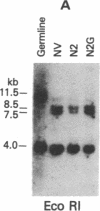
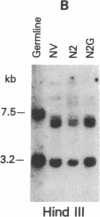
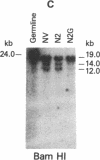
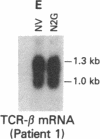
Abstract
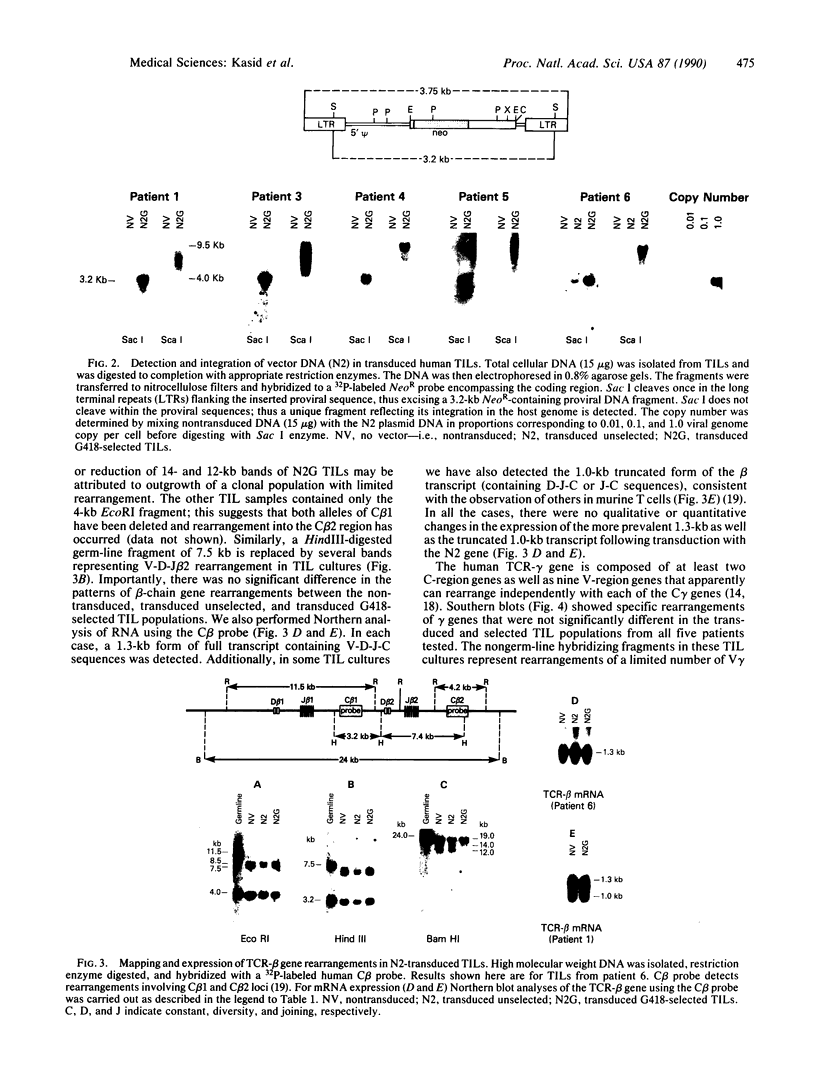
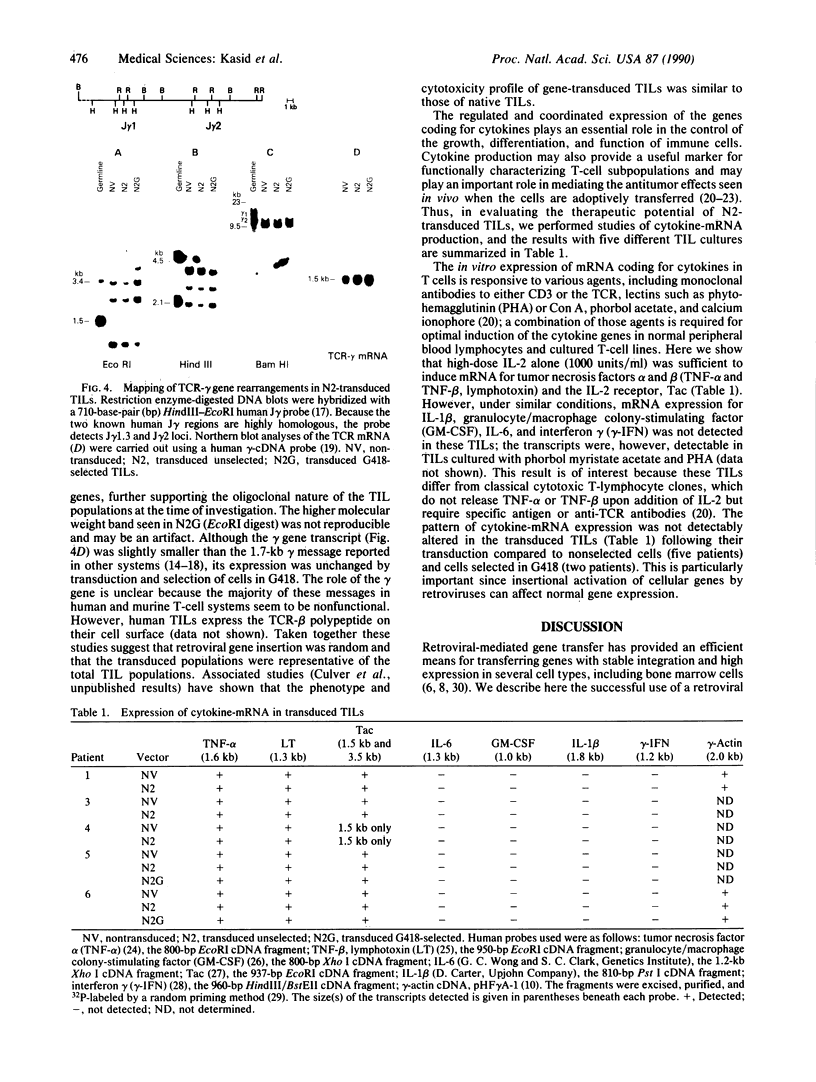
Tumor-infiltrating lymphocytes (TILs) are cells generated from tumor suspensions cultured in interleukin 2 that can mediate cancer regression when adoptively transferred into mice or humans. Since TILs proliferate rapidly in vitro, recirculate, and preferentially localize at the tumor site in vivo, they provide an attractive model for delivery of exogenous genetic material into man. To determine whether efficient gene transfer into TILs is feasible, we transduced human TILs with the bacterial gene for neomycin-resistance (NeoR) using the retroviral vector N2. The transduced TIL populations were stable and polyclonal with respect to the intact NeoR gene integration and expressed high levels of neomycin phosphotransferase activity. The NeoR gene insertion did not alter the in vitro growth pattern and interleukin 2 dependence of the transduced TILs. Analyses of T-cell receptor gene rearrangement for beta- and gamma-chain genes revealed the oligoclonal nature of the TIL populations with no major change in the DNA rearrangement patterns or the levels of mRNA expression of the beta and gamma chains following transduction and selection of TILs in the neomycin analog G418. Human TILs expressed mRNA for tumor necrosis factors (alpha and beta) and interleukin 2 receptor P55 but not for interleukin 1 beta, granulocyte/macrophage colony-stimulating factor, interleukin 6, and interferon gamma when grown with high-dose interleukin 2 without subsequent activation with mitogen or specific antigen. This pattern of cytokine-mRNA expression was not significantly altered following the transduction of TILs. The NeoR gene-transduced TILs could thus be used to follow the trafficking and survival of TILs in vivo, and clinical protocols using these transduced TILs in cancer patients have begun. The studies demonstrate the feasibility of TILs as suitable cellular vehicles for the introduction of therapeutic genes into patients receiving autologous TILs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Belldegrun A., Kasid A., Uppenkamp M., Topalian S. L., Rosenberg S. A. Human tumor infiltrating lymphocytes. Analysis of lymphokine mRNA expression and relevance to cancer immunotherapy. J Immunol. 1989 Jun 15;142(12):4520–4526. [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Iwashima M., Wettstein D. A., Kaplan K. B., Elliott J. F., Born W., Davis M. M. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987 Dec 24;330(6150):722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cornetta K., Anderson W. F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J Virol Methods. 1989 Feb;23(2):187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fisher B., Packard B. S., Read E. J., Carrasquillo J. A., Carter C. S., Topalian S. L., Yang J. C., Yolles P., Larson S. M., Rosenberg S. A. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989 Feb;7(2):250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Perkins A., Heimer E. P., Cullen B. R. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Kasid A., Bell G. I., Director E. P. Effects of transforming growth factor-beta on human lymphokine-activated killer cell precursors. Autocrine inhibition of cellular proliferation and differentiation to immune killer cells. J Immunol. 1988 Jul 15;141(2):690–698. [PubMed] [Google Scholar]
- Kasid A., Director E. P., Rosenberg S. A. Induction of endogenous cytokine-mRNA in circulating peripheral blood mononuclear cells by IL-2 administration to cancer patients. J Immunol. 1989 Jul 15;143(2):736–739. [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Quertermous T., Murre C., Dialynas D., Duby A. D., Strominger J. L., Waldman T. A., Seidman J. G. Human T-cell gamma chain genes: organization, diversity, and rearrangement. Science. 1986 Jan 17;231(4735):252–255. doi: 10.1126/science.3079918. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L. The gamma delta T cell receptor and class Ib MHC-related proteins: enigmatic molecules of immune recognition. Cell. 1989 Jun 16;57(6):895–898. doi: 10.1016/0092-8674(89)90326-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Rosenberg S. A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989 May 15;142(10):3714–3725. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]