Abstract
Background
Extrinsic labeling techniques are typically used to measure fractional absorption of zinc (FAZextrinsic) but none have been adequately evaluated.
Objective
To compare determination of the quantity of zinc absorbed (TAZextrinsic) using measurements of FAZextrinsic with results of simultaneous determinations of dietary zinc absorbed (TAZmetabolic) that are not dependent on labeling ingested food with an extrinsic tracer (modified metabolic balance technique).
Design
70Zn was administered orally with all meals for 6 consecutive days to 21 healthy, free-living adult women consuming a constant diet. 68Zn and 67Zn were administered intravenously. FAZextrinsic was measured using a dual isotope tracer ratio technique and multiplied by dietary zinc to give TAZextrinsic TAZmetabolic was determined by addition of net absorption of zinc and endogenous fecal zinc, the latter determined by an isotope dilution technique.
Results
TAZextrinsic and TAZmetabolic were 3.0 ± 1.1mg/day and 3.1 ± 1.1 mg/day respectively, paired t-test p = 0.492. The correlation coefficient for TAZextrinsic and TAZmetabolic was 0.91, and for FAZextrinsic and FAZmetabolic was 0.95. A Bland Altman analysis indicated a bias of 0.07, and the limits of agreement of −0.86 to 1.01 for TAZextrinsic and TAZmatabolic
Conclusion
These results from two independent methods provide reasonable validation of our extrinsic labeling technique for a wide range of composite diets.
Keywords: Zinc, absorption, extrinsic labeling, dietary zinc, stable isotope
Introduction
The advent of zinc stable isotope techniques has facilitated the measurement of zinc absorption from diets in human subjects. Concurrently, the demand for measurements of zinc absorption has grown paralleling the recent growth of interest in human zinc nutrition [1–4]. These measurements are almost invariably made utilizing an extrinsic labeling technique, despite limited and even questionable evidence that the absorption of this extrinsic tracer mimics the absorption of zinc endogenous to the food in the test meal [5–9].
Measurements of the quantity of dietary zinc absorbed by intrinsic labeling of foods are cumbersome, relying on tracer labeling of individual food components during production. This approach is expensive and limited at best to a few selected food items. There is an alternative approach which requires neither extrinsic nor intrinsic labeling but which provides a means of determining the absorption of dietary zinc. This alternative technique, referred to as the “modified metabolic balance technique,” requires metabolic balance measurements to determine net absorption of zinc together with isotopic measurement of intestinal excretion of endogenous zinc. The sum of these two measurements gives tqe quantity of dietary zinc absorbed [10]. This approach, too, is cumbersome as it requires quantitative metabolic collections of all feces and accurate collection of duplicate diets. However, for the determination of absorption of dietary zinc it does have several advantages. It is relatively inexpensive, can be applied to the widest possible range of composite meals, and can be used for determining the quantity of zinc absorbed over an entire day or for longer periods. Because this method is awkward and depends on very accurate metabolic collections, it is not surprising that extrinsic labeling techniques are those that are almost universally applied in practice. Extrinsic labeling techniques vary widely. For example, the isotopic label may be mixed in with the meal, when homogeneity of mixing is obviously vital as is accurate measurement of plate waste. After a series of pilot studies, we have, for many years, administered the extrinsic zinc label in an aqueous solution of zinc sulfate. The quantity given is proportional to the total quantity of endogenous zinc in each meal. The subject starts to sip this solution of extrinsic zinc label approximately half way through each meal and continues to sip for the remainder of the meal. This method, as with all extrinsic labeling techniques, assumes that the food’s endogenous zinc and the extrinsic zinc label mix in a common pool in the stomach. Our timing of the dose is to assure that there is substantial food in the stomach when the isotopic solution starts to enter that organ. The measurement achieved with extrinsic labeling is that of fractional absorption of zinc. Measurement of the total quantity of zinc ingested is also necessary to determine the quantity of zinc absorbed.
The objective of this study was to compare the results of simultaneous measurement of zinc absorption by the modified metabolic balance technique (TAZmetabolic) and our extrinsic labeling technique (TAZextrinsic), taking advantage of a more extensive study of zinc homeostasis in healthy women in Colorado [11].
Subjects and methods
Study design
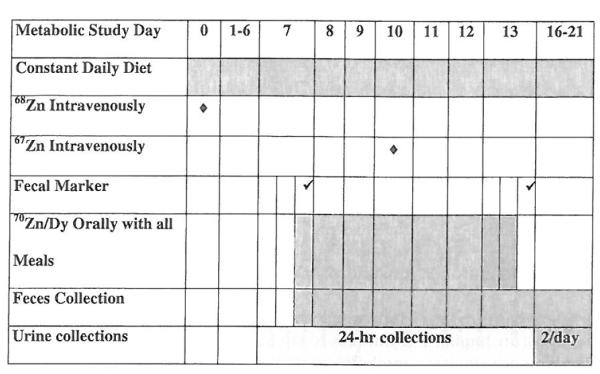
This study was a part of a larger zinc homeostasis study. Free-living, apparently healthy subjects were studied. Fecal excretion of endogenous zinc (EFZ) was measured by an isotope dilution technique [12] and was added to net absorption of zinc (NAZ) to determine TAZmetabolic. NAZ was determined from the difference between total dietary zinc and total fecal zinc for the 6-day metabolic period. FAZextrinsic was determined by a dual isotope tracer ratio technique (DITR) [12–15], in which extrinsic zinc stable isotope label was administered with each meal for the same 6-day period. The study timeline is summarized in Figure l.
Figure 1.
Study timeline
Subjects
Twenty-one female volunteers, 21–49 years of age (median 33.0) with a mean ± SD body mass index (kg/m2) of 24.1 ± 4.2 participated in the experiment. These healthy volunteers were recruited from the Denver metropolitan area. The study was approved by the Colorado Multiple Institutional Review Board. Written consent was obtained from all participants.
Study diet
Immediately following enrollment, participants met with a member of the staff of the General Clinical Research Center nutrition research team to receive instruction in the completion of a 7-day diet record. The 7-day diet record was analyzed by using the Nutrition Data System for Research, version 4.04_32 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Mean ± SD daily zinc intake was 11.4 ± 5.7 mg. A constant 3-day rotating diet with energy, zinc, and phytate intakes based on this record and. in consultation with the participant was then designed specifically for each subject. In particular, the constant daily diet was designed to provide an amount of dietary zinc similar to a subject’s usual zinc intake as indicated by analysis of the diet records. The median (range) of intakes of energy were 1907 (1402–2353) kcal/day; protein 76 (54–103) g/day; zinc 7.3 (5.2–28.2) mg/day, and phytate 778 (255–2075) mg/day. This constant diet was consumed from 7 days prior to the metabolic period through completion of the collection period. The meals were prepared in the General Clinical Research Center (GCRC) kitchen and collected on a daily basis by participants. During the 7-day metabolic study period, all meals were consumed under supervision either in the GCRC or in the Investigators’ nutrition laboratories. Duplicate dietsfrom each day were also collected for_ laboratory analyses of total zinc in the diet.
Isotope preparation and administration
Enriched stable zinc isotopes were obtained from Trace Science International, Ontario, Canada. Accurately weighed quantities of each isotopically enriched preparation were dissolved in 0.5 mol H2S04/L and then diluted in triply deionized water to prepare a stock solution. For preparation of orally administered doses, the stock solution of enriched 70Zn was diluted and titrated to pH 3.0 with metal-free ammonium hydroxide. This solution was filtered through a 0.22-μm filter to make a sterile solution. For preparation of intravenously administered doses of 67Zn and 68Zn, sterile techniques were used. The stock solutions were diluted and adjusted to pH 6.0, then filtered through a 0.22-μm filter to make a sterile solution. The University of Colorado Hospital Clinical Lab performed aerobic and anaerobic cultures to ensure sterility of the product. The solution was also tested for pyrogens prior to use. Concentrations of zinc in the isotope preparations were determined in triplicate by atomice absorption spectrophotometry and concentration measurements were adjusted for the different atomic weights of the preparations.
An accurately weighed quantity of sterile 68Zn (≈4 mg) was administered intravenously 7 days before the metabolic period. This tracer was used for the isotope dilution technique to determine endogenous fecal zinc. The tracer was administered over a thirty-minute interval via a scalp vein needle in a superficial forearm vein with a 3-way closed stopcock system. This allowed rinsing of the delivery syringe twice with N-saline contained in a second sterile syringe. An accurately weighed quantity of 67Zn (≈1 mg) was administered intravenously over a ten-minute interval on day 10 at 9:00 am. The timing of the 67Zn intravenous administration was optimized to ensure the accuracy of the dual tracer FAZextrinsic determinations by means of a simulation of the tracer administration and sampling protocols using a compartmental model of human zinc metabolism [16]. The simulation was performed by using WinSAAM 3.0 software (University of Pennsylvania, Kennett Square, PA).
A total of approximately 1~2 mg 70Zn (accurately weighed) was administered, orally divided between all meals for 6 days commencing with dinner on the first day of metabolic period and continuing through lunch on the sixth day of metabolic period. The traccr was administered in a solution starting halfway through the meal and sipped through the second half of the meal. The zinc-free plastic tube containing the isotope was rinsed three times with zinc-free water and this water was also administered orally to the participants. The amount given with each meal was roughly proportional to the total zinc in different meals typical for each subject. Dietary zinc intake ranged from 5.2 to 28.2 mg/day with an average of 11.4 ± 7.2 mg/day. The quantity of oral 70Zn tracer administered with meals was 0.2 to 0.3 mg/day or a maximum of 5.8 % of zinc in the diet.
One capsule containing ≈ 50 mg of brilliant blue was administered immediately prior to the first dose of 70Zn and again 6 days later at the same time, to mark the fecal collection period. Approximately 0.050 mg of the non-absorbable rare earth element dysprosium (Dy) (accurately measured) was added to each 70Zn oral dose administered with a meal during the metabolic period and measured subsequently in the fecal samples to monitor the completeness of collections.
Sample collection
All stools were collected from the time of the first 70Zn-labeled feed until the second fecal marker passed completely. Feces were collected separately and quantitatively in plastic bags. Complete 24-hour urine collections were collected in a zinc-free plastic container from days 7–13 during the metabolic period. Additionally, timed, clean-void midstream urine samples were collected twice daily from days 16 to 21. The times for each collection were noted on the specimen cup and log sheets. Duplicate meals were collected during the 6-day metabolic periods. Any plate waste food was collected. A baseline fecal and urine specimen were obtained prior to administration of any isotopic label. All samples were frozen at −20 °C until analysis.
Sample preparation and analyses
Accurately weighed duplicate aliquots of homogenized feces and whole-day food samples were dried separately to constant weight in an electric oven. The dried samples were then ashed in a muffle furnace at 450°C for 24 hours. A few drops of concentrated nitric acid were added to the ash, which was then heated on a hot plate before re-digesting at 450°C for 24 hours.
Ashed fecal and food samples were reconstituted quantitatively in 50 mL of 6 M HCl. The concentration of total zinc in these reconstituted fecal and food samples was determined on a diluted aliquot with an atomic absorption spectrophotometer fitted with a deuterium arc background correction lamp (Perkin-Elmer Corporation, Norwalk, CT.).
For the measurements of zinc stable isotope ratios in fecal samples, the inorganic elements were removed from reconstituted ashed samples by ion-exchange chromatography with AG-1 ion exchange resin (Bio-Rad Laboratories, Richmond, CA) [12,17].
Urine samples were digested in using an MDA-2000 microwave sample preparation system (CEM Corp., Mathews, NC). A 5-mL urine sample was placed into an Advanced Composite Vessel, combined with 1mL of concentrated HN03, and the pressure was gradually increased to a maximum pressure of 120 psi. Total digestion time was ≈ 90 minutes. Digested samples were transferred to a beaker, evaporated to dryness on a hot block, and reconstituted in 2 mL ammonia acetate buffer (pH = 5.6), then zinc in the sample was purified by its chelation with trifluoroacetylacetone and extraction of the chelate with hexane [18].
Isotope enrichment was determined by measurement of isotope ratios 67Zn/66Zn, 68Zn/66Zn, and 70Zn/66Zn by inductively coupled plasma mass spectrometry (ICP-MS) (VG Plasma Quad 3; VG Elemental, Cheshire, United Kingdom) [19]. Ratios were then converted to enrichment by a mathematical matrix. Tracer enrichment was defined as all zinc in the sample from the isotopically enriched tracer preparation, divided by the total zinc in the sample.
After reconstitution of the digested fecal aliquots, Dy concentration of the samples was measured using ICP-MS [20].The total amount of Dy measured in the fecal samples collected during the metabolic study was compared to the amount of Dy administered orally in order to calculate recovery of Dy and to monitor completeness of fecal collection in these free-living study participants.
Data processing
Total dietary zinc (TDZ) was calculated for each of the 6 days in the metabolic period using the laboratory analyses of the zinc content of the duplicate diet samples. These values were then averaged to obtain the mean TDZ intake.
FAZextrinsic was determined for each of the 12 urine specimens obtained during study days 16-21 using the following calculation and the results were averaged to obtain a mean FAZextrinsic for the subject [12,13].
Where 70Zn was the orally administered, and 67Zn the intravenously administered, tracer.
TAZextrinsic was then determined from the product of FAZextrinsic and total dietary zinc (TDZ) in mg/d.
All variables are in units of mg/day.
EFZ was determined using the following calculation: EFZ = Σ(F×f)/(u×d) [12]
Where: F is total fecal zinc (mg) and f is the enrichment of intravenously administered 68Zn tracer in each individual fecal sample collected between the markers, the products of which are then summed; u is average 68Zn enrichment in urine during the inter-marker period; and d is the duration of the period (6 days).
Statistical analysis
All results are presented as mean ± SD unless otherwise stated. All statistical analyses were performed by using GRAPHPAD PRISM (version 4.0; GraphPad Software Inc, San Diego, CA).
Results
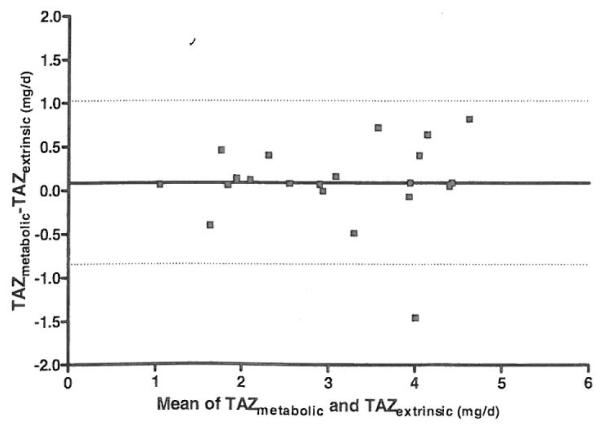
Results are summarized in Table I. TAZextrinsic and TAZmetabolic were 3.0 ± 1.1mg/day and 3.1 ± 1.1mg/ day, respectively. A paired t-test indicated that there was not a significant difference between TAZextrinsic and TAZmetabatic (p = 0.492). There was a high correlation between them (r = 0.91) and linear regression analysis (Figure 2) showed a slope and y intercept not significantly different from 1 and 0, respectively. A Bland-Altman analysis of the agreement between TAZextrinsic and TAZmetabolic (Figure 3) showed a mean difference of 0.07 with limits of the agreement of −0.86 to 1.01. FAZextrinsic and FAZmetabolic were 0.30 ± 0.10 and 0.30 ± 0.12, respectively. The correlation coefficient for FAZextrinsic and FAZmetabolic was 0.95. Dy recovery in the stools was 99.5 ± 4.2 %.
Table I.
Measurements of TDZ, oral dose, TFZ, EFZ, NAZ, AZ, FAZ
| TDZ (mg/d) |
Oral dose (mg/d) |
EFZ (mg/d) |
TFZ (mg/d) |
NAZ (mg/d) |
TAZmetabolic
(mg/d) |
FAZextrinsic
(mg/d) |
FAZmetabolic | FAZextrinsic |
|---|---|---|---|---|---|---|---|---|
| 11.4 ± 7.2 | 0.29 ± 0.05 | 2.73 ± 0.80 | 11.4 ± 6.7 | 0.37 ± 1.25 | 3.1 ± 1.1 | 3.0 ± 1.1 | 0.30 ± 0.12 | 0.30 ± 0.10 |
TDZ, total dietary zinc
EFZ, endogenous fecal zinc by isotope dilution technique
TFZ, total fecal zinc
NAZ, net absorbed zinc
TAZmetabolic, zinc absorbed determined by NAZ and EFZ
TAZextrinsic, zinc absorbed using FAZextrinsic
FAZmetabolic, fractional absorption of dietary zinc
FAZextrinsic, fractional absorption of extrinsic labeling zinc by measurement d, day
Figure 2.
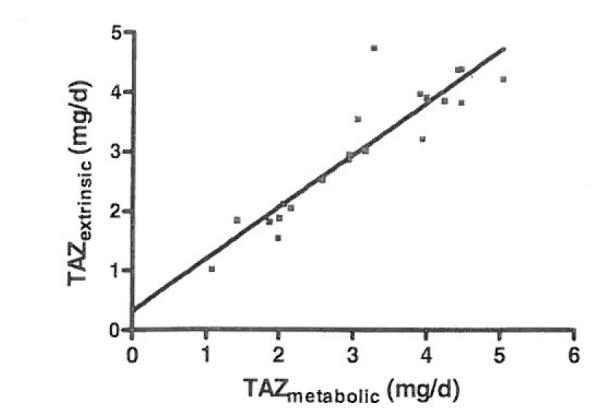
Linear regression analysis of the extrinsic labeling method compared with the quantity of absorbed zinc determined by the metabolic method
Figure 3.
Bland-Altman plot of agreement between metabolic and extrinsic labeling methods for determining quantity of zinc absorbed each day. Figure shows the bias (solid line) and the 95 % limits of agreement (dotted line).
Discussion
Previous research undertaken with the goal of validating extrinsic labeling techniques to measure zinc absorption have relied on comparison with absorption of isotope used to intrinsically label zinc in foods. Early pilot research using zinc stable isotopes for this purpose was undertaken by Evans and Johnson [8] and by Ketelson et al. in rats [5] and by Janghorbani and Young [21] in human studies. There were mixed results when solid foods were intrinsically labeled [5]. Evans and Johnson [8] and, later, Serfass and colleagues [6,7] in human studies, compared results of extrinsic labeling using milks or milk-based formulas. These provide a relatively simple food for extrinsic labeling. Overall, results were encour-aging for the use of extrinsic labeling in this liquid medium. However, studies have been limited and evaluation of the accuracy of extrinsic labeling for determining zinc absorption from a variety of self-selected diets has never been undertaken.
Neither of the two methods compared in this study can be regarded as a gold standard. Indeed, it is difficult to conceive of a gold standard for measuring total absorption of dietary zinc over an entire day or longer. The dietary method used here has a notable advantage over intrinsic isotopic labeling of dietary zinc in that it can be applied readily to the widest possible range of diets. This is not only a general advantage, but is clearly an advantage in this study of women consuming a diet based on their habitual diets, which covered a wide range of usual preferences. But it does depend on subtracting one large number from another quite similar large number to determine net absorption of zinc and depends on accurate collection of fecal samples. Moreover, it is labor-intensive and relatively expensive because of the time required for participant training, minimizing the risk of and monitoring for non-compliance, and laboratory processing of diet and fecal samples. In comparison, extrinsic labeling techniques, especially those utilizing dual isotope tracer ratio measurements [12,13] are relatively simple with respect to both sample collection and preparation. The question is, are they accurate?
We have already had reassurance that results using our dual isotope tracer ratio (DITR) technique based on measurements of isotopic enrichment in urine [12–15] for measuring absorption of extrinsic zinc label compare well with results of other techniques, including deconvolution and data obtained from compartmental modeling [22]. The other major question is whether our extrinsic labeling technique provides an accurate measure of the absorption of dietary zinc that has been extrinsically labeled. As we do not have a gold standard we cannot, theoretically, be certain using the design employed in this study. However, the only shared data for these two techniques is the quantity of zinc ingested from the test meals. All other data and techniques were different for the two methods. Therefore, the very similar results are not explicable on the basis of study design and are extraordinarily unlikely to be the result of pure chance if both techniques were inaccurate. Thus, these results provide strong evidence for the validity of both methods. In particular, they give support to the premise that our extrinsic labeling technique provides an acceptable technique for determining the quantity of dietary zinc absorbed from the labeled meals.
In conclusion, the results of this study provide reassurance that a relatively simple technique for extrinsic labeling of total human diets with zinc stable isotopes provides a reliable and practical means of determining the quantity of zinc absorbed from these diets. This not only confers needed legitimacy on multiple studies already published by us and other investigators using extrinsic labeling of diets in studies of zinc homeostasis, but also provides reassurance that this methodology is acceptable for future zinc absorption studies.
Acknowledgements
We thank all the subjects who participated in the study. We gratefully acknowledge the contribution of Therese Ida and the dieticians, nurses, and kitchen staff of the General Clinical Research Center at the University of Colorado Hospital for their assistance with the conduct of this study. We also thank Gary Grunwald for statistical advice.
This research was supported by USDA 2003–00900; GCRC M01RR00051; MOlRR00069–41; the Nestle Foundation PN0312–121; NIH K24 RR018357; CNRU #5P30 ; and the Science and Technology Commission of Shanghai Municipality 03JC14042, 08PJ14082.
List of abbreviations
- TAZmetabolic
zinc absorbed determined by NAZ and EFZ
- TAZextrinsic
zinc absorbed using FAZextrinsic
- FAZmetabolic
fractional absorption of dietary zinc
- FAZextrinsic
fractional absorption of extrinsic labeling zinc by DITR measurement
- TDZ
total dietary zinc
- EFZ
endogenous fecal zinc by isotope dilution technique
- TFZ
total fecal zinc
- NAZ
net absorbed zinc
- DITR
dual isotope tracer ratio technique
References
- 1.Hambidge M. Human zinc deficiency. J. Nutr. 2000;130:1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 2.Hambidge M. Underwood memorial lecture: Human zinc homeostasis: Good but not perfect. J. Nutr. 2003;133:1438S–1442S. doi: 10.1093/jn/133.5.1438S. [DOI] [PubMed] [Google Scholar]
- 3.Hambidge KM, Krebs NF, Westcott JE, Miller LV. Changes in zinc absorption during development. J. Pediatr. 2006;149:S64–S68. doi: 10.1016/j.jpeds.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 4.International Zinc Nutrition Consultative Group . Assessment of the risk of zinc status in populations and options for the control of zinc deficiency. International Nutrition Foundation for United Nations University Press; Boston, MA: 2004. [Google Scholar]
- 5.Ketelsen SM, Stuart MA, Weaver CM, Forbes RM, Erdman JW., Jr. Bioavailability of zinc to rats from defatted soy flour, acid-precipitated soy concentrate and neutralized soy concentrate as determined by intrinsic and extrinsic labeling techniques. J. Nutr. 1984;114:536–542. doi: 10.1093/jn/114.3.536. [DOI] [PubMed] [Google Scholar]
- 6.Egan CB, Smith FG, Houk RS, Serfass RE. Zinc absorption in women: Comparison of intrinsic and extrinsic stable-isotope labels. Am. J. Clio. Nutr. 1991;53:547–553. doi: 10.1093/ajcn/53.2.547. [DOI] [PubMed] [Google Scholar]
- 7.Serfass RE, Ziegler EE, Edwards BB, Houk RS. Intrinsic and extrinsic stable isotopic zinc absorption by infants from formulas. J. Nutr. 1989;119:1661–1669. doi: 10.1093/jn/119.11.1661. [DOI] [PubMed] [Google Scholar]
- 8.Evans GW, Johnson PE. Determination of zinc availability in foods by the extrinsic label technique. Am. J. Clin. Nu tr. 1977;30:873–878. doi: 10.1093/ajcn/30.6.873. [DOI] [PubMed] [Google Scholar]
- 9.Fairweather-Tait SJ, Dainty J. Use of stable isotopes to assess the bioavailability of trace elements: A review. Food Additives Contaminants. 2002;19:939–947. doi: 10.1080/02652030110087474. [DOI] [PubMed] [Google Scholar]
- 10.Jackson MJ, Jones DA, Edwards RH, Swainbank IG, Coleman ML. Zinc homeostasis in man: Studies using a new stable isotope-dilution technique. Br. J. Nutr. 1984;51:199–208. doi: 10.1079/bjn19840024. [DOI] [PubMed] [Google Scholar]
- 11.Sheng XY, Hambidge KM, Miller LV, Bailey K, Gibson RS, Westcott JE, Lei S, Grunwald G, Krebs NF. Major parameters of zinc homeostasis in adult women with a wide range of habitual zinc intake. FASEB J. 2008;22:697–691. [Google Scholar]
- 12.Krebs N, Miller LV, Naake VL, Lei S, Westcott JE, Fennessey PV, Hambidge KM. The use of stable isotope techniques to assess zinc metabolism. J. Nutr. Biochem. 1995;6:292–307. [Google Scholar]
- 13.Friel JK, Naake VL, Jr., Miller LV, Fennessey PV, Hambidge KM. The analysis of stable isotopes in urine to determine the fractional absorption of zinc. Am. J. Clin. Nutr. 1992;55:473–477. doi: 10.1093/ajcn/55.2.473. [DOI] [PubMed] [Google Scholar]
- 14.Shames DM, Woodhouse LR, Lowe NM, King JC. Accuracy of simple techniques for estimating fractional zinc absorption in humans. J. Nutr. 2001;131:1854–1861. doi: 10.1093/jn/131.6.1854. [DOI] [PubMed] [Google Scholar]
- 15.Sparacino G, Shames DM, Vicini P, King JC, Cobelli C. Double isotope tracer method for measuring fractional zinc absorption: Theoretical analysis. Am.J. Physiol. Endo. Metab. 2002;282:E679–687. doi: 10.1152/ajpendo.00113.2001. [DOI] [PubMed] [Google Scholar]
- 16.Miller LV, Krebs NF, Hambidge KM. Development of a compartmental model of human zinc metabolism: Identifiability and multiple studies analyses. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000;279:R1681–R1684. doi: 10.1152/ajpregu.2000.279.5.R1671. [DOI] [PubMed] [Google Scholar]
- 17.Peirce PL, Hambidge KM, Goss CH, Miller LV, Fennessey PV. Fast atom bombardment mass spectrometry for the determination of zinc stable isotopes in biological samples. Anal. Chem. 1987;59:2034–2037. doi: 10.1021/ac00144a006. [DOI] [PubMed] [Google Scholar]
- 18.Veillon C, Patterson KY, Moser-Veillon PB. Digestion and extraction of biological materials for zinc stable isotope determination by inductively coupled plasma mass spectrometry. J. Anal. Atomic Spectro. 1996;11:727–730. [Google Scholar]
- 19.Hambidge KM, Huffer JW, Raboy V, Grunwald GK, Westcott JL, Sian L, Miller LV, Dorsch JA, Krebs NF. Zinc absorption from low-phytate hybrids of maize and their wild-type isohybrids. Arn. J. Clin. Nutr. 2004;79:1053–1059. doi: 10.1093/ajcn/79.6.1053. [DOI] [PubMed] [Google Scholar]
- 20.Sheng XY, Harnbidge KM, Krebs NF, Lei S, Westcott JE, Miller LV. Dysprosium as a nonabsorbable fecal marker in studies of zinc horneo-stasis. Arn. J. Clin. Nutr. 2005;82:1017–1023. doi: 10.1093/ajcn/82.5.1017. [DOI] [PubMed] [Google Scholar]
- 21.Janghorbani M, Istfan NW, Pagounes JO, Steinke FH, Young VR. Absorption of dietary zinc in man: Comparison of intrinsic and extrinsic labels using a triple stable isotope method. Arn. J. Clin. Nutr. 1982;36:537–545. doi: 10.1093/ajcn/36.3.537. [DOI] [PubMed] [Google Scholar]
- 22.Harnbidge KM, Miller L, Krebs NF, Westcott J, Grunwald G. Accuracy of simple techniques for estimating fractional zinc absorption in humans. J. Nutr. 2002;132:322–323. doi: 10.1093/jn/132.2.322. [DOI] [PubMed] [Google Scholar]