ABSTRACT
Cryptococcus neoformans is an environmentally ubiquitous fungal pathogen that primarily causes disease in people with compromised immune systems, particularly those with advanced AIDS. There are estimated to be almost 1 million cases per year of cryptococcal meningitis in patients infected with human immunodeficiency virus, leading to over 600,000 annual deaths, with a particular burden in sub-Saharan Africa. Amphotericin B (AMB) and fluconazole (FLC) are key components of cryptococcal meningitis treatment: AMB is used for induction, and FLC is for consolidation, maintenance and, for occasional individuals, prophylaxis. However, the results of standard antifungal susceptibility testing (AFST) for AMB and FLC do not correlate well with therapeutic outcomes and, consequently, no clinical breakpoints have been established. While a number of explanations for this absence of correlation have been proffered, one potential reason that has not been adequately explored is the possibility that the physiological differences between the in vivo infection environment and the in vitro AFST environment lead to disparate drug susceptibilities. These susceptibility-influencing factors include melanization, which does not occur during AFST, the size of the polysaccharide capsule, which is larger in infecting cells than in those grown under normal laboratory conditions, and the presence of large polyploid “titan cells,” which rarely occur under laboratory conditions. Understanding whether and how C. neoformans differentially expresses mechanisms of resistance to AMB and FLC in the AFST environment compared to the in vivo environment could enhance our ability to interpret AFST results and possibly lead to the development of more applicable testing methods.
KEYWORDS: Cryptococcus neoformans, amphotericin B, antifungal susceptibility testing, fluconazole
INTRODUCTION
Cryptococcus neoformans is a fungus responsible for causing lung infections that have the potential to disseminate to the central nervous system (CNS) in the absence of adequate immune control and cause cryptococcal meningitis. This infection, which is most common among AIDS patients, is uniformly fatal when left untreated. With antifungal treatment, 10-week fatality for AIDS patients in developed countries is around 9%, while fatality in sub-Saharan Africa has been estimated to be as high as 70% (1). In 2009, it was estimated that there were 958,000 HIV-related cases per year worldwide, resulting in nearly 625,000 deaths, a disproportionate number of which came from sub-Saharan Africa and South and Southeast Asia (1).
Cryptococcal meningitis treatment occurs in three stages: induction, consolidation, and maintenance. Recommended induction therapy consists of amphotericin B (AMB) plus flucytosine, while fluconazole (FLC) is considered the optimal agent for the consolidation and maintenance phases (2). FLC may also be used as an alternative agent for induction (2), a substitution that is particularly common in resource-poor areas where AMB may be inaccessible or difficult to administer (3). Given the high death rates of patients undergoing treatment for cryptococcal meningitis, clinical practice would greatly benefit from the ability to predict those drugs to which an infection is likely to respond and at what doses. This would be particularly advantageous if it could be used to limit highly toxic and expensive AMB treatment of those patients unlikely to respond to FLC, thereby reducing costs, preventing unnecessary side effects, and preserving drug stocks in areas where availability is low. Many Candida species, for example, have demonstrated that antifungal susceptibility testing (AFST) can be an effective tool for predicting treatment outcome and therefore in guiding prescribing decisions. Based on updated, species-specific breakpoints from the Clinical and Laboratory Standards Institute (CLSI), data compiled from a number of studies show that 92% of fluconazole-susceptible Candida albicans, Candida tropicalis, and Candida parapsilosis isolates were successfully treated with fluconazole, while the same was true for only 37% of resistant isolates (4). Similar success has been achieved in correlating AFST results with treatment outcomes with other azoles, such as voriconazole and itraconazole (4). Unfortunately, no such predictions have been adequately substantiated on the basis of standard AFST of C. neoformans.
Current guidelines from the CLSI recommend testing the susceptibility of C. neoformans isolates by using a microdilution method in which suspensions of colonies are inoculated into RPMI 1640 broth containing increasing dilutions of antifungal agent, incubating at 35°C for 70 to 74 h, and visually determining the MIC against an antifungal-free growth control well (5). However, several studies have failed to find any correlation between MICs from CLSI-recommended methods and treatment outcome for either AMB or FLC (6–10), and there is little evidence that these standard assessments of susceptibility have clinical applicability (7, 11). Some research suggests that different protocols may be more successful at predicting clinical outcomes for FLC and AMB. In the case of FLC, a few studies have suggested that results from alternative susceptibility testing methods, including microdilution in yeast nitrogen base (YNB) broth and Etests, may have more clinical relevance (9, 10, 12, 13). For AMB, one research group has had some success in predicting patient response by using a method in which inoculum sizes are adjusted to reflect a patient's fungal burden (8, 11). Nonetheless, the absence of a clear link between MICs from a standard CLSI methodology and patient outcomes has undermined the efficacy of performing AFST on C. neoformans and made it impossible to establish clinical breakpoints (14).
Multiple reasons have been hypothesized for the lack of clear correlations between elevated antifungal MICs and cryptococcal meningitis treatment failure. Separate analyses of risk factors for FLC or AMB treatment failures in AIDS patients found that markers of high cryptococcal burden were strong predictive factors (9, 15), implying that perhaps the effects of varied fungal burdens mask less prominent effects of drug susceptibility (11). Additionally, the finding that multiple C. neoformans strains are found in 18% of cryptococcosis patients (16) has led to the suggestion that correlating an MIC to treatment outcome may be impeded by the concurrent presence of strains with different levels of susceptibility, of which only one is tested (14). One study used a murine model to suggest that the pharmacodynamics of FLC may prevent appropriate concentrations of drug from being reached and maintained in the cerebrum (17). It has also been proposed that AMB may work in part through its positive immunomodulatory effects, which would not be reflected in an MIC (18).
There is yet another potential explanation for the lack of correlation between FLC and AMB MICs and treatment outcome that has not been thoroughly explored: that differences between conditions in human tissues and in in vitro AFST result in fungal physiological states that manifest large differences in susceptibility to FLC and AMB. This paper reviews a significant body of literature showing various factors that have profound effects on C. neoformans susceptibility to polyenes and azoles (Table 1). These factors include melanization, which does not occur in RPMI broth, the polysaccharide capsule, which is much larger in vivo than under normal in vitro conditions, the presence of large, polyploid “titan cells,” which grow in the host but not in normal cultures, and also a variety of expression changes that occur in the host and have been hypothesized to affect ergosterol (Fig. 1). A more thorough understanding of the different compositional, morphological, and transcriptional characteristics induced by the host environment compared to the AFST broth environment and the extent to which they alter susceptibility to antifungal drugs may help us interpret the results of AFST or inform efforts to develop more clinically relevant testing protocols.
TABLE 1.
Changes induced in vivo alter antifungal susceptibility
| In vivo-induced change | Antifungal agent | Effect(s) on susceptibility | Proposed mechanism(s) | Reference(s) |
|---|---|---|---|---|
| Melanization | AMB | Decrease | Drug binding, permeability reduction | 19, 22–26 |
| FLC | Decrease | Unknown | 22 | |
| Capsule enlargement | AMB | Decrease | Protection against ROS | 36, 44, 45 |
| FLC | Increase or decrease (possibly size dependent) | For susceptibility increase, increased drug uptake via hydrophilic interactions; for susceptibility decrease, mechanism unknown | 36, 44 | |
| Titan cells | FLC | Decrease | In titan cells, mechanism unknown; in fluconazole-grown titan cell offspring, aneuploidy | 52 |
| Macrophage-induced gene expression | FLC | Potential decrease | Upregulation of CDC50 and ACL1 | 59, 60 |
| AMB | Potential decrease | Upregulation of CDC50 | 59 | |
| Antibody-induced gene expression | AMB | Conditional increase | Increased ergosterol production (antibody dependent and likely strain dependent) | 61, 62 |
FIG 1.
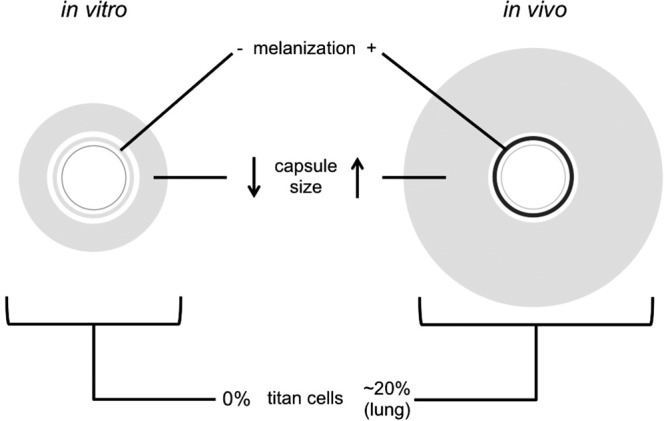
Illustration of compositional and morphological responses to the in vitro and in vivo environments discussed in the review. C. neoformans grown in RPMI for antifungal susceptibility tests lacks melanin in its cell wall, due to the absence of phenolic precursors in the medium, grows capsules 2 to 4 μm thick (45), and is not known to manifest as titan cells. Cells grown in vivo display melanized cell walls (20, 21) and grow capsules averaging 20 and 8 μm thick in lung and brain, respectively (37). Titan cells make up ∼20% of C. neoformans cells found in lungs and a smaller, but observable, portion of cells in the brain (50). Diagram was not drawn to scale.
MELANIZATION
Among the many differences between in vitro AFST conditions and the host environment, one of the most notable is the absence of phenolic substrates, such as l-3,4-dihydroxyphenylalanine (l-DOPA). These substrates, which are converted into melanin by C. neoformans laccase, have been reported to form black precipitates upon combination with antifungals in RPMI; these precipitates prevent assessment of growth (19), thereby preventing such substrates from being included in AFST broth. Melanin is a well-established virulence factor in C. neoformans that, in addition to protecting the yeast from a variety of environmental and host stresses, influences the susceptibility of many fungal species to antifungal agents (18). There is abundant evidence that melanization increases C. neoformans resistance to AMB, and some research suggests that it also affects FLC susceptibility.
C. neoformans melanizes in host tissues (20, 21), presumably by scavenging phenolic precursors that are polymerized into melanin. Several studies have demonstrated that melanized C. neoformans can withstand higher concentrations of AMB than nonmelanized C. neoformans. Traditional AFST cannot detect differences between melanized and nonmelanized cells, due to the inability of melanized cells inoculated into broth lacking phenolic substrates to produce melanized offspring, but time-kill assays can be used to assess the effects of melanization on drug susceptibility (18). Time-kill assay results have repeatedly shown that significantly more melanized than nonmelanized cells remain viable after incubation with various concentrations of AMB (19, 22–24). This observation remains true whether cells are nonmelanized due to incubation without l-DOPA or through laccase deficiency (19). Though the extent to which melanized cells outsurvive nonmelanized cells appears to differ by strain and drug concentration, almost all studies showed at least a 2-fold difference, with many assays showing over 6 times higher numbers of surviving melanized cells (19, 22–24). This suggests that the practical effect of melanization on AMB susceptibility in vivo is likely to be considerable.
The mechanism by which melanin inhibits AMB was investigated via binding assays, in which AMB was incubated with melanin. The melanin was then removed by centrifugation, and the antifungal agent was used in antifungal susceptibility tests or killing assays, yielding much higher MICs, and much less killing, from AMB incubated with melanin than in tests without melanin (19, 22). When AMB was incubated with different doses of melanin, a dose-dependent relationship emerged in which higher doses of melanin particles led to higher AMB MICs (22). Additionally, one study conducted elemental analysis of melanin after incubation with AMB and found a shift in C:N ratios (19). These results led the authors of both studies to conclude that melanin increases C. neoformans resistance by binding to AMB (19, 22), and in their review of the effects of melanization in microbes, Nosanchuk and Casadevall hypothesized that this binding is likely to inhibit AMB from reaching its targets (18). In addition, cell wall melanization results in a reduction in its permeability as a result of smaller pore sizes (25, 26). Consequently, melanization may also block the entry of large molecules, such as AMB, by decreasing the porosity of the C. neoformans cell wall (18, 25, 26), in addition to its binding effects. Finally, it has also been suggested that melanin's noted antioxidant properties (27–29) could protect C. neoformans from the oxidative stress (30) that has been demonstrated to contribute to AMB's toxicity (30–32).
An inverse relationship between melanization and AMB susceptibility is suggested by in vivo observations as well. In vitro laccase activity of C. neoformans strains isolated from cases of HIV-associated cryptococcal meningitis was found to be significantly negatively associated with the rate of fungal clearance from the human patients during AMB-based antifungal therapy (33). Given that melanin is a virulence factor responsible for protection against phagocytosis and killing by macrophages, among other host defenses (18), it is possible that the inverse relationship between laccase activity and clearance occurred independently of any effects on AMB activity. Nonetheless, these results are consistent with the notion that melanization may increase the resistance of C. neoformans to AMB and that in failing to account for the effects of melanization, CLSI AFST is rendered less effective at accurately assessing a strain's likelihood of being cleared by AMB, as has been previously suggested (18, 19).
Melanization has also been demonstrated to reduce the efficacy of FLC, but the magnitude of this effect is unclear. A time-kill assay showed significantly higher survival of melanized cells only in comparatively high concentrations of FLC (8 μg/ml) (22), while lower FLC concentrations had no significant effect (19, 22). However, as noted by van Duin et al., FLC is fungistatic rather than fungicidal and showed little killing, suggesting that the inhibitory effects of melanization on FLC activity would be unlikely to be adequately demonstrated in an assay designed to distinguish survival rates rather than growth rates. The same binding assays previously described for AMB showed no evidence of binding to FLC (19, 22), suggesting that the inhibitory effect of cryptococcal melanin on FLC activity unlikely acts through the same mechanism as has been hypothesized for AMB. It is possible that melanin increases resistance to FLC without binding the antifungal agent, perhaps by inhibiting its entry into the cell instead.
CAPSULE SIZE
Another major difference between C. neoformans during infection compared to in vitro conditions is capsule size. The polysaccharide capsule is an important virulence factor with harmful effects on the human immune system (34). Standard laboratory culture conditions, including growth on RPMI, generally produce C. neoformans cells with relatively small capsules (35, 36). However, cells from murine infections tend to have significantly larger capsules (37), and similar phenomena have been shown in ex vivo human cerebrospinal fluid (CSF) (38). Furthermore, there is no observed correspondence between the extent of an isolate's capsule growth in vitro and in vivo (34). The mechanisms by which capsule expansion inside the host is achieved are yet to be completely elucidated, but several factors affect capsule size, including CO2 concentration (39), nutrient concentration (40), pH (40), iron availability (41), Mg2+ concentration (42), the presence of certain phospholipids (43), and osmotic pressure (34).
Three separate studies have demonstrated that larger capsules protect C. neoformans from AMB. These studies have induced growth of large capsules comparable to those found in vivo by using low-nutrient media (36) or by adding NaHCO3 to the medium, and then incubating the cells in 5% CO2 (44, 45). Cells with enlarged capsules were then compared to normally grown cells with small capsules of the same strain or strains in killing assays (36), time-kill assays (45), or via the CLSI broth microdilution methodology (44). The killing assays showed significantly lower rates of killing of cells with enlarged capsules than of cells with small capsules between AMB concentrations of 0.06 and 0.25 μg ml−1 after 3 h (36). In time-kill assays performed on a set of clinical strains in 1 μg ml−1 of AMB, 100% and 75% of strains grown under capsule-enlarging conditions survived after 6 and 72 h, respectively, while only 35% and 0% of the same strains survived after the same respective durations of exposure under normal growth conditions (45). Finally, the geometric mean of AMB MICs determined by broth microdilution for a collection of clinical isolates was significantly higher when the isolates were induced to grow large capsules (44). The protective effect of these capsules was further confirmed by one of the research groups through the comparison of a wild-type strain versus an isogenic acapsular strain with a disrupted cap59 gene; the researchers found that the acapsular strain was more susceptible to AMB than the wild type (36).
It is generally accepted that AMB's primary mechanism of fungicidal activity is via binding to ergosterol, leading to the formation of pores in the fungal cell membrane. However, as previously mentioned, AMB induces oxidative damage and intracellular accumulation of reactive oxygen species (ROS) in several species of yeast, including C. neoformans (30–32). Furthermore, by inhibiting ROS production, Candida tropicalis could be rendered less susceptible to AMB, suggesting that “ROS accumulation is a universal action mechanism of [AMB]” (32). In addition to testing the effects of capsule size on antifungal susceptibility, Zaragoza et al. also demonstrated that C. neoformans cells with large capsules were protected against killing by H2O2-induced ROS and that isolated capsular polysaccharide could protect cells with small capsules, likely by acting as an antioxidant. Therefore, it is probable that greater production of capsular polysaccharide protects against AMB-induced ROS in the same manner and, given the higher levels of capsule in vivo than in vitro, is likely to play a role in the limited efficacy of in vitro testing to assess in vivo AMB clearance (36).
The role of capsule size in mediating FLC susceptibility is less clear. In an Etest comparison of wild-type and acapsular strains, it was determined that the acapsular mutant was actually highly resistant to FLC, and the authors theorized that the hydrophilic properties of both the capsule and FLC could increase drug uptake into the cell (36). In contrast, the broth microdilution method showed significantly higher FLC MICs among isolates with enlarged capsules compared to those grown under normal conditions (44). Interestingly, the results from the two methods agreed for all other drugs tested by both methods, finding lower amphotericin B susceptibilities with higher levels of capsule (as noted above) and no differences for other azoles. It is unclear whether the apparent difference in the capsule's effect on FLC susceptibility reflected in these two studies is a function of the different capsule sizes of the cells studied, suggesting that perhaps a small capsule leads to more FLC susceptibility than either a large capsule or no capsule at all, or that perhaps the different methods used to achieve the capsule sizes caused the apparent differences in FLC susceptibility. If the latter were the case, it would be plausible that either the growth conditions of the cells with enlarged capsules when tested by broth microdilution or the disruption of cap59 in the acapsular mutants could have led to the stimulation of pathways that altered FLC MICs and masked any effect of capsule size. It is worth noting that the Etest was conducted with C. neoformans serotype D lab strain isolates, while the microdilution assays used serotype A clinical isolates. As capsule composition differs between the two serotypes (46), it is possible that this difference also contributed to the contradictory results. Though further research is required to understand the nature of the relationship between capsule size and fluconazole susceptibility, there is evidence that the former may play a role in regulating the latter, and if this is the case, whether the effect is negative or positive, it likely contributes to the difference between in vivo and in vitro fluconazole susceptibility.
CELLULAR GIGANTISM (TITAN CELLS)
Titan cells are abnormally large, polyploid C. neoformans cells formed in the host environment but not under normal laboratory conditions (47, 48). In addition to their size, which can be as large as 100 μm in diameter, compared to the 5- to-7-μm diameter common for cells grown in vitro, titan cells have several structural differences. These differences include a denser, more highly cross-linked capsule and a thicker cell wall (48). Additionally, titan cells frequently contain four or eight (and sometimes more) copies of their genome within one nucleus, compared to the haploidy of most C. neoformans cells (48). Titan cells have been more extensively described in infected lungs (49), where they have been reported to make up about 20% of cells (50), though this percentage varies, possibly in part based on the immune response of the host (47). However, they have also been identified in human brain abscess aspirates (51) and in low levels in mouse brains (50). Though titan cells are believed to be too large to disseminate from the lung to the brain, their production greatly increases the dissemination of typical cells to the CNS (48). This has been hypothesized to occur either by inducing a Th2-driven response that less effectively combats the infection or by the production of haploid daughter cells, which have been shown to be more resistant to various kinds of stress, including those encountered within macrophages (48, 52).
Titan cells are significantly less susceptible to high concentrations of FLC than are typical cells (52). Perhaps more interesting, given titan cells' possible role in seeding the host with more readily disseminated daughter cells, is the fact that typically sized progeny of titan cells grown in the presence of FLC were significantly more likely to survive and propagate in FLC than the progeny of typically sized cells grown under the same conditions (52). Almost all (97.9%) strains derived from daughters of FLC-stressed titan cells were found to contain extra copies of entire (95.8%) or partial (2.1%) chromosomes on top of their background, which was usually haploid, with a notable minority (25%) of diploids (52). This was in contrast to the offspring of titan cells grown without FLC, which were haploid and apparently typical. Of the aneuploid strains tested, all had higher MICs than the parental strain used to produce the original titan cells. Chromosomes 1 and 4 were the most commonly duplicated, with fewer strains also containing extra copies of chromosomes 6, 8, and 10. Duplication of chromosomes 1 and 4 and, to a lesser extent, chromosomes 6 and 10, have all been reported to contribute to FLC resistance (53–55). Chromosome 1 contains ERG11, FLC's target, and AFR1, an efflux pump whose overexpression is associated with azole resistance, and both of these are believed to contribute to the decreased susceptibility of cells with multiple copies of chromosome 1 (53). Given this evidence, it is probable that titan cells contribute to the difficulty of clearing some cryptococcal meningitis infections caused by strains that display in vitro FLC susceptibility. This likely occurs both through the increased resistance of the titans cells themselves, as well as through the production of FLC-resistant aneuploid daughter cells during FLC exposure.
There is currently no direct evidence that titan cells or their offspring contribute to in vitro-in vivo differences in susceptibility to AMB. However, reports of C. neoformans developing heteroresistance to FLC through aneuploidy are fairly common, but none of these strains has been reported to display AMB resistance as well (though it is unclear whether they have been screened for susceptibility to this antifungal agent). Nonetheless, given that titan cells are less susceptible to oxidative stress than typical cells (47), with very thick cell walls and alterations in their capsules that could affect their permeability, it is plausible that they are less vulnerable to AMB. This topic merits further exploration.
TRANSCRIPTIONAL DIFFERENCES
While the above morphological differences between C. neoformans cells grown in RPMI and those found in infections appear to play a considerable role in modulating drug susceptibility, they likely represent just a portion of the total transcriptomic differences between cells in these two environments. Though no studies have been performed to compare these transcriptional consequences of growth in RPMI versus in vivo growth, some of their differences may be inferred through research that has examined the impact of specific components of the host immune response on C. neoformans gene expression.
Macrophage-induced gene expression.
One clear difference between the in vivo and in vitro environments encountered by C. neoformans during infection and in AFST, respectively, is the presence of host immune cells, including macrophages. Macrophages play a large role in the host's defense against cryptococcal infection but also have the potential to facilitate the spread of the organism through the body (56), given that C. neoformans is a facultative intracellular pathogen that can survive and replicate inside macrophages (57). Additionally, intracellular C. neoformans may encounter different concentrations of antifungals than extracellular C. neoformans (58). Incubation of C. neoformans with macrophages has myriad effects on the expression profile of C. neoformans, and at least two of these genes have the potential to alter susceptibility to FLC, and possibly AMB. Cdc50 is a flippase subunit believed to regulate ergosterol distribution and trafficking for Saccharomyces cerevisiae (59). A study recently demonstrated that C. neoformans cocultured with macrophage-like J774 cells overexpressed CDC50 to a 6-fold-higher level than C. neoformans cells grown on YNB (59). The same study showed that C. neoformans CDC50 mutants were hypersensitive to FLC (16 to 1 μg/ml) and also displayed somewhat higher susceptibility to AMB (2 to 0.5 μg/ml). These authors hypothesized that this effect was likely due to differences in distribution of ergosterol in the cell membranes of mutants.
ATP-citrate lyase converts citrate to acetyl coenzyme A (CoA), which is necessary for ergosterol biosynthesis. Comparison of transcripts from C. neoformans cells cultured with and without macrophage-like J774A.1 cells found that transcripts from the gene encoding ATP-citrate lyase, ACL1, were upregulated 13-fold (60). C. neoformans ACL1-deficient strains were 4 times less susceptible to FLC, and the study authors hypothesized that this was due to altered sterol and lipid synthesis resulting from lower acetyl-CoA levels in the mutants (60). Though this study did not measure AMB susceptibility of the mutants, it is plausible, given the drug's targeting of ergosterol, that ACL1 expression could alter susceptibility to this antifungal as well. While it is unclear whether overexpression of CDC50 or ACL1 would impact the antifungal susceptibility of the isolate to the same extent that underexpression did, it is plausible that the increased expression of one or both of these genes in the presence of macrophages contributes to increased FLC or AMB resistance in the host.
Antibody-induced gene expression.
Classically, antibodies are understood to combat infection by acting on microbes externally, either promoting immune mechanisms such as phagocytosis or complement activation or neutralizing binding sites required for the initiation of pathogenesis. However, recent studies have provided compelling evidence that antibody binding can have direct effects on C. neoformans by altering gene expression and metabolism. Research on serotype A laboratory strain H99 found that incubation with three different capsule-binding monoclonal antibodies (18B7, an IgG1, and 12A1 and 13F1, both IgMs) induced three distinct sets of transcriptional changes of different magnitudes, compared with the transcriptional profiles elicited by incubation with nonbinding antibodies of the same isotype (61). Of particular interest was the finding that binding by 18B7, but neither of the capsule-binding IgMs, increased the susceptibility of C. neoformans to AMB, delaying the onset of growth in 0.125 μg/ml AMB by over a day compared to incubation with the control IgG1 and by over 2 days compared to the antibody-free control. This increase in susceptibility corresponded to an increase in the ergosterol content of cells incubated with 18B7, which the authors hypothesized could be responsible for the increased AMB susceptibility by providing more ergosterol for the drug to bind in the cell membrane (61). While fluconazole susceptibility was not tested, an increase in ergosterol synthesis could plausibly impact this as well. Interestingly, a second study followed up on these results by comparing the effects of 18B7 binding on H99 and two serotype D strains, and the researchers found that the three strains displayed distinct transcriptional responses to the antibody, with much fewer expression changes in the serotype D strains than in serotype A H99 when incubated for the same amount of time (62).
The mechanism by which antibody binding alters transcription levels is not known, but it has been hypothesized to be dependent on antibody-mediated conformational changes to the capsule that put stress on the cell wall, leading to signal transduction by membrane proteins (62). The different transcriptomes induced by different antibodies with H99, and in different strains with 18B7, may be explained by the distinct binding patterns of the antibodies on the cells, plausibly activating different transduction pathways (61, 62). These variations in response both by strain and by antibody, and the limited number of either for testing, make it difficult to predict whether naturally occurring patient antibodies can be expected to have effects on the antifungal susceptibilities of clinical strains, and further research is merited. Nonetheless, the absence of antibodies from AFST broth represents another difference from the in vivo environment that may lead to the inaccurate assessment of resistance levels.
Transcriptomes of C. neoformans in RPMI versus in vivo.
One of the major gaps in the current knowledge of the differences between the host environment and the AFST environment is that the expression profiles of isolates under the two conditions have not been studied in comparison to one another. While the transcriptional consequences of individual environmental factors can be and have been studied in vitro, the in vivo environment is likely too complex and dynamic to allow adequate estimates of the conditions to which infecting microbes are subjected, and the result of integrating the expression pathways stimulated by innumerable different variables cannot be reliably anticipated. In light of this, experiments measuring gene expression in the host environment are necessary to fully understand the behavior of C. neoformans in the human brain (63). To this end, analysis has been conducted to compare growth in in vivo in human CSF, ex vivo in human CSF, and in vitro in YPD broth (64). The study in question revealed 20 genes that were significantly upregulated both in vivo and ex vivo in CSF compared to YPD and 6 genes that were significantly upregulated just in in vivo in CSF compared to the other two conditions. Among these, several have the potential to be mediators of FLC or AMB susceptibility, including CFO1 (65, 66), ENA1 (67), a 1,4-alpha-glucan-branching enzyme (68), and a gene encoding an unknown MFS transporter, a family of proteins that has been implicated in multidrug resistance via drug efflux (69). While it is likely that some of these genes are also upregulated compared to the expression levels in cells grown under CLSI AFST conditions, it is unclear to what extent the results can be considered generalizable, particularly given the many differences between YPD and RPMI broth, including carbon source (dextrose versus glucose) and pH (6.5 versus 7), the different temperatures at which the isolates were grown (37°C versus 35°C) (5, 64, 70), and the variability in C. neoformans growth under different conditions (71).
CONCLUSIONS
The lack of statistical correlation between C. neoformans MICs generated by CLSI-recommended AFST methods and treatment outcomes for both AMB and FLC has left physicians with little guidance as to what treatment is likely to be successful. While there are several factors that are likely to play a role in this matter, there is abundant evidence that physiological differences between C. neoformans cells growing in a host and under laboratory conditions can result in significant differences in susceptibility to antifungal drugs. Melanization and large polysaccharide capsules can each increase resistance to AMB and may also alter susceptibility to FLC. Titan cells are less susceptible to FLC and when exposed to the drug produce aneuploid offspring that are even more resistant to this antifungal agent. Additionally, studies suggest that genes upregulated in vivo, including some regulating ergosterol synthesis and distribution, may alter susceptibility to FLC, AMB, or both. However, there is currently no research comparing gene expression during human infection to that induced during AFST in RPMI. It is worth noting that expression of these traits is likely to vary based on both strain genetics and host immune response, and therefore the level to which they moderate drug susceptibility will also vary, potentially in a manner that can nonetheless be predicted.
Looking forward, the body of knowledge amassed on this topic provides an opportunity to improve upon current methods both for assessing the susceptibility of C. neoformans to currently used antifungals and for screening new compounds for their potential for treating C. neoformans infections. Ideally, both of these goals could be served through the development of media that induces the morphological and transcriptional profiles observed during infection by mimicking the host environment, perhaps by utilizing the existing information on the conditions that induce melanization, enlarged capsule growth, and titan cell formation. The susceptibility of C. neoformans grown in such media to a given antifungal would likely be more predictive of the clinical success of treatment with that antifungal, leading to clinically relevant AFST of individual infecting strains as well as candidate antifungal screening methods more likely to reveal compounds that will have high efficacy in vivo. Even in the absence of such an ideal growth medium, efforts should be made to ensure that the cells used in initial screening resemble those responsible for infections, to the extent that is possible, lest drugs targeting survival and virulence responses such as those discussed above, necessary for growth under the high-stress, low-nutrient conditions of the host but not necessary for growth in nutrient-replete medium, be overlooked. A few publications have described utilizing an approach similar to this by screening drugs using nutrient-deficient media (72, 73); however, those authors did not describe the morphology of the cells or include l-DOPA in their media, so it is unclear how similar the cells and conditions tested were to those that occur in vivo. One of these publications noted that the compounds found differed from those identified by another group's drug screen conducted in nutrient-replete media, and the former authors hypothesized that these differences may have been due to the alternative pathways utilized under the two conditions (73), supporting the idea that mimicking in vivo growth conditions may yield substantively different drug screening results.
In addition to attempting to alter AFST protocols such that they better mimic in vivo growth conditions, another approach to predicting the clinical outcome of treatment of specific C. neoformans infections with either AMB or FLC would be to identify genetic markers and expression patterns associated with treatment failure, such that clinical isolates might be screened for their presence. A similar approach has been developed for Candida glabrata, based on the detection of a number of FKS1 and FKS2 mutations commonly implicated in echinocandin resistance (74). Thus far, relatively little research has focused on the genetic and transcriptional basis of resistance in C. neoformans, and to our knowledge such research has exclusively used in vitro MICs, as opposed to clinical outcomes, to define resistance. However, higher expression of at least one gene, the copper transporter gene CTR4, in vitro has been associated with virulence in humans and in mice (75), suggesting that similar analyses could be made for genes associated with treatment failure as well.
We appreciate that changing the established antifungal drug testing protocols to induce changes in fungal cells that resemble those observed in vivo would entail considerable effort that in essence would defeat the notion of a standardized system. Furthermore, we are aware that different strains are likely to respond to changing testing conditions differently, adding additional complexity to any attempt to more closely mimic in vivo conditions. However, we note that when treatment can be administered as recommended, most individuals with cryptococcosis do respond to antifungal therapy, and this type of specialized testing could be reserved for those cases where the disease is refractory to therapy. In this regard, the chronicity of cryptococcosis provides the advantage of time, which can be used for additional testing under the conditions suggested above, and this testing could be carried out in specialized laboratories that develop this technical expertise. Consequently, we believe that the knowledge summarized in our review provides actionable information to improve antifungal susceptibility testing for C. neoformans.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen M-H, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicanic T, Wood R, Bekker L-G, Darder M, Meintjes G, Harrison TS. 2005. Antiretroviral roll-out, antifungal roll-back: access to treatment for cryptococcal meningitis. Lancet Infect Dis 5:530–531. doi: 10.1016/S1473-3099(05)70197-3. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Dannaoui E, Abdul M, Arpin M, Michel-Nguyen A, Piens MA, Favel A, Lortholary O, Dromer F. 2006. Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob Agents Chemother 50:2464–2470. doi: 10.1128/AAC.01520-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C-H, Chang T-Y, Liu J-W, Chen F-J, Chien C-C, Tang Y-F, Lu C-H. 2012. Correlation of anti-fungal susceptibility with clinical outcomes in patients with cryptococcal meningitis. BMC Infect Dis 12:1–9. doi: 10.1186/1471-2334-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen RA, Bauer M, Brouwer AE, Sanchez A, Thomas AM, Rajanuwong A, Chierakul W, Peacock SJ, Day N, White NJ, Rinaldi MG, Harrison TS. 2007. In vitro-clinical correlations for amphotericin B susceptibility in AIDS-associated cryptococcal meningitis. Antimicrob Agents Chemother 51:343–345. doi: 10.1128/AAC.00742-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witt MD, Lewis RJ, Larsen RA, Milefchik EN, Leal MAE, Haubrich RH, Richie JA, Edwards JE, Ghannoum MA. 1996. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis 22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]
- 10.Jessup CJ, Pfaller MA, Messer SA, Zhang J, Tumberland M, Mbidde EK, Ghannoum MA. 1998. Fluconazole susceptibility testing of Cryptococcus neoformans: comparison of two broth microdilution methods and clinical correlates among isolates from Ugandan AIDS patients. J Clin Microbiol 36:2874–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen RA, Bauer M, Pitisuttithum P, Sanchez A, Tansuphaswadikul S, Wuthiekanun V, Peacock SJ, Simpson AJH, Fothergill AW, Rinaldi MG, Bustamante B, Thomas AM, Altomstone R, Day NPJ, White NJ. 2011. Correlation of susceptibility of Cryptococcus neoformans to amphotericin B with clinical outcome. Antimicrob Agents Chemother 55:5624–5630. doi: 10.1128/AAC.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aller AI, Martin-Mazuelos E, Lozano F, Gomez-Mateos J, Steele-Moore L, Holloway WJ, Gutiérrez MJ, Recio FJ, Espinel-Ingroff A. 2000. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob Agents Chemother 44:1544–1548. doi: 10.1128/AAC.44.6.1544-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. 2006. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis 43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 14.Espinel-Ingroff A, Aller AI, Canton E, Castanon-Olivares LR, Chowdhary A, Cordoba S, Cuenca-Estrella M, Fothergill A, Fuller J, Govender N, Hagen F, Illnait-Zaragozi MT, Johnson E, Kidd S, Lass-Florl C, Lockhart SR, Martins MA, Meis JF, Melhem MSC, Ostrosky-Zeichner L, Pelaez T, Pfaller MA, Schell WA, St Germain G, Trilles L, Turnidge J. 2012. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 56:5898–5906. doi: 10.1128/AAC.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson PA, Bauer M, Leal MAE, Evans SG, Holtom PD, Diamond DM, Leedom JM, Larsen RA. 1999. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis 28:82–92. doi: 10.1086/515074. [DOI] [PubMed] [Google Scholar]
- 16.Desnos-Ollivier M, Patel S, Spaulding AR, Charlier C, Garcia-Hermoso D, Nielsen K, Dromer F. 2010. Mixed infections and in vivo evolution in the human fungal pathogen Cryptococcus neoformans. mBio 1:e00091-10. doi: 10.1198/mBio.00091-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudan A, Livermore J, Howard SJ, Al-Nakeeb Z, Sharp A, Goodwin J, Gregson L, Warn PA, Felton TW, Perfect JR, Harrison TS, Hope WW. 2013. Pharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpoints. Antimicrob Agents Chemother 57:2793–2800. doi: 10.1128/AAC.00216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosanchuk JD, Casadevall A. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Duin D, Casadevall A, Nosanchuk JD. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother 46:3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosanchuk J, Rosas A, Lee S, Casadevall A. 2000. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355:2049–2050. doi: 10.1016/S0140-6736(00)02356-4. [DOI] [PubMed] [Google Scholar]
- 21.Feldmesser M, Kress Y, Casadevall A. 2001. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 1258:33–2355. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda R, Sugita T, Jacobson ES, Shinoda T. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol Immunol 47:271–277. doi: 10.1111/j.1348-0421.2003.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 23.Liaw SJ, Wu HC, Hsueh PR. 2010. Microbiological characteristics of clinical isolates of Cryptococcus neoformans in Taiwan: serotypes, mating types, molecular types, virulence factors, and antifungal susceptibility. Clin Microbiol Infect 16:696–703. doi: 10.1111/j.1469-0691.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Casadevall A. 1994. Growth of Cryptococcus neoformans in presence of l-DOPA decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother 38:2648–2650. doi: 10.1128/AAC.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenman HC, Nosanchuk JD, Webber JBW, Emerson RJ, Camesano TA, Casadevall A. 2005. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 44:3683–3693. doi: 10.1021/bi047731m. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson ES, Ikeda R. 2005. Effect of melanization upon porosity of the cryptococcal cell wall. Med Mycol 43:327–333. doi: 10.1080/13693780412331271081. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson ES, Tinnell SB. 1993. Antioxidant function of fungal melanin. J Bacteriol 175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson ES, Emery HS. 1991. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol 173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Casadevall A. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun 62:3004–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangalli-Leite F, Scorzoni L, Mesa-Arango AC, Casas C, Herrero E, Soares Mendes Gianinni MJ, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. 2011. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect 13:457–467. doi: 10.1016/j.micinf.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Sokol-Anderson ML, Brajtburg J, Medoff G. 1986. Amphotericin B-induced oxidative damage and killing of “Candida albicans.” J Infect Dis 154:76–83. [DOI] [PubMed] [Google Scholar]
- 32.Mesa-Arango AC, Trevijano-Contador N, Román E, Sánchez-Fresneda R, Casas C, Herrero E, Argüelles JC, Pla J, Cuenca-Estrella M, Zaragoza O. 2014. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother 58:6627–6638. doi: 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabiiti W, Robertson E, Beale MA, Johnston SA, Brouwer AE, Loyse A, Jarvis JN, Gilbert AS, Fisher MC, Harrison TS, May RC, Bicanic T. 2014. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest 124:2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. 2009. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H, Chang YC, Varma A, Kwon-Chung KJ. 2009. Regulatory diversity of TUP1 in Cryptococcus neoformans. Eukaryot Cell 8:1901–1908. doi: 10.1128/EC.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera J, Feldmesser M, Cammer M, Casadevall A. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun 66:5027–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson EJ, Najjuka G, Rolfes MA, Akampurira A, Jain N, Anantharanjit J, Von Hohenberg M, Tassieri M, Carlsson A, Meya DB, Harrison TS, Fries BC, Boulware DR, Bicanic T. 2014. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis 209:74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granger DL, Perfect JR, Durack DT. 1985. Virulence of Cryptococcus neoformans: regulation of capsule synthesis by carbon dioxide. J Clin Invest 76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaragoza O, Casadevall A. 2004. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online 6:10–15. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. 1993. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis 167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- 42.Rathore SS, Raman T, Ramakrishnan J. 2016. Magnesium ion acts as a signal for capsule induction in Cryptococcus neoformans. Front Microbiol 7:325. doi: 10.3389/fmicb.2016.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. 2011. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog 7:e1002047. doi: 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale RG, Pascuccelli V, Afeltra J. 2012. Influence of capsule size on the in vitro activity of antifungal agents against clinical Cryptococcus neoformans var. grubii strains. J Med Microbiol 61:384–388. doi: 10.1099/jmm.0.036152-0. [DOI] [PubMed] [Google Scholar]
- 45.Córdoba S, Afeltra J, Vitale RG. 2011. Evaluation of the in vitro activity of amphotericin B by time-kill curve methodology against large and small capsulate C. neoformans isolates. Diagn Microbiol Infect Dis 71:260–262. doi: 10.1016/j.diagmicrobio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Cherniak R, Sundstrom JB. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 62:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog 6:e1000945. doi: 10.1371/journal.ppat.100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaragoza O, Nielsen K. 2013. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol 16:409–413. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JM, Zhou Q, Cai HR, Zhuang Y, Zhang YF, Xin XY, Meng FQ, Wang YP. 2014. Clinicopathological features of pulmonary cryptococcosis with cryptococcal titan cells: a comparative analysis of 27 cases. Int J Clin Exp Pathol 7:4837–4846. [PMC free article] [PubMed] [Google Scholar]
- 50.Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman JH, Dromer F, Nielsen KN. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Love GL, Boyd GD, Greer DL. 1985. Large Cryptococcus neoformans isolated from brain abscess. J Clin Microbiol 22:1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerstein AC, Fu MS, Mukaremera L, Li Z, Ormerod KL, Fraser JA, Berman J, Nielsen K. 2015. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 6:e01340-15. doi: 10.1128/mBio.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. 2010. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6:e1000848. doi: 10.1371/journal.ppat.1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngamskulrungroj P, Chang Y, Hansen B, Bugge C, Fischer E, Kwon-Chung KJ. 2012. Characterization of the chromosome 4 genes that affect fluconazole-induced disomy formation in Cryptococcus neoformans. PLoS One 7:e33022. doi: 10.1371/journal.pone.0033022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semighini CP, Averette AF, Perfect JR, Heitman J. 2011. Deletion of cryptococcus neoformans aif ortholog promotes chromosome aneuploidy and fluconazole-resistance in a metacaspase-independent manner. PLoS Pathog 7:e1002364. doi: 10.1371/journal.ppat.1002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabiiti W, May RC. 2012. Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans. Future Microbiol 7:1297–1313. doi: 10.2217/fmb.12.102. [DOI] [PubMed] [Google Scholar]
- 57.Feldmesser M, Kress Y, Novikoff P, Casadevall A. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68:4225–4237. doi: 10.1128/IAI.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wildfeuer A, Laufen H, Haferkamp O. 1990. Interaction of fluconazole and human phagocytic cells. Uptake of the antifungal agent and its effects on the survival of ingested fungi in phagocytes. Arzneimittelforschung 40:1044–1047. [PubMed] [Google Scholar]
- 59.Huang W, Liao G, Baker GM, Wang Y, Lau R, Paderu P, Perlin DS. 2016. Lipid flippase subunit Cdc50 mediates drug resistance and virulence in Cryptococcus neoformans. mBio 7:e00478-16. doi: 10.1128/mBio.00478-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffiths EJ, Hu G, Fries B, Caza M, Wang J, Gsponer J, Gates-Hollingsworth MA, Kozel TR, De Repentigny L, Kronstad JW. 2012. A defect in ATP-citrate lyase links acetyl-CoA production, virulence factor elaboration and virulence in Cryptococcus neoformans. Mol Microbiol 86:1404–1423. doi: 10.1111/mmi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. 2010. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest 120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClelland EE, Casadevall A. 2012. Strain-related differences in antibody-mediated changes in gene expression are associated with differences in capsule and location of binding. Fungal Genet Biol 49:227–234. doi: 10.1016/j.fgb.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upadhya R, Donlin MJ, Lodge JK. 2014. Cryptococcus at work: gene expression during human infection. mBio 5:e01097-14. doi: 10.1128/mBio.01097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Toffaletti DL, Tenor JL, Litvintseva AP, Fang C, Mitchell TG, McDonald TR, Nielsen K, Boulware DR, Bicanic T, Perfect JR. 2014. The Cryptococcus neoformans transcriptome at the site of human meningitis. mBio 5:e01087-13. doi: 10.1128/mBio.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung WH, Hu G, Kuo W, Kronstad JW. 2009. Role of ferroxidases in iron uptake and virulence of Cryptococcus neoformans. Eukaryot Cell 8:1511–1520. doi: 10.1128/EC.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Cho YJ, Do E, Choi J, Hu G, Cadieux B, Chun J, Lee Y, Kronstad JW, Jung WH. 2012. A defect in iron uptake enhances the susceptibility of Cryptococcus neoformans to azole antifungal drugs. Fungal Genet Biol 49:955–966. doi: 10.1016/j.fgb.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung KW, Strain AK, Nielsen K, Jung KH, Bahn YS. 2012. Two cation transporters Ena1 and Nha1 cooperatively modulate ion homeostasis, antifungal drug resistance, and virulence of Cryptococcus neoformans via the HOG pathway. Fungal Genet Biol 49:332–345. doi: 10.1016/j.fgb.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reese AJ, Yoneda A, Breger JA, Beauvais A, Liu H, Griffith CL, Bose I, Kim MJ, Skau C, Yang S, Sefko JA, Osumi M, Latge JP, Mylonakis E, Doering TL. 2007. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol 63:1385–1398. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa C, Dias PJ, Sá-Correia I, Teixeira MC. 2014. MFS multidrug transporters in pathogenic fungi: do they have real clinical impact? Front Physiol 5:197. doi: 10.3389/fphys.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.BD Diagnostics. 2000. Yeast extract-peptone-dextrose (YPD) agar yeast extract-peptone-dextrose (YPD) broth. DifcoTM and BBLTM manual of microbiological culture media, 2nd ed BD Diagnostics, Sparks, MD. [Google Scholar]
- 71.Zaragoza O, Mesa-Arango AC, Gómez-López A, Bernal-Martínez L, Rodríguez-Tudela JL, Cuenca-Estrella M. 2011. Process analysis of variables for standardization of antifungal susceptibility testing of nonfermentative yeasts. Antimicrob Agents Chemother 55:1563–1570. doi: 10.1128/AAC.01631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabjohns JLA, Park Y-D, Dehdashti J, Henderson C, Zelazny A, Metallo SJ, Zheng W, Williamson PR. 2014. A high throughput screening assay for fungicidal compounds against: Cryptococcus neoformans. J Biomol Screen 19:270–277. doi: 10.1177/1087057113496847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dehdashti SJ, Abbott J, Nguyen DT, McKew JC, Williamson PR, Zheng W. 2013. A high-throughput screening assay for assessing the viability of Cryptococcus neoformans under nutrient starvation conditions. Anal Bioanal Chem 405:6823–6829. doi: 10.1007/s00216-013-7134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Nagasaki Y, Kordalewska M, Press EG, Shields RK, Nguyen MH, Clancy CJ, Perlin DS. 2016. Rapid detection of FKS-associated echinocandin resistance in Candida glabrata. Antimicrob Agents Chemother 60:6573–6577. doi: 10.1128/AAC.01574-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waterman SR, Hacham M, Hu G, Zhu X, Park Y, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, Singh N, Williamson PR. 2007. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest 117:794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]


