Abstract
Identifying sites of protein-ligand interaction is important for structure-based drug discovery and understanding protein structure-function relationships. Mass spectrometry (MS) has emerged as a useful tool for identifying residues covalently modified by ligands. Current methods use database searches that depend on acquiring interpretable fragmentation spectra (MS2) of peptide-ligand adducts. This is problematic for identifying sites of hydrophobic ligand incorporation in integral membrane proteins (IMPs) where poor aqueous solubility and ionization of peptide-ligand adducts and collision-induced adduct loss hinder the acquisition of quality MS2 spectra. To address these issues, we developed a Fast Ligand Identification (FLI) tag that can be attached to any alkyne-containing ligand via Cu(I)-catalyzed cycloaddition. FLI-tag adds charge to increase solubility and ionization, and utilizes stable isotope labeling for MS1 level identification of hydrophobic peptide-ligand adducts. FLI-tag was coupled to an alkynecontaining neurosteroid photolabeling reagent and used to identify peptide-steroid adducts in MS1 spectra via the stable heavy isotope pair. Peptide-steroid adducts were not identified in MS2-based database searches because collision-induced adduct loss was the dominant feature of CID fragmentation, but targeted analysis of MS1 pairs using electron transfer dissociation (ETD) markedly reduced adduct loss. Using FLI-tag and ETD we identified Glu73 as the site of photo-incorporation of our neurosteroid ligand in the IMP, mVDAC1, and top-down MS confirmed a single site of photolabeling.
Graphical Abstract
Protein-ligand interactions play a fundamental role in cellular function. Endogenous and exogenous small molecules can act as agonists, antagonists, or allosteric modulators of protein function, but the specific sites to which these ligands bind are often unknown. This is especially true for allosteric modulators of integral membrane proteins (IMPs), which often interact with the hydrophobic transmembrane domains1. While the identification of allosteric ligand binding sites on IMPs can enable both structure-based drug discovery and an improved understanding of IMP structure-function relationships, methods for identifying sites of allosteric interaction are limited. Common approaches include photoaffinity labeling (PAL) coupled with either Edman degradation2–4 or mass spectrometry (MS)5,6, X-ray crystallography7,8, or NMR spectroscopy9. PAL-MS can identify protein-ligand interactions using far smaller amounts of protein than crystallography, NMR or Edman degradation10,11, making it an appealing approach for membrane proteins that may be difficult to purify, difficult to crystallize, or too large for NMR3,11. While PAL-MS has proven a useful method for identifying protein-ligand interactions, there are several unique challenges for identifying the specific site(s) within a protein that are labeled.
In PAL, a functional analogue of the ligand containing a photoactivatable group (usually a diazirine, butyrophenone, diazoester, arylazide, or arylketone) is covalently attached to its protein binding site via wavelength specific ultraviolet irradiation of the protein-ligand mixture12. Initially, ligands were made with reporter tags (such as a fluorophore, radioactive molecule, or biotin) that aided in the identification of the proteins to which the ligand was covalently cross-linked. More recently, alkyne containing ligands have been developed, allowing for downstream click-chemistry attachment13,14 and enrichment15,16 of the reporter tag. This has expanded the repertoire of reporter tags, and thus improved identification and purification of labeled proteins17. However, identifying the specific site(s) of interaction between an IMP and hydrophobic ligand remains challenging. In the current study we address the additional potential of clickable reporter tags to improve the identification of labeling sites in IMPs with amino acid level resolution.
Improvements in sample preparation and instrument sensitivity of tandem mass spectrometry have enabled residue level identification of some PAL sites6,18–21, but the routine identification of peptide-ligand adducts has been hindered by multiple challenges characteristic of hydrophobic ligands and peptides. These challenges include: 1) inability to collect an MS2 spectrum because of insource fragmentation or limited data dependent acquisition (DDA) of peptide-adducts with low MS1 intensity and; 2) inability to interpret the MS2 spectra due to significant collision cell adduct loss and/or limited peptide fragmentation. Decreased MS1 intensity of hydrophobic peptide-ligand adducts can result from poor aqueous solubility, poor ionization, poor chromatographic separation and the inefficient photolabeling inherent to many PAL reagents. Existing methods, such as enrichment tags, are useful for identification of ligand –binding proteins in complex proteomic samples, but they currently do not offer any advantage for residue-level MS analysis of hydrophobic peptide-ligand adducts from membrane proteins that are already purified or for samples in which spectral simplification is unnecessary. Indeed, there are limited examples of enrichment tags enabling identification of binding sites for hydrophobic ligands in integral membrane proteins22. To improve amino acid level site identification of ligand photolabeling within purified single proteins and protein complexes, we have developed a fast ligand identification (FLI) tag. This clickable tag incorporates 1) a stable heavy isotope, which enables identification of labeled peptides at the MS1 level, independent of DDA, adduct loss and database searches, and 2) increased charge and aqueous solubility, aiding in sample preparation, chromatography, and ionization.
EXPERIMENTAL
Synthesis of FLI-tag
FLI-tag was synthesized using standard solid phase peptide synthesis methods. A detailed description of synthesis is available in supplementary material.
Click chemistry addition of FLI-tag to MQ115
Synthesis of MQ115 is described in supplementary material. The copper(I) catalyzed azide/alkyne cycloaddition (click chemistry reaction) was performed as previously described14, with minor modifications. Briefly, 10µM MQ115 in 100µL 50% tertiary butanol/water was mixed with 2mM sodium ascorbate, 0.1mM TBTA, and 1mM CuSO4. Reaction was initiated with 0.5mM FLI-tag (1:1 ratio of light to heavy di-peptide) and turned for 12–16hrs at 22°C. The samples were dried via vacuum centrifugation, re-suspended in 90% ACN, 5%FA and diluted to 10%ACN, 5%FA. The click product was sequentially extracted with 2× C4 tips (Glygen, Colombia MD). Samples were eluted from tips with 90%ACN 1%FA, dried via vacuum centrifugation, and re-suspended in 10%ACN 1%FA for LC/MS/MS analysis on an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) utilizing the same parameters as the samples run on the Orbitrap Elite (Supplementary material).
Non-specific photolabeling of YGGFLRF and click addition of FLI-tag
The synthesis of KK152 has previously been described23. YGGFLRF (purchased from American peptides) was diluted to 0.1µg/µL in 800µL 60%ACN. The peptide was incubated with 10µM KK152 on ice for 1hr. Using a photoreactor, the mixture was irradiated in a quartz cuvette at >320nM UV-light for 5 min. Photolabeled peptides were stored at 4°C. An aliquot was clicked to FLI-tag and prepared for LC/MS/MS analysis as described in supplementary material.
VDAC expression and purification
Mouse voltage-dependent anion channel-1 (mVDAC1) was expressed and purified as previously described24. In short, his-tagged mVDAC1 was expressed in Escherichia coli. Inclusion bodies were isolated, solubilized and purified using a Talon affinity column. mVDAC1 was refolded using n-dodecyl-N,N-dimethylamine-N-oxide and applied to a Superdex 75 column for isolation of a homogenous protein population.
Reconstitution into bicelles and KK123 photolabeling of VDAC
mVDAC1 was mixed with a 35% solution of bicelles (2.8:1 molar ratio of DMPC:CHAPSO) in a 4:1 (v/v) ratio, and incubated on ice for 1hr. The sample was then diluted to 100µL with 1% bicelles in 1× PBS. KK123 (synthesis previously described23) was then added to the desired concentration and incubated on ice in the dark for 1 hr. The sample was then irradiated in a quartz cuvette with >320nm UV light for 5 min using a photoreactor as previously described25.
Click addition of FLI-tag to KK123 photolabeled VDAC for bottom-up mass spectrometric analysis
45µg VDAC (0.7µg analyzed per LC/MS/MS experiment) was reconstituted into bicelles and photolabeled with 10µM KK123. The sample was clicked to FLI-tag and sequentially digested with endoproteases LysC and Chymotrypsin. Digests were desalted on C18 stage tips and prepared for LC/MS/MS analysis on Orbitrap Elite. Details of digestion and sample preparation are described in supplementary material.
Gel-based Competition Assay
mVDAC1 was reconstituted into bicelles and photolabeled with 1µM KK123 in the absence or presence of 10µM or 30µM allopregnanolone. Samples were clicked overnight to TAMRA azide, run on a Trisglycine gel, and analyzed for fluorescence. Detailed methods are described in supplementary material.
Top-down mass spectrometry
120µg of mVDAC1 was reconstituted in bicelles and photolabeled as described above. The photolabeled sample was reduced with 250mM DTT, precipitated with chloroform/methanol/water as previously described with some modifications26, and analyzed by ESIMS. Detailed methods are described in supplementary material.
Pair search and reporter ion analysis
LC/MS/MS data was converted to .mzml via MSConvert. The OpenMS27 featurefindermultiplex tool was used with the parameters listed in supplementary material Table 2. The search results were processed in KNIME to exclude any pairs with the integral of intensity differences greater than 25%. A feature list was created in KNIME and exported to excel. The features with charge state >=2 were manually analyzed in Xcalibur for correct charge state assignment and expected isotope distribution. Additionally, the feature needed to be present in a minimum of 2 consecutive MS1 spectra to be included. Any pairs that did not meet these criteria were excluded. See supplementary tables 3–4 for a list of features identified by pair search but manually excluded, and a list of accepted features. The final feature list contained 23 pairs. A reporter ion search was conducted via an extracted ion chromatogram for 371.21–371.23 (expected m/z for z=1 light reporter ion), and 381.22–381.23 (expected m/z for z=1 heavy reporter ion). Retention times and features were compared between the two searches.
MS2 based software searches
VDAC LC/MS/MS data was searched against an in-house database containing the mVDAC1 sequence (UniProtKB-Q60932) modified with a His6 tag and filtered at a 1% false discovery rate using PEAKS 8.0 (Bioinformatics Solution Inc.). Met oxidation and Cys carbamidomethylation were included in each sample as variable modifications. Customized modifications were created for the add weight of KK123+FLI-tag (light, m/z=672.4322), KK123+FLI-tag (heavy, m/z= 682.4404) and KK123 without FLI-tag (m/z=372.2664).
RESULTS AND DISCUSSION
Synthesis of FLI-tag
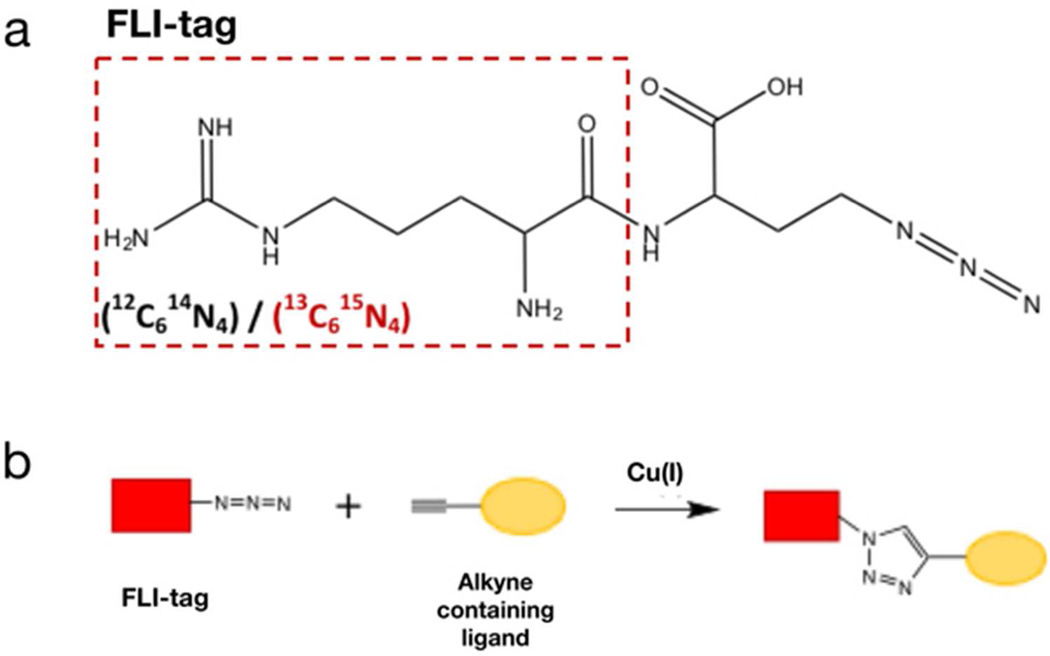
Standard peptide coupling reactions were used to synthesize a di-peptide tag with N-terminal arginine and C-terminal azidohomoalanine, an azide containing non-natural amino acid. Synthesis of the same tag with 13C6 15N4 arginine introduced a heavy isotope with a 10 Da shift (Figure 1a). Products were validated by LC/MS and had the expected mass (m/z=301.3, m/z=311.3) [data not shown]. The di-peptide tag, termed Fast Ligand Identification (FLI) tag, allows for identification of ligand adducts at the MS1 level, via its stable heavy isotope pairs.
Figure 1.
FLI-tag structure and click reaction. A) FLI-tag is a dipeptide with an N-terminal arginine (red box) and C-terminal azide. The dipeptide tag was synthesized with both light (12C6 14N4, black) and heavy (13C6 15N4, red) arginine. The C-terminal azide on FLI-tag allows for click-chemistry attachment to any alkyne containing ligand. B) A cartoon illustrating the Copper(I) catalyzed cyclo-addition (click chemistry) of the azide containing FLI-tag (red rectangle) to the alkyne containing ligand (yellow oval).
Click Chemistry addition of FLI-tag to neurosteroid
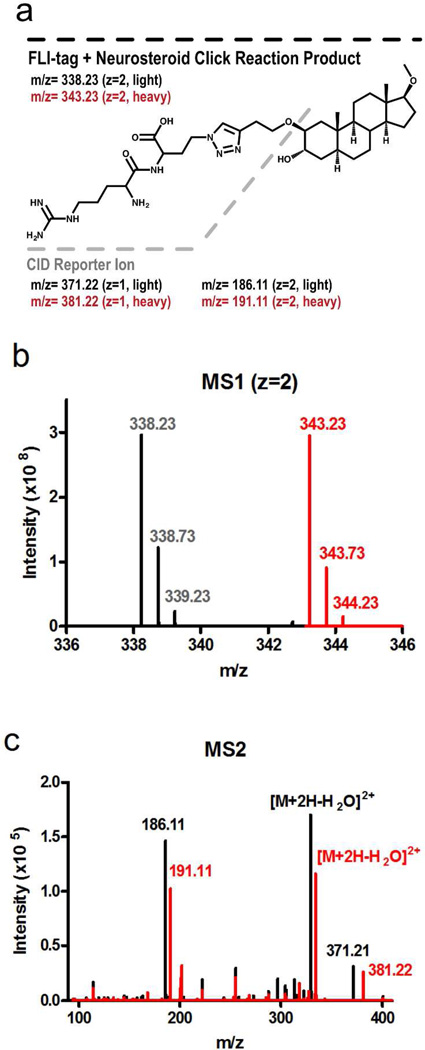
The C-terminal azide in FLI-tag allows for copper(I) catalyzed cycloaddition to any alkyne-containing ligand (Figure 1b). To demonstrate that FLI-tag conjugated to a hydrophobic ligand generated the predicted MS1 pair, FLI-tag was clicked to an alkyne containing pregnane derivative, (2β,3α,5α,17β)-2-(But-3-ynyloxy)-17-methoxy-androstan-3-ol (MQ115), to give the product shown in Figure 2a. MQ115 does not contain any titratable charges, and by itself is not ionized by positive mode electrospray ionization (ESI). Click addition of FLI-tag (in a 1:1 light to heavy dipeptide ratio) results in the MS1 isotope pair 338.2 and 343.2, with the expected 5 m/z mass shift for doubly charged ions (Figure 2b). The features contain the appropriate isotope distribution for doubly charged ions and are of approximately equal intensity, supporting the 1:1 light to heavy ratio for FLI-tag addition. Fragmentation by collision induced dissociation (CID) of 338.2 or 343.2 reveals doubly-charged features at 186.1 (light) or 191.1 (heavy) and singly-charged features at 371.2 (light) and 381.2 (heavy) corresponding to fragmentation at the butynyloxy-oxygen. These features have the potential to serve as MS2 reporter ions if observed in the CID spectra of most multiply-charged peptide-ligand-FLI-tag adducts. Loss of water as well as some additional fragmentation of the steroid were also observed in the CID spectra (Figure 2c). Click addition of FLI-tag to the alkyne containing neurosteroid resulted in the expected MS1 level pair and demonstrated possible MS2 reporter ions.
Figure 2.
Click chemistry addition of FLI-tag to a neurosteroid. A) An illustration of FLI-tag clicked to MQ115 with the expected m/z features for the light (338.23, z=2) and heavy (343.23, z=2) product. The dotted line indicates the site of CID fragmentation that results in the formation of a reporter ion. The expected m/z of the MS2 reporter ion features are listed: light z=1 (371.22), light z=2 (186.11), heavy z=1 (381.22), and heavy z=2 (191.11). B) MS1 (z=2) pair of the click reaction product. C) An overlay of light (black) and heavy (red) MS2 spectra corresponding to the MS1 features in figure 2b. Features where the heavy (red) MS2 is right shifted from the light (black) MS2 contain the arginine residue of FLI-tag. Features where the heavy and light MS2’s overlap do not contain the N-terminal arginine of FLI-tag.
Click Chemistry addition of FLI-tag to photolabeled peptide
To examine the utility of FLI-tag in the identification of peptide adducts, YGGFLRF was photolabeled with KK152, an enantiomeric 6-diazirine analogue of MQ11523 (Figure 3a) and coupled to FLI-tag. The expected MS1 features of peptide-steroid-FLI-tag adducts were identified for z=2, z=3 (Figure 3b, d) and z=4 features as isotope pairs with a mass shift of 10 Da and an m/z shift corresponding to charge state. The retention time of the peptide-adduct was 38 min without FLI-tag and 29 min with FLI-tag. This retention time shift is consistent with enhanced aqueous solubility following FLI-tag addition. CID-MS2 spectra for each charge state contain an abundant feature at m/z=859.4, corresponding to adduct loss. MS2s of the 3+ (Figure 3c) and 4+ charge states contain reporter ions as major features. MS2s for the 2+ charge state lack abundant reporter ions but give more fragmentation of the intact peptide-ligand adduct, including site-defining ions placing KK152 on the C-terminal phenylalanine (Figure 3e). These findings demonstrate the prominence of collision cell adduct loss for a peptide photolabeled with a steroid ligand. CID fragmentation of the photolabeled peptide without FLI-tag is similar, with major features representing adduct loss and site-defining ions as minor features (Figure S1). FLI-tag facilitates the identification of peptide adducts with an MS1 isotope pair and MS2 reporter ion, while enhancing peptide adduct solubility and charge state.
Figure 3.
Click addition of FLI-tag to a peptide-steroid adduct. A) Scheme illustrating non-specific photolabeling of peptide YGGFLRF with a diazirine and alkyne-containing neurosteroid (yellow oval). Irradiation of the sample resulted in a loss of N2 and covalent attachment of the ligand to YGGFLRF. FLI tag (red box) was attached via Cu(I) catalyzed click chemistry. B) MS1 (z=3) pair of the peptide-steroid-FLI-tag adduct. C) An overlay of light (black) and heavy (red) MS2 spectra for MS1 features in figure 3b. The most abundant features are loss of adduct, adduct, and reporter ions. A few (relatively low intensity) y ions were also identified. D) MS1 pair of the z=2 peptide-adduct. E) Overlay of light (black) and heavy (red) MS2 spectra for MS1 features in figure 3d. The most abundant features are adduct and loss of adduct. Site-defining y and b ions were also identified and had a relative increase in intensity compared to higher charge states. Reporter ions were minor features and are not labeled.
FLI-tag assisted identification of a neurosteroid labeling site in mVDAC-1
To determine the utility of FLI-tag in identifying hydrophobic ligand labeling sites on IMPs, we utilized VDAC, a protein that is efficiently labeled by neurosteroid analogue photolabeling reagents25, but for which the sites of neurosteroid incorporation have not been identified at the residue level. Mouse voltage-dependent anion channel-1 (mVDAC1) was purified from E. coli, reconstituted into bicelles, and photolabeled with KK123, a 6-diazirinyl analogue of MQ11523. To confirm that KK123 photolabels VDAC, 10µg of VDAC was labeled with 10µM KK123, clicked to TAMRA azide and analyzed by SDS-PAGE and fluorescent imaging. The gel shows a fluorescent band at ~35kDA when VDAC is photolabeled with KK123 and then clicked to TAMRA (Figure S2). To determine whether KK123 labeling of VDAC represents a binding site for endogenous neurosteroids, mVDAC1 was photolabeled with 1uM KK123 in the presence of increasing concentration of allopregnanolone. As shown in Figure S2, allopregnanolone causes a concentration dependent reduction in photolabeling, consistent with competitive inhibition.
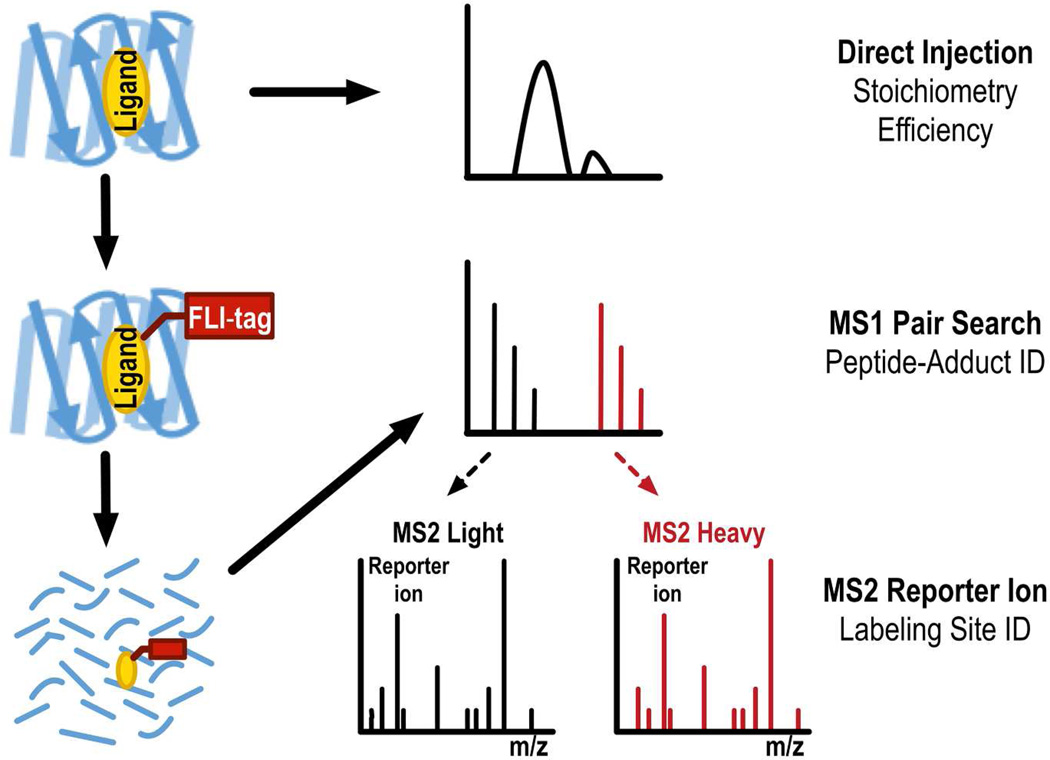
After confirming labeling at the protein level, we used peptide level (bottom-up) mass spectrometry in conjunction with FLI-tag to identify the sites of KK123 incorporation and protein level (top-down) mass spectrometry to determine the stoichiometry and efficiency of KK123 modification of VDAC (Figure 4). For bottom-up mass spectrometric analysis, mVDAC1 was photolabeled with 10µM KK123, clicked or not clicked to FLI-tag and sequentially digested using endoproteases LysC and Chymotrypsin. These enzymes were chosen to avoid potential digestion of FLI-tag’s arginine residue and optimize sequence coverage of IMPs28, key targets for future applications of FLI-tag.
Figure 4.
A flowchart depicting the utility of top-down and bottom-up mass spectrometry in identifying sites of ligand incorporation. The ligand (yellow oval) is covalently attached to the protein via photo-incorporation. Labeling stoichiometry and labeling efficiency are estimated by top-down mass spectrometry of the intact protein. Alternatively, photolabeled protein is clicked to FLI-tag (red rectangle), digested with endoproteases, and analyzed by bottom-up LC/MS/MS. An MS1 level pair search provides sensitive peptide-adduct identification. Directed acquisition and/or manual analysis of the corresponding MS2 spectra enables labeling site identification at the amino acid level. Reporter ions (m/z=371.2, m/z=381.2) in the MS2 spectra add specificity to the sensitive MS1 pair search.
An MS2-based search of the LC/MS/MS data using PEAKS software (version 8.0) demonstrated 95% sequence coverage of mVDAC1 with an FDR of <1% (Figure S3). Using the add-weights of KK123 and KK123+FLI-tag as variable modifications, no KK123 modified residues were detected by PEAKS in either the FLI-tag clicked or control samples. An MS1 pairs search of the FLI-tag sample identified 23 pairs clustered at retention times 24–28 min, 30–38 min, and 43–46 min. Each cluster represented multiple charge states and modifications of a single FLI-tag containing feature. Within the 24– 28 min and 30–38 min clusters, adduct oxidation led to slightly decreased retention times while loss of water increased retention times, consistent with each modification’s effect on hydrophobicity (Figure 5a). The pairs in the 43–46 min cluster likely represent free, photolyzed KK123 clicked to FLI-tag. Extracted ion chromatograms of the control sample (photolabeled but not clicked to FLI-tag) identified the same features but without FLI-tag, indicating that low peptide-adduct solubility was not responsible for failed identification of peptide adducts in the PEAKS search. Thus, using an automated MS1 pairs search, FLI-tag permitted the rapid identification of peptide adducts, which are otherwise not readily found using standard search methods. The 12 min decrease in retention time between control and FLI-tag samples demonstrates the added benefit of FLI-tag in increasing the aqueous solubility of peptide-adducts.
Figure 5.
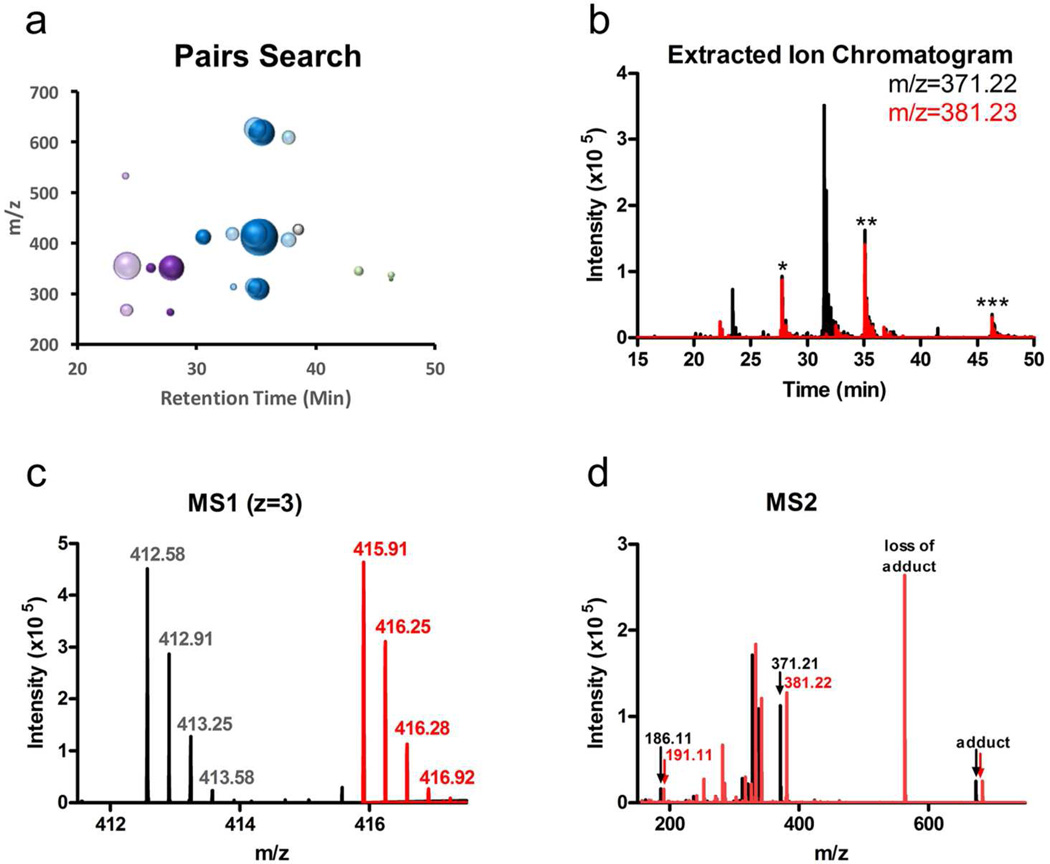
FLI-tag assisted identification of KK123 labeled peptides in mVDAC1. A) Peptides identified from the MS1 level pair search using the OpenMS FeatureFinderMultiplex tool. Purple circles represent peptide-adducts corresponding to TEK+KK123+FLI-tag, blue circles represent peptide-adducts corresponding to TEKW+KK123+FLI-tag, and green circles are consistent with freeKK123+FLI-tag. Pale circles represent peptide adducts with oxidation or water loss. The single grey circle represents a pair that was identified in the MS1 search, but did not have a corresponding MS2 spectrum. B) An extracted ion chromatogram (XIC) of z=1 reporter ion features (black=m/z=371.21 (light reporter ion), red= m/z=381.22 (heavy reporter ion). Peptide-adducts are identified at retention times where the XIC’s of light and heavy reporter ions overlap (*TEK+KK123+FLI-tag. **TEKW+KK123+FLI-tag. ***KK123+FLI-tag). C) Example of an MS1 pair (z=3) identified from pair search (and reporter ion XIC) at 35min. D) An overlay of light (black) and heavy (red) MS2 spectra corresponding to the MS1 features in figure 5c. The most abundant features are loss of adduct, adduct, and reporter ions. Site-defining ions were not confidently identified for the z=3 pair.
The MS2 spectra collected for both the FLI-tag and control samples were dominated by features of the intact peptide minus adduct, with minimal fragmentation of the peptide itself. The small number and intensity of peptide fragment ions explains why search algorithms did not identify the peptide-KK123 adducts. This finding emphasizes the importance of MS1 level identification of peptide-ligand adducts. The MS2 spectra of features identified in the MS1 level pair search also contained the same reporter ions observed in the model peptide experiments. Pairs of reporter ions in MS2 spectra were identified at the same three retention times as the MS1 pairs (Figure 5b) with multiple charge states for each peptide-KK123-FLI-tag species. Observation of the reporter ion strongly supports peptide-ligand adduct identification. While an MS1 pair search alone can be sufficient for identification of adducts, the coupling of MS1 pairs and MS2 reporter ions adds specificity to the sensitive MS1 level detection afforded by FLI-tag.
Analysis of the MS2 spectra revealed two unique neurosteroid-peptide adducts, TE*K and TEK*W. No other peptide adducts were identified using an isotope pair or reporter ion search, suggesting that KK123 modifies VDAC at a single site. High charge state precursors gave reporter ions (z>=3) (Figure 5c–d), while z=2 precursors gave site-defining ions that localized KK123 photolabeling to Glu73 (Figure 6a). However, these site-defining ions were low intensity due to collision cell adduct loss. We analyzed another set of samples using electron transfer dissociation (ETD), which significantly reduced adduct loss and increased the relative intensity of site-defining ions (Figure 6b). For the +FLI-tag sample, the improvement was significant enough for the PEAKS algorithm to place both heavy and light KK123+FLI-tag modifications on Glu73. In contrast, PEAKS did not confidently localize KK123 modification to Glu73 in the control sample (KK123 with no FLI-tag). Overall, the identification of this site was made possible by FLI-tag’s heavy isotope pair, enabling an MS1 level analysis, and FLI-tag’s added charge, improving ETD fragmentation.
Figure 6.
Identification of site defining ions in peptide TEKW placing KK123 on Glu73 in mVDAC1. A) Overlay of light (black) and heavy (red) CID-MS2 spectra corresponding to the z=2 pair (m/z= 618, m/z=623) identified at 35 min in the pair search and reporter ion XIC. The MS2 spectra of the z=2 features show substantial adduct loss, but also show low intensity site-defining ions placing KK123 on Glu73. B) Overlay of ETD-MS2 spectra. ETD fragmentation results in decreased adduct loss and increased relative intensity of site-defining ions compared to CID fragmentation.
Mass spectrometry of intact mVDAC1 photolabeled with KK123
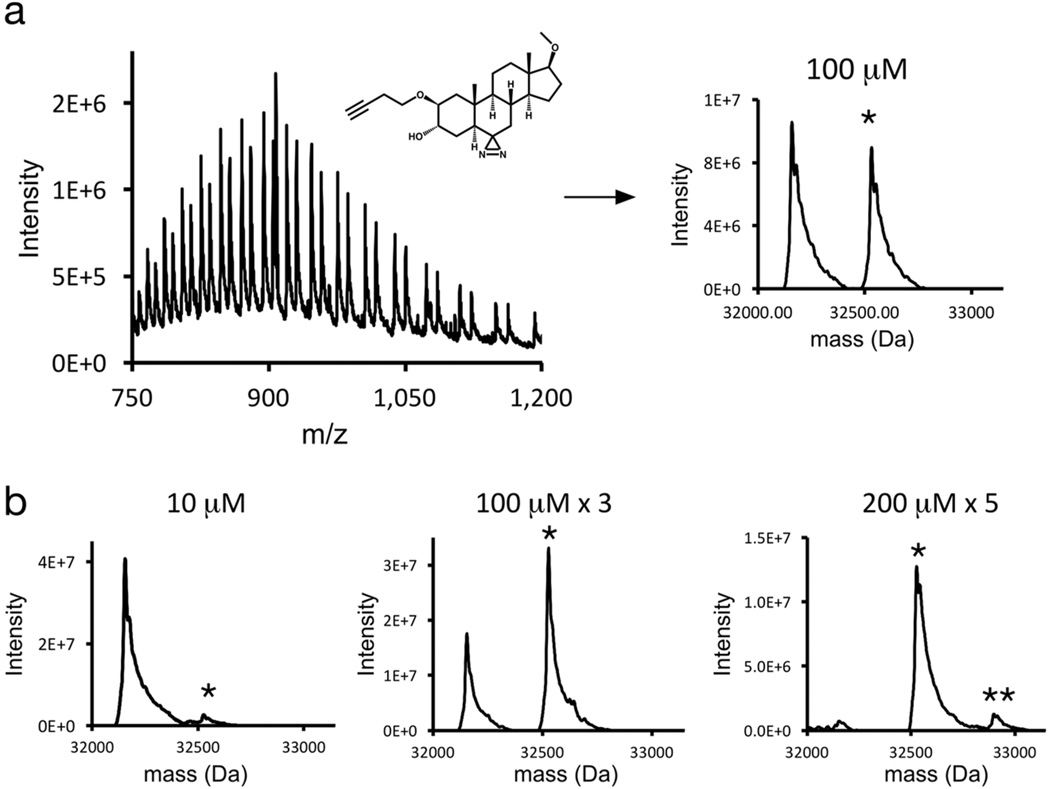
To confirm that bottom-up analysis identified all of the significant sites of KK123 incorporation, mass spectrometry of intact photolabeled mVDAC1 (top-down mass spectrometry) was performed to estimate the photolabeling efficiency and stoichiometry. Figure 7a shows a spectrum of intact mVDAC1 photolabeled with 100µM KK123. The deconvoluted spectrum demonstrates unlabeled mVDAC1 (average mass 32,154 Da) and singly labeled mVDAC1 (average mass 32,527 Da). Isotopic resolution was obtained on the Orbitrap analyzer, which yielded an unlabeled mVDAC1 monoisotopic mass of 32,134.14 Da (0.9 ppm mass accuracy) and a singly labeled mVDAC1 monoisotopic mass of 32,506.30 Da (11 ppm mass accuracy; Figure S4. At 10µM, KK123 photolabeled ~5% of VDAC protein, indicating low affinity binding of KK123 to VDAC (low occupancy), low photolabeling efficiency or both (Figure 7b). With more extensive photolabeling, a doubly labeled mVDAC1 species is not observed until nearly 100% of protein is photolabeled (Figure 7b). This pattern of photolabeling is suggestive of one predominant photolabeled site. Fragmentation of the photolabeled species using HCD also localizes the photolabeled site to the Glu73 region (Figure S5). These findings demonstrate the utility of top-down MS as a complementary approach to bottom-up MS for characterizing ligand-photolabeled IMPs29, and confirm that no significant peptide-ligand adducts were missed using FLI-tag.
Figure 7.
Top-down mass spectrometry of KK123-labeled mVDAC1. A) Spectrum of intact mVDAC1 photolabeled with 100µM KK123. Deconvolution of the full spectrum reveals two predominant species corresponding to mVDAC1 and mVDAC1 labeled with a single KK123. Asterisks indicate number of KK123 molecules labeling mVDAC1. B) Deconvoluted spectra of mVDAC1 photolabeled at the indicated concentrations and number of times.
The role of GLU73 in the structure-function relationship of mVDAC1
Glu73, may be a functionally important site of neurosteroid action (Figure S6). This residue is located in the transmembrane region of the β4 strand and is facing out into the lipid bilayer24. Interestingly, Glu73 is adjacent to K74, which disrupts the otherwise alternating pattern of hydrophilic and hydrophobic residues in the VDAC beta sheet, and is thought to destabilize the N-terminal region30. Glu73 has been hypothesized to play a role in voltage-dependent or ruthenium red-dependent gating, hexokinase-I (HK-I) induced channelclosure, and HK-1 mediated inhibition of apoptosis31. Furthermore, mutations of Glu73 alter the local membrane thickness30 suggesting that this residue may play an important role in mediating putative effects of steroids and other lipids on VDAC structure and function. Neurosteroids have neuroprotective effects32, and neurosteroid modulation of VDAC may contribute to this action25. KK123 labeling of Glu73 suggests that neurosteroids bind to VDAC at a different site than cholesterol or propofol, which have been shown to photolabel and/or bind elsewhere in the protein9,20. One caveat to our results is that aliphatic diazirines can show preference for labeling nucleophilic amino acids2. As such, it is possible that neurosteroid photolabeling of Glu73 results from photochemically preferential labeling of an acidic amino acid located at the lipid-protein interface. Since the neurosteroid allopregnanolone inhibits KK123 photolabeling of Glu73, we favor the interpretation that neurosteroids bind to VDAC at this site. This raises the interesting possibility that neurosteroids may play a role in VDAC function and mitochondrial physiology.
CONCLUSION
Mass spectrometry coupled with PAL is a powerful tool for the identification of protein-ligand interactions, but it has been difficult to obtain amino acid level resolution of ligand binding sites in IMPs33. This is due, at least in part, to difficulty in obtaining interpretable fragmentation spectra (MS2) of peptide-ligand adducts. The low efficiency of most photolabeling reagents, coupled with the poor aqueous solubility of hydrophobic ligand/hydrophobic peptide adducts can result in low intensity MS1 features of peptide-ligand adducts. Since only the most abundant features are isolated and fragmented using data-dependent acquisition (DDA), MS2 spectra of the peptide-ligand adducts may not be obtained. Current automated search methods cannot detect these adducts in the absence of MS2 spectra. Additionally, even if MS2 spectra are obtained, the most abundant CID features may be the result of adduct loss rather than peptide bond fragmentation. Without sufficient peptide bond fragmentation MS2-based software searches are unable to identify the peptide-ligand adducts and determine the residues at which photo-incorporation occurs. These challenges of low peptide-adduct abundance and adduct loss necessitate methods for MS1 level identification.
We have taken advantage of the mass shift of stable heavy isotope labels (SHLs) to develop a method for MS1 level identification of peptide-ligand adducts. SHLs have previously been used for adduct verification21, quantitative comparisons34–36, enrichment of labeled proteins37,38, and detection of small molecules39 and chemically crosslinked peptides40,41. With the exception of small molecule identification, each of these applications rely on MS2-based software searches, limiting analysis to features with interpretable MS2 spectra. Additionally, they have not addressed challenges specific to hydrophobic ligands, such as low charge state and poor aqueous solubility. In a few instances, attempts to improve identification of labeled sites have been made in which the ligands were synthesized in both an isotopically light and heavy form, followed by MS2-based analysis or manual interpretation of the LC/MS1 spectra for isotope pairs42–44. While this approach improves identification, it requires synthesis of a new label for every ligand. By incorporating the isotope labels in an azide-containing tag, FLI-tag enables MS1 level identification of any alkyne-containing ligand via downstream click chemistry attachment. Additionally, the use of OpenMS FeatureFinder-Multiplex tool to search for isotope pairs, with subsequent parsing of the pairs by intensity ratio, enables automated peptide-ligand adduct identification at the MS1 level. The masses and retention times of the peptide-ligand adducts can inform follow up experiments using directed MS2 acquisition. Finally, hydrophobic peptides from IMPs are often low charge state (1+ or 2+), and FLI-tag increases the charge state of these species, which improves fragmentation by ETD45,46. While this study focuses on sites of neurosteroid photo-incorporation in VDAC, FLI-tag can be used to identify sites of covalent incorporation for any alkyne-containing ligand.
In summary, FLI-tag facilitates the identification of hydrophobic peptide-adducts by increasing aqueous solubility and enabling MS1 level identification. Identification of isotope pairs allows for directed manual interpretation of MS2 spectra or targeted acquisition of MS2 spectra. In addition, the increased charge state of peptide-adducts with FLI-tag may facilitate alternative fragmentation methods that reduce collision-induced adduct loss such as ETD and ECD47. The compatibility of FLI-tag with any alkyne-containing ligand makes it a broadly applicable tool for the identification of hydrophobic ligand binding sites in IMPs.
Supplementary Material
Acknowledgments
We would like to thank B. Manion for his help with initial VDAC labeling experiments, J. Whitelegge for the many helpful discussions concerning top-down mass spectrometry, L. Niles and O. Kohlbacher for their help with OpenMS and KNIME. Funding for this work is supported by the National Institutes of Health (R01GM108799 to A.S.E and D.F.C.) and the Taylor Family Institute for Innovative Psychiatric Research (funding to A.S.E. and D.F.C.) This work was also supported by the NSF-Graduate Research Fellowship DGE-1143954 (to M.M.B) and NIH T32-GM108539 (to W.W.C).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Figure S1, MS2 spectrum of YGGFLRF+KK123; Figure S2, KK123 labeling of mVDAC1 with and without excess allopregnanolone; Figure S3, Outline of PEAKS (MS2 database search) sequence coverage. Figure S4, High resolution spectra of intact VDAC; Figure S5, Amino acid sequence of VDAC showing b and y fragment ions identified from HCD spectra of VDAC photo-labeled with KK123; Figure S6, Previously published mVDAC1 crystal structure (PDB 3EMN)24 showing position of Glu73 in membrane; Table S1, Fragment ion identifications for HCD of VDAC photo-labeled with KK123 based on the VDAC sequence with KK123 labeling of E73; Table S2, Parameters used with the OpenMS FeatureFinderMultiplex tool; Table S3, MS1 pair search ID’s that were rejected by manual interpretation; Table S4, Accepted features identified in MS1 pair search; Detailed methods on: FLI-tag synthesis, Non-specific photo-labeling of YGGFLRF and click addition of FLI-tag, Click addition of FLI-tag to KK123 photolabeled VDAC for bottom-up mass spectrometric analysis, Gel based competition assay with allopregnanolone, Liquid chromatography and high resolution tandem mass spectrometry, Top down mass spectrometry, Synthesis of MQ115, and supporting references. (PDF)
Author Contributions
The manuscript was written through contributions of all authors. MMB contributed to the conception, design, acquisition and analysis of data, synthesis of FLI-tag, and writing of manuscript. WWC contributed to the design, acquisition and analysis of data, and writing of manuscript. ASE contributed to the conception, design and writing of manuscript. LB and JA purified mVDAC1. KK, MQ, and DFC synthesized the photoaffinity ligands used in the manuscript (KK123, MQ115). JWJ contributed to the synthesis and purification of FLI-tag. ZC contributed to the analysis and interpretation of data. All authors reviewed and approved the content of the final manuscript.
REFERENCES
- 1.Conn PJ, Lindsley CW, Meiler J, Niswender CM. Nat Rev Drug Discov. 2014;13:692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das J. Chem Rev. 2011;111:4405–4417. doi: 10.1021/cr1002722. [DOI] [PubMed] [Google Scholar]
- 3.Hamouda AK, Jayakar SS, Chiara DC, Cohen JB. J Mol Neurosci. 2014;53:480–486. doi: 10.1007/s12031-013-0150-1. [DOI] [PubMed] [Google Scholar]
- 4.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Admas TH, Bernat V, Heinrich MR, Tschammer N. Chem Med Chem. 2016;11:575–584. doi: 10.1002/cmdc.201500573. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Mol Pharmacol. 2012;82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 8.Laurent B, Murail S, Shahsavar A, Sauguet L, Delarue M, Baaden M. Structure. 2016;24:595–605. doi: 10.1016/j.str.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory KJ, Velagaleti R, Thal DM, Brady RM, Christopoulos A, Conn PJ, Lapinsky DJ. ACS Chem Biol. 2016;11:1870–1879. doi: 10.1021/acschembio.6b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qvit N, Monderer-Rothkoff G, Ido A, Shalev DE, Amster-Choder O, Gilon C. Biopolymers. 2008;90:526–536. doi: 10.1002/bip.21010. [DOI] [PubMed] [Google Scholar]
- 12.Brunner J. Annu Rev Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 13.van Swieten PF, Leeuwenburgh MA, Kessler BM, Overkleeft HS. Org Biomol Chem. 2005;3:20–27. doi: 10.1039/b412558d. [DOI] [PubMed] [Google Scholar]
- 14.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Ravindran MS, Wenk MR. Methods Mol Biol. 2017;1491:75–85. doi: 10.1007/978-1-4939-6439-0_6. [DOI] [PubMed] [Google Scholar]
- 16.Gubbens J, de Kroon AI. Mol Biosyst. 2010;6:1751–1759. doi: 10.1039/c003064n. [DOI] [PubMed] [Google Scholar]
- 17.Lapinsky DJ. Bioorg Med Chem. 2012;20:6237–6247. doi: 10.1016/j.bmc.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Weiser BP, Eckenhoff RG. J Biol Chem. 2015;290:8559–8568. doi: 10.1074/jbc.M114.620732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW. J Biol Chem. 2004;279:37964–37972. doi: 10.1074/jbc.M405137200. [DOI] [PubMed] [Google Scholar]
- 20.Weiser BP, Bu W, Wong D, Eckenhoff RG. FEBS Lett. 2014;588:4398–4403. doi: 10.1016/j.febslet.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, Townsend RR, Fuchs K, Sieghart W, Evers AS, Franks NP. Nat Chem Biol. 2013;9:715–720. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windsor K, Genaro-Mattos TC, Miyamoto S, Stec DF, Kim HY, Tallman KA, Porter NA. Chem Res Toxicol. 2014;27:1757–1768. doi: 10.1021/tx500229h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Shu HJ, Krishnan K, Qian M, Taylor AA, Covey DF, Zorumski CF, Mennerick S. Neuropharmacology. 2016;108:193–206. doi: 10.1016/j.neuropharm.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. Proc Natl Acad Sci U S A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darbandi-Tonkabon R, Manion BD, Hastings WR, Craigen WJ, Akk G, Bracamontes JR, He Y, Sheiko TV, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. J Pharmacol Exp Ther. 2004;308:502–511. doi: 10.1124/jpet.103.058123. [DOI] [PubMed] [Google Scholar]
- 26.Wessel D, Flugge UI. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 27.Rost HL, Sachsenberg T, Aiche S, Bielow C, Weisser H, Aicheler F, Andreotti S, Ehrlich HC, Gutenbrunner P, Kenar E, Liang X, Nahnsen S, Nilse L, Pfeuffer J, Rosenberger G, Rurik M, Schmitt U, Veit J, Walzer M, Wojnar D, Wolski WE, Schilling O, Choudhary JS, Malmstrom L, Aebersold R, Reinert K, Kohlbacher O. Nat Methods. 2016;13:741–748. doi: 10.1038/nmeth.3959. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZW, Fuchs K, Sieghart W, Townsend RR, Evers AS. Mol Cell Proteomics. 2012;11:M111 011445. doi: 10.1074/mcp.M111.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan CM, Souda P, Bassilian S, Ujwal R, Zhang J, Abramson J, Ping P, Durazo A, Bowie JU, Hasan SS, Baniulis D, Cramer WA, Faull KF, Whitelegge JP. Mol Cell Proteomics. 2010;9:791–803. doi: 10.1074/mcp.M900516-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villinger S, Briones R, Giller K, Zachariae U, Lange A, de Groot BL, Griesinger C, Becker S, Zweckstetter M. Proc Natl Acad Sci U S A. 2010;107:22546–22551. doi: 10.1073/pnas.1012310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- 32.Frank C, Sagratella S. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:1117–1126. doi: 10.1016/s0278-5846(00)00124-x. [DOI] [PubMed] [Google Scholar]
- 33.Robinette D, Neamati N, Tomer KB, Borchers CH. Expert Rev Proteomics. 2006;3:399–408. doi: 10.1586/14789450.3.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Tallman KA, Porter NA, Liebler DC. Anal Chem. 2015;87:2535–2541. doi: 10.1021/ac504685y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chahrour O, Cobice D, Malone J. J Pharm Biomed Anal. 2015;113:2–20. doi: 10.1016/j.jpba.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Santamarina S, Boronat S, Domenech A, Ayte J, Molina H, Hidalgo E. Nat Protoc. 2014;9:1131–1145. doi: 10.1038/nprot.2014.065. [DOI] [PubMed] [Google Scholar]
- 37.Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MB, Bachovchin DA, Mowen K, Baker D, Cravatt BF. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian Y, Martell J, Pace NJ, Ballard TE, Johnson DS, Weerapana E. Chembiochem. 2013;14:1410–1414. doi: 10.1002/cbic.201300396. [DOI] [PubMed] [Google Scholar]
- 39.Chu JM, Qi CB, Huang YQ, Jiang HP, Hao YH, Yuan BF, Feng YQ. Anal Chem. 2015;87:7364–7372. doi: 10.1021/acs.analchem.5b01614. [DOI] [PubMed] [Google Scholar]
- 40.Collins CJ, Schilling B, Young M, Dollinger G, Guy RK. Bioorg Med Chem Lett. 2003;13:4023–4026. doi: 10.1016/j.bmcl.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 41.Goshe MB, Smith RD. Curr Opin Biotechnol. 2003;14:101–109. doi: 10.1016/s0958-1669(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 42.Pinkse MW, Rijkers DT, Dostmann WR, Heck AJ. J Biol Chem. 2009;284:16354–16368. doi: 10.1074/jbc.M808521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sachon E, Tasseau O, Lavielle S, Sagan S, Bolbach G. Anal Chem. 2003;75:6536–6543. doi: 10.1021/ac034512i. [DOI] [PubMed] [Google Scholar]
- 44.Tomohiro T, Morimoto S, Shima T, Chiba J, Hatanaka Y. Angew Chem Int Ed Engl. 2014;53:13502–13505. doi: 10.1002/anie.201408580. [DOI] [PubMed] [Google Scholar]
- 45.Mikesh LM, Ueberheide B, Chi A, Coon JJ, Syka JE, Shabanowitz J, Hunt DF. Biochim Biophys Acta. 2006;1764:1811–1822. doi: 10.1016/j.bbapap.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elviri L. In Tandem Mass Spectrometry - Applications and Principles. 2012:161–178. [Google Scholar]
- 47.Frey BL, Ladror DT, Sondalle SB, Krusemark CJ, Jue AL, Coon JJ, Smith LM. J Am Soc Mass Spectrom. 2013;24:1710–1721. doi: 10.1007/s13361-013-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.