ABSTRACT
The aims of this study were to describe the blood plasma (BP) and seminal plasma (SP) pharmacokinetics of tenofovir (TFV) in HIV-1-infected men, to assess the role of genetic polymorphism in the variability of TFV transfer into the male genital tract, and to evaluate the impact of TFV SP exposure on seminal plasma HIV load (spVL). Men from the Evarist-ANRS EP 49 study treated with TFV as part of their antiretroviral therapy were included in the study. A total of 248 and 217 TFV BP and SP concentrations from 129 men were available for the analysis. For pharmacogenetic assessment, a total of 121 single nucleotide polymorphisms (SNP) were genotyped. Data were analyzed using a nonlinear mixed-effects modeling approach. TFV pharmacokinetics were best described by a two-compartment model for BP and by an effect compartment with different input and output constants for SP. TFV exposures (area under the concentration-time curve from 0 to 24 h [AUC0–24]) were higher in SP than in BP (median AUC0–24, 7.01 versus 2.97 mg · liter−1 · h, respectively). The median (range) SP-to-BP AUC0–24 ratio was 2.24 (0.53 to 34.13). After correction for multiple testing, none of the SNPs were significantly associated with the TFV transfer rate constant. The impact of the TFV SP AUC0–24 or TFV SP-to-BP AUC0–24 ratio on spVL was not significant (P = 0.808 and 0.768, respectively). This is the first population model describing TFV pharmacokinetics in the male genital tract. TFV SP concentrations were higher than BP concentrations. Despite TFV SP exposures being higher than BP exposures, an spVL was detectable for 12.2% of the men.
KEYWORDS: tenofovir, population pharmacokinetics, seminal plasma, genital tract
INTRODUCTION
Sexual transmission of human immunodeficiency virus (HIV) is the main route of HIV infection spreading. The reduction of HIV replication in semen could play an important role in the sexual transmission of HIV. Using combined antiretroviral (ARV) treatments (cART), some studies have reported a parallel decrease in HIV RNA levels in blood plasma (BP) and seminal plasma (SP) (1–3). However, other studies have described a discordance between HIV BP viral load and SP HIV RNA load (spVL) (4–6). Thus, despite suppression of HIV replication in BP reflected by undetectable HIV RNA levels, spVL was detectable for some patients receiving cART (7). This discordance suggests a compartmentalization of HIV replication in the male genital tract, where the distribution of antiretroviral drugs could be a determinant of HIV shedding (7). A poor drug distribution in this compartment could lead to suboptimal concentrations, allowing HIV replication. The distribution of antiretroviral drugs in the genital tract has been reported to be drug specific and highly variable among individuals even for the same drug (7–9). For some protease inhibitors, SP concentrations have been reported to be lower than BP concentrations, whereas indinavir SP concentrations have been reported to be much higher (9). For nonnucleoside reverse transcriptase inhibitors, nevirapine and efavirenz SP-to-BP ratios were around 0.6 and less than 0.1, respectively (9). Nucleoside reverse transcriptase inhibitors accumulate to various extents in the male genital tract, with SP-to-BP ratios of 2.28 and 6.67 for lamivudine and zidovudine, respectively (9, 10). Available data on tenofovir (TFV) SP concentrations are sparse, although this drug is recommended as a preferred choice in first-line regimens for HIV treatment and postexposure prophylaxis and a large body of evidence from trials using TFV-based regimens now supports the concepts of “treatment as prevention” and “preexposure prophylaxis” (11, 12). Four studies have suggested an accumulation of TFV in the male genital tract to different extents (13–16). Mean TFV SP-to-BP concentrations ratios have been estimated at 2.8 (n = 4 men) and 3.3 (n = 15 men) for various sampling times (13, 14). Mean TFV SP-to-BP concentration ratios at 24 h (C24) were estimated at 4.4 for a single dose and 5.1 at steady state (n = 9 men) (15). Thus, the TFV SP-to-BP concentration ratio appears to be variable depending on the time elapsed between drug intake and sampling. Another study has focused on a TFV single-dose administration and has reported an SP-to-BP exposures ratio of 1.0 (16). However, that study included a small number of subjects (n = 8 men) and examined only the final phase of decay of TFV pharmacokinetics (PK) (from 24 h to 14 days). Furthermore, the blood-testis barrier, composed mainly of Sertoli cells, is responsible for the protection of developing germ cells from exposure to xenobiotics. Several efflux transporters for which TFV is potentially a substrate, such as P-gp and MRP4, have been shown to be present and active at the blood-testis barrier (17). Therefore, any genetic polymorphism of one of those carriers could modify SP concentrations and explain a part of the variability observed in TFV transfer into the male genital tract and thus possibly HIV shedding in this compartment.
No study has reported the penetration of TFV in the male genital tract as the SP-to-BP exposure ratio at steady state under the conditions of chronic once-daily administration. The aims of our work were (i) to describe TFV BP and SP pharmacokinetics by a population approach, (ii) to evaluate the TFV distribution in the male genital tract by use of an SP-to-BP exposure ratio at steady state, (iii) to assess the role of genetic polymorphism in the variability of TFV transfer into the male genital tract, and (iv) to assess the effect of TFV exposure levels in the male genital tract on spVL.
RESULTS
Demographic data.
Data from 129 and 123 men were available for BP and SP analyses, respectively. Table 1 summarizes the patients' demographic and biological characteristics. Tenofovir disoproxil fumarate (TDF) was associated with emtricitabine (FTC) for 94.5% of men and with abacavir for 4.7% of men. TDF was combined with either one nonnucleoside reverse transcriptase inhibitor, one ritonavir-boosted protease inhibitor, or raltegravir in 49%, 31%, and 14% of these men, respectively. The most frequent combination was TDF plus FTC plus efavirenz (55 men out of 129). Ninety-five percent of patients respected the 48-hour abstinence period before semen sampling, and 13% had clinical symptoms suggestive of potential sexually transmitted infections.
TABLE 1.
Demographic and biological characteristics of the HIV-infected men in the study
| Parametera | Median (range) |
|---|---|
| Age (yr) | 43 (27–63) |
| BW (kg) | 73 (46–108) |
| SCR (μmol · liter−1) | 78 (28–113) |
| CLCR (ml · min−1) | 113 (67–368) |
Abbreviations: BW, body weight; SCR, serum creatinine; CLCR, creatinine clearance.
Population pharmacokinetics.
A total of 248 TFV BP and 217 TFV SP concentrations at steady state were available for the analysis. A two-compartment model with first-order absorption and elimination with an effect compartment linked to the central compartment satisfactorily described the TFV plasma and seminal plasma concentrations. For seminal plasma, the best fits and objective function value (OFV) were obtained by estimating different input and output rate constants for the effect compartment. Adding a transit compartment between the central and effect compartments did not improve the fit. The pharmacokinetic parameters of this model were the absorption rate constant (ka), apparent elimination clearance (CL/F), central volume of distribution (Vc/F), intercompartmental clearance (Q/F), peripheral volume of distribution (Vp/F), BP-to-SP transfer rate constant (k1e), and SP elimination rate constant (ke1), with F being the unknown bioavailability.
The residual variability was described by a proportional error model for both BP and SP. Interindividual variabilities were retained for CL/F, Q/F, and ke1. For BP pharmacokinetics, the most significant decrease in OFV was obtained by adding the effect of lopinavir/ritonavir (LPV/r) on CL/F. After the inclusion of LPV/r on CL/F, the addition of creatinine clearance (CLCR) on CL/F significantly decreased the OFV. The effect of darunavir/ritonavir on CL/F was significant for the upward phase but not for the backward phase. Thus, the final covariate model for BP modeling was CL/F = θCL/F × βLPV/r × (CLCR/113)βCLCR, with θCL/F the typical value of CL/F for a patient with a median CLCR value of 113 ml · min−1 and no coadministration of LPV/r. For TFV SP modeling, the effects of covariates on ke1 did not significantly decrease the OFV. Table 2 summarizes the final population pharmacokinetic estimates for the BP and SP model, including the relative standard errors.
TABLE 2.
TFV population pharmacokinetic parameters for plasma and seminal plasma
| Parametera | Estimate | RSE (%) |
|---|---|---|
| Structural model | ||
| ka (h−1) | 1.35 | 41 |
| CL/F (liters · h−1) | 45.8 | 3 |
| Vc/F (liters) | 268 | 16 |
| Q/F (liters · h−1) | 197 | 39 |
| Vp/F (liters) | 1630 | 43 |
| βLPV/r on CL/F | 0.591 | 13 |
| βCLCR on CL/F | 0.269 | 34 |
| k1e (h−1) | 0.0963 | 28 |
| ke1 (h−1) | 0.0339 | 29 |
| Statistical model | ||
| ω CL/F | 0.146 | 48 |
| ω Q/F | 1.94 | 45 |
| ω ke1 | 0.889 | 7 |
| σplasma | 0.263 | 8 |
| σseminal plasma | 0.374 | 7 |
Abbreviations: ka, absorption rate constant; CL/F, apparent elimination clearance; Vc/F, apparent central volume of distribution; Q/F, apparent intercompartmental clearance; Vp/F, apparent peripheral volume of distribution; F, unknown bioavailability; βLPV/r on CL/F, influential factor of lopinavir/ritonavir coadministration on CL/F; βCLCR on CL/F, influential factor of creatinine clearance on CL/F; k1e, plasma to seminal plasma transfer rate constant; ke1, seminal plasma elimination rate constant; ω, interindividual variability estimates; σplasma, proportional residual variability estimate for plasma concentrations; σseminal plasma, proportional residual variability estimate for seminal plasma concentrations.
Model evaluation and validation.
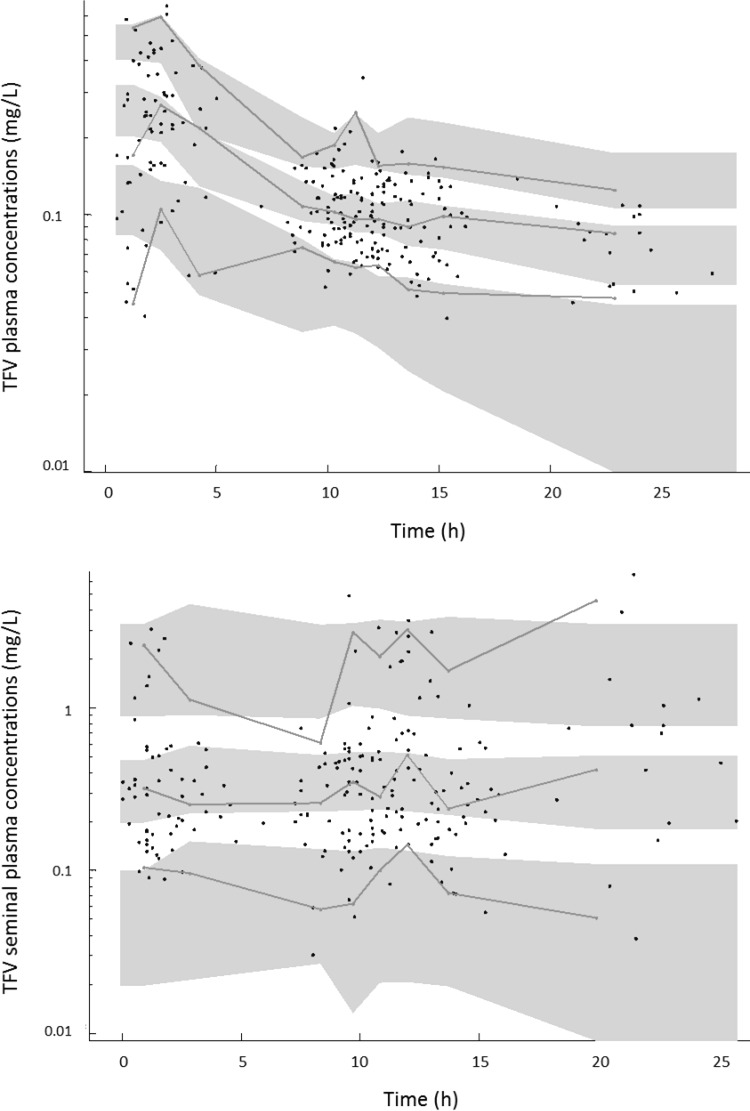
Table 2 summarizes the final model parameters. All the parameters were well estimated given the low relative standard error (RSE) values. The visual predictive check (VPC) of the final model shows that the 5th, 50th, and 95th percentiles of observed data are well included in the 90% confidence interval (CI) of the 5th, 50th, and 95th simulated percentiles both for BP and SP (Fig. 1).
FIG 1.
Validation of the final model by the visual predictive check (VPC), showing comparison between the 5th, 50th, and 95th percentiles of observed data (lines), the 90% confidence interval of simulated percentiles (areas), and the observed data (circles) for TFV plasma concentrations (top panel) and TFV seminal plasma concentrations (bottom panel) on a log scale.
TFV blood plasma and seminal plasma exposures.
Both the TFV BP area under the concentration-time curve from 0 to 24 h (AUC0–24) and C24 were higher for patients receiving LPV/r than for patients without LPV/r coadministration (Mann-Whitney-Wilcoxon test, P < 10−4). The median BP AUC0–24 was 5.10 mg · liter−1 · h and 2.95 mg · liter−1 · h for patients receiving LPV/r or not, respectively. Seminal AUC0–24 and C24 were close between patients receiving LPV/r or not (Mann-Whitney-Wilcoxon test, P = 0.61 and 0.67, respectively).The median SP AUC0–24 was 6.95 mg · liter−1 · h for patients without LPV/r and 7.70 mg · liter−1 · h for patients receiving LPV/r.
TFV concentrations in the male genital tract were higher than BP concentrations. The median C24 (range) was 0.26 (0.03 to 4.42) mg · liter−1 for SP and 0.074 (0.030 to 0.190) mg · liter−1for BP. TFV BP and SP AUC0–24 and SP-to-BP exposures ratios are summarized in Table 3. The median (range) TFV BP AUC0–24 was 2.97 (2.08 to 7.06) mg · liter−1 · h, the median (range) TFV SP AUC0–24 was 7.01 (1.25 to 107.3) mg · liter−1 · h, and the median (range) SP-to-BP AUC0–24 ratio was 2.24 (0.53 to 34.1). TFV penetration in the male genital tract was highly variable, with a SP-to-BP AUC0–24 ratio coefficient of variation (CV) of 125%. A total of 28 SP-to-BP AUC0–24 ratios out of 217 were lower than 1 (12.9% of ratios). TFVSP AUC0–24 values were higher than BP AUC0–24 values for 87.1% of the men.
TABLE 3.
TFV plasma and seminal plasma exposures
| Parameter | Plasma AUC0–24 | Seminal plasma AUC0–24 | SP-to-plasma AUC0–24 ratio |
|---|---|---|---|
| Sample size | 248 | 217 | 217 |
| Median (mg · liter−1 · h) | 2.97 | 7.01 | 2.24 |
| Range (mg · liter−1 · h) | 2.08–7.06 | 1.25–107.29 | 0.53–34.13 |
Association between genetic polymorphism and TFV transfer into the male genital tract.
Among the 129 patients who gave consent for genetic analysis and for whom germ line DNA was available, 122 were included in the genetic analysis (1 patient had genotyping of insufficient quality, with 60% of the single nucleotide polymorphisms [SNPs] genotyped, and 6 patients had a DNA quantity not suitable for a large-scale genotyping). From the 121 SNPs initially selected, 3 SNPs were filtered out after quality control due to a call rate of <90% (1 SNP, i.e., rs17300696 in the ABCC4 gene) or to a minor allele frequency of <10% (2 SNPs, i.e., rs45610534 and rs45573936 in the ABCC4 and SLC29A1 genes, respectively). Finally, 118 SNPs were included in the genetic analysis (see Table S1 in the supplemental material). The η-shrinkage of the TFV transfer constant rate (ke1) was 0.067, indicating that the empirical Bayesian estimates were reliable. In order to use a normally distributed outcome, the square root value of the ke1 parameter was used to test the association with genetic polymorphism (Shapiro-Wilk normality test, P = 0.14). From the 118 SNPs tested, 85 effective independent tests were actually estimated; the significance threshold was then fixed to 0.0006. After correction for multiple testing, none of the SNPs were significantly associated with the TFV transfer rate constant (see Fig. S1 in the supplemental material). Interestingly, the most significant SNP was rs10508017 in the ABCC4 gene, coding for a transport protein in the blood-testis barrier in a recessive genetic model (P value = 0.001).
Impact of TFV exposures on seminal plasma HIV load.
A seminal plasma HIV load was detectable (>100 copies · ml−1) for 15 patients out of 123 (12.2%) and for 17 samples out of 215 (7.9%). The median (range) for the detectable HIV load in seminal plasma was 242 (118 to 1475) copies · ml−1. Median SP AUC0–24 and SP-to-BP AUC0–24 ratios were lower for patients with detectable spVL than for patients with undetectable spVL (4.78 versus 7.02 mg · liter−1 · h for SP AUC0–24; 1.58 versus 2.28 for SP-to-BP exposure ratio) but the difference did not reach significance (P = 0.32 and P = 0.25, respectively, by the Wilcoxon signed-rank test). No association between TFV distribution in the male genital tract and spVL was shown by the receiver operating characteristic (ROC) curve analyses. The areas under the ROC curves (95% CI) were very low: 0.57 (0.43 to 0.71) for seminal plasma AUC0–24 values and 0.58 (0.44 to 0.73) for SP-to-BP AUC0–24 ratios. Using mixed-effects logistic regressions with random effect on individual, the impact of TFV distribution in the male genital tract, evaluated by SP AUC0–24 and SP-to-BP AUC0–24 ratio, on spVL was not significant (P = 0.81 and 0.77, respectively). The influence of TFV distribution in the male genital tract remained not significant after adjusting the multivariate logistic regression on confounding factors (clinical symptoms of potential sexually transmitted infections and herpes infection) (Table 4). The effect of combined TFV-FTC seminal plasma AUC0–24 or SP-to-plasma AUC0–24 ratios on spVL was not significant (P value for interaction = 0.98 for SP AUC0–24; P value for interaction = 0.73 for SP-to-BP AUC0–24 ratios).
TABLE 4.
Mixed-effects logistic regressions for spVL detectability
| Parametera | P value |
|---|---|
| TFV SP AUC0–24 | 0.808 |
| TFV SP AUC0–24 | 0.809 |
| + STI symptoms | 0.965 |
| TFV SP AUC0–24 | 0.816 |
| + Herpes infection symptoms | 0.180 |
| TFV SP-to-plasma AUC0–24 ratio | 0.768 |
| TFV SP-to-plasma AUC0–24 ratio | 0.768 |
| + STI symptoms | 0.965 |
| TFV SP-to-plasma AUC0–24 ratio | 0.775 |
| + Herpes infection symptoms | 0.179 |
Abbreviations: SP, seminal plasma; STI, sexually transmitted infections.
DISCUSSION
This is the first TFV BP and SP population pharmacokinetic study. Concentrations were satisfactorily described by a two-compartment model connected to an effect compartment with different input and output constants. The effects of CLCR and LPV/r coadministration on TFV BP pharmacokinetics were significant, as previously published (18–20).The final model was evaluated by visual predictive check, and the BP AUC0–24 was close to previous published values in adults (mean AUC0–24 = 2.95 mg · liter−1 · h versus 3.0 mg · liter−1 · h [18] or 2.87 mg · liter−1 · h [19]). Despite the sparse nature of the PK data, no significant shrinkage was pointed out, especially regarding the seminal plasma elimination rate constant, indicating that individual parameters were reasonably identified. TFV SP concentrations were higher than TFV BP concentrations, with a median SP C24 of 0.26 · liter−1 compared to a median C24 of 0.074 mg · liter−1 in BP. One TFV single-dose study has reported median TFV SP and BP C24 values of 0.023 mg · liter−1 and 0.041 mg · liter−1, respectively (16). The discrepancy between the median C24 values probably results from the steady-state condition and an accumulation of TFV in the male genital tract with repeated administrations. Other studies have reported higher TFV concentrations in SP, with SP-to-BP concentration ratios of 2.8 and 3.3 (13, 14). The mean C24 ratio at steady state was reported to be 5.1 (15), close to the mean C24 ratio at steady state of 6.6 estimated in our study. Because these concentrations ratios are highly variable depending on the time elapsed between drug intake and sampling, the SP-to-BP AUC ratio would be more representative of the penetration in the male genital tract. The median SP-to-BP exposures ratio was 2.24 (25th to 75th percentile, 1.47 to 4.17). Patterson et al. have reported an SP-to-BP exposure ratio of 1.0 (25th to 75th percentile, 0.6 to 1.4). However, they reported AUC from 24 h to 14 days for a single-dose administration and focused only on the final decay phase after 24 h (16).
Regarding other tissues, the TFV distributions in the cerebrospinal fluid and female genital tract have been reported. Low TFV cerebrospinal fluid concentrations have been reported, with concentrations being 5% of the BP concentrations (21). The TFV distribution in the female genital tract has been evaluated by noncompartmental analyses, and TFV cervicovaginal fluid-to-BP AUC ratios were estimated at 0.75 and 2.6, respectively (16, 22). Another study using a modeling approach has reported a cervicovaginal fluid-to-BP AUC ratio of 1.6 (23). Thus, the distribution of TFV appears to be variable and specific for each tissue.
The TFV distribution in the male genital tract was variable, with a substantial interindividual variability (0.89) for the SP elimination rate constant (ke1). No effect of any available covariate (including genetic polymorphism) could explain the interindividual variability on ke1. The TFV SP-to-BP AUC0–24 ratio was highly variable, with a coefficient of variation of 125%. Accordingly, 12.9% of SP-to-BP AUC0–24 ratios were lower than 1, showing that TFV seminal plasma AUC0–24 values were not systematically higher than BP AUC0–24 values.
TFV is eliminated mainly by the kidney, via a combination of glomerular filtration and active tubular secretion involving transporter proteins. TFV has been described to be a substrate of organic anion transporter 1 (OAT1), and multidrug resistance-associated proteins 4 (MRP4) (24) and 7 (MRP7) (25). A genetic polymorphism in the ABCC4 gene (MRP4) has been associated with higher intracellular concentrations of TFV-diphosphate (26). Another study has shown that genetic variability in the ABCC10 gene (MRP7) may influence TFV renal tubular transport and contribute to the development of tubular dysfunction (25).Thus, genetic polymorphisms in the renal transporters of TFV could influence TFV pharmacokinetics. Robillard et al. have studied the expression of membrane transporters in human Sertoli cells, one of the main constituents of the blood-testis barrier. The expression and activity of MRP4 have been confirmed (17). Interestingly, in our study, the most significant SNP associated with TFV transfer to the male genital tract was rs10508017 in the ABCC4 gene, coding for the MRP4 transport protein. However, this SNP did not cross the prespecified significance level (P = 0.001). Further studies will be needed to confirm this hypothesis.
In the Evarist study, BP HIV RNA was undetectable for all enrolled men, while SP HIV RNA was detectable in 7.9% of samples. This discordance between BP HIV RNA level and SP HIV RNA was close to the mean percentage previously reported (10%) (7). TFV penetration in the male genital tract was variable, and TFV SP AUC0–24 values were lower than TFV BP AUC0–24 values in 12.9% of the men. However, the low SP AUC0–24 ratios were not associated with SP HIV RNA positivity. In a previous study, FTC SP AUC0–24 values were higher than the efficacy target FTC BP AUC0–24 in more than 99% of men and were not reported as the main factor influencing spVL positivity (27). The TFV distribution in the male genital tract was more variable than the FTC distribution (CVs of SP-to-BP AUC0–24 ratios of 125 and 54.7%, respectively), with ratios lower than 1 more prevalent (12.9% versus <1%). The influence of the TFV SP AUC0–24 or SP-to BP AUC0–24 ratio on spVL was not significant. This remained not significant when symptoms of sexually transmitted diseases, previously associated with seminal plasma HIV shedding in men treated by cART, were taken into account (5). Similarly, the impact of TFV-FTC exposures on spVL was not significant. The nonsignificant results regarding exposure and response may be due to the fact that only 8% patients had detectable seminal viral load. A lack of power cannot be ruled out in our study. Aside from the lack of power, the absence of association between spVL and TFV penetration in the male genital tract could be due to the assessment of only a part of the overall drug combination. Therefore, these results suggest a substantial effect of other concomitant ARV drug exposures in seminal plasma. Viral particle transfer coming from another compartment could also explain these results. Actually, it was not shown in our study that the measured spVLs were produced locally; therefore, these viruses can also reflect a residual replication somewhere in the body, particularly in another compartment with perhaps insufficient drug levels. In the same way, Ghosn et al. have reported that the size of the blood HIV-1 reservoir was highly associated with spVL detection (28).
In conclusion, this work presents the first population model describing TFV pharmacokinetics in blood plasma and seminal plasma in a large population of HIV-1-infected men. TFV seminal plasma concentrations were higher than blood plasma concentrations. TFV accumulated in the seminal plasma in a majority of men (87.8%). The median SP-to-BP AUC0–24 ratio was 2.24. Despite TFV SP exposures higher than BP exposures in a majority of men, an spVL was detectable for 12.2% of patients.
MATERIALS AND METHODS
Study.
The Evarist-ANRS EP 49 study's main objective was to evaluate the proportion of men having an undetectable blood HIV RNA BP VL (<50 copies · ml−1) and a concomitant detectable viral load in semen (28). Men older than 18 years having sex with men, infected by HIV-1, receiving the same antiretroviral treatment for at least 3 months, and having an undetectable BP viral load (<50 copies · ml−1) for at least 6 months were eligible. The Evarist-ANRS EP 49 study protocol was approved by the Ethics Committee of Bicêtre Hospital. All the patients have signed written informed consent forms.
Patients, treatment, and sampling.
The present study population included HIV-1-infected men from the Evarist-ANRS EP 49 study receiving tenofovir disoproxil fumarate (TDF) as part of their cART. Patients received a 300-mg dose of TDF once daily. Two paired blood and semen samples were planned to be collected at two different visits (at day 0 and day 30). The time between drug intake and sampling times was variable among patients. The median (interquartile range) for the delay between dose intake and blood sampling was 11.1 h (3.7 h to 13.4 h) and 10.7 h (4.0 h to 12.5 h), respectively, for day 0 and day 30. The delay between dose intake and semen sampling was 10.5 h (3.5 h to 13 h) and 10 h (4.2 h to 12.1 h), respectively, for day 0 and day 30. The semen sampling was done 35 min (15 to 74.8 min) before the blood sampling. The seminal plasma HIV RNA load (lower limit of quantification [LLOQ], 100 copies · ml−1) was measured during these visits. For each man, demographic (body weight and age) and biological (serum creatinine) characteristics were recorded. Creatinine clearance (CLCR) was estimated using the Cockcroft-Gault formula. Associated antiretroviral drugs, respect of a 48-hour abstinence period before semen sampling, and clinical symptoms of potential sexually transmitted infections were also recorded.
Analytical method.
Tenofovir BP and SP concentrations were measured using a validated high-performance liquid chromatography-tandem mass spectrometry method (29). The LLOQ was 5 ng · ml−1 and 3.13 ng · ml−1 in BP and SP, respectively. A calibration curve was constructed in either spiked blank BP or SP over concentration ranges of 5 to 1,000 ng · ml−1 (BP) and 3.13 to 1,000 ng · ml−1 (SP). Serum creatinine concentrations were measured using the colorimetric method of Jaffe on a Cobas 8000 (Roche/Hitachi).
Genetic analysis.
Written consent for genetic analysis has been obtained from 123 patients for whom the plasma TDF concentration has been measured. DNAs were extracted from blood using a Qiagen kit following the manufacturer's instructions (Qiagen GmbH, Germany). Genes coding for transport proteins for which TFV is known to be a substrate were primarily selected (30). Among these genes, only those that were expressed in the blood-testis barrier were finally selected for further genetic analysis (http://www.proteinatlas.org/). The selected genes (proteins) were as follows: SLC28A2 (CNT-2), SLC29A1 (OAT-1), SLC29A2 (OAT-2), SLC22A1 (OCT-1), SLC22A2 (OCT-2), ABCC2 (MRP-2), ABCC4 (MRP-4), and ABCC10 (MRP-7).
We selected tag SNPs (tSNPs) capturing the common genetic variations of these 8 genes based on variation catalogued in dbSNP from the European population, using the LD TAG SNP selection tool of the SNPinfo web server (31) with the following parameters: (i) a minor allele frequency (MAF) of >20% and (ii) a linkage disequilibrium threshold of r2 > 0.8.
In addition to these tSNPs, a few SNPs were also included based on literature review. Both rs3742106 and rs1751034 in ABCC4 have been correlated with higher plasma tenofovir concentrations (32, 33), as well as rs11854484 in SLC28A2 (CNT-2) (34). Furthermore, rs9349256 and rs2125739 in ABCC10 were associated with kidney tubular dysfunction in patients treated with TDF (25). Finally, a total of 121 SNPs composed of tSNPs and SNPs from the literature were genotyped (Integragene, Evry, France) using Fluidigm technology.
Population pharmacokinetic modeling strategy.
Data were analyzed by a population approach, using the nonlinear mixed-effect modeling software program Monolix version 4.1.4 (available at www.lixoft.eu). Parameters were estimated using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov chain Monte Carlo (MCMC) procedure (35). The maximum number of iterations for each stage (K1 and K2) was fixed at 500 and 200, respectively. The number of Markov chains was fixed at 5 for all estimations.
In a first step, the TFV BP concentrations were modeled and pharmacokinetic (PK) parameters were estimated. Then, these parameters were fixed in order to investigate TFV SP PK modeling. In a final step, all the BP and SP parameters were estimated.
One- and two-compartments models with linear absorption and elimination were investigated as structural models to describe TFV BP pharmacokinetics. The addition of both an additional compartment linked to the central compartment by a first-order process and an effect compartment was tested to describe TFV SP pharmacokinetics. The effect compartment was modeled as a virtual compartment of negligible volume, not modifying the compartmental model in BP. Several models were explored: (i) the effect compartment was connected to the central compartment by a first-order process with the same or different input and output constants, (ii) a transit compartment was inserted between the central and effect compartments, and (iii) the effect compartment was connected to the BP peripheral compartment.
Several error models (proportional, additive, and mixed) were tested to describe the residual variabilities (ε). Interindividual variabilities (IIV or η) were assumed to be exponential. The effect of patient continuous covariates on pharmacokinetic parameters was tested according to a power model centered on the median. The effect of categorical covariates was tested according to the equation CL/F = θCL/F × βCOco, where θCL/F is the typical value of apparent elimination clearance for a subject without the covariate, CO = 0 for the reference value of CL/F, CO = 1 for the value of CL/F with the covariate, and βCO is the estimated influential factor of the binary covariate. The effect of combined antiretroviral drugs was tested individually, e.g., the effect of lopinavir alone, or by pharmaceutical class, e.g., the effect of protease inhibitor class. The influence of covariates was evaluated via an upward-backward model building procedure.
The objective function value (OFV) was used to test different hypotheses regarding the structural model, the structure of the variance-covariance matrix for IIV, the residual variability models, and the covariate effect(s) on pharmacokinetic parameter(s). A covariate was finally retained in the model if its effect was biologically plausible, if it produced a reduction in the variability of the pharmacokinetic parameter (IIV), and if the OFV was decreased by at least 3.84 (χ2 with 1 df, P < 0.05) in the upward phase and was increased by more than 6.63 in the backward phase (χ2 with 1 df, P < 0.01).
Model evaluation.
Goodness of fit was evaluated using the following graphs: observed concentrations versus population and individual predictions, weighted residuals versus time, and weighted residuals versus population predictions.
For validation, a visual predictive check (VPC) was performed (36). The model was used to simulate 500 vectors of parameters for each sampling time. Simulated TFV concentrations and observed data were compared: the 5th, 50th, and 95th percentiles of observed data were overlaid on the 90% confidence interval of the 5th, 50th, and 95th simulated percentiles, and a visual inspection was performed.
Association between genetic polymorphism and TFV transfer into the male genital tract.
SNPs that passed the quality control checks, i.e., (i) Hardy-Weinberg equilibrium with P > 0.05, (ii) frequency test (MAF > 0.1), and (iii) call rate >90%, were further tested for association with the TFV transfer rate constant (derived from the estimated individual pharmacokinetic parameters). Using a linear regression model, all possible genetic models were tested (additive, dominant, and recessive). Accounting for correlation due to linkage disequilibrium between the different SNPs tested, the number of independent tests was then estimated using the Genetic Type I Error Calculator software (37) in order to derive the significance threshold for multiple testing issues.
TFV BP and SP exposures.
TFV blood plasma (BP) and seminal plasma (SP) exposures (AUC0–24) were derived from the estimated individual pharmacokinetic parameters. Distribution in the male genital tract was assessed by calculating SP-to-BP AUC0–24 ratios. The percentage of exposure ratios lower than 1 was calculated.
Impact of TFV exposures on SP HIV RNA load.
To assess the association between TFV SP AUC0–24 or TFV SP-to-BP AUC0–24 ratio and spVL, ROC curves were determined using R software (38). The area under the ROC curve value associated with its 95% confidence interval was estimated. Mixed-effects logistic regressions with random effect on individuals were performed in order to evaluate the impact of TFV SP AUC0–24, TFV SP-to-BP AUC0–24 ratio, and potentially significant cutoffs obtained from the ROC curves on spVL (39). Using the results of a previous study on emtricitabine (FTC) penetration in the male genital tract (27), the impact of TFV-FTC seminal plasma AUC0–24 or SP-to-BP AUC0–24 ratios on spVL was also investigated.
Supplementary Material
ACKNOWLEDGMENTS
All authors report no disclosures or conflicts of interest.
We greatly thank all the patients and staff of the HIV clinical centers who participated in the study.
The French National Agency for Research on AIDS and Viral Hepatitis (ANRS) sponsored the Evarist study. This study also received financial support from SIDACTION.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02062-16.
REFERENCES
- 1.Chan DJ, McNally L, Batterham M, Smith DE. 2008. Relationship between HIV-RNA load in blood and semen in antiretroviral-naïve and experienced men and effect of asymptomatic sexually transmissible infections. Curr HIV Res 6:138–142. doi: 10.2174/157016208783885074. [DOI] [PubMed] [Google Scholar]
- 2.Chan DJ, Ray JE, McNally L, Batterham M, Smith DE. 2008. Correlation between HIV-1 RNA load in blood and seminal plasma depending on antiretroviral treatment status, regimen and penetration of semen by antiretroviral drugs. Curr HIV Res 6:477–484. doi: 10.2174/157016208785861177. [DOI] [PubMed] [Google Scholar]
- 3.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, Boggian K, Cohen MS, Fiscus SA, Eron JJ. 2000. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS 14:117–121. [DOI] [PubMed] [Google Scholar]
- 4.Sheth PM, Kovacs C, Kemal KS, Jones RB, Raboud JM, Pilon R, la Porte C, Ostrowski M, Loutfy M, Burger H, Weiser B, Kaul R. 2009. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS 23:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 5.Politch JA, Mayer KH, Welles SL, O'Brien WX, Xu C, Bowman FP, Anderson DJ. 2012. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 26:1535–1543. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorello G, la Porte C, Pilon R, Zhang G, Karnauchow T, MacPherson P. 2009. Discordance in HIV-1 viral loads and antiretroviral drug concentrations comparing semen and blood plasma. HIV Med 10:548–554. doi: 10.1111/j.1468-1293.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor S, Davies S. 2010. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 5:335–343. doi: 10.1097/COH.0b013e32833a0b69. [DOI] [PubMed] [Google Scholar]
- 8.Trezza CR, Kashuba ADM. 2014. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: implications for HIV prevention. Clin Pharmacokinet 53:611–624. doi: 10.1007/s40262-014-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Else LJ, Taylor S, Back DJ, Khoo SH. 2011. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther (Lond) 16:1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 10.Dumond JB, Reddy YS, Troiani L, Rodriguez JF, Bridges AS, Fiscus SA, Yuen GJ, Cohen MS, Kashuba ADM. 2008. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. J Acquir Immune Defic Syndr 48:156–162. doi: 10.1097/QAI.0b013e31816de21e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Partners PrEP Study Team. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L-G, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng J-H, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx Study Team. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosn J, Chaix M-L, Peytavin G, Rey E, Bresson J-L, Goujard C, Katlama C, Viard J-P, Tréluyer J-M, Rouzioux C. 2004. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS 18:1958–1961. doi: 10.1097/00002030-200409240-00014. [DOI] [PubMed] [Google Scholar]
- 14.Lowe SH, van Leeuwen E, Droste JAH, van der Veen F, Reiss P, Lange JMA, Burger DM, Repping S, Prins JM. 2007. Semen quality and drug concentrations in seminal plasma of patients using a didanosine or didanosine plus tenofovir containing antiretroviral regimen. Ther Drug Monit 29:566–570. doi: 10.1097/FTD.0b013e31811fef29. [DOI] [PubMed] [Google Scholar]
- 15.Vourvahis M, Tappouni HL, Patterson KB, Chen Y-C, Rezk NL, Fiscus SA, Kearney BP, Rooney JF, Hui J, Cohen MS, Kashuba ADM. 2008. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr 47:329–333. doi: 10.1097/QAI.0b013e3181632cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba ADM. 2011. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robillard KR, Hoque T, Bendayan R. 2012. Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 18.Jullien V, Tréluyer J-M, Rey E, Jaffray P, Krivine A, Moachon L, Lillo-Le Louet A, Lescoat A, Dupin N, Salmon D, Pons G, Urien S. 2005. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob Agents Chemother 49:3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. 2006. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr 43:278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 20.Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, Delahunty T, Bushman LR, Fletcher CV. 2008. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther 83:265–272. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 21.Best BM, Letendre SL, Koopmans P, Rossi SS, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Capparelli EV, Ellis RJ, Grant I. 2012. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr 59:376–381. doi: 10.1097/QAI.0b013e318247ec54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, Bridges AS, Stewart PW, Cohen MS, Kashuba ADM. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumond JB, Nicol MR, Kendrick RN, Garonzik SM, Patterson KB, Cohen MS, Forrest A, Kashuba ADM. 2012. Pharmacokinetic modelling of efavirenz, atazanavir, lamivudine and tenofovir in the female genital tract of HIV-infected pre-menopausal women. Clin Pharmacokinet 51:809–822. doi: 10.1007/s40262-012-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pushpakom SP, Liptrott NJ, Rodríguez-Nóvoa S, Labarga P, Soriano V, Albalater M, Hopper-Borge E, Bonora S, Di Perri G, Back DJ, Khoo S, Pirmohamed M, Owen A. 2011. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis 204:145–153. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Novoa S, Labarga P, Soriano V. 2009. Pharmacogenetics of tenofovir treatment. Pharmacogenomics 10:1675–1685. doi: 10.2217/pgs.09.115. [DOI] [PubMed] [Google Scholar]
- 27.Valade E, Tréluyer J-M, Illamola SM, Bouazza N, Foissac F, De Sousa Mendes M, Lui G, Chenevier-Gobeaux C, Suzan-Monti M, Rouzioux C, Assoumou L, Viard J-P, Hirt D, Urien S, Ghosn J, Evarist-ANRS EP 49 Study Group . 2015. Emtricitabine seminal plasma and blood plasma population pharmacokinetics in HIV-infected men in the Evarist-ANRS EP 49 study. Antimicrob Agents Chemother 59:6800–6806. doi: 10.1128/AAC.01517-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosn J, Leruez-Ville M, Blanche J, Delobelle A, Beaudoux C, Mascard L, Lecuyer H, Canestri A, Landman R, Zucman D, Ponscarme D, Rami A, Viard J-P, Spire B, Rouzioux C, Costagliola D, Suzan-Monti M, Evarist-ANRS EP 49 Study Group . 2014. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis 58:1763–1770. doi: 10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- 29.Illamola S, Valade E, Hirt D, Dulioust E, Wolf J, Tréluyer J. 2016. Each matrix is singular. Development and validation of a LC-MS/MS method for the quantification of tenofovir and emtricitabine in plasma and seminal plasma. J Chromatogr B 1033-1034:234–241. doi: 10.1016/j.jchromb.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Michaud V, Bar-Magen T, Turgeon J, Flockhart D, Desta Z, Wainberg MA. 2012. The dual role of pharmacogenetics in HIV treatment: mutations and polymorphisms regulating antiretroviral drug resistance and disposition. Pharmacol Rev 64:803–833. doi: 10.1124/pr.111.005553. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Taylor JA. 2009. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res 37:W600–W605. doi: 10.1093/nar/gkp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rungtivasuwan K, Avihingsanon A, Thammajaruk N, Mitruk S, Burger DM, Ruxrungtham K, Punyawudho B, Pengsuparp T. 2015. Influence of ABCC2 and ABCC4 polymorphisms on tenofovir plasma concentrations in Thai HIV-infected patients. Antimicrob Agents Chemother 59:3240–3245. doi: 10.1128/AAC.04930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. 2008. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr 47:298–303. doi: 10.1097/QAI.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 34.Calcagno A, Cusato J, Marinaro L, Trentini L, Alcantarini C, Mussa M, Simiele M, D'Avolio A, Di Perri G, Bonora S. 2016. Clinical pharmacology of tenofovir clearance: a pharmacokinetic/pharmacogenetic study on plasma and urines. Pharmacogenomics J 16:514–518. doi: 10.1038/tpj.2015.71. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn E, Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1038. doi: 10.1016/j.csda.2004.07.002. [DOI] [Google Scholar]
- 36.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M-X, Yeung JMY, Cherny SS, Sham PC. 2012. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet 131:747–756. doi: 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314. [Google Scholar]
- 39.Bates D, Maechler M, Bolker B, Walker S. 2015. lme4: fitting linear mixed-effects models using Eigen and S4. J Stat Software 67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.