ABSTRACT
A retrospective study was conducted in a large sample of acutely hospitalized older patients who underwent therapeutic drug monitoring during levofloxacin treatment. The aim was to assess the population pharmacokinetics (popPK) and pharmacodynamics of levofloxacin among older patients. PopPK and Monte Carlo simulation were performed to define the permissible doses in older patients according to various degrees of renal function. Classification and regression tree (CART) analysis was used to detect the cutoff 24-hour area under the concentration-time curve (AUC24)/MIC ratio that best correlated with the clinical outcome. The probability of target attainment (PTA) of this value was calculated against different pathogens. A total of 168 patients were included, and 330 trough and 239 peak concentrations were used for the popPK analysis. Creatinine clearance (CrCL) was the only covariate that improved the model fit (levofloxacin CL = 0.399 + 0.051 × CrCLCKD-EPI [creatinine clearance estimated by means of the chronic kidney disease epidemiology]). Drug doses ranged between 500 mg every 48 h and 500 mg every 12 h in relation to different renal functions. The identified cutoff AUC24/MIC ratio (≥95.7) was the only covariate that correlated with a favorable clinical outcome in multivariate regression analysis (odds ratio [OR], 20.85; 95% confidence interval [CI], 1.56 to 186.73). PTAs were optimal (>80%) against Escherichia coli and Haemophilus influenzae, borderline against Staphylococcus aureus, and suboptimal against Pseudomonas aeruginosa. The levofloxacin doses defined in our study may be effective for the treatment of infections due to bacterial pathogens, with an MIC of ≤0.5 mg/liter in older patients with various degrees of renal function, while minimizing the toxicity risk. Conversely, the addition of another active antimicrobial should be considered whenever treating infections caused by less susceptible pathogens.
KEYWORDS: fluoroquinolones, personalized therapy, safety, efficacy, population pharmacokinetics
INTRODUCTION
Levofloxacin is a fluoroquinolone antibiotic with one of the broadest spectra of activity, encompassing both Gram-negative and Gram-positive organisms and atypical and anaerobic bacteria (1). Accordingly, it has been used for many years for the treatment of a variety of infections, such as community-acquired pneumonia (CAP), skin and soft tissue infections, urinary tract infections, and acute exacerbation of chronic bronchitis and sinusitis (2, 3).
Levofloxacin is a moderately lipophilic drug that is mainly renally eliminated as an unchanged moiety. A linear relationship between drug clearance (CL) and creatinine clearance (CrCL) has been demonstrated (4). From a pharmacodynamic point of view, it has been shown that the most relevant predictor of fluoroquinolone efficacy in clinical settings is the 24-hour area under the concentration-time curve (AUC24)/MIC ratio. Different AUC24/MIC ratios have been proposed as optimal targets depending on the invading pathogen. Although an AUC24/MIC ratio of 25 to 30 may suffice for infections due to Streptococcus pneumoniae (5), values of 100 to 125 have been recommended for efficacy against those due to Gram-negative pathogens (6, 7). Interestingly, an AUC24/MIC target of ≥87 was associated with microbiological eradication of both Gram-positive and Gram-negative pathogens among 47 patients who were treated with levofloxacin for nosocomial pneumonia (8). However, it should be noticed that in this study levofloxacin was combined with other agents in patients infected with Pseudomonas aeruginosa (ceftazidime or piperacillin-tazobactam) or with methicillin-resistant Staphylococcus aureus (MRSA) (vancomycin) (8). Similarly, combination therapy was also present in the retrospective analysis by Schentag et al. (7).
Fluoroquinolones are among the most frequently used antimicrobials for the treatment of community-acquired infections, which account for a significant number of emergency visits and hospitalizations among older adults. Older patients may be at increased risk of adverse drug reactions (ADRs), mainly because of the pathophysiological changes associated with aging processes and/or of polypharmacy (9). High frequencies of tendinopathy and of tendon ruptures in older patients were associated with aging, impairment of renal function, and corticosteroid coadministration (10, 11).
Accordingly, since levofloxacin toxicity is dose dependent (12), from a safety perspective, dosage adjustments in older patients with varying degrees of renal impairment should be warranted in order to avoid drug-related toxicity (13, 14).
The primary aim of this study was to describe the population pharmacokinetics (popPK) and pharmacodynamics (PD) of high-dose levofloxacin in a large sample of acutely hospitalized older patients in order to estimate the permissible doses that would produce safe and effective exposure in older patients with various degrees of renal function.
RESULTS
Patient characteristics.
One hundred and sixty-eight acutely hospitalized older patients were included in this study. Demographic and clinical data are summarized in Table 1. The majority of patients were males (103/168; 61.3%), and the median (interquartile range [IQR]) age of the study population was 81 years (IQR, 76 to 88). Community-acquired pneumonia, urinary tract infections, and acute exacerbation of chronic bronchitis accounted for most of the bacterial infections requiring levofloxacin treatment (118/168; 70.2%). Levofloxacin was administered mainly orally (145/168; 86.3%) for a median length of treatment of 10 days. Favorable clinical outcomes were reported in 73.2% of cases (123/168).
TABLE 1.
Population characteristics
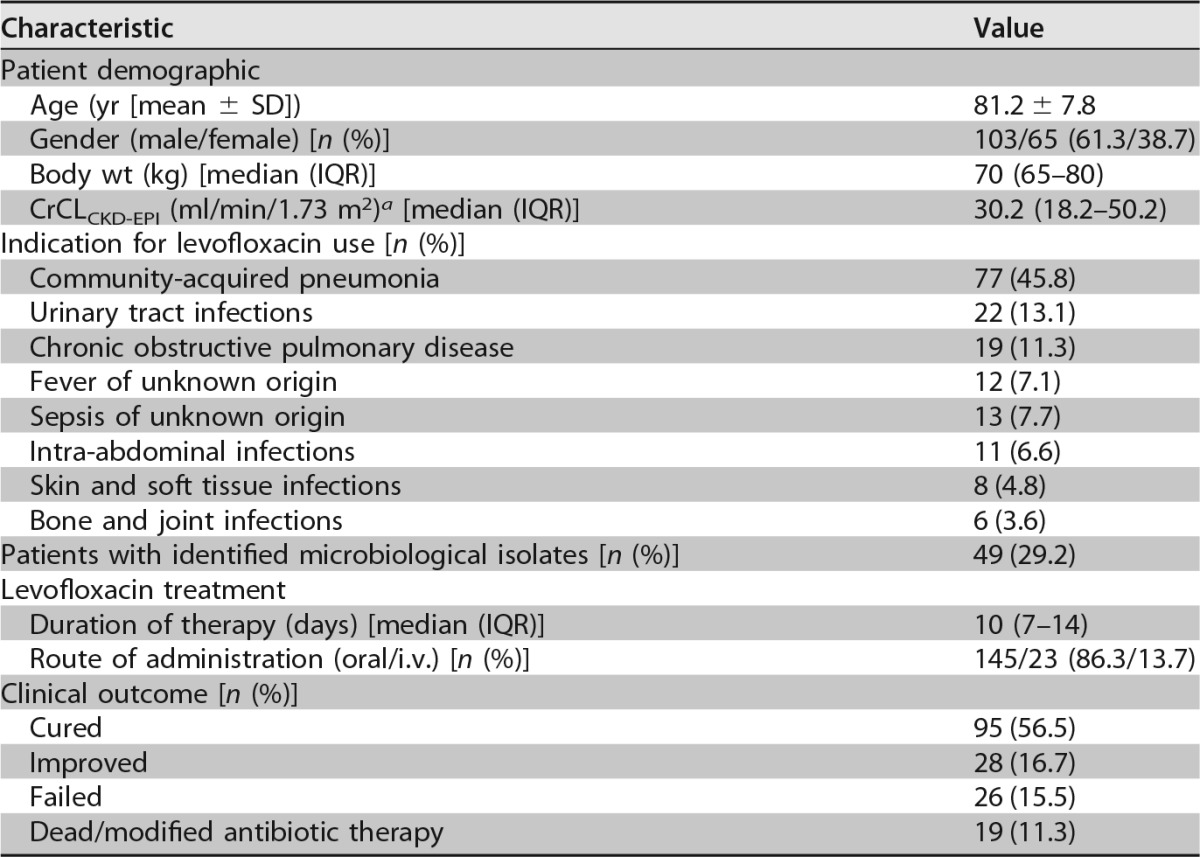
a At first TDM.
Population pharmacokinetic analysis.
A total of 569 levofloxacin plasma concentrations (330 trough and 239 peak concentrations) were included in the population analysis. A two-compartment linear model, with first-order input (for orally administered doses) and first-order clearance from the central compartment, best described the levofloxacin concentrations. Compartments were connected by first-order intercompartmental rate constants.
The only covariate that improved the model fit was CrCLCKD-EPI (objective function value [OFV] reduction from 2,125 to 2,086; P < 0.01). The final model for clearance was as follows: levofloxacin CL = 0.399 + 0.051 × CrCLCKD-EPI, where CL is the value of levofloxacin clearance and CrCLCKD-EPI is the creatinine clearance estimated by means of the chronic kidney disease epidemiology (CKD-EPI) formula.
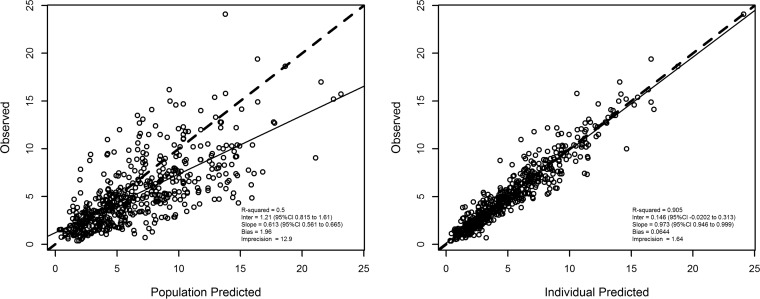
Figure 1 shows the diagnostic plots for the final covariate model. After maximum a posteriori probability (MAP)-Bayesian estimation, the observed-versus-predicted plot had an intercept and slope that were close to zero and 1, respectively (observed = 0.146 + 0.973 × predicted [r2 = 0.905; P < 0.01]). The bias and precision were acceptable (bias, 0.064 mg/liter, and precision, 1.64 mg/liter).
FIG 1.
Diagnostic plot for the final covariate model. Shown are observed versus population predicted plasma concentrations (left) and individual predicted plasma concentrations (right). Solid lines refer to linear regression between observed and predicted concentrations. Dashed lines are the identity lines between observed and predicted concentrations.
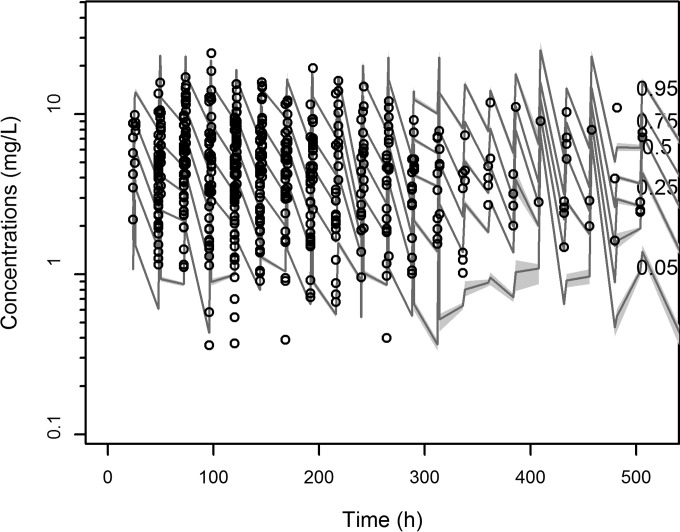
The mean (±standard deviation [SD]) and the median pharmacokinetic parameter estimates for the final covariate model are shown in Table 2. The distribution of the observed concentrations was consistent with that of the predicted concentrations, as suggested by the visual predictive check (VPC) plot (Fig. 2). The normal distribution of normalized prediction distribution errors (NPDEs) (P = 0.115 in the Shapiro-Wilk normality test) confirmed the adequacy of the model for dosing simulations.
TABLE 2.
Parameter estimates for the final population pharmacokinetic model of levofloxacin in older patients
| Unit | ka (h−1) | kcp (h−1) | kpc (h−1) | CL (liters/h) | Vca (liters) | Fos (%) | Tlag (h) |
|---|---|---|---|---|---|---|---|
| Mean | 16.15 | 0.63 | 1.77 | 2.53 | 52.95 | 0.83 | 1.47 |
| SD | 13.47 | 0.85 | 0.52 | 1.46 | 21.57 | 0.21 | 0.65 |
| Coefficient of variation | 83.41 | 133.52 | 29.47 | 57.84 | 40.73 | 24.83 | 43.95 |
| Median | 9.91 | 0.04 | 2.00 | 2.20 | 61.25 | 0.98 | 1.87 |
Vc, volume of the central compartment.
FIG 2.
Visual predictive check of levofloxacin plasma concentrations versus time for the final covariate model. Gray shading displays predicted intervals of simulated data.
Monte Carlo simulation for estimation of levofloxacin doses predicting optimal target drug exposure in older patients with various degrees of renal function.
Table 3 shows the distributions of probabilities of simulated patients with underexposure, optimal target exposure, and overexposure at the various permissible doses of levofloxacin. The regimens that were associated with the highest proportion of optimal target exposure and the lowest risk of under- and/or overexposure were as follows: 500 mg every 48 h for CrCLCKD-EPI values of <20 ml/min/1.73 m2, 750 mg every 48 h for CrCLCKD-EPI values of 20 to 39 ml/min/1.73 m2, 500 mg every 24 h for CrCLCKD-EPI values of 40 to 59 ml/min/1.73 m2, 750 mg every 24 h for CrCLCKD-EPI values of 60 to 79 ml/min/1.73 m2, and 500 mg every 12 h for CrCLCKD-EPI values of >80 ml/min/1.73 m2. Nevertheless, >20% risk of underexposure could be expected when using 500 mg every 24 h or 750 mg every 24 h in patients with CrCLCKD-EPI values of 40 to 59 and 60 to 79 ml/min/1.73 m2, respectively. Similarly, >10% risk of overexposure could be observed when using 500 mg every 48 h or 500 mg every 12 h in patients with CrCLCKD-EPI values of <20 and >80 ml/min/1.73 m2, respectively.
TABLE 3.
Probabilities of achieving underexposure, normal target exposure, and overexposure with different levofloxacin dosing regimens in older patients in relation to different classes of renal function
| Levofloxacin regimen (mg) | Probabilitya |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–19 |
20–39 |
40–59 |
60–79 |
>80 |
|||||||||||
| <50 | 50–160 | >160 | <50 | 50–160 | >160 | <50 | 50–160 | >160 | <50 | 50–160 | >160 | <50 | 50–160 | >160 | |
| 125 every 48 h | 91.8 | 8.2 | 0.0 | 99.8 | 0.2 | 0.0 | 99.8 | 0.2 | 0.0 | 99.9 | 0.1 | 0.0 | 100.0 | 0.0 | 0.0 |
| 250 every 48 h | 48.5 | 50.5 | 1.0 | 91.4 | 8.6 | 0.0 | 99.0 | 1.0 | 0.0 | 99.6 | 0.4 | 0.0 | 99.9 | 0.1 | 0.0 |
| 500 every 48 h | 6.4 | 77.2 | 16.4 | 32.2 | 67.0 | 0.8 | 81.6 | 18.4 | 0.0 | 95.7 | 4.3 | 0.0 | 97.2 | 2.8 | 0.0 |
| 750 every 48 h | 1.4 | 53.9 | 44.7 | 7.2 | 86.2 | 6.6 | 42.2 | 57.2 | 0.6 | 79.6 | 20.0 | 0.4 | 89.0 | 11.0 | 0.0 |
| 500 every 24 h | 2.3 | 50.3 | 47.4 | 5 | 81.3 | 13.7 | 22.2 | 76.0 | 1.8 | 59.2 | 40.1 | 0.7 | 78.7 | 21.0 | 0.3 |
| 750 every 24 h | 1.1 | 17.1 | 81.8 | 1.7 | 51.3 | 47.0 | 5.8 | 82.8 | 11.4 | 23.2 | 73.1 | 3.7 | 50.3 | 47.6 | 2.1 |
| 500 every 12 h | 0 | 3.6 | 96.4 | 0.2 | 12.3 | 87.5 | 0.1 | 39.0 | 60.9 | 1.5 | 70.1 | 28.4 | 2.8 | 82.8 | 14.4 |
Probability of achieving underexposure (AUC24 < 50 mg · h/liter), normal target exposure (AUC24 between 50 and 160 mg · h/liter), and overexposure (AUC24 > 160 mg · h/liter) with different levofloxacin dosing regimens in older patients in relation to different classes of renal function. The classes of renal function (ml/min/1.73 m2) are shown in the top row, and those of levofloxacin AUC24 (mg · h/liter) are shown in the bottom row in the header.
PK/PD analysis.
Forty-nine patients had documented bacterial infections, but only 41 of them (83.7%) were eligible for the PK/PD analysis (4 had to be excluded because of infections caused by levofloxacin-resistant pathogens, 3 because of death from other causes, and 1 because of stopping therapy for adverse events). Most of the eligible patients received levofloxacin as monotherapy (56.1%) and had favorable clinical outcomes (75.6%).
Blood and urine accounted for most of the primary sources of infection (80.5%). The bacteria most frequently yielded were Escherichia coli, S. aureus, and P. aeruginosa, which accounted overall for 65.1% (28/43) of the isolates (Table 4).
TABLE 4.
Bacterial pathogens (n = 43 from 41 patients) included in the pharmacokinetic/pharmacodynamic analysis
| Pathogen | No. of isolates | MIC range (mg/liter) |
|---|---|---|
| Escherichia coli | 12 | 0.03–4 |
| Staphylococcus aureus | 9 | 0.125–0.5 |
| Pseudomonas aeruginosa | 7 | 0.25–2 |
| Klebsiella pneumoniae | 4 | 0.06–1 |
| Haemophilus influenzae | 2 | 0.03 |
| Klebsiella oxytoca | 2 | 0.06–1 |
| Staphylococcus epidermidis | 2 | 0.25–4 |
| Enterobacter aerogenes | 1 | 0.125 |
| Streptococcus pneumoniae | 1 | 1 |
| Staphylococcus saprophyticus | 1 | 0.5 |
| Staphylococcus schleiferi | 1 | 0.25 |
| Staphylococcus capitis | 1 | 0.25 |
A cutoff value of ≥95.7 for the total AUC24/MIC ratio was identified as a valuable predictor of a favorable clinical outcome in classification and regression tree (CART) analysis. Among the five patients whose AUC24/MIC ratios were below this breakpoint, a positive clinical outcome observed was in only one case (20%). Conversely, of the 36 patients with AUC24/MIC ratios of ≥95.7, positive clinical outcomes were observed in 30 (83.3%) cases. The area under the receiver operating characteristic (ROC) curve for this cutoff value was high (0.79).
Among the various covariates that were tested by univariate analysis for potential relationships with favorable clinical outcomes (age, gender, weight, CrCLCKD-EPI, route of levofloxacin administration, AUC24/MIC ratio of ≥95.7, length of levofloxacin treatment, and cotreatment with other antimicrobials), only weight (P = 0.117; log-likelihood = −21.399) and an AUC24/MIC ratio of ≥95.7 (P < 0.05; log-likelihood = −19.328) were predictive of a favorable clinical outcome. In the multivariate logistic regression analysis, only an AUC24/MIC ratio of ≥95.7 was definitely associated with a favorable clinical outcome (odds ratio [OR], 20.85; 95% confidence interval [CI], 1.56 to 186.73; P < 0.05; log-likelihood = −16.828).
PTA and cumulative fraction of response (CFR) at the cutoff AUC24/MIC ratio associated with a favorable clinical outcome.
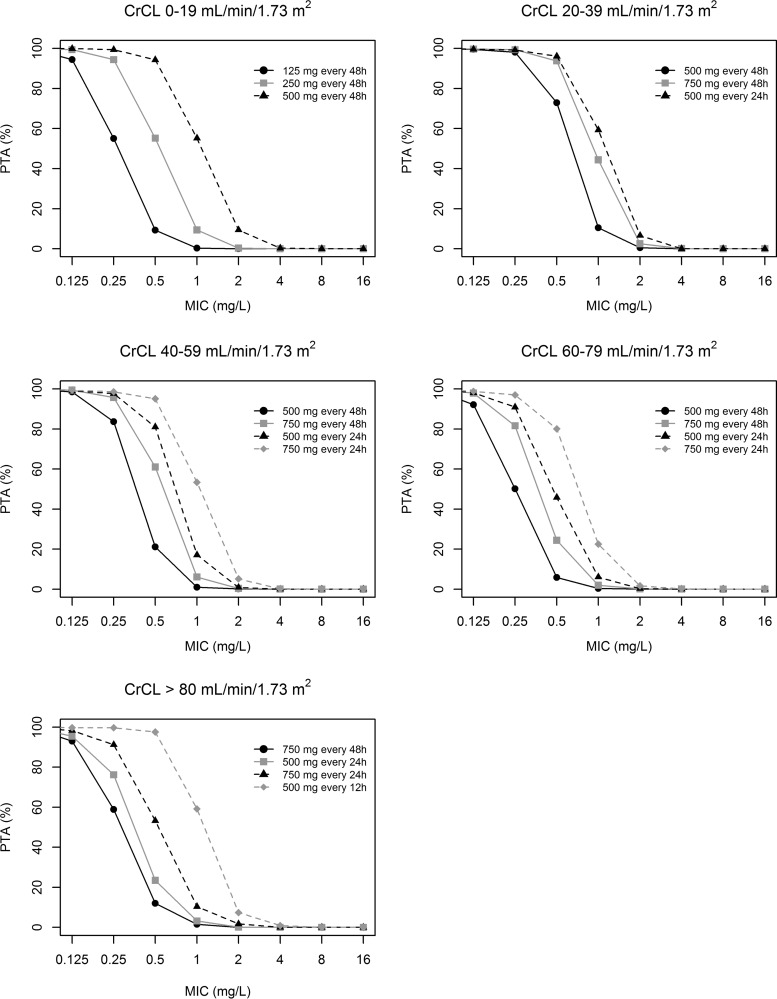
Figure 3 shows the probability of achieving an AUC24/MIC ratio cutoff value of ≥95.7 with the various permissible doses of levofloxacin. The analysis showed that the permissible levofloxacin doses may achieve optimal PTAs only against those pathogens with levofloxacin MICs of ≤0.5 mg/liter.
FIG 3.
Probabilities of achieving an AUC24/MIC value of ≥95.7 with the various permissible doses of levofloxacin in relation to different degrees of renal function and susceptibility of the invading pathogen.
Table 5 summarizes the levofloxacin doses that resulted in effective AUC24 values in older patients in relation to different degrees of susceptibility of the pathogens to levofloxacin.
TABLE 5.
Permissible dosing regimens of levofloxacin granting optimal PTA in older patients in relation to different degrees of renal function and of the susceptibility of the invading bacterial pathogen
| MIC (mg/liter) | Dosing regimen (mg) for class of renal function (ml/min/1.73 m2): |
||||
|---|---|---|---|---|---|
| 0–19 | 20–39 | 40–59 | 60–79 | >80 | |
| 0.125 | 125 every 48 h | 500 every 48 h | 500 every 48 h | 500 every 48 h | 750 every 48 h |
| 0.25 | 250 every 48 h | 500 every 48 h | 500 every 48 h | 750 every 48 h | 750 every 24 h |
| 0.5 | 500 every 48 h | 750 every 48 h | 500 every 24 h | 750 every 24 h | 500 every 12 h |
Table 6 shows the CFRs of the permissible doses of levofloxacin against the bacterial pathogens that were most frequently yielded in our study population (E. coli, S. aureus, H. influenzae, and P. aeruginosa). Although optimal CFRs were always achieved against S. aureus, H. influenzae, and E. coli, this was never the case against P. aeruginosa.
TABLE 6.
Cumulative fractions of response of the permissible doses of levofloxacin against the invading pathogens more frequently yielded in the study population according to their EUCAST MIC distributions
| Class of renal function (ml/min/1.73 m2) | Levofloxacin dose (mg) | CFR |
|||
|---|---|---|---|---|---|
| S. aureus | H. influenzae | E. coli | P. aeruginosa | ||
| 0–19 | 125 every 48 h | 59.89 | 99.66 | 82.06 | 16.48 |
| 250 every 48 h | 77.03 | 99.78 | 85.07 | 40.36 | |
| 500 every 48 h | 81.59 | 99.85 | 87.34 | 62.24 | |
| 20–39 | 500 every 48 h | 79.22 | 99.79 | 85.80 | 47.07 |
| 750 every 48 h | 81.26 | 99.84 | 87.12 | 59.63 | |
| 500 every 24 h | 81.49 | 99.85 | 87.43 | 63.08 | |
| 40–59 | 500 every 48 h | 71.28 | 99.73 | 83.45 | 25.81 |
| 750 every 48 h | 77.73 | 99.78 | 85.26 | 42.03 | |
| 500 every 24 h | 79.42 | 99.81 | 86.16 | 50.72 | |
| 750 every 24 h | 81.13 | 99.84 | 87.28 | 61.63 | |
| 60–79 | 500 every 48 h | 57.19 | 99.65 | 81.57 | 14.41 |
| 750 every 48 h | 70.61 | 99.73 | 83.52 | 26.68 | |
| 500 every 24 h | 74.86 | 99.76 | 84.55 | 36.08 | |
| 750 every 24 h | 79.16 | 99.81 | 86.20 | 51.22 | |
| >80 | 750 every 48 h | 60.72 | 99.67 | 82.12 | 18.21 |
| 500 every 24 h | 67.91 | 99.71 | 83.27 | 25.50 | |
| 750 every 24 h | 75.51 | 99.77 | 84.90 | 39.43 | |
| 500 every 12 h | 81.67 | 99.85 | 87.52 | 63.81 | |
DISCUSSION
In this study, we addressed the issue of dosing optimization with levofloxacin in acutely hospitalized older patients, among whom the attainment of optimal pharmacodynamic targets of efficacy with fluoroquinolones should be balanced against safety concerns.
Population pharmacokinetic modeling provided robust estimates of the pharmacokinetic parameters in our population. The final model explained almost 91% of the variability of drug concentrations over time with acceptable bias and precision. The pharmacokinetic estimates for levofloxacin in the study population are quite different from those previously described in other cohorts. The mean CL of levofloxacin in our population was consistently lower (2.53 liters/h) than that observed among healthy volunteers (15), adult patients with normal renal function (8, 16, 17), and elderly patients with CAP (18). Of note, this is in agreement with the fact that most of our patients, unlike those in the other studies, were very old (mean age, 81.2 years) and had impaired renal function (median CrCLCKD-EPI, 30.4 ml/min/1.73 m2).
The fact that CrCLCKD-EPI was the only covariate that improved the model fit is similar to previous findings in elderly patients (19). This suggests that estimation of renal function by means of this formula should be considered mandatory in older patients for calculating appropriate dose adjustments of levofloxacin in order to avoid drug overexposure. Interestingly, our Monte Carlo simulations provided a detailed stratification of dose adjustments of levofloxacin in relation to different levels of renal function in older patients. It is worth noting that in patients with severe renal impairment (CrCLCKD-EPI < 40 ml/min/1.73 m2), the levofloxacin dosage must be more than halved in order to avoid overexposure.
Our approach, by targeting drug exposure in all of the patients within a desired range similar to that observed in subjects with normal renal function, may minimize the risk of exposure-dependent toxicity among older patients. This is in agreement with a recent Japanese study showing that adjustments of the levofloxacin dose in relation to the degree of renal function may help to decrease the incidence of adverse events in elderly patients (14). In this regard, it is worth mentioning that among our study population no patients suffered from tendinopathy or had to stop therapy because of chondrotoxicity (data not shown).
The opportunity to define permissible doses of levofloxacin in older patients was further strengthened by the findings of two recent reviews showing that levofloxacin is the fluoroquinolone associated with the highest risk of causing tendon damage (10, 12). This may further strengthen the valuable role that a real-time therapeutic drug monitoring (TDM)-guided approach to levofloxacin dosage adjustments may have in preventing drug-related toxicity in older patients.
Our approach still ensured that patients had a high probability of having favorable clinical outcomes. The relatively high cutoff value of the AUC24/MIC ratio identified by CART analysis as a valuable predictor of clinical efficacy among our study population (≥95.7) was similar to that reported previously by Drusano et al. among patients with nosocomial pneumonia (8). This might be explained by the fact that most of the bacterial clinical isolates included in our analysis, similar to what occurred in the Drusano et al. study, were Gram-negative pathogens, which were shown to require much higher pharmacodynamic thresholds than Gram-positive pathogens.
Importantly, our pharmacodynamic analyses suggested that pathogens with an MIC of ≤0.5 mg/liter are adequately treated. However, even if this value is lower than the EUCAST clinical breakpoint for susceptibility for levofloxacin against Gram-negative and Gram-positive pathogens, which is set to 1 mg/liter (20), it corresponds to that of USCAST for S. aureus and E. coli. In both cases, this raises potential concerns about the efficacy of levofloxacin monotherapy in some settings. Results similar to ours were reported in a population pharmacokinetic analysis of 38 adult Korean patients. In that study, a levofloxacin regimen of 250 and 500 mg once daily in patients with CrCL values of 20 to 50 and >50 ml/min, respectively, resulted in an AUC24/MIC ratio of >100 only against pathogens with an MIC up to and including 0.5 mg/liter (17). Conversely, in another study, it was shown that dosing regimens of 125, 250, and 500 mg once daily were predicted to ensure a PTA of >90% against pathogens with an MIC of up to 2 mg/liter in patients with CrCL values of <20, 20 to 50, and >50 ml/min, respectively (21). In addition, it is worth mentioning that our study is unique in that PTAs were estimated for various doses of levofloxacin that were different in relation to various degrees of renal function. This step, in our opinion, should be considered mandatory at this time in order to prevent exposure-related toxicity with levofloxacin in older patients (12).
When looking at species-specific CFRs, the optimal CFR in older patients may be predicted in relation to the permissible doses against E. coli and H. influenzae, whereas borderline CFR may be achieved against S. aureus. This offers the opportunity to speculate that levofloxacin may still represent a valuable therapeutic weapon in older patients for the treatment of urinary tract infections, which are frequently caused by E. coli. Similarly, levofloxacin may be valuable in the treatment of hematogenous discitis, which may be frequently caused by methicillin-susceptible S. aureus. Conversely, only suboptimal CFRs were observed against P. aeruginosa, and this means that currently levofloxacin should not be considered an effective antipseudomonal monotherapy.
This study has several limitations. The retrospective design, lack of evaluation of microbiological eradication in assessing the clinical outcome, and use of combination antimicrobial therapy are all relevant considerations. As far as the population analysis is concerned, we recognize that the estimate of ka might not be robust enough, due to the limited variability in the sampling times of peak concentrations. Additionally, we recognize that our definition of overexposure is arbitrary, but we strongly believe that this approach may be helpful in containing the risk of exposure-dependent toxicity with levofloxacin. Finally, we acknowledge that our PK/PD analysis was based mainly on Gram-negative pathogens, and this could mean that the identified cutoff AUC24/MIC target is probably too high for S. pneumoniae, a pathogen for which an AUC24/MIC of >30 is commonly accepted as the pharmacodynamic target of efficacy. Nevertheless, the large patient sample size and the heterogeneity of the patients' diagnoses could strengthen the generalizability of our results.
In conclusion, our study is unique in that it defined for the first time the permissible doses of levofloxacin that should be administered to older patients with various degrees of renal function in order to minimize the risk of exposure-dependent toxicity. Additionally, it highlights that these doses might be effective only when treating infections due to bacterial pathogens with MICs of ≤0.5 mg/liter, which could have implications for in vivo susceptibility clinical breakpoints.
MATERIALS AND METHODS
Study design.
This was a retrospective study conducted between May 2007 and December 2012 among older patients aged ≥65 years who were admitted to the First Division of Internal Medicine of the Santa Maria della Misericordia University Hospital of Udine, Udine, Italy, and who underwent TDM of levofloxacin at the Institute of Clinical Pharmacology of the same hospital. The study was approved by the Regional Ethics Committee. Informed written consent was waived due to the retrospective and observational nature of the study.
Patients received levofloxacin because of documented or suspected bacterial infection. The use of additional antimicrobial agents was permitted at the discretion of the treating physician (ceftazidime, piperacillin-tazobactam, or meropenem for suspected and/or proven infections by Gram-negative pathogens; vancomycin or teicoplanin for suspected and/or proven infections by MRSA).
The dosage of levofloxacin was initially chosen by the attending physician and subsequently adjusted on the basis of TDM-guided clinical pharmacological advice that was made promptly available in the hospital intranet. TDM of levofloxacin is routinely performed at our hospital, with target concentrations of 1 to 3 mg/liter for trough concentrations and 6 to 9 mg/liter for peak concentrations (which were collected 2 h after oral administration or 1.5 h after intravenous [i.v.] administration), respectively. These concentrations correspond to AUC24 values between 50 and 160 mg · h/liter, which is the range of exposures normally observed with the standard high dose of 500 mg every 12 h (which is licensed in Italy) in subjects with normal renal function (7, 15, 22, 23). This TDM-guided approach, by maintaining exposure within the expected normal range, is finalized to prevent theoretical overexposure (arbitrarily defined as an AUC24 of >160 mg · h/liter) and may aid in minimizing the risk of exposure-dependent toxicity in older patients, which is definitely the population at greater risk of toxicity during levofloxacin therapy (11).
The following demographic and clinical data were retrieved from each patient's medical record: age, gender, weight, height, type and site of infection, bacterial clinical isolate (whenever available) with the MIC of levofloxacin, underlying disease(s), serum creatinine, levofloxacin dose, route of administration and TDM data, and cotreatment with any other drug. Baseline and end-of-therapy C-reactive protein (CRP) levels were also collected. Creatinine clearance was estimated by means of the CKD-EPI formula (CrCLCKD-EPI) (24).
Blood samples for TDM were collected at least 48 h after starting levofloxacin. Levofloxacin concentrations were analyzed by means of a validated high-performance liquid chromatography (HPLC) method with UV detection, as previously described (4). Precision and accuracy were assessed by performing replicate analyses of quality control samples against calibration standards. Intra- and interassay coefficients of variation were always less than 10%. The lower limit of detection was 0.1 mg/liter.
Assessment of clinical outcomes.
Clinical outcomes were defined as cured, improved, unchanged, or failed according to the treatment response assessed at the end of therapy by the attending physician. A patient was classified as cured if signs and symptoms of infection disappeared at the end of therapy, as improved in cases of partial clinical response associated with significant decrease in CRP values from baseline, or as unchanged or failed in cases of absence of clinical response at the end of therapy. Patients who were cured and improved were considered to have a successful clinical outcome.
Population pharmacokinetic modeling.
One- and two-compartment models were developed and fitted using the nonparametric adaptive grid (NPAG) approach included in the Pmetrics package for R (Los Angeles, CA, USA) (25). The base-weighting scheme was developed by use of a polynomial function that relates the drug concentration to the standard deviation of the observations, using the between-day assay variability data. MAP-Bayesian parameter estimates for levofloxacin were determined for each patient in the data set and were used for describing the pharmacokinetic parameters (ka [first-order transfer rate constant of absorption], kcp and kpc [first-order intercompartmental transfer rate constants connecting the central and peripheral compartments, respectively], CL [total clearance of levofloxacin], V[volume of distribution], Fos [oral bioavailability of levofloxacin], and Tlag [time delay between drug administration and first observed concentration]) for each patient in the population.
First, we developed a basic model without covariates by using the building data set, which was parameterized only for clearance (CL) and volume of distribution (V). Subsequently, we tested covariates that were deemed clinically relevant. Only those covariates that significantly increased the log-likelihood value of the covariate model (i.e., twice the difference in log-likelihood values for the covariate versus the base model with the appropriate degrees of freedom assessed against a χ2 distribution) were retained for further analysis.
The model performance was further evaluated by assessing the goodness of fit of the observed-predicted plot, the coefficient of determination of the linear regression of the observed-predicted values, and the OFV of each run. Additionally, a VPC and NPDEs were also determined. The VPC compares the observed concentrations overlaid with model-predicted concentration-time profiles; 95% of the observed concentrations should reside within the 95% CI derived from model predictions. NPDEs provide a quantitative assessment of the final model and are considered a better evaluation tool than a plot of weighted residuals, especially when dealing with models with covariates (26). NPDEs should be normally distributed when the model is appropriately fitted.
Monte Carlo simulation for estimation of levofloxacin doses predicting optimal target drug exposure in older patients with various degrees of renal function.
One-thousand-subject Monte Carlo simulations were conducted using Pmetrics to estimate the AUC24 values achievable with various candidate regimens of levofloxacin (125 mg every 48 h, 250 mg every 48 h, 250 mg daily, 500 mg every 48 h, 750 mg every 48 h, 500 mg daily, 750 mg daily, and 500 mg every 12 h) for different levels of renal function (0 to 19, 20 to 39, 40 to 59, 60 to 79, and >80 ml/min/1.73 m2).
In order to define the permissible levofloxacin doses in the study population, we considered desirable in this population the achievement of the exposure range that was observed in healthy volunteers with normal renal function with the standard high dose of 500 mg every 12 h (AUC24, 50 to 160 mg · h/liter) (14, 15, 22). Consistently, an AUC24 value of <50 mg · h/liter was defined as underexposure, an AUC24 value between 50 and 160 mg · h/liter was defined as optimal target exposure, and an AUC24 value of >160 mg · h/liter was defined as overexposure. Permissible doses were defined as those producing less than 10% probability of causing both drug underexposure and overexposure in each class of renal function. The identified levofloxacin doses were considered sufficiently safe for clinical use in this population and were subsequently tested in the pharmacokinetic/pharmacodynamic analysis.
PK/PD analysis.
AUC24/MIC ratios were calculated for all of the patients who yielded bacterial clinical isolates and were tested for levofloxacin susceptibility. Considering that levofloxacin is approximately 30% plasma protein bound, all the pharmacodynamic targets were multiplied by a factor of 0.7 in order to obtain the free targets (fAUC24/MIC), which were then included in the PK/PD analysis.
Logistic regression analysis was used to explore the relationship between drug exposure and other clinical factors and the probability of a clinical outcome. For those patients who had antimicrobial combination therapy, we created a dichotomous categorical variable. Covariates with a P value of <0.20 in the univariate analysis were deemed of potential clinical relevance and included in the multivariate model on the basis of a forward stepwise approach.
CART analysis was used to develop a prediction model for detecting the cutoff value of the AUC24/MIC ratio that best correlated with a favorable clinical outcome in the study population. Subsequently, the validity of the identified cutoff value was tested by means of ROC analysis.
PTA and CFR at the cutoff AUC24/MIC ratio associated with a favorable clinical outcome.
We estimated the PTA of the identified cutoff value of the AUC24/MIC ratio in relation to the various levofloxacin doses. The CFR (27) was then assessed against the bacterial species that were more frequently isolated in the study population. The optimal CFR was defined as ≥80% of subjects within the desired AUC24/MIC range.
Statistical analysis.
The Kolmogorov-Smirnov test was used to assess whether data were normally or nonnormally distributed. Accordingly, the mean plus SD or median with IQR was used in the descriptive statistics. Categorical variables were compared by the χ2 test or Fisher's exact test, while continuous variables were compared using the Student t test or Mann-Whitney test. A P value of <0.05 was required to achieve statistical significance. All statistical analyses were performed using Systat version 13 (Systat Software, Inc.).
ACKNOWLEDGMENTS
This study was conducted as part of our routine work.
We declare that we have no conflicts of interest related to this work.
REFERENCES
- 1.Blondeau JM. 1999. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther 21:3–42. doi: 10.1016/S0149-2918(00)88266-1. [DOI] [PubMed] [Google Scholar]
- 2.Anderson VR, Perry CM. 2008. Levofloxacin: a review of its use as a high-dose, short-course treatment for bacterial infection. Drugs 68:535–565. doi: 10.2165/00003495-200868040-00011. [DOI] [PubMed] [Google Scholar]
- 3.Noreddin AM, Elkhatib WF. 2010. Levofloxacin in the treatment of community-acquired pneumonia. Expert Rev Anti Infect Ther 8:505–514. doi: 10.1586/eri.10.35. [DOI] [PubMed] [Google Scholar]
- 4.Pea F, Di Qual E, Cusenza A, Brollo L, Baldassarre M, Furlanut M. 2003. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin Pharmacokinet 42:589–598. doi: 10.2165/00003088-200342060-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose PG, Grasela DM, Grasela TH, Passarell J, Mayer HB, Pierce PF. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother 45:2793–2797. doi: 10.1128/AAC.45.10.2793-2797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazzola M, Matera MG, Donnarumma G, Tufano MA, Sanduzzi A, Marchetti F, Blasi F. 2005. Pharmacodynamics of levofloxacin in patients with acute exacerbation of chronic bronchitis. Chest 128:2093–2098. doi: 10.1378/chest.128.4.2093. [DOI] [PubMed] [Google Scholar]
- 7.Schentag JJ, Meagher AK, Forrest A. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 2. Human trials. Ann Pharmacother 37:1478–1488. [DOI] [PubMed] [Google Scholar]
- 8.Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis 189:1590–1597. doi: 10.1086/383320. [DOI] [PubMed] [Google Scholar]
- 9.Davies EA, O'Mahony MS. 2015. Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol 80:796–807. doi: 10.1111/bcp.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabyat RM, Raisch DW, McKoy JM, Bennett CL. 2015. Fluoroquinolone-associated tendon-rupture: a summary of reports in the Food and Drug Administration's adverse event reporting system. Expert Opin Drug Saf 14:1653–1660. doi: 10.1517/14740338.2015.1085968. [DOI] [PubMed] [Google Scholar]
- 11.Nicolle LE. 1999. Quinolones in the aged. Drugs 58(Suppl 2):S49–S51. [DOI] [PubMed] [Google Scholar]
- 12.Bidell MR, Lodise TP. 2016. Fluoroquinolone-associated tendinopathy: does levofloxacin pose the greatest risk? Pharmacotherapy 36:679–693. doi: 10.1002/phar.1761. [DOI] [PubMed] [Google Scholar]
- 13.Furlanut M, Brollo L, Lugatti E, Di Qual E, Dolcet F, Talmassons G, Pea F. 2003. Pharmacokinetic aspects of levofloxacin 500 mg once daily during sequential intravenous/oral therapy in patients with lower respiratory tract infections. J Antimicrob Chemother 51:101–106. doi: 10.1093/jac/dkg035. [DOI] [PubMed] [Google Scholar]
- 14.Tachi T, Teramachi H, Asano S, Tanaka K, Fukuta M, Osawa T, Aoyama S, Yasuda M, Mizui T, Goto C, Tsuchiya T. 2013. Impact of levofloxacin dose adjustments by dispensing pharmacists on adverse reactions and costs in the treatment of elderly patients. Pharmazie 68:977–982. [PubMed] [Google Scholar]
- 15.Chow AT, Fowler C, Williams RR, Morgan N, Kaminski S, Natarajan J. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob Agents Chemother 45:2122–2125. doi: 10.1128/AAC.45.7.2122-2125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Wong FA, Corrado M. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother 42:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiem S, Ryu SM, Lee YM, Schentag JJ, Kim YW, Kim HK, Jang HJ, Joo YD, Jin K, Shin JG, Ghim JL. 2016. Population pharmacokinetics of levofloxacin in Korean patients. J Chemother 28:308–313. doi: 10.1179/1973947815Y.0000000033. [DOI] [PubMed] [Google Scholar]
- 18.Noreddin AM, Marras TK, Sanders K, Chan CK, Hoban DJ, Zhanel GG. 2004. Pharmacodynamic target attainment analysis against Streptococcus pneumoniae using levofloxacin 500 mg, 750 mg and 1000 mg once daily in plasma (P) and epithelial lining fluid (ELF) of hospitalized patients with community acquired pneumonia (CAP). Int J Antimicrob Agents 24:479–484. doi: 10.1016/j.ijantimicag.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Deguchi T, Nakane K, Yasuda M, Shimizu T, Monden K, Arakawa S, Matsumoto T. 2010. Microbiological outcome of complicated urinary tract infections treated with levofloxacin: a pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents 35:573–577. doi: 10.1016/j.ijantimicag.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 20.EUCAST. 2016. Clinical breakpoints. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden. [Google Scholar]
- 21.Leroy B, Uhart M, Maire P, Bourguignon L. 2012. Evaluation of fluoroquinolone reduced dosage regimens in elderly patients by using pharmacokinetic modelling and Monte Carlo simulations. J Antimicrob Chemother 67:2207–2212. doi: 10.1093/jac/dks195. [DOI] [PubMed] [Google Scholar]
- 22.Child J, Mortiboy D, Andrews JM, Chow AT, Wise R. 1995. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother 39:2749–2751. doi: 10.1128/AAC.39.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien SC, Wong FA, Fowler CL, Callery-D'Amico SV, Williams RR, Nayak R, Chow AT. 1998. Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers. Antimicrob Agents Chemother 42:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flamant M, Haymann JP, Vidal-Petiot E, Letavernier E, Clerici C, Boffa JJ, Vrtovsnik F. 2012. GFR estimation using the Cockcroft-Gault, MDRD study, and CKD-EPI equations in the elderly. Am J Kidney Dis 60:847–849. doi: 10.1053/j.ajkd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brendel K, Comets E, Laffont C, Laveille C, Mentre F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother 55:601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]