ABSTRACT
Hearing loss and nephrotoxicity are associated with prolonged treatment duration and higher dosage of amikacin and kanamycin. In our tuberculosis center, we used therapeutic drug monitoring (TDM) targeting preset pharmacokinetic/pharmacodynamic (PK/PD) surrogate endpoints in an attempt to maintain efficacy while preventing (oto)toxicity. To evaluate this strategy, we retrospectively evaluated medical charts of tuberculosis (TB) patients treated with amikacin or kanamycin in the period from 2000 to 2012. Patients with culture-confirmed multiresistant or extensively drug-resistant tuberculosis (MDR/XDR-TB) receiving amikacin or kanamycin as part of their TB treatment for at least 3 days were eligible for inclusion in this retrospective study. Clinical data, including maximum concentration (Cmax), Cmin, and audiometry data, were extracted from the patients' medical charts. A total of 80 patients met the inclusion criteria. The mean weighted Cmax/MIC ratios obtained from 57 patients were 31.2 for amikacin and 12.3 for kanamycin. The extent of hearing loss was limited and correlated with the cumulative drug dose per kg of body weight during daily administration. At follow-up, 35 (67.3%) of all patients had successful outcome; there were no relapses. At a median dose of 6.5 mg/kg, a correlation was found between the dose per kg of body weight during daily dosing and the extent of hearing loss in dB at 8,000 Hz. These findings suggest that the efficacy at this lower dosage is maintained with limited toxicity. A randomized controlled trial should provide final proof of the safety and efficacy of TDM-guided use of aminoglycosides in MDR-TB treatment.
KEYWORDS: pharmacokinetics, pharmacodynamics, amikacin, TDM, tuberculosis, kanamycin, therapeutic drug monitoring
INTRODUCTION
Amikacin and kanamycin are almost similar aminoglycosides and are both are considered very useful as second line injectable drugs for the treatment of multidrug-resistant tuberculosis (MDR-TB) (1). MDR-TB is caused by Mycobacterium tuberculosis resistant to at least isoniazid and rifampin. Although the in vitro activity of amikacin and kanamycin appeared to be high against M. tuberculosis (2, 3), the early bactericidal activity was low (4). In addition, extremely resistant tuberculosis (XDR-TB) is resistant to at least one aminoglycoside and any fluoroquinolone.
According to World Health Organization (WHO) guidelines, aminoglycosides are administered in a dose of 15 mg/kg/day with a maximum of 1,000 mg daily in the treatment of patients with MDR-TB (5). Although cross-resistance between amikacin and kanamycin is thought to be nearly complete (6, 7), isolates resistant to one aminoglycoside may still be susceptible to the other aminoglycoside, and in vitro susceptibility should therefore be evaluated for each drug (8). The toxicity of aminoglycosides is profound and permanent, and hearing loss and nephrotoxicity have been observed in 8 to 37% of the patients receiving these drugs for any period of time (9–11). These adverse effects may worsen with prolonged treatment and higher dosage (1). In a study based on data of 28 TB patients in Botswana treated with 15 to 25 mg/kg amikacin daily, 7 patients developed hearing loss. The cumulative area under the curve (AUC) and the duration of amikacin treatment were predictors of hearing loss (12).
Aminoglycosides are not metabolized; renal excretion is the only elimination pathway. Patients with increased serum creatinine values and/or nephrotoxic comedication run a higher risk for encountering nephrotoxicity (1). Because of these serious adverse events, monitoring is advised and should consist of a baseline evaluation (audiogram, vestibular testing, Romberg testing, and serum creatinine measurement) and a monthly evaluation during treatment (questionnaire regarding auditory or vestibular symptoms and serum creatinine) (1). Aminoglycoside-related ototoxicity generally manifests first at high frequencies, sometimes without the patients noticing their hearing loss (13). Regular monitoring gives the opportunity to alter the provided therapy in order to prevent more extensive hearing loss.
Pharmacokinetic (PK) and pharmacodynamic (PD) parameters have increasingly gained attention in the development of drugs and treatment of TB in recent years (14). Data regarding PK and PD parameters in TB are, however, scarce. For other bacterial infections, predominantly Gram-negative infections, e.g., caused by Pseudomonas aeruginosa, the maximum concentration (Cmax)/MIC ratio is the most relevant PK/PD parameter to assess the efficacy of aminoglycosides (15, 16). In addition, it was shown that PK parameters of aminoglycosides may vary, and the patients may benefit from individualized treatment (17–21). In our TB Center, we used PK/PD parameters targeting a surrogate endpoint of a Cmax/MIC ratio of >20 to maintain efficacy while preventing (oto)toxicity Therefore, we performed a retrospective survey to evaluate the PK parameters of amikacin and kanamycin to detect predictors for PK parameters, as well as efficacy and toxicity.
RESULTS
Patient characteristics.
Eighty patients with a median age of 30.5 years (interquartile range [IQR] = 25.0 to 39.0 years) met the inclusion criteria; 37 (46.3%) patients were female, and 43 (53.8%) were male. Patient characteristics at baseline are presented in Table 1. Drug susceptibility testing was performed for all patients. All except three patients had a favorable outcome. One patient stopped due to drug addiction related problems, and two patients were transferred to other hospitals without follow-up. The blood levels of 57 patients (71%) were retrievable from the patient files.
TABLE 1.
Patient characteristics at baseline (n = 80)a
| Parameter | No (%) of patients or median (IQR) |
|
|---|---|---|
| Amikacin | Kanamycinb | |
| General characteristics | ||
| Male (%) | 26 (48.1) | 17 (65.4) |
| Female (%) | 28 (51.9) | 9 (34.6) |
| Age (yr) | 30 (25–39) | 31 (25–40) |
| Wt (kg) | 61.4 (55.2–68.4) | 57.2 (50.0–68.2) |
| BMI (kg/m2) | 21.2 (19.4–23.6) | 20.5 (18.5–22.4) |
| Ethnicity (%) | ||
| European | 7 (13.0) | 2 (7.7) |
| Asian | 17 (31.5) | 4 (15.4) |
| African | 14 (25.9) | 12 (46.2) |
| Other | 14 (25.9) | 7 (26.9) |
| Unknown | 2 (3.7) | 1 (3.8) |
| Tuberculosis | ||
| Localization (%) | ||
| Pulmonary | 42 (77.8) | 19 (73.1) |
| Extrapulmonary | 6 (11.1) | 3 (11.5) |
| Both pulmonary and extrapulmonary | 6 (11.1) | 4 (15.4) |
| Drug susceptibility | ||
| MDR (%) | 52 (96.3) | 26 (100) |
| XDR (%) | 2 (3.7) | 0 |
| Comorbidity | ||
| Diabetes mellitus type 1 (%) | 3 (5.6) | 1 (3.8) |
| Diabetes mellitus type 2 (%) | 3 (5.6) | 1 (3.8) |
| HIV coinfection (%) | 4 (7.4) | 4 (15.4) |
| Creatinine level at baseline | 64.0 (50.8–77.3) | 69.5 (51.3–77.3) |
The results are presented as median with interquartile range (IQR) in parentheses or as the number (%) of patients. BMI, body mass index; MDR, multidrug resistant; XDR, extensively drug resistant; HIV, human immunodeficiency virus.
One patient used amikacin but switched to kanamycin. This patient was included in the kanamycin results, since this aminoglycoside treatment represented the largest treatment period.
Pharmacokinetic and pharmacodynamics.
All patients but one started with a daily dosing regimen with a median dose of 400.0 mg (IQR = 400.0 to 568.2 mg) with a median duration of 85 days (IQR = 60 to 111 days). From these patients, 36 patients continued their aminoglycoside treatment—after the initial daily treatment—in a five-times-weekly regimen, with a median dose of 400.0 mg (IQR = 387.5 to 500.0 mg) and a median duration of 61 days (IQR = 56 to 78 days). One patient did not receive the first daily dosing schedule and was treated with the five-times-weekly regimen from the start. After this five-times-weekly regimen, 27 patients received a three-times-weekly regimen with a median dose of 400.0 mg (IQR = 350.0 to 500.0 mg), with a median duration of 61 days (IQR = 54 to 82 days). Four patients immediately received the three-times-weekly regimen after the daily regimen. At the start of the treatment (the first 3 weeks), the dosage was changed in 18 patients based on serum concentrations. The dosage of aminoglycosides was increased in 3 patients with 100 mg each, and the dosage was decreased in 15 patients with a mean decrease of 102 ± 57 mg. The comedication(s) used is displayed in Table 2.
TABLE 2.
Anti-TB medication (n = 80)
| Medication | No. (%) of patients |
|---|---|
| Fluoroquinolones | |
| Levofloxacin | 21 (26.3) |
| Moxifloxacin | 57 (71.3) |
| Second-line injectable agents | |
| Amikacin | 54 (67.5) |
| Kanamycin | 25 (31.3) |
| Both amikacin and kanamycin | 1 (1.3) |
| Capreomycin | 2 (2.5) |
| Other core second-line agents | |
| Linezolid | 62 (77.5) |
| Protionamide | 52 (65.0) |
| Clofazimine | 64 (80.0) |
| Cycloserine | 8 (10.0) |
| Add-on agents (D1) | |
| Pyrazinamide | 32 (40.0) |
| Ethambutol | 58 (72.5) |
| Add-on agents (D3) | |
| Thioacetazone | 7 (8.8) |
| Others/not classified | |
| Rifabutin | 11 (13.8) |
| Clarithromycin | 11 (13.8) |
| Azithromycin | 3 (3.8) |
| Co-trimoxazole | 7 (8.8) |
| Ciprofloxacin | 4 (5.0) |
| Ertapenem | 7 (8.8) |
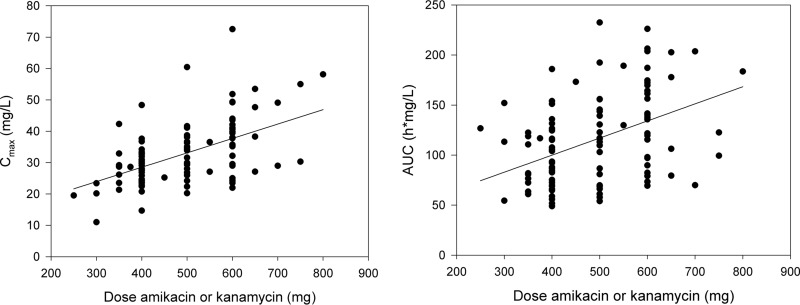
Treatment details are displayed in Table 3. All Cmax levels and AUCs are displayed in Fig. 1. The trough level was <3 mg/liter in all patients. The Cmax and AUC correlated both with the dose per kg of body weight (r = 0.53 and 0.25, P < 0.05). The Cmax and AUC were both not significantly different between both aminoglycosides (P = 0.86 and 0.61). The median dose per kg of body weight was slightly, yet significantly, higher in male patients (6.7 mg/kg) than in female patients (6.0 mg/kg; P = 0.025) for both aminoglycosides.
TABLE 3.
Treatment details and side effects
| Parameter or side effect | No. (%) of patients or median (IQR) |
|
|---|---|---|
| Amikacin | Kanamycina | |
| Common parameters | ||
| Duration (days) of hospital stay | 92.5 (67.3–162.3) | 110.0 (90.5–186.5) |
| Duration (days) of treatment with aminoglycosides | 138.0 (69.8–187.0) | 104.0 (82.0–179.8) |
| Creatinine level (μmol/liter) after 90 days of treatment | 80.0 (66.0–93.0) | 77.0 (62.0–100.5) |
| Creatinine level (μmol/liter) after 180 days of treatment | 82.0 (70.0–95.0) | 83.5 (67.8–101.5) |
| Observed side effectsa | ||
| Nephrotoxicity | 11 (22.9) | 9 (34.6) |
| Ototoxicity | 4 (9.1) | 5 (21.7) |
Nephrotoxicity is defined as a serum creatinine level >1.5 times the baseline serum creatinine level at any time during treatment; ototoxicity is defined as reduced hearing at any frequency >20 dB, as determined by audiometry at any time during treatment compared to the baseline. One patient used both amikacin and kanamycin. The patient is included in the kanamycin results, since this aminoglycoside treatment represented the largest treatment period.
FIG 1.
(Left) Correlation between dose of amikacin or kanamycin per kg of body weight (mg/kg) and Cmax (mg/liter). (Right) Correlation between dose of amikacin or kanamycin per kg of body weight (mg/kg) and the AUC (h·mg/liter).
The median treatment duration with amikacin was 166 days (IQR = 78 to 202 days) with a median cumulative dose of 791.0 mg/kg (IQR = 522.0 to 1,281.6 mg/kg). With kanamycin, the median treatment duration was 124 days (IQR = 82 to 193 days), with a median cumulative dose of 860.7 mg/kg (IQR = 569.2 to 1,337.5 mg/kg). The treatment duration and cumulative dose were not significantly different between the two aminoglycosides (P = 0.650 and P = 0.945) or between genders (P = 0.813 and P = 0.265).
The median MICs for amikacin and kanamycin were, with or without resistant cases (MIC > 5 mg/liter), 1.0 mg/liter (range, 1 to 20 mg/liter; n = 67) and 2.5 mg/liter (range, 1 to 20 mg/liter; n = 12), respectively. The achieved mean weighted Cmax/MIC was 25.0 for both aminoglycosides. With amikacin, the mean weighted Cmax/MIC was 31.2, while a mean weighted Cmax/MIC of 12.3 was obtained using kanamycin. When using individual MIC values, the mean individual Cmax/MIC ratio was 26.0, and the mean individual weighted Cmax/MIC ratio was 25.3 (n = 25) for both aminoglycosides together. The mean cumulative AUC0–24 values were 15,205 mg/liter·h·days for amikacin and 15,518 mg/liter·h·days for kanamycin.
Adverse events and clinical outcome.
The serum creatinine levels for 20 patients (25.0%) were considered elevated, as displayed in Table 3. All except six patients were classified as a grade 1 toxicity, five patients were classified as grade 2 toxicity, and 1 patient was classified as grade 3 toxicity according to the common toxicity criteria (CTC) (22).
The total dose (P = 0.230), duration (P = 0.301), weighted Cmax (P = 0.824), cumulative AUC (P = 0.970), age (P = 0.404), body weight at the start of the treatment (P = 0.121), and body mass index (BMI) were all nonsignificantly related to the occurrence of nephrotoxicity. All coadministered drugs were also nonsignificantly related to nephrotoxicity (P > 0.05, Fisher exact test), except for the drug co-trimoxazole (P = 0.01, n = 7), ethambutol (P = 0.034, n = 58), and levofloxacin (P = 0.044, n = 21). Cycloserine also seemed to be correlated with the occurrence of nephrotoxicity (P = 0.02, Fisher exact test). Five patients on cycloserine developed some nephrotoxicity. The nephrotoxicity had occurred already before the start of the cycloserine treatment.
Regression analysis on the different grades of nephrotoxicity and the factors mentioned did not reveal independent predictors for toxicity (Table 4). Furthermore, no significant increase of the incidence of nephrotoxicity was observed with diabetes mellitus type 2 (Mann-Whitney U test P = 0.404). The relation between diabetes mellitus type 1 and nephrotoxicity showed a nonsignificant trend (P = 0.079). In addition, we performed several probit models in order to establish possible factors associated with the occurrence and extent of nephrotoxicity. However, the cumulative AUC, weighted trough, and treatment duration did not correlate with the occurrence and extent of nephrotoxicity.
TABLE 4.
Spearman correlations of different factors predicting nephrotoxicity
| Classification |
P |
|||
|---|---|---|---|---|
| Total dose | Total duration | Dose (mg/kg) | Baseline serum creatininec | |
| CTC > 50% binarya | 0.226 | 0.313 | 0.159 | 0.000 |
| CTC > 50% regressionb | 0.200 | 0.321 | 0.220 | 0.001 |
That is, a serum creatinine level above 50% of the baseline at any moment during treatment, as defined by the common toxicity criteria (22).
That is, regression analysis on the different categories in toxicity, as defined by the common toxicity criteria (22).
Both P values were significant at a 95% significance level.
Audiometry results were available in 70 patients (87.5%), generally at the start of the aminoglycoside treatment and thereafter every 3 to 4 weeks. The results of the audiometry showed hearing loss in 9 patients (11.3%, Table 3), predominantly at higher frequencies (4,000 and 8,000 Hz). The mean hearing loss was 37.5 dB (range, 25.0 to 50.0) at 4,000 Hz and 46.1 dB (range, 25.0 to 70.0) at 8,000 Hz. The cumulative dose (P = 0.421), dose per kg of body weight (P = 0.741), duration (P = 0.644), body weight (P = 0.978), gender (P = 0.386), age (P = 0.155), and BMI (P = 0.432) did not correlate with the occurrence of ototoxicity.
The AUC0–24, weighted Cmax, and duration of therapy did not relate to the occurrence or extent of ototoxicity using Probit models. Also, the weighted Cmax was not related to the occurrence and extent of hearing loss (P > 0.05). Furthermore, none of all coadministered drugs correlated with ototoxicity (P > 0.132). The administration of cycloserine was also not correlated with the occurrence of ototoxicity (P = 0.66, Fisher exact test). In all, eight patients used cycloserine; one of these patients experienced hearing loss.
Regression analysis was performed on the extent of hearing loss at 8,000 Hz in decibels (dB) of all patients with hearing loss (n = 9). The dose received during the daily regimen was correlated with hearing loss in dB at 8,000 Hz (P = 0.004, R = 0.851).
Data on clinical outcome were available of 52 patients. Of all patients, 35 (67.3%) had successful outcomes, 15 patients were lost to follow-up (28.8%), and 2 patients (3.8%) died within the follow-up period of 2 years. None of the patients had a documented treatment failure or relapse. Simple linear regression between the weighted Cmax/MIC and time to sputum and culture conversion did not reveal any linear relationship (P = 0.44 and 0.64, respectively). In addition, we performed a classification and regression tree (CART) analysis to establish any links between the Cmax/MIC ratio, the cumulative dosage, and the time to sputum and culture conversion. However, this did not yield any significant results.
DISCUSSION
This study showed a low level of hearing loss in the investigated cohort, predominately in high frequencies as expected. The treatment outcome in patients receiving aminoglycosides given in a lower therapeutic drug monitoring (TDM)-guided dose, was good. This may be explained by the fact that the dosage was guided by the Cmax/MIC value in individual patients, since the Cmax/MIC correlates to the efficacy of aminoglycosides (23, 24). Although retrospective in nature, these findings are important since amikacin and kanamycin form the cornerstone of today's MDR-TB treatment.
A recent prospective study using CART analysis showed that a cumulative AUC of amikacin above 87,232 mg/liter·h·day significantly increases the probability of ototoxicity to 10% (12). This study in 28 patients, 10 of whom had earlier aminoglycoside exposure, found audiometry-confirmed hearing loss in 7 (25%) of the patients studied. The peak and trough concentration of amikacin did not correlate with the occurrence of ototoxicity. By using blood concentration-guided dosing, our mean cumulative AUC was well below this threshold of 87,232 mg/liter·h·day, which could explain the relatively low incidence of ototoxicity in our population. This should be an argument for minimizing the cumulative AUC during aminoglycoside treatment.
The occurrence of ototoxicity varies among different studies. According to the study of Peloquin et al. (25), the incidence of hearing loss after treatment with aminoglycosides was 37%. de Jager et al. found an incidence of 21.3% during treatment (9). This is higher than in our study, with an incidence of hearing loss in 11.3% of all patients. No difference in demographics was found between the group with and without ototoxicity. Therapeutic parameters, particularly dose, cumulative dose, duration, and Cmax, were all nonsignificantly correlated with ototoxicity, making ototoxicity prediction with these parameters impossible. The lack of relationship between Cmax and the daily dose is consistent with a previous study (25).
Based on the findings presented above, regular audiometry should be common practice (26). This regular audiometry could be difficult in programmatic settings due to logistical problems or lack of equipment and trained personnel. However, it has been shown that audiological monitoring using a smartphone connected to headphones, preferably with passive noise canceling, correlates well with professional audiometry (27, 28). This could be a viable option in developing countries. When there is evidence of ototoxicity, a possible solution could be to administer the aminoglycosides five times or even three times a week, according to WHO guidelines (29, 30), or to exclude aminoglycosides from the regimen and use another anti-TB drug, such as capreomycin. The effect of this dosing regimen on the clinical efficacy has, however, not been established. When reducing the dose, recommendations on the Cmax/MIC ratio need to be taken into account to avoid loss of efficacy (31, 32).
The prevalence of nephrotoxicity in our study was comparable with an earlier report from our center (16.8%) (9) and with a report by Peloquin et al. (11.6%) (25). No significant influence of different factors on either the occurrence or the extent of nephrotoxicity was found. This finding is in line with the earlier study of Peloquin (11). The results of the current cohort are in contrast to those reported in an earlier study from January 1995 to July 2000 performed at our center (9). In the earlier cohort, the total dose and duration of the aminoglycoside therapy were significantly correlated with nephrotoxicity. Applied doses in the earlier study were, however, >2-fold higher than the dose used in our study (750 to 1,000 mg versus 400 mg). It is, however, questionable whether the serum creatinine level is the right tool to measure nephrotoxicity. An increase in serum creatinine could also be related to increased muscle mass and weight gain, which is often seen during successful TB treatment.
The use of co-trimoxazole was correlated with the occurrence of nephrotoxicity. Co-trimoxazole, a combination of trimethoprim and sulfamethoxazole, is known to increase the serum creatinine level, since trimethoprim decreases the tubular secretion of creatinine (33, 34). This finding is supported by the fact that a clear time relationship between the co-trimoxazole administration and the elevation in serum creatinine was detected in 5 of 6 patients. The serum creatinine value has, however, limited predictive value during treatment with co-trimoxazole due to the specific inhibition of clearance of the creatinine molecule.
The dosage applied in our study is a 2-fold lower than the 15-mg/kg dose recommended by the WHO (5), and yet the outcome was favorable in the vast majority of patients, and in those with an unfavorable outcome, aminoglycoside dosage was not a predictor of poor outcome. All but three patients completed their treatment and were well when discharged after a median of 150.5 days of treatment. This showed that the therapy provided was effective. This is supported by the finding that of all patients with follow-up data, 35 (67.3%) did not have a relapse after 2 years. We therefore hypothesize that the dose of aminoglycosides can be decreased, taking into consideration that the Cmax/MIC recommendations are met, when they are coadministered with another highly active medication, such as linezolid, clofazimine, and moxifloxacin, without an apparent loss of efficacy.
Dosing based on the Cmax/MIC of aminoglycosides should be used rather than dosing based on body weight in order to improve treatment outcomes and to reduce toxicity, since lower dosing based on serum concentrations resulted in less toxicity and good treatment outcome. Another recent study confirmed that the Cmax/MIC is a driver of effective treatment (24). This means that analytical techniques in order to analyze amikacin or kanamycin in serum with high-throughput rates should be made available in all TB programs to deliver fast and accurate results. In addition, simple drug susceptibility testing in order to establish a precise MIC value should also be available (35). Both PK and PD analysis requires trained and experienced personnel with equipment. However, it would be feasible to centralize these facilities in order to concentrate knowledge and reduce costs.
With accurate dosing based on the Cmax/MIC, the cumulative AUC can be minimized in order to reduce ototoxicity. It should be noted that the cumulative AUC threshold value of 87,232 mg/liter·h·days was established in a prospective study with only 28 patients (12), and its validity needs to be tested in larger cohorts. With our proposed limited sampling strategy, the AUC0–24 can be predicted with only two serum samples (36), which can be analyzed in a centralized laboratory in order to estimate the AUC0–24. Treating physicians should be aware of the patients' cumulative AUC0–24 in order to reduce or possibly avoid hearing loss. It should be noted that the trough level of aminoglycosides should not be used to change the dose and to assess the risk of ototoxicity. In addition, there is a large variation in Cmax and AUC0–24 (and thus efficacy and toxicity) as shown in Fig. 1, which cannot be explained by the administered dose alone. This is an additional reason to use PK/PD-guided dosing.
One limitation of this study was the rather imprecise method used to determine the MIC. We used an MIC for amikacin of <1 mg/liter as simply 1 mg/liter in our statistical analysis; however, it has been shown that many isolates have MICs below 1 mg/liter for amikacin (37). Therefore, the weighted Cmax/MIC could be higher for amikacin than reported, increasing its efficacy. In addition, the reference laboratory commonly reported MICs based on breakpoint concentrations rather than precise MICs. The Cmax/MIC calculated in this study is therefore a “worst case scenario,”since the actual MIC could be lower than the breakpoint concentration.
After more than 30 years of medical practice prescribing aminoglycosides in a dose of 15 mg/kg, we believe that a formal study is warranted between standard of care and an individualized approach based on drug susceptibility and drug concentrations. With the dosage of 6.5 mg/kg used in this study and the old breakpoint MIC of 2 mg/liter for amikacin and 5 mg/liter for kanamycin determined using the Middlebrook 7H10 agar method (38), the Cmax/MIC ratio would be 12.5 and 5. However, the median MIC found in this study is lower than the breakpoint MIC found, and sufficient Cmax/MIC ratios were reached. In vitro testing using a hollow fiber infection model should be performed to detect the optimal Cmax/MIC ratio as has already been done for other anti-TB drugs (39–42). Combining amikacin or kanamycin with other drugs in this setup seems rational since the treatment of MDR-TB is based on a treatment regimen with a combination of anti-TB drugs. An additional effect of single drugs in a multidrug regimen can therefore be evaluated. Based on these data, a new MDR-TB dosing strategy can be designed to improve efficacy, while toxicity may be reduced.
In conclusion, a lower, TDM-guided dosage of aminoglycosides resulted in an acceptable treatment outcome with relatively low percentages of hearing loss. However, this approach should be validated in a prospective randomized trial.
MATERIALS AND METHODS
In this retrospective study, we evaluated all patients with culture-confirmed MDR-TB or XDR-TB, either pulmonary or extrapulmonary, receiving amikacin or kanamycin as part of their TB treatment for at least 3 days (steady state) who were hospitalized at the Tuberculosis Centre Beatrixoord between 1 August 2000 and 16 May 2012. Only patients older than 17 years were included. Since retrospective data were collected, the Institutional Review Board of the University Medical Center Groningen waived the requirement for research subjects to give informed consent (METc 2013/492).
Data collection.
Medical history, age, sex, weight, length, ethnicity, comorbidity, type of diagnosis, localization of TB, the MICs of amikacin and kanamycin, resistance pattern, dose and duration of TB treatment, creatinine levels at baseline, and adverse events (hearing loss and renal dysfunction) were collected from the patients' medical records. Parameters such as the cumulative dose and the dose per kg of body weight were calculated based on the gathered data. The serum levels of routine TDM of amikacin and kanamycin and the MIC of the sputum isolates were also retrieved from the patients' records. Adverse events were monitored using audiometric monitoring and the determination of the serum creatinine as described below.
Serum level measurements.
Cmax samples obtained 30 min after a 1-h infusion and Cmin samples obtained immediately before infusion were collected. Amikacin concentrations were determined by a fluorescence polarization immunoassay (TDx or Architect; Abbott Laboratories, IL) with a lower limit of quantitation (LOQ) of 1.5 mg/liter. Kanamycin concentrations were determined using a validated analytical method by liquid chromatography with coupled tandem mass spectrometry (TSQ Quantum; Thermo Fisher, San Jose, CA) with an LOQ of 0.1 mg/liter (43).
Drug susceptibility testing.
The sputum isolates were subjected to drug susceptibility testing for amikacin and kanamycin at the Dutch National Mycobacteria Reference Laboratory (National Institute for Public Health and the Environment [RIVM]). The Middlebrook 7H10 agar dilution method was applied for drug susceptibility testing of the isolate(s) (44). The MIC was commonly tested at predefined breakpoints. Drug susceptibility testing was not repeated during the treatment, except when the physicians expected the development of drug resistance based on clinical nonimprovement. Sputum samples for microscopy (fluorescent staining) and culture were collected weekly and sent to the national reference laboratory for analysis.
PK/PD analysis.
The Cmax/MIC ratio and time to sputum and culture conversion were calculated and considered to be proxy parameters for efficacy. The aminoglycoside dose was adjusted based on the amikacin and kanamycin concentrations and the MIC.
Based on the peak and through levels, the AUC0–24 was estimated with the use of a validated population pharmacokinetic model using MW/Pharm 3.81 (Mediware, The Netherlands) (36). The Cmax/MIC was consecutively calculated by dividing the Cmax by the median MICs of 1 mg/liter (amikacin) and 2.5 mg/liter (kanamycin). A weighted Cmax/MIC was calculated for each patient by the following formula:
In addition, the Cmax/MIC and weighted Cmax/MIC were calculated with individual MICs when both the individual pharmacokinetic data and the corresponding individual MIC were available.
Adverse events and clinical outcome.
Adverse events of the aminoglycosides were assessed by evaluation of ototoxicity and renal function at baseline and during treatment. Audiometry was performed monthly at 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz. Hearing loss was defined as a 20-dB reduction in hearing threshold from baseline irrespective of side (right or left ear) or frequency (45). Audiometry was usually performed every 3 to 4 weeks during aminoglycoside treatment. Renal function was evaluated at least once a week by measuring creatinine in serum. Renal toxicity was defined as a more than 50% increase in the baseline serum creatinine concentration at any moment during the treatment, in accordance with the common toxicity criteria (CTC) (22). Treatment outcome was evaluated 2 years after completion of treatment using common WHO criteria (46).
Statistics.
All statistics were performed using SPSS 20 (SPSS, Virginia, IL). M. tuberculosis isolates showing no growth at <1 mg/liter were statistically analyzed as 1 mg/liter. Differences in gender and type of aminoglycoside were assessed using Mann-Whitney U tests. The determinants in nephrotoxicity and ototoxicity were also assessed using Mann-Whitney U tests, except for the gender (chi-squared test), and using another comedication (Fisher exact test). Correlations between the extent of nephrotoxicity and ototoxicity and continuous or categorical factors were calculated using the Spearman coefficient. The correlation between clearance and distribution volume and the occurrence of side effects was assessed using the Spearman coefficient. The relation between the nephrotoxicity, classified according to the CTC as binary or categorical, and the demographic data was determined by the Spearman rank-order correlation test. Relations between the weighted Cmax/MIC and time to sputum and culture conversion was assessed using simple linear regression and classification and regression tree (CHAID) analysis. All P values below 0.05 were considered significant.
REFERENCES
- 1.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA, American Thoracic Society Centers for Disease Control and Prevention and the Infectious Diseases Society. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi N, Labrousse V, Goh KS. 1996. In vitro activities of fourteen antimicrobial agents against drug-susceptible and -resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol 33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 3.de Steenwinkel JE, de Knegt GJ, ten Kate MT, van Belkum A, Verbrugh HA, Kremer K, van Soolingen D, Bakker-Woudenberg IA. 2010. Time-kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J Antimicrob Chemother 65:2582–2589. doi: 10.1093/jac/dkq374. [DOI] [PubMed] [Google Scholar]
- 4.Donald PR, Sirgel FA, Venter A, Smit E, Parkin DP, Van de Wal BW, Mitchison DA. 2001. The early bactericidal activity of amikacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 5:533–538. [PubMed] [Google Scholar]
- 5.World Health Organization. 2008. Annex 2: weight-based dosing of drugs for adults, p 20 In Anonymous guidelines for the programmatic management of drug-resistant tuberculosis: emergency update, 2008 ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.Alangaden GJ, Kreiswirth BN, Aouad A, Khetarpal M, Igno FR, Moghazeh SL, Manavathu EK, Lerner SA. 1998. Mechanism of resistance to amikacin and kanamycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother 42:1295–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. 2009. High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother 53:5064–5068. doi: 10.1128/AAC.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruuner A, Jureen P, Levina K, Ghebremichael S, Hoffner S. 2003. Discordant resistance to kanamycin and amikacin in drug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 47:2971–2973. doi: 10.1128/AAC.47.9.2971-2973.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jager P, van Altena R. 2002. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis 6:622–627. [PubMed] [Google Scholar]
- 10.Duggal P, Sarkar M. 2007. Audiologic monitoring of multidrug-resistant tuberculosis patients on aminoglycoside treatment with long-term follow-up. BMC Ear Nose Throat Disord 7:5. doi: 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Modongo C, Pasipanodya JG, Zetola NM, Williams SM, Sirugo G, Gumbo T. 2015. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother 59:6337–6343. doi: 10.1128/AAC.01050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. 2014. Long term streptomycin toxicity in the treatment of Buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 8:e2739. doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies GR, Nuermberger EL. 2008. Pharmacokinetics and pharmacodynamics in the development of anti-tuberculosis drugs. Tuberculosis 88(Suppl 1):S65–S74. doi: 10.1016/S1472-9792(08)70037-4. [DOI] [PubMed] [Google Scholar]
- 15.Kovarik JM, Hoepelman IM, Verhoef J. 1989. Once-daily aminoglycoside administration: new strategies for an old drug. Eur J Clin Microbiol Infect Dis 8:761–769. doi: 10.1007/BF02185842. [DOI] [PubMed] [Google Scholar]
- 16.Mattie H, Craig WA, Pechere JC. 1989. Determinants of efficacy and toxicity of aminoglycosides. J Antimicrob Chemother 24:281–293. doi: 10.1093/jac/24.3.281. [DOI] [PubMed] [Google Scholar]
- 17.Bartal C, Danon A, Schlaeffer F, Reisenberg K, Alkan M, Smoliakov R, Sidi A, Almog Y. 2003. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am J Med 114:194–198. doi: 10.1016/S0002-9343(02)01476-6. [DOI] [PubMed] [Google Scholar]
- 18.Matthews I, Kirkpatrick C, Holford N. 2004. Quantitative justification for target concentration intervention–parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol 58:8–19. doi: 10.1111/j.1365-2125.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rea RS, Capitano B, Bies R, Bigos KL, Smith R, Lee H. 2008. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 30:674–681. doi: 10.1097/FTD.0b013e31818b6b2f. [DOI] [PubMed] [Google Scholar]
- 20.Romano S, Fdez de Gatta MM, Calvo MV, Caballero D, Dominguez-Gil A, Lanao JM. 1999. Population pharmacokinetics of amikacin in patients with haematological malignancies. J Antimicrob Chemother 44:235–242. doi: 10.1093/jac/44.2.235. [DOI] [PubMed] [Google Scholar]
- 21.Thomson AH, Coote J, MacPherson L, Gordon J. 1992. Bayesian estimation of streptomycin pharmacokinetics. Ther Drug Monit 14:522–524. doi: 10.1097/00007691-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services and National Institutes of Health, National Cancer Institute. 2010. Common toxicity criteria (CTC), version 4.03. NIH publication 09-5410.2013. National Institutes of Health, Bethesda, MD. [Google Scholar]
- 23.Srivastava S, Modongo C, Siyambalapitiyage Dona CW, Pasipanodya JG, Deshpande D, Gumbo T. 2016. Amikacin optimal exposure targets in the hollow-fiber system model of tuberculosis. Antimicrob Agents Chemother 60:5922–5927. doi: 10.1128/AAC.00961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modongo C, Pasipanodya JG, Magazi BT, Srivastava S, Zetola NM, Williams SM, Sirugo G, Gumbo T. 2016. Artificial intelligence and amikacin exposures predictive of outcomes in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother 60:5928–5932. doi: 10.1128/AAC.00962-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peloquin CA, Berning SE, Nitta AT, Simone PM, Goble M, Huitt GA, Iseman MD, Cook JL, Curran-Everett D. 2004. Aminoglycoside toxicity: daily versus three-times-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis 38:1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 26.Melchionda V, Wyatt H, Capocci S, Garcia Medina R, Solamalai A, Katiri S, Hopkins S, Cropley I, Lipman M. 2013. Amikacin treatment for multidrug-resistant tuberculosis: how much monitoring is required? Eur Respir J 42:1148–1150. doi: 10.1183/09031936.00184312. [DOI] [PubMed] [Google Scholar]
- 27.Foulad A, Bui P, Djalilian H. 2013. Automated audiometry using apple iOS-based application technology. Otolaryngol Head Neck Surg 149:700–706. doi: 10.1177/0194599813501461. [DOI] [PubMed] [Google Scholar]
- 28.Derin S, Cam OH, Beydilli H, Acar E, Elicora SS, Sahan M. 2016. Initial assessment of hearing loss using a mobile application for audiological evaluation. J Laryngol Otol 130:248–251. doi: 10.1017/S0022215116000062. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. 2008. Anonymous guidelines for the programmatic management of drug-resistant tuberculosis: emergency update, 2008 ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 30.Lange C, Abubakar I, Alffenaar JW, Bothamley G, Caminero JA, Carvalho AC, Chang KC, Codecasa L, Correia A, Crudu V, Davies P, Dedicoat M, Drobniewski F, Duarte R, Ehlers C, Erkens C, Goletti D, Gunther G, Ibraim E, Kampmann B, Kuksa L, de Lange W, van Leth F, van Lunzen J, Matteelli A, Menzies D, Monedero I, Richter E, Rusch-Gerdes S, Sandgren A, Scardigli A, Skrahina A, Tortoli E, Volchenkov G, Wagner D, van der Werf MJ, Williams B, Yew WW, Zellweger JP, Cirillo DM. 2014. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 44:23–63. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuermberger E, Grosset J. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur J Clin Microbiol Infect Dis 23:243–255. doi: 10.1007/s10096-004-1109-5. [DOI] [PubMed] [Google Scholar]
- 32.Craig WA. 2001. Does the dose matter? Clin Infect Dis 33(Suppl 3):S233–S237. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 33.Masters PA, O'Bryan TA, Zurlo J, Miller DQ, Joshi N. 2003. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 163:402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- 34.Smilack JD. 1999. Trimethoprim-sulfamethoxazole. Mayo Clin Proc 74:730–734. doi: 10.4065/74.7.730. [DOI] [PubMed] [Google Scholar]
- 35.Heysell SK, Pholwat S, Mpagama SG, Pazia SJ, Kumburu H, Ndusilo N, Gratz J, Houpt ER, Kibiki GS. 2015. Sensititre MycoTB plate compared to Bactec MGIT 960 for first- and second-line antituberculosis drug susceptibility testing in Tanzania: a call to operationalize MICs. Antimicrob Agents Chemother 59:7104–7108. doi: 10.1128/AAC.01117-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkstra JA, van Altena R, Akkerman OW, de Lange WC, Proost JH, van der Werf TS, Kosterink JG, Alffenaar JW. 2015. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Int J Antimicrob Agents 46:332–337. doi: 10.1016/j.ijantimicag.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Ho YI, Chan CY, Cheng AF. 1997. In vitro activities of aminoglycoside-aminocyclitols against mycobacteria. J Antimicrob Chemother 40:27–32. doi: 10.1093/jac/40.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Pfyffer GE, Bonato DA, Ebrahimzadeh A, Gross W, Hotaling J, Kornblum J, Laszlo A, Roberts G, Salfinger M, Wittwer F, Siddiqi S. 1999. Multicenter laboratory validation of susceptibility testing of Mycobacterium tuberculosis against classical second-line and newer antimicrobial drugs by using the radiometric BACTEC 460 technique and the proportion method with solid media. J Clin Microbiol 37:3179–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 190:1642–1651. doi: 10.1086/424849. [DOI] [PubMed] [Google Scholar]
- 40.Drusano GL, Sgambati N, Eichas A, Brown DL, Kulawy R, Louie A. 2010. The combination of rifampin plus moxifloxacin is synergistic for suppression of resistance but antagonistic for cell kill of Mycobacterium tuberculosis as determined in a hollow-fiber infection model. mBio 1:e00139-10. doi: 10.1128/mBio.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis 201:1225–1231. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dijkstra JA, Sturkenboom MG, Hateren K, Koster RA, Greijdanus B, Alffenaar JW. 2014. Quantification of amikacin and kanamycin in serum using a simple and validated LC-MS/MS method. Bioanalysis 6:2125–2133. doi: 10.4155/bio.14.191. [DOI] [PubMed] [Google Scholar]
- 44.van Klingeren B, Dessens-Kroon M, van der Laan T, Kremer K, van Soolingen D. 2007. Drug susceptibility testing of Mycobacterium tuberculosis complex by use of a high-throughput, reproducible, absolute concentration method. J Clin Microbiol 45:2662–2668. doi: 10.1128/JCM.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brummett RE, Fox KE. 1989. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother 33:797–800. doi: 10.1128/AAC.33.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HS, Alarcon E, Cegielski JP, Grzemska M, Gupta R, Espinal M. 2005. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 9:640–645. [PubMed] [Google Scholar]