ABSTRACT
The loss of fitness in colistin-resistant (CR) Acinetobacter baumannii was investigated using longitudinal isolates from the same patient. Early CR isolates were outcompeted by late CR isolates for growth in broth and survival in the lungs of mice. Fitness loss was associated with an increased susceptibility to oxidative stress since early CR strains had reduced in vitro survival in the presence of hydrogen peroxide and decreased catalase activity compared to that of late CR and colistin-susceptible (CS) strains.
KEYWORDS: Acinetobacter, ESKAPE, catalase, fitness, mass spectrometry, mouse model, serial isolation, virulence
TEXT
Colistin resistance (CR) in Acinetobacter baumannii is due to the addition of phosphoethanolamine (1, 2) and galactosamine to the lipid A portion of the lipopolysaccharide (LPS) (3) and is mediated by the upregulation of the PmrAB two-component system (4). CR leads to the loss of fitness and virulence in laboratory-derived and clinical isolates of A. baumannii (5–7), suggesting that adapting to colistin stress may be detrimental to the survival of this pathogen. However, these studies were only evaluated in a single CR isolate and, thus, do not take into account the potential adaptations made by bacteria throughout the course of infection.
In this study, our goal was to further investigate the association between CR and fitness loss as it developed in vivo by performing a comparative analysis of five longitudinal CR A. baumannii strains that were serially obtained from the same patient following colistin therapy. Briefly, the patient was hospitalized in an intensive care unit or surgical ward after sustaining severe trauma injuries in combat operations outside of the United States (8). The patient received antibiotics for complicated infections during an initial hospitalization in Afghanistan, transient hospitalization in Germany, and definitive hospitalization in the United States. The bacterial isolates used in this study (parental, colistin-susceptible [CS] MRSN 5540 and CR clinical isolates MRSN 6268, MRSN 6269, MRSN 6270, MRSN 6271, and MRSN 6272) were obtained from successive cultures from the urinary or respiratory tract (8). CR in all of the strains was due to a point mutation in pmrA and/or pmrB (8) that resulted in the addition of phosphoethanolamine to the lipid A portion of the LPS (Fig. 1A).
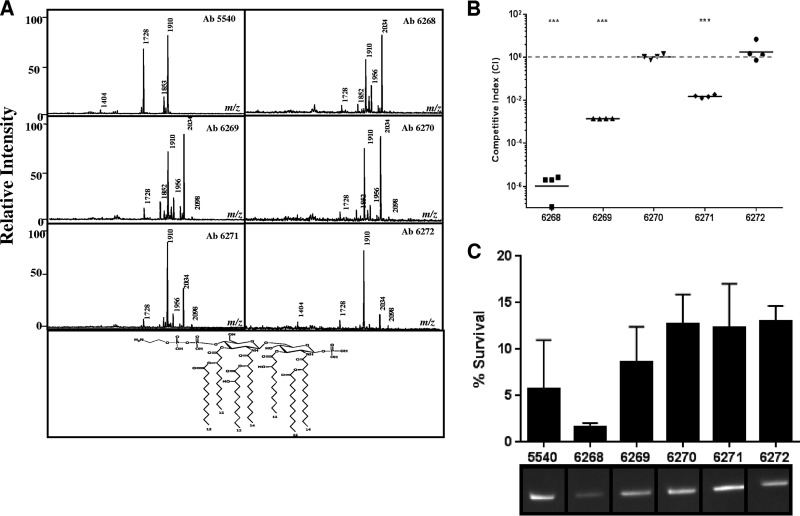
FIG 1.
Fitness loss in colistin-resistant A. baumannii was due to enhanced susceptibility to oxidative stress and a defect in catalase activity. (A) Mass spectra of lipid A of A. baumannii clinical isolates. The peak at 2,034 m/z represents phosphoethanolamine. (B) A. baumannii clinical isolates competed for growth in LB for 24 h. Each symbol represents a single replicate. Data are representative of those from three independent experiments. (C) The survival of CR A. baumannii clinical isolates in the presence of 0.001% hydrogen peroxide was measured after 2 h. The percent survival was assessed as the CFU ratio of treated and untreated samples. Total catalase activity was quantified using in-gel catalase assays. Statistical analyses of competition experiments (one sample t test) were performed using GraphPad Prism. ***, P < 0.0005.
Fitness loss was measured using competition assays. For in vitro experiments, 100 μl of bacterial inoculum was grown in 20 ml of lysogeny broth (LB) at 37°C for 24 h, and bacteria were plated as described above. Early CR isolates were also outcompeted by the CS isolate for growth in LB after 24 h, with MRSN 6268 being the least fit strain. Late CR isolates showed a steady increase in fitness, culminating with the final isolate MRSN 6272 having a competitive index (CI) of >1.0 (Fig. 1B). There was no significant difference in SDS susceptibilities of CR isolates (data not shown), indicating that the loss of fitness was not due to an inherent loss of membrane integrity.
The isolates used in this study were previously exposed to colistin during infection, which can kill A. baumannii through the production of hydroxyl radicals (9, 10). To address whether oxidative stress contributed to the fitness defect in CR isolates, we grew bacteria in the presence or absence of 0.001% hydrogen peroxide for 2 h and the percent survival rate was calculated. Early CR isolate MRSN 6268 had an ∼2% survival rate in the presence of hydrogen peroxide, while late isolates MRSN 6270 to 6272 had an ∼12% survival rate (Fig. 1C). Catalases play important roles in protecting bacteria from damage caused by reactive oxygen species by hydrolyzing hydrogen peroxide (11). Therefore, an in-gel catalase assay was performed as previously described (12) to determine whether the increased susceptibility of early isolates to oxidative stress was due to altered total catalase activity. Indeed, early CR isolate MRSN 6268 exhibited a reduced level of catalase activity compared to other CR isolates (Fig. 1C), suggesting that the increase in susceptibility to oxidative stress in early CR isolates was due to an inability to break down hydroxyl radicals. Sun et al. recently showed that hydrogen peroxide resistance in A. baumannii was mediated by KatG and KatE (13), which are also conserved in these isolates. It is possible that the expression of catalase genes in CR isolates is altered, and further characterization of these genes is needed to determine whether they play a role in decreased fitness.
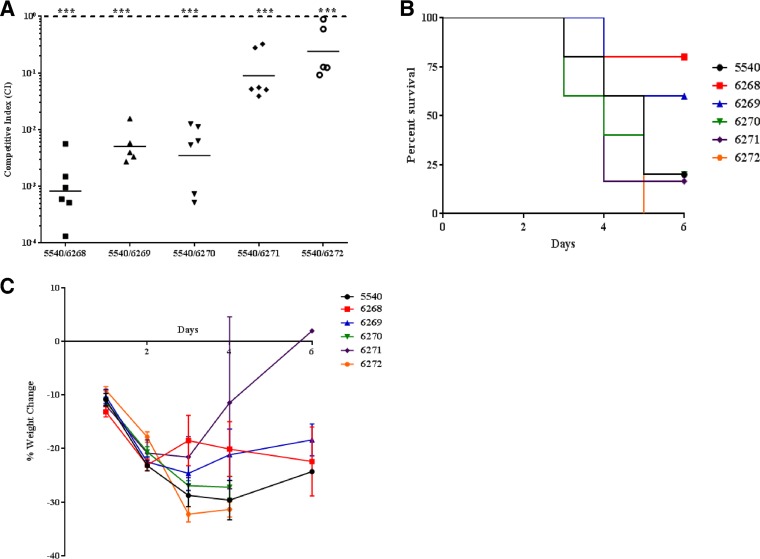
Using a neutropenic mouse model of lung infection, we investigated the in vivo fitness of longitudinal isolates. Briefly, cyclophosphamide-treated BALB/c mice were intranasally infected with 5.0 × 106 CFU of an inoculum containing a 1:1 ratio of the CS isolate and the indicated CR strain was suspended in sterile phosphate-buffered saline (PBS). The lungs were collected at 48 h, and homogenates were plated on lysogeny broth (LB) agar with and without colistin sulfate. The competitive index (CI) was calculated using the following formula: CI = (mutant CFUoutput/wild-type CFUoutput)/(mutant CFUinput/wild-type CFUinput) (14). Interestingly, early CR isolates were outcompeted by the CS strain for growth in the lungs of mice at 48 h; however, there was a steady increase in fitness of A. baumannii clinical isolates, as they were isolated from the patient longitudinally (Fig. 2A). To determine whether in vivo adaptations of latter CR isolates correlated with increased virulence, we performed survival studies. Mice were intranasally infected with 1 × 108 CFU of each strain and monitored for signs of morbidity. We found that mice infected with the CS strain had susceptibilities similar to those of mice infected with late CR isolates (Fig. 2B). Mice infected with late CR isolates had a 0% to 25% survival rate, which is in stark contrast to the 60% to 80% survival rates of mice infected with early CR isolates. Furthermore, mice that died from A. baumannii infection exhibited a >25% decrease in body weight (Fig. 2C). These data showed that increased virulence in late CR isolates correlated with increased in vivo fitness, suggesting that A. baumannii may compensate for the possible growth defect associated with developing colistin resistance (Fig. 2B). It should be noted that virulence in this model is relative since a high dose of bacterial inoculum was required to establish infection in mice that were immunosuppressed using cyclophosphamide, a drug that causes transient leukopenia in this mouse model of infection. All of the research presented was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (15).
FIG 2.
In vivo fitness adaption of colistin-resistant A. baumannii. (A) Colistin-resistant A. baumannii clinical isolates were competed for growth in the lungs of mice for 48 h. Data are combined from two independent experiments, and each symbol represents a single animal. (B) Survival curves of mice (n = 5) intranasally infected with 1 × 108 CFU of A. baumannii. (C) Weight change of mice infected intranasally with 1 × 108 CFU of A. baumannii. Data are representative of two independent animal experiments. Statistical analysis of competition experiments (one-sample t test) was performed using GraphPad Prism. ***, P < 0.0005.
Colistin resistance has been associated with in vitro growth defects in A. baumannii. Therefore, we performed growth curves of A. baumannii in nutrient-rich media (see Fig. S1 in the supplemental material) and found that MRSN 6268 and MRSN 6271 had significant defects in growth compared to MRSN 5540 up to 12 h, suggesting that the observations associated with decreased fitness and virulence in MRSN 6268 may be due to a generalized growth defect. Interestingly, MRSN 6271 had increased virulence and fitness despite the in vitro growth defect. We speculate that the growth defect, while an important contributing factor, may not impact the outcome of infection since all strains were able to grow equally at 12 h. To address the contradicting in vitro growth defect, we measured bacterial burden in the lungs of mice infected with 5 × 106 CFU at 24 h postinfection. As previously mentioned, the absence of an intact cellular immune system in neutropenic mice allows for the observation of inherent differences in growth of study isolates. There was no difference in bacterial burden in the lungs of mice infected with study isolates after 24 h, suggesting that the in vitro growth defect observed in A. baumannii may not correlate with the ability of bacteria to grow in the host.
Our findings further characterize the phenotype of CR A. baumannii clinical isolates in vivo by showing that CR isolates underwent an initial loss in fitness and virulence, partially due to an inability to cope with oxidative stress. This fitness and virulence defect was compensated for in late CR isolates, and adaptive mutations likely attribute increased fitness and virulence. The absence of single nucleotide polymorphisms (SNPs) in virulence genes of CR isolates, as revealed by whole-genome sequencing (data not shown), suggests that bacterial adaptions to increase fitness and virulence may be due to posttranslational modifications or physiological changes. Taken together, these findings highlight an area of weakness for this emerging superbug that can be potentially exploited by drug intervention.
Supplementary Material
ACKNOWLEDGMENTS
The research presented was supported and funded via multiple grants from the Military Infectious Diseases Research Program (MIDRP) and the Defense Medical Research and Development Program (DMRDP).
The findings and opinions expressed herein belong to the authors and do not necessarily reflect the official views of the WRAIR, the U.S. Army, or the Department of Defense.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00598-16.
REFERENCES
- 1.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the PmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. 2013. Unique structural modifications are present in the LPS from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hraiech S, Roch A, Lepidi H, Atieh T, Audoly G, Rolain JM, Raoult D, Brunel JM, Papazian L, Bregeon F. 2013. Impaired virulence and fitness of a colistin-resistant clinical isolate of Acinetobacter baumannii in a rat model of pneumonia. Antimicrob Agents Chemother 57:5120–5121. doi: 10.1128/AAC.00700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Rojas R, McConnell MJ, Jimenez-Mejias ME, Dominguez-Herrera J, Fernandez-Cuenca F, Pachon J. 2013. Colistin resistance in a clinical Acinetobacter baumannii strain appearing after colistin treatment: effect on virulence and bacterial fitness. Antimicrob Agents Chemother 57:4587–4589. doi: 10.1128/AAC.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Rojas R, Dominguez-Herrera J, McConnell MJ, Docobo-Perez F, Smani Y, Fernandez-Reyes M, Rivas L, Pachon J. 2011. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J Infect Dis 203:545–548. doi: 10.1093/infdis/jiq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 9.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. 2012. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother 56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson AA, Hancock RE, McGroarty EJ. 1985. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol 164:1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne LG, Diaz GA. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal Biochem 157:89–92. doi: 10.1016/0003-2697(86)90200-9. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Crowell SA, Harding CM, De Silva PM, Harrison A, Fernando DM, Mason KM, Santana E, Loewen PC, Kumar A, Liu Y. 2016. KatG and KatE confer Acinetobacter resistance to hydrogen peroxide but sensitize bacteria to killing by phagocytic respiratory burst. Life Sci 148:31–40. doi: 10.1016/j.lfs.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unsworth KE, Holden DW. 2000. Identification and analysis of bacterial virulence genes in vivo. Philos Trans R Soc Lond B Biol Sci 355:613–622. doi: 10.1098/rstb.2000.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.