ABSTRACT
Systemic candidiasis is a leading cause of nosocomial bloodstream infection with a high mortality rate despite treatment. Immune-based strategies are needed to improve outcomes. We previously reported that genetic deficiency in the chemokine receptor CCR1 improves survival and ameliorates tissue damage in Candida-infected mice. Here, we found that treatment of immunocompetent Candida-infected mice with the CCR1-selective antagonist BL5923 improves survival, decreases the kidney fungal burden, and protects from renal tissue injury.
KEYWORDS: CCR1, Candida, candidiasis, chemokine receptor
TEXT
Systemic candidiasis, most often caused by the commensal yeast Candida albicans, is a leading cause of nosocomial bloodstream infections in the United States (1–3). Vaccines are not available, and despite antifungal therapy, the mortality rate of infected patients exceeds 40% (1–3). Therefore, novel immune-based strategies are highly desirable to augment or supplement the current antifungal drug treatment of candidiasis and improve patient outcomes. Although neutrophils are critical for effective host defense during systemic candidiasis, control of the pathogen may come at the cost of tissue damage (4–6). Indeed, neutrophil-induced tissue injury during infection can be seen in some patients with renal candidiasis and in a subset of neutropenic patients with disseminated candidiasis upon neutrophil recovery (7, 8). We have previously shown that genetic deficiency of the neutrophil-targeted chemokine receptor CCR1 in mice results in attenuated renal tissue injury and improved host survival in a mouse model of systemic candidiasis of hematogenous origin (9). BL5923 is a CCR1-selective small-molecule antagonist with a favorable pharmacokinetic profile (10). It has equal potency against human, mouse, and rat CCR1 (50% inhibitory concentrations [IC50s] of 20, 22, and 28 nM, respectively) in a binding assay and inhibits CCL3-induced calcium flux (IC50 of 16 nM) and chemotaxis (IC50 of 3 nM). BL5923 lacks any significant binding to human CCR2, CCR4, CCR5, CCR6, CCR7, CXCR1, CXCR2, or CXCR3 (IC50 of >50 μM) (10). BL5923 has been shown to protect mice from diabetic nephropathy, lupus nephritis, and metastasis of colon cancer cells to the liver (11–13). Here, we aimed to reproduce the effect of genetic CCR1 deficiency with pharmacological blockade of CCR1 and examine its effect on the outcome of systemic candidiasis in immunocompetent mice in vivo.
We infected 8- to 12-week-old C57BL/6 female mice (Envigo) by intravenous injection of ∼1.25 × 105 to 1.5 × 105 blastospores of C. albicans strain SC5314. Candida-infected mice were treated twice daily for the duration of the experiment with either 50 mg/kg of BL5923 (Novartis) or vehicle (0.5% [wt/vol] hydroxyethyl cellulose [Sigma-Aldrich]) by oral gavage (11–13) starting 6 h after infection, and animal survival was monitored daily for 2 weeks. In separate experiments, Candida-infected mice were treated twice daily with either BL5923 or vehicle and euthanized at day 4 postinfection, and their kidneys were harvested and were weighted for assessment of tissue fungal burdens by CFU determination or fixed in 10% neutral buffered formalin for routine histology processing. Tissue sections were prepared and stained with hematoxylin and eosin (H&E) for histological evaluation and with periodic acid-Schiff (PAS) special stain to highlight fungal organisms. The extent of kidney histological damage was graded by a board-certified pathologist (C.R.L.), who was blind to the mouse treatment group, on a scale of 0 to 3, where 0 indicates no visible renal parenchymal damage, 1 indicates focal mild inflammation, 2 indicates patchy moderate inflammation with focal renal parenchymal damage, and 3 indicates extensive inflammation with severe renal parenchymal tissue destruction. In addition, each kidney was evaluated for the extent of renal cortical and medullary involvement by using the same scoring criteria. The amount of parenchymal hemorrhage was also quantified on a scale of 0 to 3, where 0 indicates no visible hemorrhage, 1 indicates minimal hemorrhage, 2 indicates moderate amounts of hemorrhage, and 3 indicates severe extensive hemorrhage with involvement of the entire kidney. All experiments were performed in duplicate on two independent days in accordance with the standards for humane care and treatment of research animals approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. Mouse survival was plotted by using a Kaplan-Meier curve and evaluated by the log rank (Mantel-Cox) test. Comparisons of levels of tissue injury in BL5923- and vehicle-treated mice were performed by using an unpaired t test, a Mann-Whitney U test, or a chi-square test, where appropriate. Prism software version 6.0 was used for statistical analyses, and P < 0.05 was considered statistically significant.
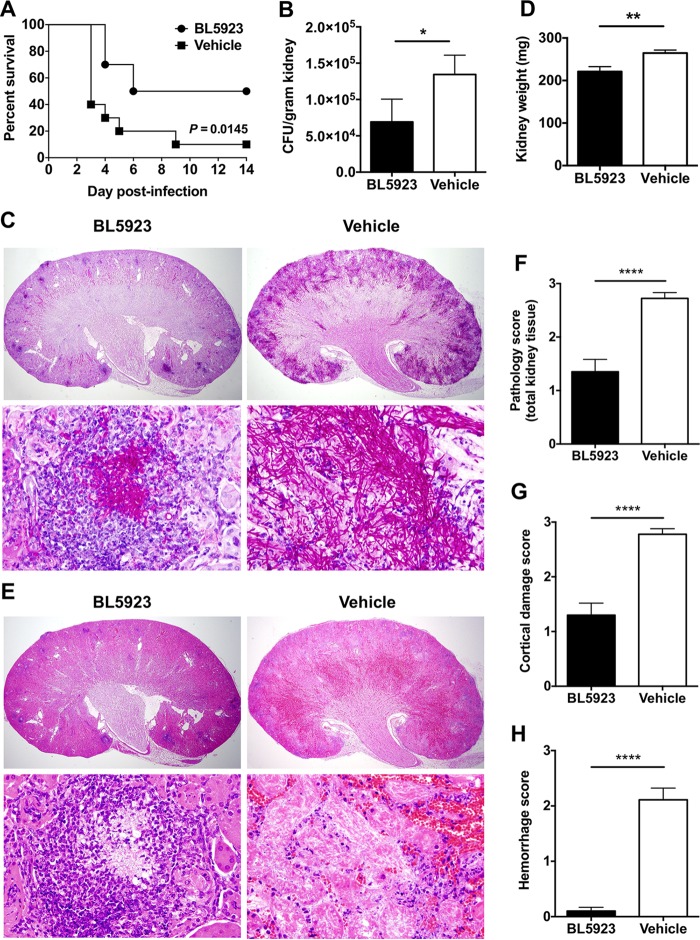
We found that Candida-infected mice treated with the CCR1-selective antagonist BL5923 had significantly improved survival at 2 weeks postinfection (50%) compared to vehicle-treated mice (10%; P = 0.0145; Fig. 1A) and had a longer median survival time of 10 days than the 3 days of vehicle-treated mice. BL5923 treatment led to significantly decreased tissue fungal burdens in the kidney (P = 0.0185; Fig. 1B), the target organ in the mouse model of systemic candidiasis (14), and less extensive renal tissue invasion by Candida pseudohyphae and hyphae, as observed in histology by using PAS stain (Fig. 1C). The improved survival and fungal control observed in Candida-infected BL5923-treated mice correlated with a less pronounced injury-induced increase in kidney weight postinfection (P = 0.004; Fig. 1D) and significantly less extensive kidney tissue damage with smaller areas of abscess formation, renal necrosis, and parenchymal destruction than vehicle-treated mice, as shown by histology with the H&E and PAS stains (Fig. 1C, E, and F; P < 0.0001). The amelioration of renal tissue injury following BL5923 treatment was particularly evident in the marked decrease in parenchymal hemorrhage and renal cortical damage (Fig. 1F to H; P < 0.0001); in agreement, while 100% (18/18) of the vehicle-treated kidneys developed capsular damage after infection, only 40% (8/20) of the BL5923-treated kidneys developed such pathology (P < 0.0001), consistent with what we previously observed in Ccr1−/− mice (9).
FIG 1.
Treatment with the CCR1-selective inhibitor BL5923 results in improved survival and decreased renal tissue injury during systemic candidiasis of hematogenous origin. (A) Percent survival at 2 weeks after infection. (B) Kidney fungal burdens at day 4 postinfection. *, P = 0.0185. (C) Histopathology at day 4 postinfection. Representative PAS-stained sections show decreased tissue invasion by Candida pseudohyphae and hyphae in BL5923-treated mice. Magnification, ×20 (upper panels) and ×600 (lower panels). (D) Kidney weights at day 4 postinfection. **, P = 0.004. (E) Histopathology at day 4 postinfection. Representative H&E-stained sections show decreased kidney tissue damage, necrosis, and hemorrhage in BL5923-treated mice. Magnifications, ×20 (upper panels) and ×600 (lower panels). (F to H) Histology scores of renal tissue damage at day 4 postinfection: F, total kidney histology score; G, cortical damage score; H, hemorrhage score. ****, P < 0.0001. The results in panels A, B, and D are from two independent experiments with 10 BL5923-treated and 10 vehicle-treated mice. The results in panels C and E to H are from two independent experiments with 20 BL5923-treated and 18 vehicle-treated kidneys. Quantitative data represent the mean ± the standard error of the mean.
In the present study, we corroborate and extend our previous observation on the pathogenic role of the chemokine receptor CCR1 in the mouse model of systemic candidiasis (9). We show that, consonant with our reported findings in mice with a genetic deficiency in CCR1, pharmacological blockade of CCR1 with the selective small-molecule CCR1 antagonist BL5923 significantly improves survival and ameliorates renal tissue injury during systemic candidiasis of hematogenous origin in nonneutropenic mice. In addition, our study provides further insight into the renal protective effects of CCR1 blockade beyond those previously observed in mouse models of diabetic nephropathy and lupus nephritis (11–13) to also include infection-associated renal injury.
Our data, although promising, are preliminary and have limitations. First, we experimented with only one strain of C. albicans, so further studies are needed to evaluate differential effects among other C. albicans strains or non-albicans Candida species. Second, we did not assess mouse survival and tissue fungal burdens/histopathology in the same set of experiments. The specificity of our observation also deserves further study of the role of pharmacological CCR1 blockade in ameliorating acute kidney injury in sepsis caused by nonfungal microorganisms and non-Candida fungal pathogens. In addition, as candidiasis also afflicts patients with immunosuppression (1), the generalizability of the pharmacological blockade of CCR1 in the setting of immunodeficiency merits further investigation.
Our preliminary data suggest that adjunct pharmacological blockade of CCR1 along with antifungal therapy may represent a potential therapeutic intervention in selected patients with systemic candidiasis, particularly those with renal involvement and papillary necrosis (8). Further animal studies are required to examine the role of adjunct CCR1 in the treatment of emerging multidrug-resistant Candida species such as Candida auris or azole- and echinocandin-resistant Candida glabrata, for which the antifungal treatment options available in the clinic are limited (15–17).
ACKNOWLEDGMENTS
D.P.K. acknowledges the Frances King Black Endowed Professorship for Cancer Research. This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
REFERENCES
- 1.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarty TP, Pappas PG. 2016. Invasive candidiasis. Infect Dis Clin North Am 30:103–124. doi: 10.1016/j.idc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS. 2014. New insights into innate immune control of systemic candidiasis. Med Mycol 52:555–564. doi: 10.1093/mmy/myu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionakis MS, Netea MG. 2013. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog 9:e1003079. doi: 10.1371/journal.ppat.1003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. 1997. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol 158:2356–2362. [PubMed] [Google Scholar]
- 7.Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, Lortholary O. 2008. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 46:696–702. doi: 10.1086/527390. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski JF Jr, Abramowsky CR. 1981. Candida-associated renal papillary necrosis. Am J Clin Pathol 75:190–194. doi: 10.1093/ajcp/75.2.190. [DOI] [PubMed] [Google Scholar]
- 9.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. 2012. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog 8:e1002865. doi: 10.1371/journal.ppat.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revesz L, Bollbuck B, Buhl T, Dawson J, Feifel R, Heng R, Hiestand P, Sparrer H, Schlapbach A, Waelchli R. 2006. Bridged piperazines and piperidines as CCR1 antagonists with oral activity in models of arthritis and multiple sclerosis. Lett Drug Des Discov 3:689–694. doi: 10.2174/157018006778631866. [DOI] [Google Scholar]
- 11.Bignon A, Gaudin F, Hemon P, Tharinger H, Mayol K, Walzer T, Loetscher P, Peuchmaur M, Berrebi D, Balabanian K. 2014. CCR1 inhibition ameliorates the progression of lupus nephritis in NZB/W mice. J Immunol 192:886–896. doi: 10.4049/jimmunol.1300123. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M, Taketo MM. 2010. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A 107:13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ninichuk V, Khandoga AG, Segerer S, Loetscher P, Schlapbach A, Revesz L, Feifel R, Khandoga A, Krombach F, Nelson PJ, Schlondorff D, Anders HJ. 2007. The role of interstitial macrophages in nephropathy of type 2 diabetic db/db mice. Am J Pathol 170:1267–1276. doi: 10.2353/ajpath.2007.060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lionakis MS, Lim JK, Lee CC, Murphy PM. 2011. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Farmakiotis D, Tarrand JJ, Kontoyiannis DP. 2014. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis 20:1833–1840. doi: 10.3201/eid2011.140685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang E, Farmakiotis D, Yang D, McCue DA, Kantarjian HM, Kontoyiannis DP, Mathisen MS. 2015. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother 70:2362–2368. doi: 10.1093/jac/dkv087. [DOI] [PMC free article] [PubMed] [Google Scholar]