phenotypic characteristics to strains that have reduced susceptibility to DAP.
E. All of the above.
TREATMENT AND OUTCOME
High-inoculum MRSA infections and exposure to VAN are well-known risk factors for the development of the VISA/hVISA phenotype (4). Additionally, VISA/hVISA isolates have been shown to concomitantly exhibit decreased DAP susceptibility and to share common genetic pathways leading to tolerance to both of these compounds (5). In our case, the unexpected finding of CPT resistance left us with very few options to treat this patient. The combination of DAP and β-lactam antibiotics has shown synergism against MRSA, despite having β-lactam MICs out of the susceptible range (6). We decided to discontinue VAN and initiate a combination of DAP (8 mg/kg daily) plus CPT (600 mg every 8 h), which resulted in clinical success.
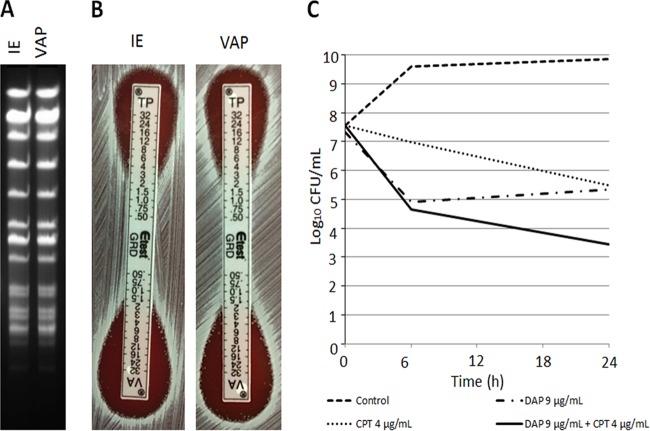
Due to the unexpected presence of CPT resistance and possibility of patient-to-patient transmission, we characterized the CPT-resistant S. aureus isolates recovered from the IE case and subsequent VAP patient. A detailed description of the methods is included in the supplemental material. The isolates obtained from both patients showed identical pulsed-field gel electrophoresis (PFGE) patterns (Fig. 1A) and antibiotic susceptibilities (Table 1). Screening of hVISA by the glycopeptide resistance detection (GRD) Etest was positive for both strains (Fig. 1B), and population analyses showed a subpopulation growing at a VAN concentration of 3 μg/ml (as is usually observed with hVISA strains). However, the calculated ratio of PAP to area under the concentration-time curve (AUC) compared to strain Mu3 was ≤9 (see Fig. S1 and Table S3 in the supplemental material). DAP and CPT were bacteriostatic in time-kill assays, but the combination of both antibiotics was synergistic, achieving bactericidal activity against the S. aureus strain recovered from the bloodstream of the IE patient (Fig. 1C). Whole-genome comparison of the strains showed the presence of 144 single nucleotide polymorphisms (SNPs) that differed between the isolates, suggesting that they were closely related strains. Additionally, both harbored SCCmec II agr type II and belonged to sequence type 105 (ST105) by multilocus sequence typing (MLST) (clonal complex 5). The analysis of resistomes predicted resistance to 5 antibiotic families and was concordant with the clinical susceptibility report (see Table S1 in the supplemental material). We found no genetic changes previously associated with DAP or VAN resistance or tolerance (see Table S2 in the supplemental material). In terms of CPT resistance, both isolates exhibited the E447K amino acid substitution in the predicted PBP2a protein encoded by the mecA gene. No other changes previously related to CPT resistance were found.
FIG 1.
PFGE, GRD test, and killing curves for S. aureus isolates. (A) SmaI restriction-PFGE results for the IE and VAP strains show identical patterns. (B) Result of GRD test for IE and VAP strains after 48 h of incubation. (C) Time-kill assays for the CPT-resistant hVISA IE strain. The CPT-resistant hVISA IE strain was grown in Mueller-Hinton broth (MHB) supplemented with DAP (9 μg/ml) alone, CPT (4 μg/ml) alone, or the DAP-CPT combination and in the absence of DAP and CPT. DAP alone and CPT alone failed to show bactericidal activity, defined as ≥3-log10-CFU/ml reduction at 24 h in comparison to the initial inoculum. However, the combination restored the bactericidal activity and showed synergistic effects (≥2-log10-CFU/ml reduction at 24 h in comparison to each antibiotic alone). The limit of detection was 10 CFU/ml. IE, infective endocarditis, VAP, ventilator-associated pneumonia; MHA, Mueller-Hinton agar, TP, teicoplanin.
In a single case, we illustrate the difficulties in treating MRSA IE when resistance emergences during therapy, showing the immense adaptability and plasticity of these multidrug-resistant organisms. The most striking feature of our case is the emergence of CPT resistance without exposure to the antibiotic and the ability of CPT-resistant organisms to be transmitted from patient to patient. Previous reports of CPT resistance emerging during therapy have been associated with the use of this antibiotic—sometimes for prolonged periods of time (3)—and to the best of our knowledge, emergence of resistance in the absence of CPT exposure has not been consistently documented. Our isolates harbored the E447K substitution in the predicted active site of PBP2a, which is one of the signature changes associated with CPT resistance (3). No changes in the allosteric domain of PBP2a or in other proteins previously involved in PBP2a-independent routes leading to CPT resistance were found (7). Our case raises the intriguing possibility that CPT resistance may be selected by β-lactams other than CPT. Particularly, our patient was previously exposed to piperacillin-tazobactam and cefepime. Another alternative is that the patient may have been colonized by a CPT-resistant strain, although this possibility is unlikely since he did not have a medical history suggestive of multiple contacts with the health care system. Importantly, CPT resistance emerged in the setting of decreased susceptibility to both VAN and DAP, suggesting that mutations in PBP 2a affecting CPT activity can be acquired in the setting of alterations in cell envelope homeostasis that mediate nonsusceptibility to VAN and DAP, posing an important therapeutic challenge. The fact that this multidrug-resistant MRSA isolate could be transmitted to another patient suggests that development of resistance did not markedly affect its fitness and the ability to spread from one host to the other. Additionally, the fact that the organism was capable of producing a different infection in a new patient suggests that no loss of virulence occurred, despite the expression of multiple resistance determinants.
The combination of DAP plus CPT has been shown to be synergistic in vitro against DAP-nonsusceptible strains of S. aureus. Exposure to β-lactams in such strains seems to increase the activity of DAP by favoring the binding of the antibiotic to the cell membrane (8). In a recent retrospective report, Sakoulas et al. analyzed 10 cases of MRSA IE and 2 cases of VISA infections (1 IE and 1 bacteremia) in which the combination of DAP plus CPT was successfully used. Of note, 2 cases included were DAP resistant, and one was an IE case due to MRSA with intermediate susceptibility to CPT (MIC, 2 μg/ml) (9). The management of our IE case was particularly complicated by the fact that the isolate was hVISA (and the patient failed VAN therapy) and was also shown to be DAP tolerant. Thus, additional CPT resistance significantly limited the therapeutic options. Despite the resistance phenotypes, we decided to use the combination of DAP plus CPT, taking theoretical advantage of the “seesaw” effect (10, 11). This rationale was strongly supported by our time-kill studies, which showed synergism using concentrations of antibiotics achievable by standard human dosing of both DAP and CPT. Moreover, recent studies have suggested that PBP1 plays an important role in this synergism, (12) and CPT has high affinity for S. aureus PBP1 (13). The clinical response was excellent, and we were able to complete therapy and successfully treat the patient without evidence of recurrence to date.
In summary, CPT resistance in the background of other multidrug-resistant phenotypes is a serious concern for the treatment of IE. Combination therapy seems to be effective against these organisms and should be seriously considered in the presence of multiple resistances.
COMMENTARY
The two patients with nosocomial infections reported by Nigo et al. from a hospital in Houston, one with endocarditis and one with ventilator-associated pneumonia, are a microcosm of a world of problems caused by methicillin-resistant Staphylococcus aureus (MRSA). These infections were caused by two very closely related sequence type 5 (ST5) multiple-drug resistant strains of the USA100 clone type, endemic in U.S. hospitals for over 30 years and still the most common type. The primary source and the mode of transmission of these strains are otherwise obscure and typical. Both strains had acquired resistance to ceftaroline due to a single canonical point mutation in mecA (3, 14, 15) under mysterious circumstances since neither patient had been previously treated with this antibiotic. It is of more than passing interest that the one other well-documented case of infection with ST5 ceftaroline-resistant MRSA was also identified in a Houston hospital 3 years earlier (3). In this case, there was a history of prior therapy with ceftaroline.
The report on a patient with endocarditis by Nigo et al. failed to clear the bacteremia with vancomycin, despite a vancomycin MIC of 1 μg/ml for the first blood isolate. The day 7 isolate had a vancomycin MIC of 2 μg/ml, a worrisome development as this occurred on therapy. Whether this was the cause or an effect of treatment failure is uncertain, particularly given that the patient with ventilator-associated pneumonia caused by a virtually identical isolate with a vancomycin MIC of 2 μg/ml responded to vancomycin. The importance of vancomycin MICs of >1 mg/ml as a predictor of treatment failure (16–28) has been hotly debated. In neither of these cases was the MIC a particularly good predictor. The patient whose initial isolate had a MIC of 1 μg/ml failed, whereas the patient whose initial isolate had a MIC of 2 μg/ml was cured.
What should be done when the patient is failing first-line therapy and choices are limited, as in the patient with endocarditis? The isolate was multiple-drug resistant, and although the daptomycin MIC was within the susceptible range, it was at the upper limit. Moreover, emergence of daptomycin resistance (technically, nonsusceptibility, but let us not mince words) on therapy does occur, may be preceded by prior therapy with a glycopeptide, and tracks with high-inoculum infections and intermediate susceptibility to vancomycin (i.e., VISA) (29, 30), including the rather ill-defined and difficult-to-test-for hVISA phenotype. The isolate did not meet strict criteria for either, but the distinction is academic given the persistently positive blood cultures, an increase in the MIC from 1 to 2 μg/ml, and a population analysis profile that just fell short of the Mu3 reference strain. These observations underscore limitations of in vitro susceptibility testing of vancomycin and argue against relying on the MIC alone for changing therapy. The choice of combination therapy with daptomycin given at 8 mg/kg once daily plus ceftaroline at 600 mg every 8 h was a reasonable one given the concern for emergence of resistance to daptomycin if used as a single agent. Ceftaroline alone was not an option because of resistance, but a β-lactam, even if there is resistance, in combination with daptomycin enhances binding of daptomycin to the bacterial cell membrane and synergistically potentiates its bactericidal effect (31–34). A theoretical added benefit is that each drug protects against emergence of higher-level resistance to the other by the seesaw effect (31), in which increasing resistance to one drug is counterbalanced by increasing susceptibility to the other. Rapid sterilization of the blood and eventual cure of the patient with endocarditis add to the admittedly anecdotal, but nevertheless compelling, data that daptomycin-ceftaroline combination therapy is an effective salvage regimen (9). Should such a regimen be used routinely as initial therapy for treatment of MRSA bacteremia and endocarditis? This question is best answered by a randomized clinical trial to determine whether outcomes are better for combination compared to single-drug therapy.
Supplementary Material
ACKNOWLEDGMENTS
C.A.A. is supported by grants from NIH NIAID (R01-AI093749, R21-AI114961, R21/R33 AI121519, and K24-AI114818). H.F.C. is supported by NIH grant R01AI100291.
This journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01235-16.
REFERENCES
- 1.Marty FM, Yeh WW, Wennersten CB, Venkataraman L, Albano E, Alyea EP, Gold HS, Baden LR, Pillai SK. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J Clin Microbiol 44:595–597. doi: 10.1128/JCM.44.2.595-597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho TT, Cadena J, Childs LM, Gonzalez-Velez M, Lewis JS II. 2012. Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother 67:1267–1270. doi: 10.1093/jac/dks006. [DOI] [PubMed] [Google Scholar]
- 3.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, Perez KK, Musick WL, Rosato AE, Musser JM. 2014. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes DM, Ward KE, LaPlante KL. 2015. Clinical implications of vancomycin heteroresistant and intermediately susceptible Staphylococcus aureus. Pharmacotherapy 35:424–432. doi: 10.1002/phar.1577. [DOI] [PubMed] [Google Scholar]
- 5.Cui L, Tominaga E, Neoh H, Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 50:1079–1082. doi: 10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard SN, Rolek KM. 2013. Evaluation of the combination of daptomycin and nafcillin against vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother 68:644–647. doi: 10.1093/jac/dks453. [DOI] [PubMed] [Google Scholar]
- 7.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-López C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. 2013. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci U S A 110:16808–16813. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. 2012. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 56:5296–5302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CYM, Rybak MJ, Kullar R, Dhand A, Rose WE, Goff DA, Bressler AM, Lee Y, Pogliano J, Johns S, Kaatz GW, Ebright JR, Nizet V. 2014. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 36:1317–1333. doi: 10.1016/j.clinthera.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. 2013. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open? Drug Resist Updat 16:73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Werth BJ, Vidaillac C, Murray KP, Newton KL, Sakoulas G, Nonejuie P, Pogliano J, Rybak MJ. 2013. Novel combinations of vancomycin plus ceftaroline or oxacillin against methicillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA. Antimicrob Agents Chemother 57:2376–2379. doi: 10.1128/AAC.02354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. β-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moisan H, Pruneau M, Malouin F. 2010. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother 65:713–716. doi: 10.1093/jac/dkp503. [DOI] [PubMed] [Google Scholar]
- 14.Alm RA, McLaughlin RE, Kos VN, Sader HS, Iaconis JP, Lahiri SD. 2014. Analysis of Staphylococcus aureus clinical isolates with reduced susceptibility to ceftaroline: an epidemiological and structural perspective. J Antimicrob Chemother 69:2065–2075. doi: 10.1093/jac/dku114. [DOI] [PubMed] [Google Scholar]
- 15.Chan LC, Basuino L, Diep B, Hamilton S, Chatterjee SS, Chambers HF. 2015. Ceftobiprole- and ceftaroline-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:2960–2963. doi: 10.1128/AAC.05004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JL, Wang JT, Sheng WH, Chen YC, Chang SC. 2010. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/L, by the broth microdilution method. BMC Infect Dis 10:159. doi: 10.1186/1471-2334-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Gao W, Christiansen KJ, Coombs GW, Johnson PD, Howden BP. 2011. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 204:340–347. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 18.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 19.Mavros MN, Tansarli GS, Vardakas KZ, Rafailidis PI, Karageorgopoulos DE, Falagas ME. 2012. Impact of vancomycin minimum inhibitory concentration on clinical outcomes of patients with vancomycin susceptible Staphylococcus aureus infections: a meta-analysis and metaregression. Int J Antimicrob Agents 40:496–509. doi: 10.1016/j.ijantimicag.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 21.Takesue Y, Nakajima K, Takahashi Y, Ichiki K, Ishihara M, Wada Y, Tsuchida T, Uchino M, Ikeuchi H. 2011. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 μg/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J Infect Chemother 17:52–57. doi: 10.1007/s10156-010-0086-0. [DOI] [PubMed] [Google Scholar]
- 22.van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin hetero-resistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6:e21217. doi: 10.1371/journal.pone.0021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price J, Atkinson S, Llewelyn M, Paul J. 2009. Paradoxical relationship between the clinical outcome of Staphylococcus aureus bacteremia and the minimum inhibitory concentration of vancomycin. Clin Infect Dis 48:997–998. doi: 10.1086/597359. [DOI] [PubMed] [Google Scholar]
- 24.de Sanctis JT, Swami A, Sawarynski K, Gerasymchuk L, Powell K, Robinson-Dunn B, Carpenter CF, Sims MD. 2011. Is there a clinical association of vancomycin MIC creep, agr group II locus, and treatment failure in MRSA bacteremia? Diagn Mol Pathol 20:184–188. doi: 10.1097/PDM.0b013e318208fc47. [DOI] [PubMed] [Google Scholar]
- 25.Chen SY, Liao CH, Wang JL, Chiang WC, Lai MS, Chie WC, Chang SC, Hsueh PR. 2014. Method-specific performance of vancomycin MIC susceptibility tests in predicting mortality of patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 69:211–218. doi: 10.1093/jac/dkt340. [DOI] [PubMed] [Google Scholar]
- 26.Baxi SM, Clemenzi-Allen A, Gahbauer A, Deck D, Imp B, Vittinghoff E, Chambers HF, Doernberg S. 2016. Vancomycin MIC does not predict 90-day mortality, readmission, or recurrence in a prospective cohort of adults with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 60:5276–5284. doi: 10.1128/AAC.00658-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. 2014. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564. doi: 10.1001/jama.2014.6364. [DOI] [PubMed] [Google Scholar]
- 28.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Howden BP, Johnson PD. 2013. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capone A, Cafiso V, Campanile F, Parisi G, Mariani B, Petrosillo N, Stefani S. 2016. In vivo development of daptomycin resistance in vancomycin-susceptible methicillin-resistant Staphylococcus aureus severe infections previously treated with glycopeptides. Eur J Clin Microbiol Infect Dis 35:625–631. doi: 10.1007/s10096-016-2581-4. [DOI] [PubMed] [Google Scholar]
- 30.Kelley PG, Gao W, Ward PB, Howden BP. 2011. Daptomycin non-susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J Antimicrob Chemother 66:1057–1060. doi: 10.1093/jac/dkr066. [DOI] [PubMed] [Google Scholar]
- 31.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. 2012. β-Lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berti AD, Theisen E, Sauer JD, Nonejuie P, Olson J, Pogliano J, Sakoulas G, Nizet V, Proctor RA, Rose WE. 2015. Penicillin binding protein 1 is important in the compensatory response of Staphylococcus aureus to daptomycin-induced membrane damage and is a potential target for β-lactam-daptomycin synergy. Antimicrob Agents Chemother 60:451–458. doi: 10.1128/AAC.02071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers HF, Basuino L, Hamilton SM, Choo EJ, Moise P. 2016. Daptomycin-β-lactam combinations in a rabbit model of daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 60:3976–3979. doi: 10.1128/AAC.00589-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.