Abstract
In recent years, human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) have emerged as a vital cell source for in vitro modeling of genetic cardiovascular disorders, drug screening, and in vivo cardiac regeneration research. Looking forward, the ability to efficiently cryopreserve hPSC-CMs without compromising their normal biochemical and physiologic functions will dramatically facilitate their various biomedical applications. Although working protocols for freezing, storing, and thawing hPSC-CMs have been established, the question remains as to whether they are optimal. In this chapter, we discuss our current understanding of cryopreservation appertaining to hPSC-CMs, and proffer key questions regarding the mechanical, contractile, and regenerative properties of cryopreserved hPSC-CMs.
Keywords: hPSC-CMs, cryopreservation, thawing, freezing, contraction, beating, cell viability, electrical coupling, engraftment, transplantation
1. Introduction
Despite advances in prevention and management, cardiovascular disease remains a leading cause of morbidity and mortality worldwide—underscoring the need for novel and improved therapies. While still critically important, animal models fail to recapitulate several key aspects of human cardiovascular physiology. Thus, the development of new cardiac therapies has been partly hindered by a lack of human cardiac tissues and isolated cardiomyocytes to study in vitro. Primary human cardiomyocytes are difficult to obtain, fail to thrive in culture, and are especially refractory to genetic manipulation. In this regard, human pluripotent stem cells (hPSCs), including human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs), have been vaunted as a promising platform to effect progress. Due to their unparalleled self-renewal capacity and differentiation potential in vitro hPSCs represent a consummate and theoretically unlimited source of cardiomyocytes for disease modeling, stem cell research, drug discovery, and cell-based regenerative therapies. In contrast to their primary counterparts, cardiomyocytes derived from hPSCs are readily amenable to long-term culture, and can be additionally manipulated for genetic modifications and tissue engineering purposes. Considering the time and cost of continuous culture, cryopreservation of hPSC-derived cardiomyocytes (hPSC-CMs) will greatly facilitate their various applications.
In this chapter, we further enumerate the uses of hPSC-CMs in both clinical and research settings and explain the value of their cryopreservation. We additionally discuss considerations and strategies for freezing, storing, and thawing methods as well as our current understanding of the phenotype and function of cryopreserved hPSC-CMs. Finally, we highlight key questions that must be rejoined before cryopreserved hPSC-CMs can be credibly regarded as an equivalent cell source when compared to their freshly-derived counterparts for in vitro modeling, drug discovery, and in vivo cardiac regeneration.
2. Applications of pluripotent stem cell-derived cardiomyocytes
hPSC-CMs have been used broadly for cardiac tissue engineering [1, 2] and developmental biology [3, 4], but the applications garnering the most attention are in disease modeling, drug development, and cardiac regeneration therapy. Therefore, in this section, we will provide a brief introduction into these areas of investigation to emphasize the importance of hPSC-CMs in biomedical research.
2.1. Disease modeling
In recent years, hPSC-CMs have emerged as a fruitful tool for modeling genetic cardiac disorders in vitro [5–7]. Although valuable, animal models cannot accurately model many aspects of human cardiac syndromes due to substantial differences in cardiac electrophysiology. While seemingly a better alternative, primary human cardiomyocytes are difficult to obtain and genetically manipulate in vitro. Furthermore, they cannot proliferate and rapidly dedifferentiate in culture exhibiting dramatic, time-dependent phenotypic changes [8]. These hurdles limit their use for disease pathway studies and drug discovery applications.
In contrast, hiPSCs derived from afflicted patient somatic cells are readily expandable in culture and can be differentiated into cardiomyocytes at any time, providing a theoretically limitless source of human diseased cells for in vitro studies. Furthermore, hPSCs are amenable to genetic manipulation prior to differentiation, enabling the use of classic molecular biology techniques to interrogate cardiac biology and disease pathophysiology. Numerous reports have established that hiPSC-CMs can recapitulate the main pathognomonic characteristics of genetic cardiac disorders including inherited arrhythmias at the single cell level, and can thus subsequently be used to gain insights into disease mechanisms [9]. Such disease models have been successfully generated from patients afflicted with disorders including long QT syndrome (LQTS) [10–12], Timothy Syndrome [13], and catecholaminergic polymorphic ventricular tachycardia (CPVT) [14, 15] (CITE MYSELF - DMM), to name a few. Moreover, in the emerging field of precision medicine, this technology could one day be used to tailor new or existing therapies to patient-specific needs [16].
2.2. Drug discovery and safety testing
The fact that hPSC-CMs exhibit key cardiac electrophysiological responses to external electrical and pharmacological stimuli at the cellular level makes them suitable for drug discovery and safety testing. Moreover, they are permissive to physiologically-relevant tissue engineering and high throughput screening platforms. While the prospect of drug discovery using hPSC-CMs is currently being explored, these cells have already been successfully applied in safety pharmacology testing to identify drug-induced cardiotoxicity [17, 18]. Numerous drugs make it through animal studies, but are withdrawn during human trials due to proarrhythmic side effects such as QT interval prolongation and torsades de pointes [19–21]. A prevailing expectation is that incorporating hPSC-CMs into early drug screens will minimize the risk of cardiotoxic events in clinical subjects, and thus reduce the rates of late-stage drug attrition [22].
2.3. Myocardial repair
Perhaps the most revolutionary prospect of hPSC-CMs is for use in cardiac regeneration therapy. Due to the heart’s limited regenerative capacity, cells lost during ischemic heart disease are not replaced intrinsically—resulting in fibrosis, reduced cardiac function, and overall morbidity [23]. Therefore, proposed strategies to restore the damaged myocardium have focused on external interventions such as cell, tissue, or full organ replacement [24]. In contrast to other non-cardiomyogenic stem cell types such as mesenchymal precursor cells that confer benefits through paracrine effects [25], transplanted hPSC-CMs also have the potential to achieve true contractile tissue regeneration [26]. Indeed, experiments in small and large animal models have demonstrated that hPSC-CMs transplanted to sites of ischemic injury survive and can electrically couple to the host myocardium [27–35]. In the case of human myocardial infarction, it is estimated that up to one billion hPSC-CMs may need to be transplanted for sufficient contractile tissue repair to be accomplished [36, 37], emphasizing the demand for efficient and scalable methods of procuring these cells for medical applications, including forthcoming clinical trials [38].
3. Considerations for the cryopreservation of pluripotent stem cell-derived cardiomyocytes
As the usage of hPSC-CMs increases, so does the demand for their long-term storage. Cryopreservation enables the storage of hPSC-CMs with desired phenotypes for later investigation and supports the establishment of clinical large-scale biobanking initiatives. Freezing may also be used as an effective strategy to attain clinically-relevant quantities of hPSC-CMs, since they can be generated in multiple batches and amassed over time, and allow sufficient time to perform quality control for cell preparations. Moreover, frozen hPSC-CMs can be used by researchers with minimal knowledge of hPSC culture or differentiation procedures, since the maintenance of pre-made hPSC-CMs is relatively inexpensive and simple compared to the time-consuming and resource-intensive process of cardiac differentiation from hPSCs. Current protocols for cryopreserving hPSC-CMs are similar to those for other cultured cell types, and thus aim to minimize the formation of damaging ice crystals. These procedures involve cell dissociation, centrifugation, re-suspension in the presence of a cryoprotective agent (CPA), and cooling in cryovials. A working protocol for the cryopreservation of hPSC-CMs has already been established [39], and thus will not be re-iterated here. However, elements of the cryopreservation procedure may differ slightly between laboratories—varying in cell handling, freezing rate, storage temperature, and choice of CPA(s). Therefore, in this section, we review the literature available regarding these variables, and compare methods for successfully freezing and storing hPSC-CMs.
3.1. Cardiac maturity
The optimal differentiation day at which to freeze hPSC-CMs has been explored, with the general consensus being that younger cells exhibit enhanced recovery from cryopreservation compared to older hPSC-CMs. This may be due, at least in part, to the fact that immature cardiomyocytes are smaller, rounder, and less structured than their mature counterparts [40–42]. Another possibility is that immature hPSC-CMs are protected by their distinct metabolic profile, which is more glycolytic in nature than mature hPSC-CMs [3, 40, 43]. Studies have reported high recovery percentages of beating hPSC-CMs frozen at days 12-30 after cardiac induction [28, 29, 44] with diminished survival observed in hPSC-CMs frozen later than 30 days [45]. In a direct comparison between day 12- and day 16-frozen cells (pre-beating and post-beating), day 12-frozen cells exhibited significantly higher post-thaw viability and fewer ultrastructural alterations than day 16-frozen hPSC-CMs [44]. Since hPSC-CMs typically initiate beating after differentiation day 14, these results suggest that improved cryopreservation outcomes may be achieved when cells are frozen at a pre-contraction stage. If this is true, the post-thaw cardiomyogenic capacity of hPSC-CMs frozen at even earlier progenitor stages should be explored.
3.2. Pro-survival treatments
hPSC-CMs that are cryopreserved for eventual cardiac transplantation are often subjected to a pro-survival protocol. The method was initially established by Laflamme and colleagues, and has been shown to enhance engraftment and survival after transplantation [34]. The protocol consists of two parts, the first of which is performed prior to cryopreservation. Approximately 24 hours prior to cryopreservation, cells are heat-shocked for 30 minutes by incubation at 43°C, then returned to 37°C in medium supplemented with 100 ng/mL IGF1 (Peprotech) and 0.2 µM cyclosporine A (Sandimmune, Novartis). The next day, hPSC-CMs are dissociated and cryopreserved as usual [29, 33, 46].
3.3. Cell dissociation
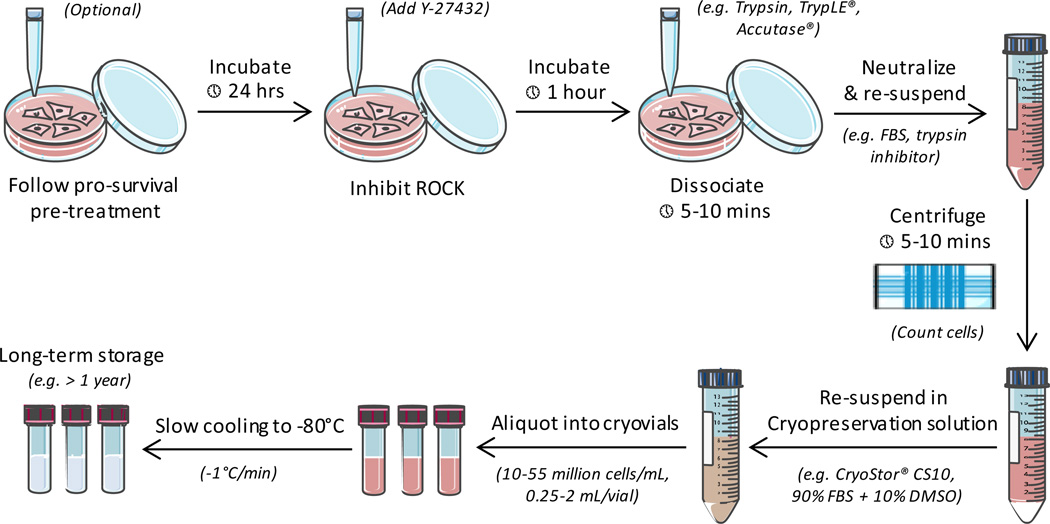
The first step in cryopreserving hPSC-CMs (excluding the pro-survival pre-treatment) involves their removal from any culture surface and enzymatic dissociation (Figure 1). Dissociation of hPSC-CMs into a single-cell suspension is imperative for minimizing the formation of damaging ice crystals, since it allows for maximal exposure of the cells to CPAs. Approximately one hour prior to enzymatic dispersion, cells may be treated with 10 µM of Y-2763, an inhibitor of the pro-apoptotic protein Rho-associated kinase (ROCK), which has been shown to promote post-cryopreservation survival of hPSC-CMs and stem cells [44, 47–50]. Efficient dissociation of hPSC-CMs can be achieved by incubation at 37°C for 5–10 minutes with Gibco® 0.25% Trypsin-EDTA, Accutase®, or the xeno-free TrypLE™ family of products (Thermo Fisher). Gentle dissociation reagents are not advised, since they require extended incubation times and are markedly less effective. If using trypsin, the enzyme should be neutralized after incubation either with serum-containing media or a chemically-defined trypsin inhibitor. Neutralized cells are then washed via centrifugation (300 g, 5 minutes) and re-suspended directly in a cryoprotective solution in preparation for freezing.
Figure 1.
General procedure for the cryopreservation of hPSC-CMs
3.4. Cryoprotective solutions
Successful freezing of hPSC-CMs requires cell concentrates to be suspended in a cryopreservation solution. Depending on the eventual application of the cells, the solution usually consists of a xeno-free, chemically-defined cryopreservation solution (e.g. CryoStor® CS10) [39, 46, 49, 50] or fetal bovine serum (FBS) containing a CPA [44, 45]. In principle, CPAs are compounds that reduce cellular damage during freezing by lowering the probability of intracellular and extracellular ice crystal formation [51–54]. A variety of cell-penetrating and membrane non-permeable CPAs are available, but dimethyl sulfoxide (DMSO) at a concentration of 10% is the most widely-used for hPSC-CM cryopreservation. Careful adherence to optimized protocols is recommended, since prolonged exposure to DMSO is assumed to be harmful to hPSC-CMs based on its clinical cardiotoxicity profile [55, 56]. The percentage of FBS in cryoprotective solutions can range from 30–90%, with improved recovery observed in preparations containing higher levels of FBS [44, 45]. If a lower percentage of FBS is used, the remaining portion is completed with hPSC-CM culture media, such as RPMI 1640 containing B27 supplement (Life Technologies).
3.5. Cell density and solution volume
Cell density and attendant solution volumes for the cryopreservation of hPSC-CMs have also been examined (Table 1). Recovery percentages of hPSC-CM preparations with cell densities of approximately 27–53 million cells per mL in CryoStor® CS10 volumes of 0.25–1.5 mL per vial were reported to be comparable [46]. Interestingly, recovery percentages in CryoStor® CS10 preparations containing 10–15 million cells per mL in volumes of 1–2 mL per vial were reported to be higher, suggesting that lower cell concentrations are preferable [49]. However, this discrepancy may be attributed to differences in methodology, since the higher density recovery percentages were quantified via flow cytometry in lieu of trypan blue exclusion.
Table 1.
Cell densities, suspension volumes, and freezing rates for hPSC-CM cryopreservation
| Reference | Cell density (cells/mL) |
Freezing volume (mL) |
Recovery rate (%), assay |
Freezing rate (Δ°C/min) |
|---|---|---|---|---|
| Xu et. al. [46] | ~ 27 million | 1.5 | 70–77, cell counting and flow cytometry |
1 until −40°C |
| ~ 53 million | 1.5 | 5 from −40°C to −80°C | ||
| 40 million | 0.25 | |||
| Zhu et.al. [39] | 40 million | 0.25 | Not reported | 1 from 0°C to −7°C |
| 0.75 from −7°C to −10°C | ||||
| 1 from −10°C to −80°C | ||||
| Chen et.al. [49] | 10 million | 1 | 84.3 ± 5.2, trypan blue exclusion |
Controlled rate program not specified |
| 15 million | 2 |
3.6. Freezing rates and storage
Cryopreservation of hPSC-CMs is generally achieved by controlled-rate or uncontrolled freezing techniques that cool samples to a temperature of −80°C before they are transferred to ultra-low temperature storage (i.e. liquid nitrogen immersion). As the name implies, controlled-rate freezing is a process whereby sample temperatures are continuously monitored by a programmable device and adjusted internally to maintain a pre-determined cooling rate. Uncontrolled freezing techniques utilize containers in which samples are surrounded by an insulating substance that is placed into a −80°C freezer to achieve an approximate cooling profile of 1°C per minute. The obvious advantage of controlled-rate freezing is that multiple freezing rate steps can be incorporated to minimize the liberation of fusion heat, which is cell-injurious [53, 57]. Studies have optimized freezing protocols for other cell types [58], but no consensus currently exists regarding the best strategy for freezing hPSC-CMs. However, a few groups have published their controlled-rate freeing protocols, which we have enumerated here (Table 1). Another aspect that remains undetermined is the durability of hPSC-CMs in a cryopreserved state. Retrospective studies have observed that hematopoietic stem cells can be effectively cryopreserved for well over a decade [59–62], but these results cannot be assumed for hPSC-CMs. Therefore, the long-term viability of hPSC-CMs at ultra-low storage temperatures should be evaluated, since this will be an important consideration in future biobanking initiatives. For very short-term storage, cryopreservation may not even be necessary, since recent evidence suggests that hPSC-CMs can be efficiently stored in hypothermic conditions (4°C) for up to 7 days without significantly compromising cell viability [63].
3.7. Thawing strategies
Rapid thawing of frozen samples minimizes recrystallization of ice, and thus provides improved cell viability compared to slow thawing [64–67]. Once samples are removed from cryogenic storage, they are dipped immediately in a warm 37°C water bath. If there is distance between the cryogenic storage vessel and the water bath, the frozen samples are transported on dry ice (−80°C) until immediately before thawing. Samples are swirled or gently shaken in the bath to ensure the entire sample thaws at a consistent rate, and removed when the last visible ice crystal has melted. To remove the cryoprotective agent from the sample, the thawed cells are thoroughly dispersed in an excess of culture media, and washed once via centrifugation (300 g, 5 minutes) before re-suspension and plating. To enhance viability, cells may be thawed in media containing 10 µM Y-2763 [47–49]. hPSC-CMs that are thawed for continued culture may be seeded onto matrigel-coated plates. In some instances, hPSC-CMs may form aggregates during the freeze/thaw cycle. Presumably, cell aggregation occurs as a result of adhesive DNA molecules released from dying cells. In these cases, addition of the endonuclease deoxyribonuclease I (DNase I) may be added to curtail the presence of free DNA fragments and cell clumps [50]. DNase I Solution (Stem Cell Technologies) is added to the media during the initial dispersal and washing step to achieve a final concentration of 0.1 mg per mL (i.e. 200 Kunitz units per mL), followed by incubation for 15 minutes at room temperature prior to proceeding with downstream applications (Figure 2).
Figure 2.
General procedure for thawing cryopreserved hPSC-CMs
The percentage of cell recovery after cryopreservation of hPSC-CMs ranges from 55% to more than 90%, with an average of ~83% [46, 49]. The method of cardiac differentiation does not appear to affect the cryopreservation outcomes of resultant hPSC-CMs, since both growth factor and small molecule-induced hPSC-CMs recover equally well [45]. The percentage of cardiomyocytes in a mixed population of hPSC-CMs also does not appear to affect post-thaw recovery, since viability has been found to be similar across cell preparations with varying degrees of cardiac purity [46].
4. Functional recovery of stem cell-derived cardiomyocytes after cryopreservation
After rapid thawing of ≤ day 30 hPSC-CMs, high cell viability and recovery of spontaneous beating can be expected. Several studies have reported data regarding post-thaw cell viability, cardiac cell purity, engraftment efficiency, and contractility parameters from frozen hPSC-CM preparations [29, 39, 44–46, 49, 68]. In general, these studies suggest that the form and function of cryopreserved hPSC-CMs are similar to their freshly-derived counterparts in the short term, but one study has reported that accelerated, irregular, and arrhythmic beating occurs during extended post-thaw culture [44]. In-depth characterization of the transcriptomic, proteomic, metabolic, epigenetic, and therapeutic properties of cryopreserved hPSC-CMs is lacking, and will be necessary to determine if these cells can be used for transplantation comparable to freshly-derived cells. In this section, we review previously reported cell recovery parameters.
4.1. Cell structure and cycling
Micro- and ultrastructural alterations in cryopreserved hPSC-CMs have been reported, but not yet linked to functional consequences. Post-thaw hPSC-CMs retain their cardiogenic features, but do exhibit features of organelle damage. Transition electron microscopic analysis of cellular components in cryopreserved hPSC-CMs revealed partially disrupted nuclei, damaged cell membranes, and loss of mitochondrial integrity [44]. However, the maturation stage of the cell appears to be critical in structural recovery, since hPSC-CMs frozen at a pre-contraction differentiation stage (day 12) exhibited less structural damage than cells frozen at a post-contraction stage (day 16). In terms of cell cycling, cryopreservation does not appear to arrest cells that are in active phases, since the expression of Ki-67 [46] and BrdU incorporation [44] were both detectable in post-thaw cryopreserved hPSC-CMs.
4.2. Mechanical and contractile properties
The majority of cryopreserved hPSC-CMs recover contractility 1–5 days post-thawing [44, 46, 49], but the question remains as to whether they recover 100% of their pre-frozen contractile capacity. An early study used perforated patch clamp to show that cryopreserved hPSC-CMs retained cardiac action potential characteristics and expected electrophysiological responses to pharmacological modulators [49]. A later study performed a comprehensive physiological analysis of cryopreserved hPSC-CMs and acutely-isolated adult rabbit ventricular cardiomyocytes, but did not directly compare the contractile properties of thawed hPSC-CMs to freshly-derived hPSC-CMs [45]. In this study, experiments were performed 3–5 days post-thaw, and demonstrated that hPSC-CMs retained a functional sarcoplasmic reticulum (SR) and robust intracellular Ca2+ handling after cryopreservation. Moreover, the study showed that cytosolic Ca2+ buffering, twitch transient amplitude, resting diastolic Ca2+ content, SR Ca2+ content, and relative contribution of SR Ca2+ flux were comparable between cryopreserved hPSC-CMs and acutely-isolated adult rabbit cardiomyocytes. Collectively, these results imply that the contractile parameters of cryopreserved hPSC-CMs are mostly uncompromised during short-term post-thaw culture. However, a separate study that examined thawed hPSC-CMs in extended culture observed profound contractile alterations that progressively deteriorated until ultimate arrest [44]. In this study, spontaneous beating frequency of post-thaw hPSC-CMs was initially similar to that of non-frozen cells, but began to accelerate 6 days after thawing to unprecedented rates of 108 beats per minute compared to 20–40 beats per minute in non-frozen cells. Furthermore, the beating became irregular 8 days after thawing, and completely arrested at post-thaw day 12.
In addition to apoptosis and anoikis, cryopreservation is known to disrupt cell-cell and cell-matrix adhesion [69–71], increase production of reactive oxygen species [72], and activate various cellular stress pathways [52, 53]. In other proliferating cell types, any losses of function may be recovered in subsequent generations [73, 74]. However, the opportunity for hPSC-CMs to recover during extended culture is forfeited, since the majority of fully differentiated cardiomyocytes have negligible cell cycle activity [75]. Therefore, it is not surprising that post-thaw functionality may be compromised during prolonged post-thaw culture. This again begs the question of whether freezing hPSC-CMs during their still-proliferative cardiac progenitor phase would result in improved post-thaw outcomes, assuming that the ultimate cardiomyogenic capacity of the cells is unaffected by the cryopreservation procedure. This is an intriguing notion, and should be further explored.
Another interesting concept is that the post-thaw environment may influence the functional recovery of cryopreserved hPSC-CMs. A study performed in standard two-dimensional (2D) culture conditions reported progressive functional decline during extended (≥ 6 days) post-thaw maintenance, while a study that maintained hPSC-CMs in a three-dimensional (3D) micro-tissue culture system did not report any beating abnormalities up to a week post-thaw [50]. Furthermore, studies that have transplanted cryopreserved hPSC-CMs into rodents presented no evidence of arrhythmias after host-graft electrical coupling [33, 50]. These results suggest that thawed hPSC-CMs are especially vulnerable to single-cell culture, but may demonstrate improved long-term outcomes in host tissues or under tissue-like conditions. This inference is corroborated by a recent study that demonstrated hPSC-CMs cultured in 3D micro-tissue aggregates also are more resistant to prolonged hypothermic (4°C) storage-induced cell injury compared to their counterparts cultured in 2D monolayers [63]. However, differences in cryopreservation methodology may have also contributed to the observed differences, so these results must be interpreted with caution.
4.3. Post-transplantation engraftment and electrical coupling
Experiments in rodents and large animal models have demonstrated that transplanted cryopreserved hPSC-CMs partially re-muscularize injured hearts and can beat synchronously with the host myocardium [28, 29, 33, 46, 50, 76]. Xu et. al. compared post-transplantation cell survival of freshly-derived and cryopreserved hPSC-CMs using an athymic rat model of acute myocardial ischemia-reperfusion. Immunohistological analysis of the injured myocardial tissue one week and four weeks after transplantation revealed similar overall graft sizes between the two hPSC-CM preparations, demonstrating comparable short and long-term engraftment efficiency at small scales [46]. Using a large-scale animal model, Chong et. al. also found no adverse impact of cryopreservation on hPSC-CM graft size in pigtail macaques [29]. Efficient host-graft electromechanical integration of cryopreserved hPSC-CMs has also been reported, with 1:1 coupling between in vivo hPSC-CM calcium fluorescence transients and the host ECG in rodents [33, 50] and non-human primates [29]. The fact that the monkey study utilized approximately one billion cells suggests cryopreservation of hPSC-CMs may be a sound strategy for scaling-up to transplantation in humans. However, in contrast to rodents, non-fatal ventricular arrhythmias were observed transiently in primates that received cryopreserved hPSC-CMs over the course of a 3-month period—although this might be due to species-specific differences in heart size and beating rate, and may not be attributed to cryopreservation per se [29].
5. Conclusions and future directions
Although current cryopreservation protocols are viable, the question still remains as to whether or not they are optimal. To adequately address this question, more studies that directly compare the physiological properties of freshly-derived and cryopreserved hiPSC-CMs and hESC-CMs are warranted. As mentioned earlier, the differentiation stage at which hPSC-CMs should be frozen for maximal post-thaw functional recovery remains to be established, and the potential for freezing cardiac progenitors should be further explored. Also imperative are studies that investigate and characterize the biology of cryopreservation-associated physiological sequelae in hPSC-CMs, since there is evidence from both in vitro [44] and in vivo [29] studies that cryopreserved hPSC-CMs acquire pro-arrhythmic tendencies during extended post-thaw time periods. Another important concern stems from evidence showing that freeze/thawed mesenchymal stromal cells [77] and natural killer cells [78, 79] show impaired immunomodulatory and therapeutic properties compared to freshly-derived cells, despite exhibiting high post-thaw viability. In light of this, a side-by-side comparison of transplanted non-frozen and cryopreserved hPSC-CMs’ ability to improve cardiac function in recipient hearts would be very informative. Finally, since frozen cells often show variable viability upon thawing, standardized or automated methods of thawing hPSC-CMs should be established.
In conclusion, the ability to successfully cryopreserve and thaw hPSC-CMs with their therapeutic potential intact would greatly facilitate their availability and distribution for preclinical studies and clinical trials of myocardial repair. While progress has been made in developing methods and characterizing freeze-thawed hPSC-CMs, further studies are needed before cryopreserved hPSC-CMs can be regarded as functionally and therapeutically equivalent to their freshly-derived counterparts for their multiple in vitro and in vivo applications.
Acknowledgments
This work was supported in part by grants GA-2014-126 from the Center for the Advancement of Science in Space and R21 HL123928 from the NIH. M.K.P. was supported by the Center for Pediatric Nanomedicine at Emory/Georgia Tech. Figures were prepared using Servier Medical Art.
Abbreviations
- hPSC-CMs
Human pluripotent stem cell-derived cardiomyocytes
- CPA
Cryoprotective agent
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- ROCK
Rho-associated kinase
References
- 1.Turnbull IC, Lieu DK, Li RA, Costa KD. Cardiac tissue engineering using human stem cell-derived cardiomyocytes for disease modeling and drug discovery. Drug Discov Today Dis Models. 2012;9(4):e219–e227. doi: 10.1016/j.ddmod.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feric NT, Radisic M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev. 2016:96110–96134. doi: 10.1016/j.addr.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birket Matthew J, Casini S, Kosmidis G, Elliott David A, Gerencser Akos A, Baartscheer A, Schumacher C, Mastroberardino Pier G, Elefanty Andrew G, Stanley EdG, Mummery Christine L. PGC-1α and reactive oxygen species regulate human embryonic stem cell-derived cardiomyocyte function. Stem Cell Reports. 2013;1(6):560–574. doi: 10.1016/j.stemcr.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, Passier R, Mummery CL. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat Biotech. 2015;33(9):970–979. doi: 10.1038/nbt.3271. [DOI] [PubMed] [Google Scholar]
- 5.Lui KO, Stachel MW, Lieu DK, Li RA, Bu L. Induced pluripotent stem cells as a disease model for studying inherited arrhythmias: promises and hurdles. Drug Discov Today Dis Models. 2012;9(4):e199–e207. [Google Scholar]
- 6.Li RA. The use of induced pluripotent stem cells for disease modeling: what are the promises and hurdles? Drug Discov Today Dis Models. 2012;9(4):e143–e145. [Google Scholar]
- 7.Davis RP, van den Berg CW, Casini S, Braam SR, Mummery CL. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol Med. 2011;17(9):475–484. doi: 10.1016/j.molmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 8.LI RK, Mickle DA, Weisel RD, Carson S, Omar SA, Tumiati LC, Wilson GJ, Williams WG. Human pediatric and adult ventricular cardiomyocytes in culture: assessment of phenotypic changes with passaging. Cardiovasc Res. 1996;32(2):362–373. doi: 10.1016/0008-6363(96)00079-x. [DOI] [PubMed] [Google Scholar]
- 9.Lieu DK, Turnbull IC, Costa KD, Li RA. Engineered human pluripotent stem cell-derived cardiac cells and tissues for electrophysiological studies. Drug Discov Today Dis Models. 2012;9(4):e209–e217. doi: 10.1016/j.ddmod.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 11.Lahti AL, Kujala VJ, Chapman H, Koivisto A-P, Pekkanen-Mattila M, Kerkelä E, Hyttinen J, Kontula K, Swan H, Conklin BR, Yamanaka S, Silvennoinen O, Aalto-Setälä K. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech. 2012;5(2):220–230. doi: 10.1242/dmm.008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, Seyfarth M, Sinnecker D, Schömig A, Laugwitz K-L. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 13.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012;4(3):180–191. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itzhaki I, Maizels L, Huber I, Gepstein A, Arbel G, Caspi O, Miller L, Belhassen B, Nof E, Glikson M, Gepstein L. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J Am Coll Cardiol. 2012;60(11):990–1000. doi: 10.1016/j.jacc.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 16.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016 doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandenius CF, Steel D, Noor F, Meyer T, Heinzle E, Asp J, Arain S, Kraushaar U, Bremer S, Class R, Sartipy P. Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J Appl Toxicol. 2011;31(3):191–205. doi: 10.1002/jat.1663. [DOI] [PubMed] [Google Scholar]
- 18.Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, Wu H, Beygui RE, Wu SM, Robbins RC, Bers DM, Wu JC. Screening drug-induced arrhythmia using human induced pluripotent stem cell–derived cardiomyocytes and low-impedance microelectrode arrays. Circulation. 2013;128(11 suppl 1):S3–S13. doi: 10.1161/CIRCULATIONAHA.112.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald JS, Robertson RT. Toxicity testing in the 21st century: a view from the pharmaceutical industry. Toxicol Sci. 2009;110(1):40–6. doi: 10.1093/toxsci/kfp088. [DOI] [PubMed] [Google Scholar]
- 20.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2(6):439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 21.Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy. 2001;21(12):1468–1472. doi: 10.1592/phco.21.20.1468.34482. [DOI] [PubMed] [Google Scholar]
- 22.Laustriat D, Gide J, Peschanski M. Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans. 2010;38(4):1051–1057. doi: 10.1042/BST0381051. [DOI] [PubMed] [Google Scholar]
- 23.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 24.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6(239):239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117(6):576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 26.Kuraitis D, Suuronen EJ, Sellke FW, Ruel M. The future of regenerating the myocardium. Curr Opin Cardiol. 2010;25(6):575–582. doi: 10.1097/HCO.0b013e32833f0318. [DOI] [PubMed] [Google Scholar]
- 27.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotech. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 28.Chong JJH, Murry CE. Cardiac regeneration using pluripotent stem cells—progression to large animal models. Stem Cell Res. 2014;13(3, Part B):654–665. doi: 10.1016/j.scr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50(19):1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 31.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1(1):9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167(3):663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 35.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagege A, Desnos M, Renaud JF, Menasche P, Puceat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 37.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47(9):1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Menasche P, Vanneaux V, Fabreguettes JR, Bel A, Tosca L, Garcia S, Bellamy V, Farouz Y, Pouly J, Damour O, Perier MC, Desnos M, Hagege A, Agbulut O, Bruneval P, Tachdjian G, Trouvin JH, Larghero J. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur Heart J. 2015;36(12):743–750. doi: 10.1093/eurheartj/ehu192. [DOI] [PubMed] [Google Scholar]
- 39.Zhu W-Z, Van Biber B, Laflamme MA. Methods for the derivation and use of cardiomyocytes from human pluripotent stem cells. Methods Mol Biol (Clifton, NJ) 2011:767419–767431. doi: 10.1007/978-1-61779-201-4_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22(14):1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31(5):829–837. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poon E, Keung W, Liang Y, Ramalingam R, Yan B, Zhang S, Chopra A, Moore J, Herren A, Lieu DK, Wong HS, Weng Z, Wong OT, Lam YW, Tomaselli GF, Chen C, Boheler KR, Li RA. Proteomic analysis of human pluripotent stem cell-derived, fetal, and adult ventricular cardiomyocytes reveals pathways crucial for cardiac metabolism and maturation. Circ Cardiovasc Genet. 2015;8(3):427–436. doi: 10.1161/CIRCGENETICS.114.000918. [DOI] [PubMed] [Google Scholar]
- 44.Kim YY, Ku SY, Liu HC, Cho HJ, Oh SK, Moon SY, Choi YM. Cryopreservation of human embryonic stem cells derived-cardiomyocytes induced by BMP2 in serum-free condition. Reprod Sci. 2011;18(3):252–260. doi: 10.1177/1933719110385130. [DOI] [PubMed] [Google Scholar]
- 45.Hwang HS, Kryshtal DO, Feaster TK, Sanchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015:8579–8588. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu C, Police S, Hassanipour M, Li Y, Chen Y, Priest C, O'Sullivan C, Laflamme MA, Zhu WZ, Van Biber B, Hegerova L, Yang J, Delavan-Boorsma K, Davies A, Lebkowski J, Gold JD. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6(1):53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heng BC. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2009;41(5):376–380. doi: 10.1016/j.tice.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23(12):2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 49.Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, Liu JC, Chai J, Gold J, Wu J, Hsu D, Couture LA. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015;15(2):365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerbin KA, Yang X, Murry CE, Coulombe KL. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS One. 2015;10(7):e0131446. doi: 10.1371/journal.pone.0131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosden RG. General principles of cryopreservation. Methods Mol Biol. 2014:1154261–1154268. doi: 10.1007/978-1-4939-0659-8_11. [DOI] [PubMed] [Google Scholar]
- 52.Hubel A. Parameters of cell freezing: implications for the cryopreservation of stem cells. Transfus Med Rev. 1997;11(3):224–233. doi: 10.1053/tmrv.1997.0110224. [DOI] [PubMed] [Google Scholar]
- 53.Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2015:12573–12619. doi: 10.1007/978-1-4939-2193-5_1. [DOI] [PubMed] [Google Scholar]
- 54.Meryman HT. Cryopreservation of living cells: principles and practice. Transfusion. 2007;47(5):935–945. doi: 10.1111/j.1537-2995.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 55.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75(3):781–786. [PubMed] [Google Scholar]
- 56.Zenhausern R, Tobler A, Leoncini L, Hess OM, Ferrari P. Fatal cardiac arrhythmia after infusion of dimethyl sulfoxide-cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol. 2000;79(9):523–526. doi: 10.1007/s002770000186. [DOI] [PubMed] [Google Scholar]
- 57.De Santis L, Coticchio G. Theoretical and experimental basis of slow freezing. Reprod Biomed Online. 2011;22(2):125–132. doi: 10.1016/j.rbmo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Balint B, Ivanovic Z, Petakov M, Taseski J, Jovcic G, Stojanovic N, Milenkovic P. The cryopreservation protocol optimal for progenitor recovery is not optimal for preservation of marrow repopulating ability. Bone Marrow Transplant. 1999;23(6):613–619. doi: 10.1038/sj.bmt.1701623. [DOI] [PubMed] [Google Scholar]
- 59.Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S, Sato K, Klein JP, Gale RP. Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant. 1999;23(4):395–396. doi: 10.1038/sj.bmt.1701580. [DOI] [PubMed] [Google Scholar]
- 60.Aird W, Labopin M, Gorin NC, Antin JH. Long-term cryopreservation of human stem cells. Bone Marrow Transplant. 1992;9(6):487–490. [PubMed] [Google Scholar]
- 61.Attarian H, Feng Z, Buckner CD, MacLeod B, Rowley SD. Long-term cryopreservation of bone marrow for autologous transplantation. Bone Marrow Transplant. 1996;17(3):425–430. [PubMed] [Google Scholar]
- 62.Veeraputhiran M, Theus JW, Pesek G, Barlogie B, Cottler-Fox M. Viability and engraftment of hematopoietic progenitor cells after long-term cryopreservation: effect of diagnosis and percentage dimethyl sulfoxide concentration. Cytotherapy. 2010;12(6):764–766. doi: 10.3109/14653241003745896. [DOI] [PubMed] [Google Scholar]
- 63.Correia C, Koshkin A, Carido M, Espinha N, Šarić T, Lima PA, Serra M, Alves PM. Effective hypothermic storage of human pluripotent stem cell-derived cardiomyocytes compatible with global distribution of cells for clinical applications and toxicology testing. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2015-0238. pii: sctm.2015-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seki S, Mazur P. Effect of warming rate on the survival of vitrified mouse oocytes and on the recrystallization of intracellular ice. Biol Reprod. 2008;79(4):727–737. doi: 10.1095/biolreprod.108.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao J, Du J, Kleinhans FW, Critser ES, Mazur P, Critser JK. The effect of collection temperature, cooling rate and warming rate on chilling injury and cryopreservation of mouse spermatozoa. J Reprod Fertil. 1995;104(2):231–236. doi: 10.1530/jrf.0.1040231. [DOI] [PubMed] [Google Scholar]
- 66.El-Naggar MM, Al-Mashat FM, Elayat AA, Sibiany AR, Ardawi MS, Badawoud MH. Effect of thawing rate and post-thaw culture on the cryopreserved fetal rat islets: functional and morphological correlation. Life Sci. 2006;78(17):1925–1932. doi: 10.1016/j.lfs.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 67.Hochi S, Semple E, Leibo SP. Effect of cooling and warming rates during cryopreservation on survival of in vitro-produced bovine embryos. Theriogenology. 1996;46(5):837–847. doi: 10.1016/s0093-691x(96)00241-5. [DOI] [PubMed] [Google Scholar]
- 68.Moriguchi H, Madson J. Autologous human cardiac stem cells transplantation for the treatment of ischaemic cardiomyopathy: first study of human-induced pluripotent stem (iPS) cell-derived cardiomyocytes transplantation. BMJ Case Rep. 2013 [Google Scholar]
- 69.Wong RC, Dottori M, Koh KL, Nguyen LT, Pera MF, Pebay A. Gap junctions modulate apoptosis and colony growth of human embryonic stem cells maintained in a serum-free system. Biochem Biophys Res Commun. 2006;344(1):181–188. doi: 10.1016/j.bbrc.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 70.Wong RC, Pebay A, Nguyen LT, Koh KL, Pera MF. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. 2004;22(6):883–889. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- 71.Ichikawa H, Yoshie S, Shirasawa S, Yokoyama T, Yue F, Tomotsune D, Sasaki K. Freeze-thawing single human embryonic stem cells induce e-cadherin and actin filament network disruption via g13 signaling. Cryo Letters. 2011;32(6):516–524. [PubMed] [Google Scholar]
- 72.Xu X, Cowley S, Flaim CJ, James W, Seymour L, Cui Z. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol Prog. 2010;26(3):827–837. doi: 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugimachi K, Sosef MN, Baust JM, Fowler A, Tompkins RG, Toner M. Long-term function of cryopreserved rat hepatocytes in a coculture system. Cell Transplant. 2004;13(2):187–195. doi: 10.3727/000000004773301799. [DOI] [PubMed] [Google Scholar]
- 74.Sosef MN, Baust JM, Sugimachi K, Fowler A, Tompkins RG, Toner M. Cryopreservation of isolated primary rat hepatocytes: enhanced survival and long-term hepatospecific function. Ann Surg. 2005;241(1):125–133. doi: 10.1097/01.sla.0000149303.48692.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285(6):H2355–H2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 76.Riegler J, Ebert A, Qin X, Shen Q, Wang M, Ameen M, Kodo K, Ong SG, Lee WH, Lee G, Neofytou E, Gold JD, Connolly AJ, Wu JC. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Reports. 2016;6(2):176–187. doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lönnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mata MM, Mahmood F, Sowell RT, Baum LL. Effects of cryopreservation on effector cells for antibody dependent cell-mediated cytotoxicity (ADCC) and natural killer cell (NK) activity in (51)Cr-release and CD107a assays. J Immunol Methods. 2014:4061–4069. doi: 10.1016/j.jim.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominguez E, Lowdell MW, Perez-Cruz I, Madrigal A, Cohen SBA. Natural killer cell function is altered by freezing in DMSO. Biochem Soc Trans. 1997;25(2):175S-S. doi: 10.1042/bst025175s. [DOI] [PubMed] [Google Scholar]