ABSTRACT
In addition to cholesterol-lowering capabilities, statins possess anti-inflammatory and immunomodulatory effects. We sought to quantify the real-world impact of different statin exposure patterns on clinical outcomes in Staphylococcus aureus bacteremia. We conducted a retrospective cohort study among hospitalized patients with positive S. aureus blood cultures receiving appropriate antibiotics within 48 h of culture collection (Veterans Affairs hospitals, 2002 to 2013). Three statin exposure groups were compared to nonusers: pretreated statin users initiating therapy in the 30 days prior to culture and either (i) continuing statin therapy after culture or (ii) not continuing after culture, and (iii) de novo users initiating at culture. Nonusers included patients without statins in the year prior to culture through discharge. Propensity score-matched Cox proportional hazards regression models were developed. We were able to balance significantly different baseline characteristics using propensity score matching for pretreated without continuation (n = 331), pretreated with continuation (n = 141), and de novo (n = 177) statin users compared to nonusers. We observed a significantly lower 30-day mortality rate (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.25 to 0.84; number needed to treat [NNT], 10) among pretreated and continued statin users, while protective effects were not observed in de novo (HR, 1.04; 95% CI, 0.60 to 1.82; NNT, undefined) or pretreated but not continued (HR, 0.92; 95% CI, 0.64 to 1.32; NNT, 47) users. In our national cohort study among patients with S. aureus bacteremia, continuation of statin therapy among incident statin users was associated with significant beneficial effects on mortality, including a 54% lower 30-day mortality rate.
KEYWORDS: anti-inflammatory and immunomodulatory effects, bacteremia, mortality, Staphylococcus aureus, statins, HMG-CoA reductase inhibitors, statins
INTRODUCTION
Statins, selective and competitive inhibitors of 3-hydroxy 3-methylglutaryl coenzyme A (HMG-CoA) reductase, are widely used for primary and secondary prevention of cardiovascular diseases (1). The anti-inflammatory, immunomodulatory, and endothelial barrier protection potential of statins have received considerable research attention (1). It has been postulated that the pleiotropic effect of statins reflects reduced pathogen invasion of host cells (2), decreased levels of proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α] and interleukin-6 [ΙL-6]), and acute-phase proteins, such as C-reactive protein (3, 4), or diminished activation of inflammatory cells (e.g., macrophages and T cells) (5, 6). In fact, a randomized double-blind placebo-controlled clinical trial among patients with bacterial infections found significant reductions in TNF-α and ΙL-6 levels in the statin group compared to the placebo group (7), and another trial observed significantly lower IL-6 and improved survival among prior statin users continuing statin therapy (8).
Staphylococcus aureus is one of the most prevalent pathogens of bacteremia (9). S. aureus bacteremia is associated with a significant burden of disease and a high case fatality rate, ranging from 20 to 30% (10). Laboratory studies have found that statins inhibit S. aureus invasion of human endothelial cells (2, 11) and enhance clearance of S. aureus by phagocytes through the induction of DNA-based extracellular traps (12). Whether these impressive laboratory observations with statins consistently result in significant real-world clinical benefits in complex patients with invasive S. aureus infections remains unclear. Even less clear is the relationship between statin therapy timing and duration and subsequent effects on mortality, including the impact of statin initiation at admission/culture, as adjunctive therapy to antibiotics. Although two large meta-analyses have demonstrated protective effects with statins, exposure periods prior to hospitalization (pretreated) and during hospitalization (continuation and de novo) vary widely (13, 14). Therefore, the purpose of this study was to compare clinical outcomes in patients with S. aureus bacteremia with various statin exposure patterns to those not exposed to statins among a large, national cohort.
(This work was presented in part at the 31st International Conference on Pharmacoepidemiology and Therapeutic Risk Management, 25 August 2015.)
RESULTS
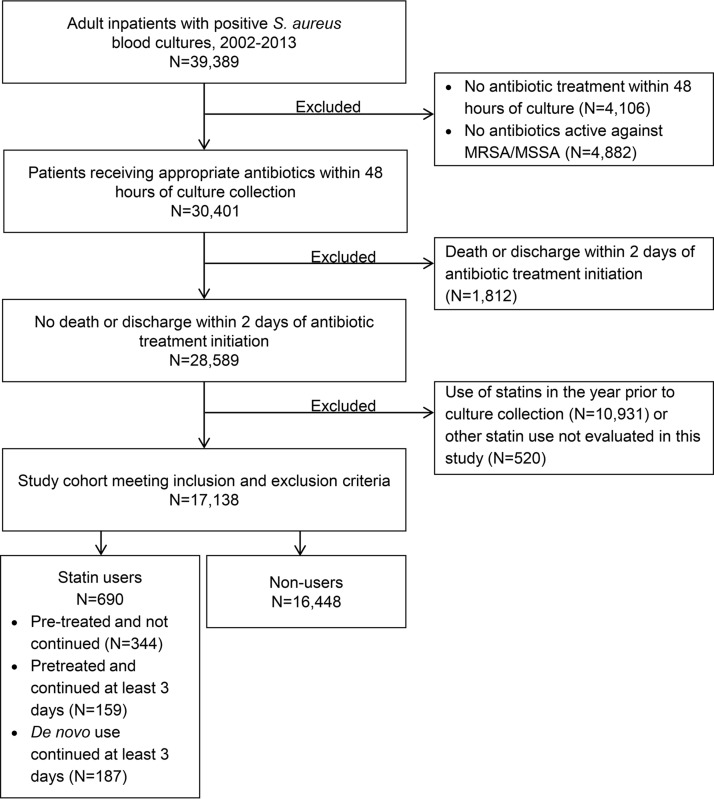
We identified 17,138 patients with S. aureus bacteremia who met our inclusion and exclusion criteria (Fig. 1). Of them, 16,448 were nonusers of statins, 344 were pretreated without continuation at culture, 159 were pretreated with continuation, and 187 were de novo users. Mean statin duration prior to culture was 7 days both among those who continued (standard deviation [SD], 6.9; median, 5; interquartile range [IQR], 3 to 10) and those who did not continue (SD, 7.7; median, 3; IQR, 1 to 11) statin therapy. Statin-exposed patients were significantly older (mean, 69.7 to 71.7 years) (Table 1) and more likely to have been in intensive care at the time of culture collection (22.7% to 29.6%) than nonusers (67 years, 19.8% in intensive care at culture; P < 0.05). Half of nonusers had methicillin-susceptible S. aureus (MSSA) and half had methicillin-resistant S. aureus (MRSA). A similar distribution was observed among the statin exposure groups, except de novo users were more likely to have MSSA (58.3% versus 50.2%; P < 0.05). Sepsis was significantly less common among the pretreated exposure groups than for nonusers (pretreated without continuation, 78.2% versus 83.2% [P < 0.05]; pretreated with continuation, 72.3% versus 83.2% [P < 0.05]).
FIG 1.
Study cohort identification. MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
TABLE 1.
Demographic and hospitalization-related characteristics in statin users and nonusers
| Characteristic | Value(s) by treatment groupa |
|||
|---|---|---|---|---|
| Unexposed (n = 16,448) | Pretreated without continuation (n = 344) | Pretreated with continuation (n = 159) | De novo (n = 187) | |
| Age (yr) | 67.0 ± 12.5 | 69.7 ± 10.9* | 71.7 ± 10.5* | 71.6 ± 11.3* |
| Body mass index | 26.6 ± 7.1 | 28.3 ± 7.1* | 27.3 ± 6.8 | 27.3 ± 6.5 |
| Demographics [no. (%)] | ||||
| Male gender | 16,068 (97.7) | 341 (99.1) | 157 (98.7) | 183 (97.9) |
| White race | 10,202 (62.0) | 250* (72.7) | 105 (66.0) | 112 (59.9) |
| Hispanic ethnicity | 1,013 (6.2) | 18 (5.2) | 7 (4.4) | 9 (4.8) |
| Yr [no. (%)] | ||||
| 2002–2005 | 6,605 (40.2) | 121 (35.2) | 54 (34.0) | 48* (25.7) |
| 2006–2009 | 5,621 (34.2) | 133 (38.7) | 59 (37.1) | 72* (38.5) |
| 2010–2013 | 4,222 (25.7) | 90 (26.2) | 46 (28.9) | 67* (35.8) |
| Admission source [no. (%)] | ||||
| Home | 14,632 (89.0) | 303* (88.1) | 145* (91.2) | 161 (86.1) |
| Hospital | 669 (4.1) | 24* (7.0) | 10* (6.3) | 14 (7.5) |
| Nursing home | 1,147 (7.0) | 17* (4.9) | 4* (2.5) | 12 (6.4) |
| Intensive care at culture | 3,262 (19.8) | 78 (22.7) | 47* (29.6) | 49* (26.2) |
| Treating specialty [no. (%)] | ||||
| General medicine | 9,807 (59.6) | 185 (53.8) | 82* (51.6) | 106* (56.7) |
| Intensive care | 3,468 (21.1) | 85 (24.7) | 50* (31.5) | 56* (29.9) |
| Surgery | 1,749 (10.6) | 47 (13.7) | 22* (13.8) | 17* (9.1) |
| Other | 1,424 (8.7) | 27 (7.8) | 5* (3.1) | 8* (4.3) |
| Region of facility [no. (%)] | ||||
| Midwest | 3,096 (18.8) | 58 (16.9) | 30* (18.9) | 39* (20.9) |
| Northeast | 2,295 (13.9) | 50 (14.5) | 14* (8.8) | 32* (17.1) |
| South | 7,372 (44.8) | 151 (43.9) | 99* (62.3) | 94* (50.3) |
| West | 3,685 (22.4) | 85 (24.7) | 16* (10.1) | 22* (11.8) |
| Source of infectionb [no. (%)] | ||||
| Catheter | 349 (2.1) | 10 (2.9) | 3 (1.9) | 2 (1.1) |
| Endocarditisc | 579 (3.5) | 8 (2.3) | 2 (1.3) | 13 (6.9) |
| Respiratory culture site | 1,216 (7.4) | 27 (7.8) | 9 (5.7) | 7 (3.7) |
| Skin and soft tissue culture site | 2,130 (12.9) | 55 (16.0) | 14 (8.8) | 25 (13.4) |
| Urine | 2,083 (12.7) | 31* (9.0) | 7* (4.4) | 31 (16.6) |
| S. aureus pathogen [no. (%)] | ||||
| MRSA infection | 8,184 (49.8) | 172 (50) | 73 (45.9) | 78* (41.7) |
| MSSA infection | 8,264 (50.2) | 172 (50.0) | 86 (54.1) | 109* (58.3) |
| Sepsis [no. (%)] | 13,676 (83.2) | 269* (78.2) | 115* (72.3) | 156 (83.4) |
Data are means ± standard deviations or number (percent) of patients. An asterisk indicates a P value of <0.05 for pairwise comparison between the statin exposure group and nonuser group.
Culture-confirmed source of infection ±24 h from culture collection unless indicated otherwise.
Source of infection identified from ICD-9-CM diagnosis codes ±24 h from culture collection.
Comorbidity scores during the hospital admission were similar between the exposed groups and nonusers (Table 2); however, there was a lower overall comorbidity burden in the year prior to the current admission among pretreated users with continuation (mean Charlson score, 2.5; SD, 2.9) and de novo users (mean Charlson score, 2.7; SD, 3.1) compared to nonusers (mean Charlson score, 3.2; SD, 3.1; P < 0.05 for both comparisons). Despite similar overall comorbidity burden between statin users and nonusers, the burden of cardiovascular diseases was significantly higher among the statin exposure groups, both during the current admission and in the previous year, as was utilization of medications for hypertension and diabetes. The overall 30-day mortality rate was 20.2% in our study population. The median time to 30-day mortality was similar between nonusers (11 days; IQR, 5 to 18; 20.3%), pretreated statin users without continuation (12 days; IQR, 6 to 18; 19.0%), and de novo users (12 days; IQR, 9 to 17; 16.6%), yet it was significantly lower among pretreated statin users with continuation of therapy (18 days; IQR, 9 to 23; 13.8%; P < 0.05).
TABLE 2.
Clinical characteristics and health service utilization in statin users and nonusers
| Characteristic | Value(s) by treatment groupa |
|||
|---|---|---|---|---|
| Unexposed (n = 16,448) | Pretreated without continuation (n = 344) | Pretreated with continuation (n = 159) | De novo (n = 187) | |
| Time to antibiotic treatment initiation from culture collection [days (IQR)] | 0 (1–0) | 0 (1–0) | 0 (1–0) | 0 (1–0) |
| Length of antibiotic therapy [days (IQR)] | 9 (15–5) | 9 (14.5–6) | 10 (14–6) | 10 (15–6) |
| Time to culture collection from admission [days (IQR)] | 0 (5–0) | 2* (9–0) | 4* (10–1) | 0* (0–0) |
| Surgery during current admission [no. (%)] | 5,808 (35.3) | 123 (35.8) | 65 (40.9) | 62 (33.2) |
| Comorbidity during current admission [no. (%)] | ||||
| Charlson score (means ± SD) | 3.2 ± 2.7 | 3.4 ± 2.6 | 3.4 ± 2.6 | 3.3 ± 2.5 |
| Alcohol abuse | 820 (5.0) | 12 (3.5) | 12 (7.6) | 10 (5.4) |
| Cancer | 1,798 (10.9) | 34 (9.9) | 13 (8.2) | 7* (3.7) |
| Cardiac arrhythmia | 2,348 (14.3) | 71* (20.6) | 32* (20.1) | 35 (18.7) |
| Cerebrovascular disease | 1,465 (8.9) | 49* (14.2) | 25* (15.7) | 38* (20.3) |
| Chronic renal disease | 1,783 (10.8) | 47 (13.7) | 23 (14.5) | 27 (14.4) |
| Chronic respiratory disease | 815 (5.0) | 15 (4.4) | 12 (7.6) | 6 (3.2) |
| Congestive heart failure | 2,924 (17.8) | 99* (28.8) | 57* (35.9) | 57* (30.5) |
| Coronary heart disease | 1,703 (10.4) | 88* (25.6) | 55* (34.6) | 53* (28.3) |
| Diabetes | 5,607 (34.1) | 170* (49.4) | 58 (36.5) | 83* (44.4) |
| Hypertension | 8,175 (49.7) | 210* (61.1) | 99* (62.3) | 111* (59.4) |
| Mild liver disease | 1,792 (10.9) | 10* (2.9) | 8*(5.0) | 8* (4.3) |
| Myocardial infarction | 860 (5.2) | 52* (15.1) | 42* (26.4) | 45* (24.1) |
| Peripheral vascular disease | 414 (2.5) | 19* (5.5) | 5 (3.1) | 4 (2.1) |
| Medication use during current admission [no. (%)] | ||||
| Antihypertensive medication | 11,590 (70.5) | 306* (88.9) | 148* (93.1) | 163* (87.2) |
| Diuretic | 7,896 (48.0) | 209* (60.8) | 87 (54.7) | 95 (50.8) |
| Diabetic medication (oral) | 1,971 (12.0) | 68* (19.8) | 17 (10.7) | 32* (17.1) |
| Insulin | 8,174 (49.7) | 229* (66.6) | 81 (50.9) | 100 (53.5) |
| Corticosteroid | 4,283 (26.0) | 99 (28.8) | 27* (17.0) | 37 (19.8) |
| H2RA/PPI | 12,656 (76.9) | 283* (82.3) | 129 (81.1) | 133 (71.1) |
| NSAID | 2,820 (17.1) | 46 (13.4) | 18 (11.3) | 29 (15.5) |
| Medical conditions in year prior to current admissionb | ||||
| Low-density lipoprotein testing [no. (%)] | 8,358 (50.8) | 220* (64.0) | 106* (66.7) | 88 (47.1) |
| Low-density lipoprotein [mg/dl (IQR)] | 83 (62–107) | 82 (60–116) | 89* (68–121) | 87 (65–120) |
| Previous alcohol abuse [no. (%)] | 632 (3.8) | 9 (2.6) | 5 (3.1) | 2* (1.1) |
| Previous cancer [no. (%)] | 897 (5.4) | 18 (5.2) | 2* (1.3) | 7 (3.7) |
| Previous cardiac arrhythmia [no. (%)] | 1,220 (7.4) | 36* (10.5) | 13 (8.2) | 12 (6.4) |
| Previous chronic renal disease [no. (%)] | 968 (5.9) | 23 (6.7) | 9 (5.7) | 10 (5.4) |
| Previous chronic respiratory disease [no. (%)] | 471 (2.9) | 9 (2.6) | 1 (0.6) | 3 (1.6) |
| Previous coronary heart disease [no. (%)] | 1,219 (7.4) | 64* (18.6) | 25* (15.7) | 19 (10.2) |
| Previous hypertension [no. (%)] | 9,313 (56.6) | 236* (68.6) | 96 (60.4) | 99 (52.9) |
| Previous mild liver disease [no. (%)] | 1,030 (6.3) | 11* (3.2) | 6 (3.8) | 8 (4.3) |
| Previous myocardial infarction [no. (%)] | 654 (4.0) | 47* (13.7) | 15* (9.4) | 15* (8.0) |
| Previous skin or subcutaneous tissue infection [no. (%)] | 892 (5.4) | 24 (7.0) | 6 (3.8) | 17* (9.1) |
| History of medication usec [no. (%)] | ||||
| Antihypertensive medication | 10,253 (62.3) | 314* (91.3) | 143* (89.9) | 93* (49.7) |
| Diuretic | 6,836 (41.6) | 210* (61.1) | 92* (57.9) | 49* (26.2) |
| Diabetic medication (oral) | 2,336 (14.2) | 98* (28.5) | 21 (13.2) | 28 (15.0) |
| Insulin | 5,330 (32.4) | 196* (57.0) | 77* (48.4) | 40* (21.4) |
| Corticosteroid | 3,880 (23.6) | 92 (26.7) | 31 (19.5) | 24* (12.8) |
| H2RA/PPI | 9,455 (57.5) | 262* (76.2) | 110* (69.2) | 59* (31.6) |
| NSAID | 3,312 (20.1) | 78 (22.7) | 23 (14.5) | 19* (10.2) |
| Influenza vaccination | 2,010 (12.2) | 44 (12.8) | 15 (9.4) | 26 (13.9) |
| Previous surgeryb | 4,956 (30.1) | 115 (33.4) | 32* (20.1) | 43* (23.0) |
| Previous hospitalizationb | 9,294 (56.5) | 220* (64.0) | 78 (49.1) | 75* (40.1) |
| Previous nursing home stayb | 1,596 (9.7) | 24 (7.0) | 9 (5.7) | 12 (6.4) |
Data are means ± standard deviations, median (interquartile range [IQR], q1-q3), or number (percent) of patients. An asterisk indicates a P value of <0.05 for pairwise comparison between the statin exposure group and nonuser group. H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug.
Present in the 1 year prior to the Staphylococcus aureus bacteremia hospitalization.
Present in the 90 days prior to the Staphylococcus aureus bacteremia hospitalization.
Baseline characteristics were balanced between statin users and nonusers within propensity score-matched pairs (pretreated without continuation, n = 331; pretreated with continuation, n = 141; de novo, n = 177). Characteristics for the propensity score models, including initial antibiotic treatment, treating specialty, MSSA/MRSA status, sepsis, statin indication, and other characteristics independently associated with the exposure groups or the outcomes, can be found in Table S1 in the supplemental material. Each model demonstrated goodness of fit, with high C statistics of 0.86 to 0.92, indicating excellent discrimination between the groups (15), and complete overlap in propensity score distributions between statin exposure groups and nonusers (pretreated without continuation, mean, 0.094; SD, 0.101; median, 0.054; IQR, 0.022 to 0.132; pretreated with continuation, mean, 0.098; SD, 0.110; median, 0.052; IQR, 0.020 to 0.137; de novo, mean, 0.076; SD, 0.095; median, 0.037; IQR, 0.016 to 0.099).
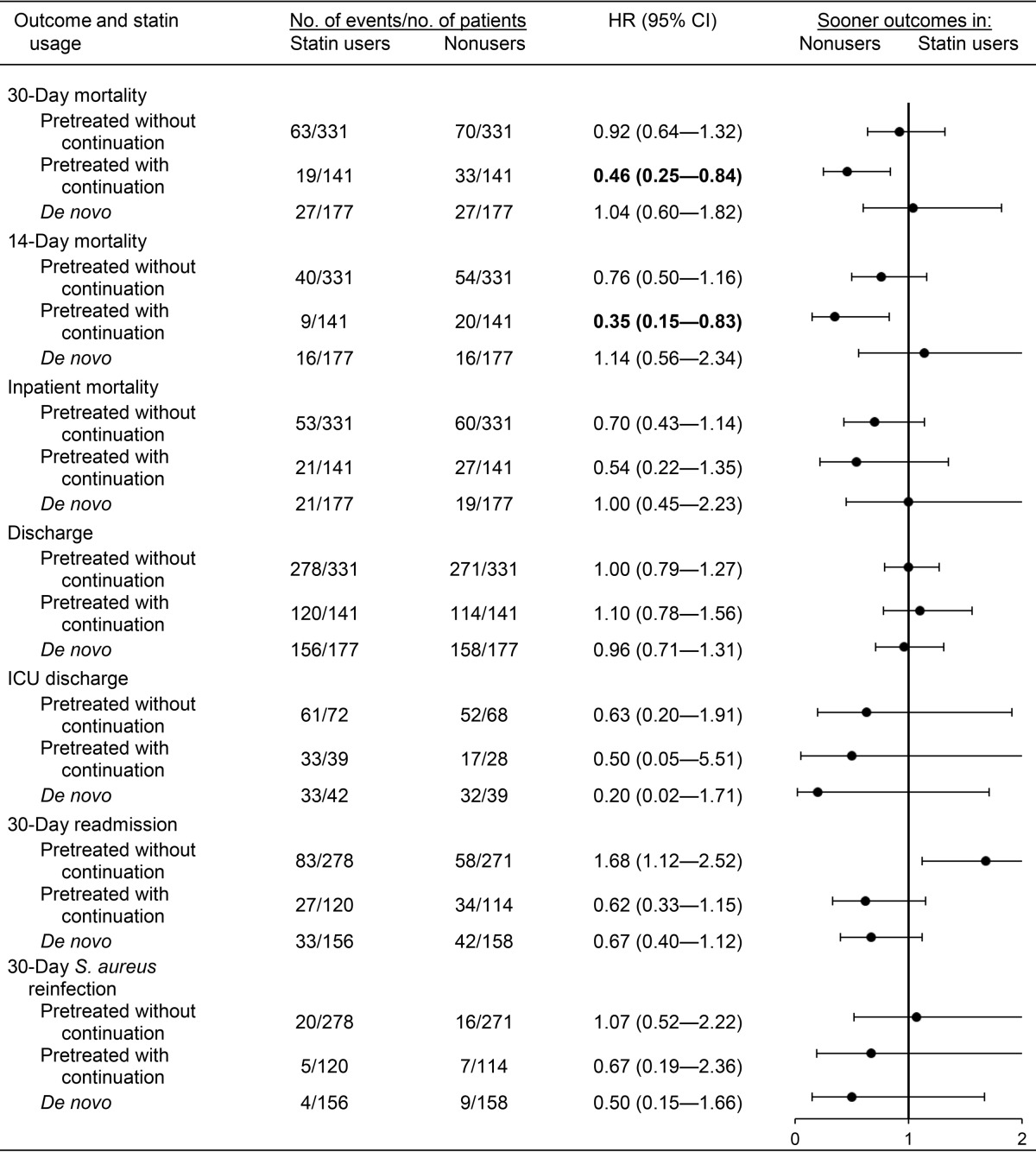
Time to event analyses comparing statin users to nonusers (reference group) are presented in Table 3. No significant differences were observed between nonusers and two of the statin exposure groups (pretreated without continuation and de novo) for any of the outcomes assessed. The rate of 30-day mortality was significantly lower in pretreated statin users with continuation than in propensity-matched nonusers (hazard ratio [HR]HR, 0.46; 95% confidence interval [CI], 0.25 to 0.84) but not among pretreated users who did not continue statin therapy after culture (HR, 0.92; 95% CI, 0.64 to 1.32) or de novo users (HR, 1.04; 95% CI, 0.60 to 1.82). Among pretreated statin users continuing statin therapy after culture, 14-day mortality was also significantly lower than that of nonusers (HR, 0.35; 95% CI, 0.15 to 0.83); however, significant differences were not observed for the other outcomes assessed, including inpatient mortality.
TABLE 3.
Clinical outcomes in propensity matched statin users and non-usersa
Propensity score matched within a 0.005 caliper range. The propensity score was derived from an unconditional logistic regression model and controlled for the variables listed in Tables S2 to S4. Boldface indicates a P value of <0.05.
Similar results were observed in sensitivity analyses utilizing propensity score quintile adjustment (Tables S2 to S4). Sensitivity analyses with inverse probability of treatment weighting (IPTW) also demonstrated significantly lower mortality rates among pretreated statin users with continuation (14-day mortality HR, 0.15; 95% CI, 0.07 to 0.32; 30-day mortality HR, 0.17; 95% CI, 0.10 to 0.30; inpatient mortality HR, 1.39; 95% CI, 1.19 to 1.62) (Tables S2 to S4). Alternatively, in IPTW analyses, statin users without continuation had significantly higher mortality than nonusers, including 14-day mortality (HR, 3.81; 95% CI, 3.26 to 4.44), 30-day mortality (HR, 2.84; 95% CI, 2.46 to 3.28), and inpatient mortality (HR, 3.76; 95% CI, 3.23 to 4.36). In de novo statin users, the 30-day readmission rate was significantly higher than that of nonusers (HR, 1.75; 95% CI, 1.11 to 2.75), as was 30-day S. aureus reinfection (HR, 12.33; 95% CI, 1.21 to 125.59).
The 30-day mortality risk difference in pretreated statin users with continuation versus nonusers was 99 per 1,000 patients (95% CI, 10 to 189 per 1,000), and the number needed to treat (NNT) was 10. For 14-day mortality, the risk difference was 78 per 1,000 patients (95% CI, 8 to 148 per 1,000) and the NNT was 13. The 14-day and 30-day survival probability curves for pretreated statin users with continuation versus nonusers can be found in Fig. 2.
FIG 2.
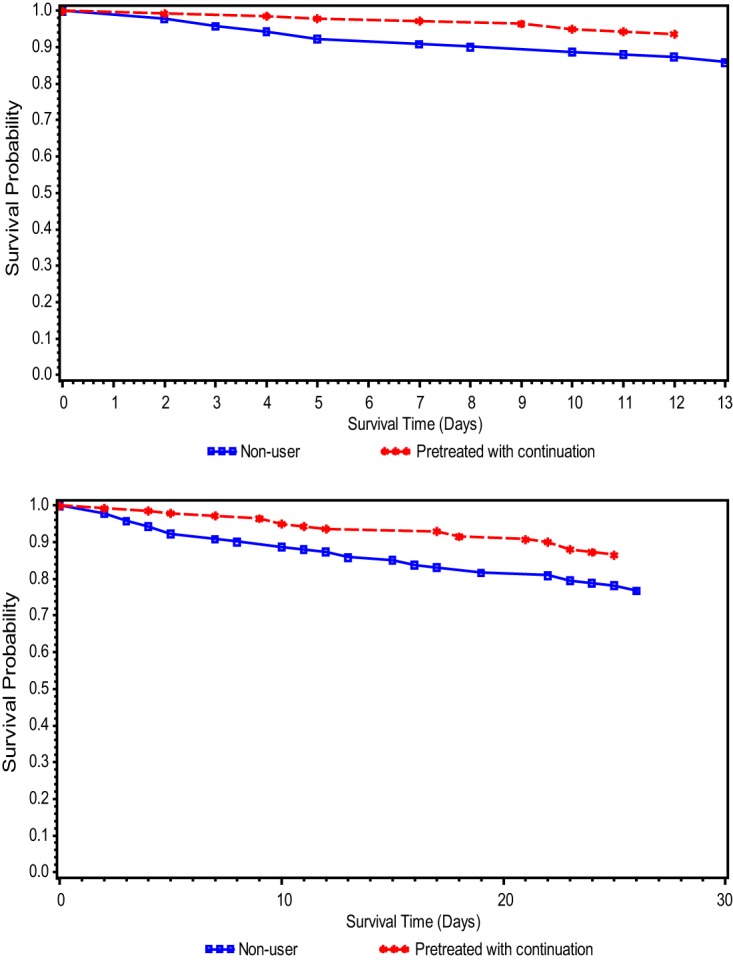
(a) Fourteen-day survival probability curve among propensity-matched statin users with continuation and nonusers. (b) Thirty-day survival probability curve among propensity-matched statin users with continuation and nonusers.
DISCUSSION
Recent statin initiation with continuation of statin therapy for at least 3 days after culture was associated with a substantial protective effect on mortality among our large, national, real-world cohort with S. aureus bacteremia. These findings were robust in our primary analyses using propensity score matching and in our sensitivity analyses using propensity score quintile adjustment and inverse probability of treatment weighting. In vitro research suggests statins confer protective effects in S. aureus bacteremia, since they (i) inhibit S. aureus invasion of human endothelial cells (2, 11); (ii) interfere with S. aureus biofilm formation (16); and (iii) enhance clearance of S. aureus by phagocytes through the induction of DNA-based extracellular traps (12). Consistent with our findings, several meta-analyses have identified protective effects with statins on all-cause mortality among patients with various types of infections. Pleiotropic effects with statins were evaluated among patients with sepsis, pneumonia, or bacteremia by pooling 20 published studies (13). The authors reported a 50% reduced mortality in statin users (pooled odds ratio [OR], 0.49; 95% CI, 0.37 to 0.61). The bacteremia-related mortality (evaluated in 4 studies out of 20) was also significantly lower in statin users (pooled OR, 0.33; 95% CI, 0.09 to 0.75). Another meta-analysis found that outpatient use of statins was associated with a 29% decreased risk of all-cause mortality in patients with any infection (pooled OR across 41 studies, 0.71; 95% CI, 0.64 to 0.78) (14).
Among the included studies in both meta-analyses, exposure periods prior to hospitalization (pretreated) and after hospitalization (continuation and de novo) varied widely, and sensitivity analyses by statin exposure timing and duration were not conducted (13, 14). Indeed, some studies have included patients with such varied statin exposures, and application of the study findings to clinical practice would not be possible. One observational study defined statin use as the presence of a statin on the day of culture regardless of previous or continued use (17). This statin exposure definition combined both prevalent (of unknown timing and duration) and incident statin users, as well as patients continuing and not continuing statin therapy. Not surprisingly, statin use in this study was not associated with reductions in 90-day mortality, intensive care unit (ICU) admission, or hospital/ICU discharge when adjusting for confounders, including indications for statin therapy, using propensity score methods (17).
In our study, pretreated patients who continued on statin therapy experienced decreased rates of mortality, while these protective effects were not observed in pretreated patients who did not continue statin therapy or in patients with de novo use. These results support statin continuation through the period of inflammation, as effects on the inflammatory response are no longer observed once the statin is discontinued (18). Similar results were observed in a multicenter randomized placebo-controlled trial of 250 patients with severe sepsis assigned to statin therapy (n = 123) or placebo (n = 127) (8). Randomization accounted for prior statin use, defined as at least 2 weeks of statin use prior to hospitalization (prevalent users) or no use in the 2 weeks before admission; those with less than 2 weeks of statin use prior to admission were excluded. Pretreated statin users assigned to statin therapy had a lower 28-day mortality (5% versus 11%; P = 0.01) than the placebo group, although like our study, inpatient mortality was not significantly lower. Further, 28-day mortality in de novo users was similar to that of the placebo group (16.3% versus 14.9%; P = 0.78). It should be noted that duration of previous statin use was not assessed in the clinical trial, and as such, variations in outcomes may have existed because of duration, although the study size was likely too small to detect any such differences (pretreated assigned to statins, n = 37; pretreated assigned to placebo, n = 40).
We only know of one other study specifically examining the effects of statins on patient mortality in S. aureus bacteremia (19). A prospective cohort study, which included 160 S. aureus bacteremia episodes from one hospital in Spain, found that the 33 statin users were less likely to die within 14 days than nonusers (adjusted OR, 0.08; 95% CI, 0.01 to 0.66), but a significant difference between groups was not observed for 30-day mortality (adjusted OR, 0.35; 95% CI, 0.10 to 1.23; P = 0.10). Statin exposure was defined as prevalent statin use at bacteremia onset, and all users had at least 1 month of previous statin therapy. Another limitation of this Spanish study, besides prevalent statin use, was that 23/33 (70%) of the statin users had a vascular catheter as the source of bacteremia, compared to only 46/127 (36%) in nonusers. Given that vascular catheters are a readily removable source of bacteremia with lower mortality rates than other sources, such a difference is difficult to ignore (20). In our study, catheter source was similar between statin exposure groups and nonusers (Table 1).
Although most observational studies have confirmed the protective effects of statins on clinical outcomes in bacterial infections (19, 21–23), there is a concern surrounding this association due to the possibility of healthy user bias (24, 25). Patients taking preventive medications, such as statins, are more likely to have healthier behaviors resulting in favorable outcomes, including lower mortality rates, than sicker patients (26, 27). A multicenter inception cohort study conducted by Yende et al. supported this trend among statin users, providing evidence that statin use was significantly associated with good health behaviors, including health insurance, good functional status, and immunizations (28). Our approach to minimizing healthy user bias in our study was 3-fold (29). First, we designed our study to include only incident statin users and to assess patients continuing statin therapy as one exposure group and those not continuing as a separate exposure group, both of which were compared to a common reference group of nonusers. Second, we included proxies for healthy behaviors in our propensity score model, including use of preventative services (e.g., vaccination and health screenings) and conditions that impact health behaviors. Third, we implemented propensity score matching to identify nonusers with similar distributions of important patient characteristics related to health. By excluding prevalent statin users, we believe our study minimized the potential for healthy user bias, as this bias is observed in chronic medication use (25).
There are limitations to our study. First, although we employed propensity score methods to address potential confounders of the association between the use of statins and clinical outcomes, we were unable to control for unmeasured confounding. These methods allowed us to balance confounders of the exposure-outcome relationship that were included in the propensity score; however, it could not control for unbalanced factors that were not measured in our study. Second, variations in point estimates were observed with propensity score matching, adjustment, and inverse probability of treatment weighting. Although propensity score matching produced the most conservative estimates, it also resulted in the greatest balance between groups. Third, we attempted to identify incident statin use in order to assess the effect of statins at the time of S. aureus infection. We defined incident use as initiation in the 30 days prior to culture, with no prior statin exposure in the previous year. As such, incident use did not necessarily mean throughout the patient's lifetime. Therefore, our estimates may not completely rule out the influence of historical statin use (beyond the window that we defined in this study) on the outcomes. Fourth, our study results should be applied carefully to the general population, since our study was conducted among veterans and approximately 98% were male. Fifth, as a retrospective study of existing data, the accuracy of operational definitions depends on the data source. Although we utilized one of the most comprehensive and accurate data sources for health outcome research available in the United States, misclassification may still occur. For example, culture source is a free text field in the microbiology data, and without mention of a catheter in that field we could not determine whether it was a catheter source. Lastly, we did not assess outcomes for specific statins or doses, which is an important area of inquiry, as some data suggest added benefit of high-potency or high-dose statins (30, 31).
Conclusions.
Our large, national, real-world cohort study showed that continuation of statins in recent initiators significantly lowered the risk of 30-day mortality in S. aureus bacteremia. By continuing statins in 10 patients, 1 death would be prevented in the 30 days after culture. New initiation of statins as adjunctive therapy to antibiotics still requires further investigation as a potential measure to optimize positive clinical outcomes and should include clinical observational research and pragmatic trials to ensure greater real-world application of the findings.
MATERIALS AND METHODS
Data source.
The Veterans Health Administration (VA) is a nationwide health care system for veterans in the United States which has utilized an electronic medical record since 1999 (32). National VA databases provide comprehensive information on patient care, including the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) (33), diagnostic and procedure codes, laboratory and microbiology results, vital signs and vital status, and pharmacy data, including barcode medication administration records for inpatients, inpatient and outpatient prescription and fill records, and medications prescribed by non-VA providers or purchased by patients at non-VA pharmacies. This study was approved by the Institutional Review Board and Research and Development Committee at the Providence Veterans Affairs Medical Center. The methods described here were prespecified in our research plan.
Study population.
We conducted a retrospective cohort study quantifying the effect of statin use on clinical outcomes among patients with S. aureus bacteremia. We identified adult patients (age, ≥18 years) admitted to VA hospitals whose blood cultures were positive for S. aureus between 1 January 2002 and 1 December 2013. We then assessed antibiotic therapy for each patient during hospital admission. We included patients who received intravenous β-lactam therapy (ampicillin-sulbactam, nafcillin, oxacillin, piperacillin-tazobactam, cefazolin, cefotetan, cefoxitin, ceftazidime, ceftriaxone, ceftaroline, ertapenem, doripenem, imipenem-cilastatin, or meropenem) or vancomycin for methicillin-susceptible S. aureus (MSSA) and vancomycin or ceftaroline for methicillin-resistant S. aureus (MRSA) within 48 h of culture collection. Due to the existing labeling guidance (drug interactions) on temporarily suspending statins in patients receiving daptomycin, we did not include patients with initial daptomycin therapy. We excluded patients who died or were discharged on the day of culture or the day after culture. We only evaluated the first admission within the study period after accounting for all inclusion and exclusion criteria.
Statin use.
All statin users were incident users not having used statins in the year prior to culture. The study was designed with this restriction criterion to avoid healthy user bias. We defined incident pretreated statin users as those initiating a statin (i.e., atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin) in the 30 days prior to culture collection. Among pretreated statin users, we included those continuing therapy for at least 3 days after culture (pretreated with continuation) and those not continuing therapy after culture (pretreated without continuation). De novo users initiated statins on the day of culture or the day after culture. Nonusers included patients without any pharmacy records for statins in the year prior to culture collection through discharge.
Outcomes.
Our primary outcome was time to 30-day mortality, defined as mortality within 30 days of the index date, i.e., the culture collection date. The secondary outcomes of interest were time to 14-day mortality (mortality within 14 days of the index date), inpatient mortality (mortality during the hospitalization), hospital discharge, intensive care unit (ICU) discharge, 30-day readmission, and 30-day S. aureus reinfection. We calculated time for each endpoint from the index date to the event date. ICU discharge was examined among patients whose cultures were taken while in the ICU. For ICU and hospital discharge, if patients died during hospital admission, we censored them on their date of death. For readmission and reinfection, we computed time from the hospital discharge date to the event date. Patients who died during admission were not included in the evaluation of postdischarge outcomes. We censored patients on their date of death if they died within 30 days after discharge.
Statistical analysis.
We assessed baseline differences between the statin exposure group and nonusers using a chi-square or Fisher's exact test for categorical variables and a t test or nonparametric Wilcoxon rank sum test for continuous variables. To generate propensity scores (the predicted probability of statin use), we developed an unconditional logistic regression model using a manual backward elimination approach (34, 35). In the final propensity score models, we checked for multicollinearity and goodness of fit and ran propensity score diagnostics (36). We performed nearest-neighbor propensity score matching within 0.005 caliper (36) and reviewed subsequent covariate balance between the matched groups (34, 35).
To quantify the effect of statin therapy on clinical outcomes, we used Cox proportional hazard regression models. Cox proportional hazard regression assumptions were assessed, including proportionality (37). These analyses were conducted separately for each statin exposure group, in which separate propensity score models were built for pretreated users with continuation, pretreated users without continuation, and de novo users. Subsequent outcomes, compared to nonusers, were assessed separately for each of these statin exposure groups. A hazard ratio above 1 indicated an increased probability of the outcome occurring sooner in the statin exposure group than in nonusers. The number needed to treat was calculated from risk differences among matched pairs. In sensitivity analyses, Cox models were adjusted for propensity score quintiles, with quintile 1 serving as the reference, and weighted by the inverse probability of treatment (38). All analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC).
Supplementary Material
ACKNOWLEDGMENTS
The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs. G.S. and V.N. have research support under a National Institutes of Health grant (1U54HD090259). A.C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We appreciate the assistance of Vrishali Lopes with data preparation and analyses.
A.C. has received research funding from Pfizer, Merck (Cubist), and The Medicines Company. T.T. and E.N. have no conflicts to disclose. G.S. has received speaking honoraria from Merck, Allergan, Sunovion, and The Medicines Company and consulting fees from Allergan and The Medicines Company. S.O. is a consultant for AtoxBio BioAegis, Arsanis, Aridia, and Battelle and has received institutional grants from Glaxo-Smith-Kline, Asahi-Kasei, Cardeas, and Ferring. V.N. has received research funding from or acted as an advisor for InhibRx, Altermune Technologies, Trius Therapeutics, Cidara Therapeutics, and Roche Pharmaceuticals. K.L. has received research funding from or acted as a scientific advisor for Allergan, Bard, Merck (Cubist), Pfizer, and The Medicines Company.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02228-16.
REFERENCES
- 1.Hennessy E, Adams C, Reen FJ, O'Gara F. 2016. Is there potential for repurposing statins as novel antimicrobials? Antimicrob Agents Chemother 60:5111–5121. doi: 10.1128/AAC.00192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn MP, Knecht SM, Rushing FL, Birdsong J, Siddall CP, Johnson CM, Abraham TN, Brown A, Volk CB, Gammon K, Bishop DL, McKillip JL, McDowell SA. 2008. Simvastatin inhibits Staphylococcus aureus host cell invasion through modulation of isoprenoid intermediates. J Pharmacol Exp Ther 326:135–143. doi: 10.1124/jpet.108.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenson RS, Tangney CC, Casey LC. 1999. Inhibition of proinflammatory cytokine production by pravastatin. Lancet 353:983–984. [DOI] [PubMed] [Google Scholar]
- 4.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. 2001. Effect of hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation 103:1933–1935. doi: 10.1161/01.CIR.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 5.Fehr T, Kahlert C, Fierz W, Joller-Jemelka HI, Riesen WF, Rickli H, Wüthrich RP, Ammann P. 2004. Statin-induced immunomodulatory effects on human T cells in vivo. Atherosclerosis 175:83–90. doi: 10.1016/j.atherosclerosis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Kwak B, Mulhaupt F, Myit S, Mach F. 2000. Statins as a newly recognized type of immunomodulator. Nat Med 6:1399. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 7.Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NJ, Douvdevani A, Amichay D, Almog Y. 2009. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 35:1255–1260. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 8.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B, Jones D, Joyce C, Kostner K, McNeil J, Nichol A, Roberts MS, Syres G, Venkatesh B, ANZ-STATInS Investigators-ANZICS Clinical Trials Group. 2013. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 9.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 10.Kern WV. 2010. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis 23:346–358. doi: 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 11.Pruefer D, Makowski J, Schnell M, Buerke U, Dahm M, Oelert H, Sibelius U, Grandel U, Grimminger F, Seeger W, Meyer J, Darius H, Buerke M. 2002. Simvastatin inhibits inflammatory properties of Staphylococcus aureus α-toxin. Circulation 106:2104–2110. doi: 10.1161/01.CIR.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- 12.Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. 2010. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janda S, Young A, FitzGerald JM, Etminan M, Swiston J. 2010. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 25:656.e657–656.e622. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Wen X, Peng J, Lu Y, Guo Z, Lu J. 2012. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS One 7:e51548. doi: 10.1371/journal.pone.0051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 16.Graziano TS, Cuzzullin MC, Franco GC, Schwartz-Filho HO, de Andrade ED, Groppo FC, Cogo-Muller K. 2015. Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One 10:e0128098. doi: 10.1371/journal.pone.0128098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung S, Pokharel R, Gong MN. 2012. Statins and outcomes in patients with bloodstream infection: a propensity-matched analysis. Crit Care Med 40:1064–1071. doi: 10.1097/CCM.0b013e31823bc9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almog Y, Novack V, Eisinger M, Porath A, Novack L, Gilutz H. 2007. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med 35:372–378. doi: 10.1097/01.CCM.0000253397.42079.D5. [DOI] [PubMed] [Google Scholar]
- 19.López-Cortés LE, Gálvez-Acebal J, del Toro MD, Velasco C, de Cueto M, Caballero FJ, Muniain MA, Pascual Á Rodríguez-Baño J. 2013. Effect of statin therapy in the outcome of bloodstream infections due to Staphylococcus aureus: a prospective cohort study. PLoS One 8:e82958. doi: 10.1371/journal.pone.0082958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose WE, Eickhoff JC, Shukla SK, Pantrangi M, Rooijakkers S, Cosgrove SE, Nizet V, Sakoulas G. 2012. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 206:1604–1611. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liappis AP, Kan VL, Rochester CG, Simon GL. 2001. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis 33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 22.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. 2006. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 32:75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 23.Hsu J, Andes DR, Knasinski V, Pirsch J, Safdar N. 2009. Statins are associated with improved outcomes of bloodstream infection in solid-organ transplant recipients. Eur J Clin Microbiol Infect Dis 28:1343–1351. doi: 10.1007/s10096-009-0787-4. [DOI] [PubMed] [Google Scholar]
- 24.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. 2006. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ 333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, Solomon DH. 2007. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol 166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 26.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. 2006. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White HD. Adherence and outcomes: it's more than taking the pills. Lancet 366:1989–1991. [DOI] [PubMed] [Google Scholar]
- 28.Yende S, Milbrandt EB, Kellum JA, Kong L, Delude RL, Weissfeld LA, Angus DC. 2011. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 39:1871–1878. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrank WH, Patrick AR, Brookhart MA. 2011. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 26:546–550. doi: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouellette DR, Moscoso EE, Corrales JP, Peters M. 2015. Sepsis outcomes in patients receiving statins prior to hospitalization for sepsis: comparison of in-hospital mortality rates between patients who received atorvastatin and those who received simvastatin. Ann Intensive Care 5:9. doi: 10.1186/s13613-015-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou SY, Chu H, Chao PW, Ou SM, Lee YJ, Kuo SC, Li SY, Shih CJ, Chen YT. 2014. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Med 40:1509–1517. doi: 10.1007/s00134-014-3418-1. [DOI] [PubMed] [Google Scholar]
- 32.Hynes DM. 2013. Overview of VA data, information systems, national databases and research uses. VA Information Resource Center, Washington, DC: http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=751. [Google Scholar]
- 33.Centers for Disease Control and Prevention. 2011. International classification of diseases, 9th revision, clinical modification. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 34.D'Agostino RB., Jr 1998. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Rubin DB. 1997. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 127:757–763. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 36.Austin PC. 2011. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. 1999. Applied survival analysis: regression modeling of time to event data. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 38.Hernán MA, Robins JM. 2017. Causal inference. Chapman & Hall/CRC, Boca Raton, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.