SUMMARY
The ability of nanoparticle surface functionalities to regulate immune responses during an immunological challenge (i. e. inflammation) would open new doors for their use in non-prophylactic therapeutics. We report here the use of functionalized 2 nm core gold nanoparticles to control the immunological responses of in vitro and in vivo systems presented with an inflammatory challenge. The results showed that NPs bearing a hydrophobic zwitterionic functionality boost inflammatory outcomes while hydrophilic zwitterionic NPs generate minimal immunological responses. Surprisingly, tetra(ethylene glycol) headgroups generate a significant anti-inflammatory response both in vitro and in vivo. These results demonstrate the ability of simple surface ligands to provide immunomodulatory properties, making them promising leads for the therapeutic usage of nanomaterials in diseases involving inflammation.
Keywords: Inflammation, prophylactic, gold nanoparticles, zwitterions
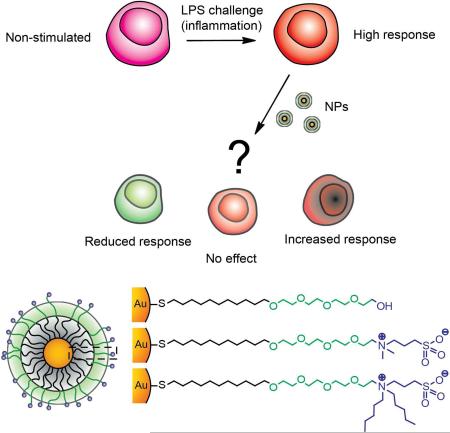
Graphical abstract
INTRODUCTION
The ability to control immune responses by tailoring the nanoparticle (NP) surface chemical identity has opened new avenues for therapeutics.1-4New vaccine formulations have been developed by the use of specific antigens at the NP surface,5 or by inducing NP recognition for adjuvancy.6 These applications rely on the capability of enhancing immune responses (adjuvancy) by either delivering the antigen of interest or by enhancing antigen presentation. 7 By the use of this prophylactic approach, vaccines against cancer, 8 hepatitis B, 9 and other diseases have been developed. 10 However, much less understood is the behavior of nanomaterials in challenged systems, for example when an inflammatory event is already present (remedial approach, Figure 1a). Controlling the immunological profile of inflammatory responses in these activated systems is important for the treatment of diseases characterized by excess inflammation,11 such as arthritis,12 atherosclerosis,13 and fibromyalgia.14
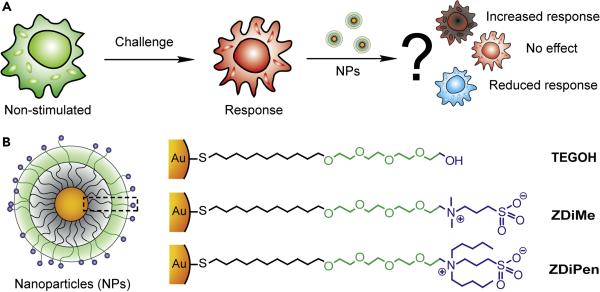
Figure 1.
(a) Cartoon depicting the non-prophylactic therapeutic approach used to study the inflammatory challenge. For our non-prophylactic studies, the challenge is comprised of a stimulation with LPS, which induces a strong inflammatory response. (b) Chemical structure of the NPs (2 nm diameter gold core) bearing different chemical groups while maintaining a net neutral charge.
Nanoparticle surface chemistry plays a central role on the recognition and the type of response that is being triggered by the immune system towards these materials.1-5 For example, the presence of surface charge greatly increases the adsorption of opsonins, forming a protein corona with concomitant recognition by the mononuclear phagocytic system. 15 This phenomenon can be overcome by the use of “stealth” functionalities (such as poly(ethylene glycol) and zwitterions) that inhibit the non-specific adsorption of proteins.16 Interestingly, these functionalities trigger other types of immune responses on their own, such as the activation of the complement system.17,18 Similarly, by decorating the NP surface with hydrophobic moieties, specific types of innate immune responses can be triggered.19,20 Despite these observations of relationships between NP surface chemistry and immune responses in unchallenged conditions, it is not clear how or which chemical functionalities can be employed to control the immunological profile of challenged systems.21
We recently developed a series of uncharged NPs having specific surface functionality with variable hydrophobicity while avoiding the formation of a protein corona. These particles with zwitterionic and non-ionic tetra(ethylene glycol) (TEG) ligands were engineered to directly study relationships between biological activities and NP surface chemistry.22 Here, we report the use of these uncharged NPs to elucidate immunological responses when an inflammatory challenge is presented. Using both in vitro and vivo studies with murine systems, we observed reduction of inflammatory responses for neutral TEG particles, dramatically increased inflammation for NPs bearing a hydrophobic zwitterionic functionality, and an unaltered response for hydrophilic zwitterionic particles. Our findings indicate that these particles are promising leads for their use in adjuvant therapy (hydrophobic NPs) and as anti-inflammatory agents (neutral NPs).
RESULTS AND DISCUSSION
Three different types of surface chemical functionalities were selected for our immunomodulation studies (Figure 1b), with a 2 nm Au core used as the scaffold for their presentation.23 TEGOH is structurally based on poly(ethylene glycol) coatings that are commonly used in nanomaterials, a functionality that can be recognized by different components of the immune system in vivo such as the complement system.16 Likewise, ZDiPen was developed with the premise that hydrophobic NPs are capable of triggering various types of innate immune responses, and have been used previously in the development of vaccines.24 This NP was selected as the most hydrophobic particle from the zwitterionic family that maintained solution stability. Finally, hydrophilic structures (like ZDiMe) have been shown to maintain a stable pro-/anti-inflammatory balance.20 We engineered all of these NPs to bear a neutral charge that dramatically decreases non-specific adsorption of proteins, thus reducing interference and allowing the study of immune responses intrinsic to the chemical functionalities (Figure S1). Likewise, to ensure the minimal interference originated by endotoxins, we performed a Limulus amebocyte lysate (LAL) gel cloth test to verify that all the NPs were free of endotoxins at the conditions of the study (below 0.03 EU/mL - 100nM NP concentration).
We generated inflammation in our in vitro and in vivo studies using lipopolysaccharide (LPS), a bacterial agent that triggers strong inflammatory responses.25 As the readout we measured the secretion of TNFα, a cytokine characteristic of pro-inflammatory cellular profiles.26 TNFα is the major target for many inflammatory diseases, and reduction of TNFα secretion is a well-established clinical treatment for inflammatory diseases.27
Our initial in vitro studies used the J774.2 murine monocyte cell line that is highly sensitive to LPS stimulation.28 These monocytes are professional phagocytes, a type of cell that interacts strongly with nanomaterials.29 Nanoparticle ZDiMe did not affect the secretion of TNFα relative to the control (Figure 2a), suggesting that NPs coated with hydrophilic zwitterionic functionalities have minimal interaction with the cells, an observation that is in agreement with the low cellular uptake of these NPs by the J774.2 cells (Figure 2b) and consistent with prior studies showing the long circulatory lifetime particles with this functionality.30
Figure 2.
(a) TNFα secretion of J774.2 cells in the presence of the NPs, with and without LPS stimulation after 3h incubation. Values normalized against the positive control (Cell + LPS). (b) Cellular uptake of the different NPs for both LPS challenged and unchallenged conditions. (c) ROS generation of J774.2 cells under the same experimental conditions (LPS challenge, 24 h). Values normalized against the normal cell response. (d) Cell viability of J774.2 after 24 h incubation with the different NPs and LPS measured by trypan blue. (e) MTS assay indicating the metabolism (vitality) of cells after 24 h exposure with NPs and LPS. Values normalized against the responses of untreated cells. (*) indicates statistically significant difference and (n.s.s.) indicates lack of statistically significant difference relative to control (Cells alone in gray or Cell+LPS in red).
In contrast to hydrophilic zwitterionic particle ZDiMe, both TEGOH and ZDiPen decreased the secretion of TNFα (Figure 2a), indicating a reduction of the inflammatory response to LPS. The cellular uptake of these two NPs was higher than ZDiMe, in agreement with previous reports depicting the greater cellular uptake of hydrophobic NPs, possibly due to a stronger interaction with the hydrophobic portions of the cell membrane (Figure 2b). However, TEG NPs were not taken up as readily as ZDiPen indicating that the anti-inflammatory effect depends on both cellular uptake and on responses caused by the functionality per se. We wanted to verify if these observations were valid in fully differentiated macrophages, a more specific type of cells that may not be sensitive to these molecular patterns. As such, studies with all of the above particles were repeated using RAW 264.7 cells. As can be observed in Figure S2, similar results were obtained for these cells, indicating that this anti-inflammatory response is generalizable among different types of macrophages.
The secretion of reactive oxygen species (ROS) is an important aspect of inflammatory responses that is directly related to the activation of immune cells.31,32 We measured endogenous ROS generation by J774.2 cells after exposure to NPs and LPS, by the use of 2’,7’-dichlorodihydrofluorescein diacetate, a pro-fluorophore that is activated by ROS.33 As shown in Figure 2c, both ZDiPen and TEG reduced the response of the cells significantly comparative to the baseline (cells alone). These results are consistent with our TNFα findings, and indicate a decrease in the activation of the cells. We quantified live/dead cells after 24 h of incubation using trypan blue to determine if the change in these responses was indeed a reduction of activity and not simply a decrease in the viability of cells. As observed in Figure 2d (and Figure S2e-f), J774.2 cells were viable for all the NPs under the conditions of the study, with live/dead assays comparable to the controls, indicating no toxic effects of the particles. We further analyzed if the viability of the cells was altered by the presence of the NPs by the use of (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H tetrazolium) (MTS). Interestingly, while ZDiMe and TEG had minimal or no effect on the metabolism of the cells, ZDiPen decreased significantly the metabolic activity as shown in Figure 2e. From these studies, we can surmise that the decrease in immune response observed upon treatment with ZDiPen arises from decrease in cell metabolism. The origin of the anti-inflammatory properties of TEG are more difficult to explain. There are two possible identified mechanisms for immunomodulation by TEG. The first is that TEG acts an antagonist for LPS, decreasing the response of the macrophage by blocking receptors. The second mechanism is that TEG binds to the LPS, blocking interactions of the LPS with the macrophage. Both options are viable in vitro, however the latter mechanism would be unlikely in vivo (vide infra), where substantial anti-inflammatory activity is observed with TEG.
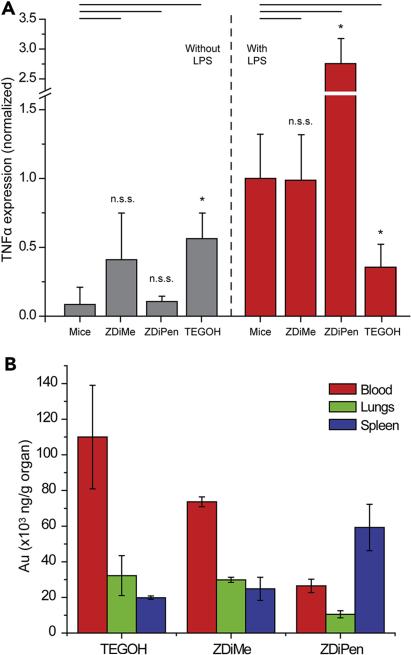
Based on the promising in vitro activity of the NPs, we next examined the in vivo relevance of these results by using LPS-challenged mice. In particular, we wanted to observe if TEGOH and ZDiPen maintained their capability to reduce inflammation, and determine if ZDiMe was not immunomodulatory. We first established a baseline of the response by measuring TNFα blood levels (overall secretion) when C57BL/6 mice were challenged by intraperitoneal (IP) injection with different concentrations of LPS. Mice were sacrificed after 2 h of the treatment and blood was extracted and processed as described in the ESI. A concentration of LPS of 200 ng/mice was chosen since this concentration was the lowest that induced a significant and robust TNFα readout (Figure S5a). We then performed IP injections of LPS, followed by injection with TEGOH, ZDiMe and ZDiPen (injection scheme in Figure S3b). In addition to TNFα levels, we also measured the concentration of gold in the different organs by inductively-coupled plasma mass spectrometry (ICP-MS), to observe how different functionalities affect the biodistribution of these NPs. As shown in Figure 3a, no significant change in the inflammatory response was observed with ZDiMe in either unchallenged or challenged mice, mirroring the results in vitro. Based on these results, it can be concluded that hydrophilic zwitterionic structures do not perturb the immune system and do not trigger a significant response. This result, coupled with the fact that these structures have been shown to possess extended blood circulation times,30 indicates the utility of hydrophilic zwitterions for the generation of non-immunogenic delivery vehicles.17
Figure 3.
(a) In vivo TNFα secretion 2 h after ZDiMe, ZDiPen and TEGOH (2.75 mg/kg) were injected in mice with and without the presence of LPS (0.01 mg/kg). (b) Nanoparticle distribution in the blood, lung and spleen of mice evidencing the fast elimination of ZDiPen comparative to the other two NPs. Accumulation in other organs shown in Figure S3c.
The results obtained for ZDiPen contrast strongly with those of ZDiMe, showing a strong pro-inflammatory response (Figure 3a). This outcome is particularly intriguing since this hydrophobic NP responded very differently in vitro, where an anti-inflammatory response was observed (Figure 2a). These in vivo results suggest the involvement of other components of the immune system in the mechanism that orchestrates the NP-induced inflammatory responses, such as the complement system, a part of the immune system hypothesized to be triggered by hydrophobic moieties.34 However, it is important to observe that the biodistribution profile was very distinct than that for ZDiMe (Figure 3b). Despite the fact that both NPs have the same surface charge and size, the presence of a hydrophobic headgroup strongly decreased the concentration of NPs in the blood while increasing levels in the spleen. This result coupled with the strong interaction with cells observed in the cellular uptake studies suggest that that the change in the in vitro/in vivo behavior possibly arises from differences in the interaction with other components of the immune system, such as the complement system or non-specific hydrophobic-driven interactions with other cells. The highly strong synergistic response obtained by the combination of LPS and ZDiPen, a level that cannot be achieved by either LPS or ZDiPen alone, indicates the potential utility of these functionalities for adjuvant therapies that would not require covalent conjugation between the antigen and the NP.35
Perhaps the most surprising result of the in vivo studies was the anti-inflammatory effect of TEGOH, which decreased the secretion of TNFα in vivo (Figure 3a) relative to Cells+LPS, a result that mimics our findings in vitro. Interestingly, while TEGOH triggered an inflammatory response in unchallenged systems on its own (an effect that has been observed in the past for similar NPs36), these levels are maintained in the presence of LPS suggesting a “buffering” effect of the stimuli. Likewise, the biodistribution of TEG was similar to that of the hydrophilic ZDiMe, indicating that this particle is not eliminated as quickly as the hydrophobic ZDiPen. This result suggests that the immunological response does not necessarily correlate with biodistribution, in the same way that cellular uptake does not correlate with TNF secretion in vitro. Recent studies have reported the generation of antibodies against PEG-like structures when NPs are injected in the body, indicating the interaction of this type of functionalities with components of the immune system involved in adaptive immunity (i.e. immune cells such as macrophages), a process that is related to the extended of the PEG chain and the size of the particle. Since in our case the immunological response was observed both in vitro and in vivo, however, a cell-mediated mechanism is probably directing the response. This mechanism is consistent with the potential role of TEG as an antagonist for LPS binding and activation, a possibility that is under exploration. While binding of LPS to TEG and subsequent interaction cannot be ruled out, the separate injections of these two agents makes this possibility less likely. The fact that there is a decrease in inflammatory response indicates the potential of these NPs for use in anti-inflammatory therapies, a property that was not observed in the previous studies performed in the absence of an inflammatory stimulus.35
CONCLUSION
We have demonstrated that surface chemistry of nanoparticles can be used to generate pro- and anti-inflammatory responses in cells and animals challenged with LPS. These studies provide important clues for the therapeutic use of nanomaterials in diseases involving inflammation, as well as input for the design of non-immunogenic materials. Hydrophilic zwitterionic motifs provide minimally immunogenic coverages suitable for delivery applications. In contrast, hydrophobic zwitterionic particles are strongly pro-inflammatory in LPS-challenged systems, suggesting their utility in adjuvant applications. Finally, PEG-like structures have anti-inflammatory properties that emerge with challenged systems, providing a potential role for these motifs in combatting inflammation in diseases such as rheumatoid arthritis and atheroschlerosis. Taken together, these findings demonstrate the ability of chemically “simple” functionality to provide lead structures for the use of NPs in non-prophylactic applications, an important step towards the development of a new generation of immunotherapies.
Supplementary Material
Bigger Picture statement.
Nanoparticle-based immunotherapies have to date been aimed towards the development of prophylactic therapeutics such as vaccines. However, nanomaterials have the potential to reduce or enhance immunological challenges already present in the body. Here, we use engineered nanoparticles to demonstrate how pre-existing inflammatory effects can be reduced or boosted by the chemistry of the nanoparticles surface. These findings open new avenues for non-prophylactic applications of NPs, a critical step towards the development of a new generation of immunotherapies.
Highlights.
□ Functionalized AuNPs modulate the immune response under the LPS challenge.
□ ZDiPen AuNPs boost inflammatory response.
□ ZDiMe AuNPs generate minimal immunological response.
□ TEGOH AuNPs generate a significant anti-inflammatory response.
Acknowledgements
This work was supported by the grants from the NIH (GM077173) and the Center for Hierarchical Manufacturing (CMMI-1025020), and NIH CA166009 and the NMSS grant is RG5151.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Conceptualization: V. M. R., B. A. O., and D. F. M.; Investigation: Y. L., D. F. M., and F. A.; Recourses: S. H., P. P., and B. D.; Writing-Original Draft: D. F. M. and Y.L.; Writing-Review & Editing B. A. O., and V. M. R.; Supervision: B. A. O., and V. M. R.
Conflicts of Interest: The authors declare no competing financial interests.
Supporting Information Available: Nanoparticle synthesis and characterization, detailed experimental methods, and additional figures.
REFERENCES AND NOTES
- 1.Moon JJ, Huang B, Irvine DJ. Engineering nano-and microparticles to Tune Immunity. Adv. Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsabahy M, Wooley KL. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013;42:5552–5576. doi: 10.1039/c3cs60064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 4.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nat. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 5.Bastús NG, Sánchez-Tilló E, Pujals S, Farrera C, Kogan MJ, Giralt E, Celada A, Lloberas J, Puntes V. Peptides conjugated to gold nanoparticles induce macrophage activation. Mol. Immunol. 2009;46:743–748. doi: 10.1016/j.molimm.2008.08.277. [DOI] [PubMed] [Google Scholar]
- 6.Slütter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider YJ, Riet EV, Préat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release. 2009;138:113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Serda RE. Particle platforms for cancer immunotherapy. Int. J. Nanomedicine. 2013;8:1683–1696. doi: 10.2147/IJN.S31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Geest BG, Willart MA, Hammad H, Lambrecht BN, Pollard C, Bogaert P, De Filette M, Saelens X, Vervaet C, Remon JP, Grooten J. Polymeric multilayer capsule-mediated vaccination induces protective immunity against cancer and viral infection. ACS. Nano. 2012;6:2136–2149. doi: 10.1021/nn205099c. [DOI] [PubMed] [Google Scholar]
- 9.Chong CSW, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS, Tyrrell DL, Samuel J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Release. 2005;102:85–99. doi: 10.1016/j.jconrel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Saltzman WM, Mellmand I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JR. TNF-mediated inflammatory disease. J. Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 12.Choy EHS, Panayi GS. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N. Engl. J. Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 13.Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia—increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012;242:33–38. doi: 10.1016/j.jneuroim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am. J. Cardiol. 2000;86:3–8. doi: 10.1016/s0002-9149(00)01339-4. [DOI] [PubMed] [Google Scholar]
- 15.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;301:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, Jiang S. Super-hydrophilic zwitterionic poly (carboxybetaine) and amphiphilic non ionic poly (ethylene glycol) for stealth nanoparticles. Nano. Today. 2012;7:404–413. [Google Scholar]
- 17.Yang W, Liu S, Bai T, Keefe AJ, Zhang L, Ella-Menye J, Li Y, Jiang S. Poly(carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano. Today. 2014;9:10–16. [Google Scholar]
- 18.Reddy ST, Viles AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Yin Y, Wang L, Zhang W, Chen X, Yang X, Xu J, Ma G. Surface hydrophobicity of microparticles modulates adjuvanticity. J. Mater. Chem. B. 2013;1:3888–3896. doi: 10.1039/c3tb20383b. [DOI] [PubMed] [Google Scholar]
- 20.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis JS, Roy K, Keselowsky BG. Materials that harness and modulate the immune system. MRS. Bull. 2014;39:25–34. doi: 10.1557/mrs.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyano DF, Saha K, Prakash G, Yan B, Kong H, Yazdani M, Rotello VM. Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyano DF, Rotello VM. Langmuir. 2011;27:10376–10385. doi: 10.1021/la2004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shima F, Akagi T, Uto T, Akashi M. Manipulating the antigen-specific immune response by the hydrophobicity of amphiphilic poly (γ-glutamic acid) nanoparticles. Biomaterials. 2013;34:9709–9716. doi: 10.1016/j.biomaterials.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 25.Juskewitch JE, Platt JL, Knudsen BE, Knutson KL, Brunn GJ, Grande JP. Disparate roles of marrow- and parenchymal cell-derived TLR4 signaling in murine LPS-induced systemic inflammation. Sci. Rep. 2012;2:918. doi: 10.1038/srep00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz E, Patel DD, Hartung T, Schwartz DA. Toll-like receptor 4 (TLR4)-deficient murine macrophage cell line as an in vitro assay system to show TLR4-independent signaling of bacteroides fragilis lipopolysaccharide. Infect. Immun. 2002;70:4892–4896. doi: 10.1128/IAI.70.9.4892-4896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann M, Maini RN. TND defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 28.Jones KJ, Perris AD, Vernallis AB, Worthington T, Lambert PA, Elliott TSJ. Induction of inflammatory cytokines and nitric oxide in J774.2 cells and murine macrophages by lipoteichoic acid and related cell wall antigens from Staphylococcus epidermidis. J. Med. Microbiol. 2005;54:315–321. doi: 10.1099/jmm.0.45872-0. [DOI] [PubMed] [Google Scholar]
- 29.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliver. Rev. 1995;17:31–48. [Google Scholar]
- 30.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nanoparticles. Plos. One. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive Oxygen Species Promote TNFα-Induced Death and Sustained JNK Activation by Inhibiting MAP Kinase Phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 32.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu Z-J, Arcaro KF, Rotello VM. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage vy cationic gold nanoparticles. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seong S-Y, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, Yang T, Qiu S, Ma G. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35:6086–6097. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, Kim SH, Kim HO, Lim YT, Chung BH, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharm. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.