Abstract
Tooth loss is a significant health issue that affects the physiological and social aspects of everyday life. Missing teeth impair simple tasks of chewing and speaking, and can also contribute to reduced self-confidence. An emerging and exciting area of regenerative medicine based dental research focuses on the formation of bioengineered whole tooth replacement therapies that can provide both the function and sensory responsiveness of natural teeth. This area of research aims to enhance the quality of dental and oral health for those suffering from tooth loss. Current approaches use a combination of dental progenitor cells, scaffolds and growth factors to create biologically based replacement teeth to serve as improved alternatives to currently used artificial dental prosthetics. This article is an overview of current progress, challenges, and future clinical applications of bioengineered whole teeth.
Keywords: Odontogenesis, Tooth Loss, Cell Differentiation, Odontoblasts, Ameloblast, Dentin
Introduction
As a highly prevalent disease, tooth loss affects over 158 million people worldwide [1]. Craniofacial birth defects, poor dental hygiene, battlefield injuries, accidental and intentional traumatic injuries all can contribute to tooth loss. Currently, artificial dental implants are the most commonly used tooth replacement therapy. Unfortunately, dental implants are prone to failures, and are associated with complications such as tissue and bone loss around the implant site, fracture, peri-implantitis, infections and inflammation. [2, 3]. All of these issues highlight the clinical need for dental implant alternatives, including biologically based replacement teeth as superior alternatives to artificial dental implants [4, 5].
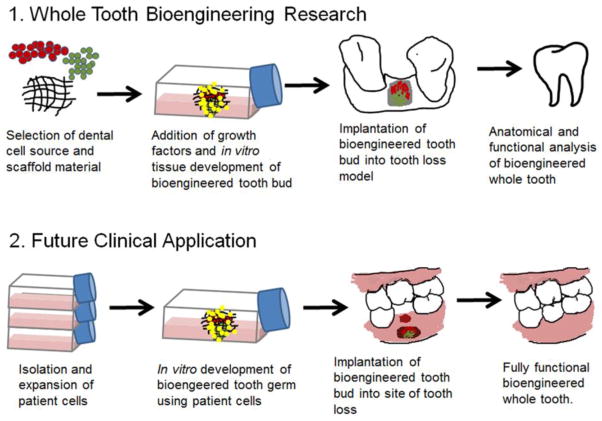
Ideally, bioengineered teeth would be generated using autologous dental cells extracted from an individual patient, such as those harvested from an extracted wisdom tooth, which would then be expanded in in vitro tissue culture. Once sufficient numbers of cells are generated, they would then be incorporated within a scaffold, and implanted at the site of tooth loss, where it would be expected to develop, erupt and function like a natural tooth. This regenerative therapy approach will only become a reality in the clinic once extensive investigation identifies postnatal dental cells sources, appropriate scaffold materials and fabrication, and inductive factors that can be readily used for devising bioengineered teeth that resemble natural teeth (Figure 1).
Figure 1. Models for whole tooth bioengineering.
1) Research Objective. In order for whole tooth bioengineering to become a reality in a clinical setting, extensive research must be conducted. This research includes identifying suitable cell sources and scaffold materials that support the in vitro development of a bioengineered tooth bud for implantation into a tooth loss model. 2) Future Clinical Applications.. Once appropriate materials have been validated, they should be easily translatable for clinical application of using patients own cells to correct an area of tooth loss by regenerating a fully functional tooth.
Natural teeth are highly complex organs composed of hard mineralized tissues, including enamel, dentin, and cementum, and soft tissues including dental pulp and periodontal ligament [6]. All of these tissues originate from the ectoderm-derived dental epithelium and the neural crests derived dental mesenchyme, whose early interactions initiate and subsequently support reciprocal and reiterative signaling throughout tooth development [7, 8]. The initiation of tooth development is defined by a thickening of the oral epithelium that then invaginates into the underlying dental mesenchyme. The surrounding dental mesenchyme then condenses, leading to morphogenesis of the dental epithelium and dental cell differentiation [6, 9, 10]. Dental epithelial derived ameloblasts are the differentiated cells that are responsible for enamel production, while differentiated dental mesenchymal derived odontoblasts produce dentin [5, 8, 11, 12]. The process of tooth development is regulated by the interactions of the dental tissues – dental epithelium and dental mesenchyme. It has been shown that if this interaction is prevented, tooth development will not progress [13–15]. It has also been demonstrated by classical tissue recombinant studies that the odontogenetic potential, or the instructional capability of the dental tissues and cells, is conserved even after tissue dissociation and in vitro culture [16]. These concepts that drive natural tooth development provide an instructive guide for optimal conditions that can be used to create bioengineered whole teeth.
Dental Cell Sources for Whole Tooth Bioengineering
Embryonic dental stem cells, harvested from mice and/or rats, have commonly been used in many historic and current tooth regeneration studies due to their significant odontogenic potential. However, human embryonic cells cannot be used as a clinically relevant human dental cell source due to unavoidable ethical issues, potential for immune reaction and rejection, and malignant potential. Current embryonic dental stem cell alternatives focus on using postnatal dental stem and progenitor cells isolated from adult dental epithelial and dental mesenchymal tissues. Prior published reports have demonstrated the odontogenic potential of postnatal (adult) dental epithelial and dental mesenchymal single cell suspensions, including the ability to produce anatomically accurate tooth crowns consisting of dentin, pulp and enamel. [17–19].
Today, the most common source of dental mesenchymal stem cells being used for tooth regeneration research is postnatal dental pulp stem cells (DPSCs). The dental pulp contains an enriched population of stem cells that can be easily isolated. In numerous studies, DPSC have been shown to differentiate into odontoblasts and osteoblasts, and to form pulp, dentin, and cementum tissues respectively [20–23]. Stem cells of human exfoliated deciduous teeth (SHED) can be isolated from the pulp of primary human teeth, and have demonstrated capacity to differentiate into odontoblasts, and to produce dentin-like and pulp-like tissues [24, 25]. SHED can be extracted from a very accessible source – human baby teeth - and have the ability to provide an adequate number of cells for regenerative dental applications [24]. Stem cells of the apical pallia (SCAP) are isolated from pulp tissue located within open roots of developing baby teeth [26], and have been shown to differentiate into odontoblasts and osteoblasts, and to form dentin-like structures. [27]. Dental follicle precursor cells (DFPCs) are mesenchymal cells that surround and enclose the developing tooth bud, and which will eventually contribute to the periodontal ligament and cementum tissues [28]. DFPCs are able to differentiate into cementoblasts that form cementum, and to periodontal ligament-like tissues [28], and have been found to be suitable for dentin regeneration [29]. Similarly, Periodontal Ligament Stem Cells (PDLSCs) have been shown to differentiate into cementum forming cementoblasts, as well as periodontal ligament-like tissues [30]. In addition, when PDLSCs were combined with DPSCs, root-like and dentin-like structures were formed [20].
Various tissue sources have also been investigated to successfully generate dental epithelial cells that can differentiate into enamel secreting ameloblasts. For example, dental epithelial cell rests of Malassez (ERM) have the ability to differentiate into ameloblast like cells and to produce enamel when combined with dental pulp cells [31]. Another study showed that when cells from the enamel organ were combined with dental mesenchymal cells, enamel-dentin structures were formed [32]. It has also been shown that skin epithelial cells have the ability to express ameloblasts markers when cultured with dental pulp cells [33]. Finally, adult human gingival cells have the ability to form enamel structures when combined with dental mesenchymal cells [34]. Any or all of these dental epithelial cell sources may prove promising for effective whole tooth tissue engineering applications.
Recently, investigations using induced pluripotent stem cells (iPSCs) for tooth regeneration research have increased. These cells are pluripotent and therefore have the ability to develop into a variety of cells types [35–37]. It has been shown that gingival cells, SHED, SCAP, DSCPs, and periodontal ligament cells can all be used to create iPSCs [38–40]. In addition, iPSCs have been shown to exhibit the ability to differentiate into ameloblast-like and odontoblast-like cells [41, 42].
The field of tooth tissue engineering and regenerative dentistry has investigated this wide variety of cell types to identify sources that can easily be accessed and utilized for clinical dental applications. The knowledge gained from the use of the cells mentioned above has helped to further our understanding and appreciation of how they can be combined and utilized to advance whole tooth bioengineering research. Also, it was recently reported that a scaffold free method can be used to examine the usefulness of various cell sources for the use in tooth regenerative studies [43]•• In addition, a recent study has demonstrated that recombination of post-natal dental epithelial and dental mesenchymal tissues have the ability to form tooth structures, offering an alternative to single cell suspension techniques in whole tooth regeneration [44].••
Scaffold Materials and Bioprinitng for Tooth Tissue Engineering
Appropriate selection of scaffold materials is very important for regenerative dental applications, as the microenvironment provides cellular support and mechanical cues that affect cell behavior. For example, it has been shown that hydrogel scaffold stiffness can influence the fate of mesenchymal stem cells [45]. In addition, scaffold materials must allow cellular attachment, spreading, proliferation, and differentiation to allow the development of the desired tissues. Furthermore and ideally, scaffold degradation rate should match the rate of extracellular matrix (ECM) deposition by the cells, in order to ensure robust formation and durability of the bioengineered tissue [46]. An extensive variety of natural and synthetic scaffold materials have been investigated for tooth regeneration applications [46–48]. One group of materials that has been examined are poly-L-lactic acid (PLLA)/polylactic-co-glycolic acid (PLGA) polymers [49–51]. PLLA, PLGA and their derivatives are synthetic polymers that can be readily prepared. Hydrogel based materials such as collagen, gelatin, and alginate are highly tunable, and have been used to successfully bioengineer various dental tissues [21, 22, 32, 52]. Silk-based materials have also shown promise in providing an environment that can support osteo-dentin like mineralized tissue formation, but further optimizations are needed to enhance bioengineered dental tissue formation [53, 54]. A combination of these and other novel materials may eventually be used to successfully engineer the wide variety of hard and soft tissues that comprise the natural tooth.
The size and shape of scaffold materials can be easily and meticulously generated with the use of 3D printing [55]. This fabrication method deposits material layer by layer until a desired 3D structure is produced [56]. Today, 3D printers are able to dispense plastic, ceramics, biomaterials, and even cells in a highly organized manner [56–58]. It has been suggested that 3D printing can be utilized in regenerative medicine to aide in the creation of complex bioengineered tissues and organs [58, 59]. In addition, 3D printing can offer customizations on a patient to patient basis [56, 60].
Incorporation of Growth Factors
Growth factors are soluble proteins that direct the development of various tissues and organs. Several important growth factors are involved in natural tooth development, including bone morphogenetic protein (BMP), fibroblastic growth factor (FGF), and transforming growth factor beta 1 (TGFβ1) [5, 8, 46, 61, 62]. The addition of these factors to bioengineered tooth constructs can therefore be used to enhance the successful generation of bioengineered whole teeth.
BMP4 is thought to play an important role in tooth morphogenesis by activating transcription factors in the dental mesenchyme [63, 64]. BMP4, in combination with BMP2 and BMP7, regulate cell proliferation, tooth patterning and crown shape [65–67]. Additionally, BMP4 is involved in ameloblast differentiation and tooth root formation [68, 69]. Furthermore, it has been suggested that loss of bmp4 gene expression may account for the lack of teeth in birds [70]. Roles for Bmp signaling in both dental mesenchymal and dental epithelial cell differentiation was further demonstrated by the differentiation of iPSCs into both odontoblastic and ameloblastic lineages, respectively, by the addition of exogenous BMP4. FGF signaling has been shown to be required for tooth morphogenesis [71]. Decreased FGF signaling prevents tooth development [71, 72]. TGFβ1 can induce odontoblast differentiation, pulp and dentin formation [73–76], and has been used to enhance the differentiation DPSCs into odontoblasts in vitro [21]. These studies emphasize that selective incorporation of combinations of these growth factors into novel bioengineered tooth constructs could be used to enhance dental cell differentiation, and dental tissue and whole tooth formation.
Host Implant Models for Tooth Tissue Engineering
Small animals such as mice, rats, ferrets and rabbits are ideal for in vivo tooth regeneration studies that include a large number of samples, as their maintenance is more cost effective than large animals. Usual implantation sites in small animals, such as subcutaneous pockets and renal capsules, are selected based on their high vascular availability. Tooth extraction socket/implantation sites of smaller animals may be difficult to perform and analyze because the operation area is small and delicate and their dentition is not similar to humans. Normally larger animals are used for more advanced studies of tooth construct jaw implants. Mini-pigs are commonly used for tooth/alveolar bone implantation studies because they have a dentition similar to humans.[77, 78] It has been suggested that the implantation site is important as it may influence the morphology of the bioengineered tooth [21]. Therefore, knowing how, when and where to place the bioengineered tooth implant can greatly affect its outcome.
Current Progress and Challenges in Whole Tooth Regeneration
The ideal bioengineered whole tooth would mimic the development, function and appearance of a natural tooth. To date, only a handful of studies have demonstrated the successful generation of fully functional bioengineered teeth, by implanting bioengineered tooth constructs composed of embryonic dental cells that were implanted and grown in mice tooth extraction sites [79–81]. As already mentioned, the clinical relevance of these studies is hindered by the fact that embryonic stem cells, versus adult stem cells were used. Nevertheless, these studies can be used to guide strategies to generate bioengineered whole teeth for the use of human tooth replacement. One of the earliest successful whole tooth regeneration studies used single cell suspensions of postnatal dental cells to engineer whole tooth crowns consisting of dental pulp, dentin, enamel, and tooth root tissues [49]. These anatomically correct tooth crowns were imperfect, in that they were very small and did not conform to the size and shape of the scaffold. Since then, additional studies have focused on identifying appropriate sources of adult dental cells, on appropriate and optimal scaffold materials, and on growth factor combinations that can properly direct the regeneration of functional bioengineered tooth. A recent report describes the use of a gelatin-chondroitin-hyaluronan scaffold seeded with postnatal dental cells implanted into a healed mandibular tooth extraction site of an adult Lanyu miniature pig, to successfully generate enamel-like tissues, dentin, cementum, and developing tooth roots [21]••. Further improvements to this model, including validation that the purported bioengineered tooth was in fact not a natural pig replacement tooth, as well as functional analysis of these bioengineered teeth, would significantly improve the significance of this study.
Today, the major challenges facing the field of whole tooth bioengineering are identifying reliable sources of dental epithelial cells for clinical applications, and optimizing methods to fabricate scaffolds that can promote and accommodate the organized growth of all of the various hard and soft dental tissues, to form functional bioengineered teeth of specified size and shape. Additionally, bioengineered teeth must be sufficiently vascularized and integrated within the recipient anatomy. Overcoming these challenges may eventually contribute to emerging alternatives such as is bio-hybrid teeth, composed of both bioengineered living tissue and artificial materials [20, 82]•.
Conclusions and the Future of Whole Tooth Tissue Engineering
Whole tooth bioengineering is an exciting field that has emerged to provide an alternative to dental prosthesis currently used to treat the large numbers of people suffering from tooth loss. Although dental prosthetics historically have been the hallmark of tooth replacement therapy, associated complications reveal the need for significant improvements. The field of whole tooth bioengineering research has demonstrated distinct accomplishments during its relatively short life. However, current research efforts must be directed to focus on the challenges and limitations that currently block our ability to reliably create clinically relevant bioengineered replacement teeth. Still, recent accomplishments indicate that despite the fact that teeth are complex organs composed of a wide variety of soft and hard tissues, whole tooth bioengineering for human tooth replacement is indeed possible, and in fact is the future of dentistry.
Footnotes
Conflict of Interest
Elizabeth E. Smith declares that she has no conflict of interest.
Pamela C. Yelick reports that she has two patents pending, one relevant to the field of study, and one that is not.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Elizabeth E. Smith, Department of Cell, Molecular, and Developmental Biology, Sackler School of Graduate Biomedical Sciences, Tufts University School Medicine, Department of Orthodontics, Tufts University School of Dental Medicine
Pamela C. Yelick, Director, Division of Craniofacial and Molecular Genetics, Professor, Department of Orthodontics, Tufts University School of Dental Medicine, Department of Biomedical Engineering, Tufts University, Department of Cell, Molecular, and Developmental Biology, Sackler School of Graduate Biomedical Sciences Tufts University School of Medicine, 136 Harrison Avenue, M824, Boston MA 02111.
References
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenstein G, Cavallaro J, Romanos G, Tarnow D. Clinical Recommendations for Avoiding and Managing Surgical Complications Associated With Implant Dentistry: A Review. Journal of Periodontology. 2008;79:1317–1329. doi: 10.1902/jop.2008.070067. [DOI] [PubMed] [Google Scholar]
- 3.Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008;19:119–130. doi: 10.1111/j.1600-0501.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 4.Yen AH, Yelick PC. Dental Tissue Regeneration – A Mini-Review. Gerontology. 2011;57:85–94. doi: 10.1159/000314530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai WF, Lee JM, Jung HS. Molecular and engineering approaches to regenerate and repair teeth in mammals. Cell Mol Life Sci. 2014;71:1691–1701. doi: 10.1007/s00018-013-1518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. Journal of Cell Science. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 7.Thesleff I, Nieminen P. Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol. 1996;8:844–850. doi: 10.1016/s0955-0674(96)80086-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: growth factors, transcription factors, and stem cells. Cell Res. 2005;15:301–316. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- 9.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 10.Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140A:2530–2535. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- 11.Ruch JV, Lesot H, Karcher-Djuricic V, Meyer JM, Olive M. Facts and Hypotheses Concerning the Control of Odontoblast Differentiation. Differentiation. 1982;21:7–12. doi: 10.1111/j.1432-0436.1982.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 12.Jussila M, Thesleff I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thesleff I, Vainio S, Jalkanen M. Cell-matrix interactions in tooth development. The International Journal of Developmental Biology. 1989;33:91–97. [PubMed] [Google Scholar]
- 14.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 15.Glasstone S. The development of tooth germs on the chick chorio-allantois. J Anat. 1954;88:392–399. [PMC free article] [PubMed] [Google Scholar]
- 16.Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 17.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered Teeth from Cultured Rat Tooth Bud Cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 18.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11 doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 19.Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. Bioengineered Dental Tissues Grown in the Rat Jaw. J Dent Res. 2008;87:745–750. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao ZH, Hu L, Liu GL, Wei FL, Liu Y, Liu ZH, Fan ZP, Zhang CM, Wang JS, Wang SL. Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. Journal of Dental Research. 2016 doi: 10.1177/0022034516639260. [DOI] [PubMed] [Google Scholar]
- 21••.Yang KC, Kitamura Y, Wu CC, Chang HH, Ling TY, Kuo TF. Tooth Germ-Like Construct Transplantation for Whole-Tooth Regeneration: An In Vivo Study in the Miniature Pig. Artif Organs. 2016;40:E39–E50. doi: 10.1111/aor.12630. This recent study is of major importance because it showed the successful generation of erupted bioengineered teeth in a porcine tooth loss model. Implanted tooth buds were composed of gelatin-chrodroitin-hyaluronan scaffolds seeded with differentiated odontoblast and osteoblasts and gingival epithelial cells. This investigation supports the proposal for using adult autologous cells for whole tooth bioengineering in future clinical applications. [DOI] [PubMed] [Google Scholar]
- 22.Bhoj M, Zhang C, Green DW. A First Step in De Novo Synthesis of a Living Pulp Tissue Replacement Using Dental Pulp MSCs and Tissue Growth Factors, Encapsulated within a Bioinspired Alginate Hydrogel. J Endod. 2015;41:1100–1107. doi: 10.1016/j.joen.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth. J Endod. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Sedgley CM, Botero TM. Dental Stem Cells and Their Sources. Dent Clin North Am. 2012;56:549–561. doi: 10.1016/j.cden.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, Liu Y, Li Y, Zhou Y, Zhou J, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708–6723. doi: 10.1016/j.biomaterials.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Seo B-M, Miura M, Gronthos S, Mark Bartold P, Batouli S, Brahim J, Young M, Gehron Robey P, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. The Lancet. 364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 31.Shinmura Y, Tsuchiya S, Hata K-i, Honda MJ. Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. J Cell Physiol. 2008;217:728–738. doi: 10.1002/jcp.21546. [DOI] [PubMed] [Google Scholar]
- 32.Honda MJ, Shinohara Y, Hata KI, Ueda M. Subcultured Odontogenic Epithelial Cells in Combination With Dental Mesenchymal Cells Produce Enamel–Dentin-Like Complex Structures. Cell Transplant. 2007;16:833–847. doi: 10.3727/000000007783465208. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Jiang M, Hao W, Liu W, Tang L, Liu H, Jin Y. Skin epithelial cells as possible substitutes for ameloblasts during tooth regeneration. J Tissue Eng Regen Med. 2013;7:934–943. doi: 10.1002/term.1485. [DOI] [PubMed] [Google Scholar]
- 34.Angelova Volponi A, Kawasaki M, Sharpe PT. Adult Human Gingival Epithelial Cells as a Source for Whole-tooth Bioengineering. J Dent Res. 2013 doi: 10.1177/0022034513481041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Karagiannis P, Eto K. Ten years of induced pluripotency: from basic mechanisms to therapeutic applications. Development. 2016;143:2039–2043. doi: 10.1242/dev.138172. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Zhang Y, Chen S, Cai J, Pei D. Application of iPS Cells in Dental Bioengineering and Beyond. Stem Cell Reviews and Reports. 2014;10:663–670. doi: 10.1007/s12015-014-9531-2. [DOI] [PubMed] [Google Scholar]
- 38.Egusa H, Okita K, Kayashima H, Yu G, Fukuyasu S, Saeki M, Matsumoto T, Yamanaka S, Yatani H. Gingival Fibroblasts as a Promising Source of Induced Pluripotent Stem Cells. PLoS One. 2010;5:e12743. doi: 10.1371/journal.pone.0012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada N, Wang B, Lin NH, Laslett AL, Gronthos S, Bartold PM. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J Periodontal Res. 2011;46:438–447. doi: 10.1111/j.1600-0765.2011.01358.x. [DOI] [PubMed] [Google Scholar]
- 40.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GTJ. iPS Cells Reprogrammed From Human Mesenchymal-Like Stem/Progenitor Cells of Dental Tissue Origin. Stem Cells Dev. 2009;19:469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Liu YF, Zhang J, Duan YZ, Jin Y. Ameloblasts serum-free conditioned medium: bone morphogenic protein 4-induced odontogenic differentiation of mouse induced pluripotent stem cells. J Tissue Eng Regen Med. 2016;10:466–474. doi: 10.1002/term.1742. [DOI] [PubMed] [Google Scholar]
- 42.Ozeki N, Mogi M, Kawai R, Yamaguchi H, Hiyama T, Nakata K, Nakamura H. Mouse-Induced Pluripotent Stem Cells Differentiate into Odontoblast-Like Cells with Induction of Altered Adhesive and Migratory Phenotype of Integrin. PLoS One. 2013;8:e80026. doi: 10.1371/journal.pone.0080026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43••.Kuchler-Bopp S, Bécavin T, Kökten T, Weickert JL, Keller L, Lesot H, Deveaux E, Benkirane-Jessel N. Three-dimensional Micro-culture System for Tooth Tissue Engineering. J Dent Res. 2016;95:657–664. doi: 10.1177/0022034516634334. This recent study is of major importance because it demonstrates that that tooth organogenesis can be achieved by incorporating scaffold free single cell suspensions with the hanging drop method. Since a considerably low amount of cells were used, as compared to traditional hanging drop methods, this approach can be used for high-throughput screening of dental cell sources from normal and even pathologic tissue for tooth development and regenerative medicine studies. [DOI] [PubMed] [Google Scholar]
- 44••.Zhang W, Vázquez B, Yelick PC. Bioengineered post-natal recombinant tooth bud models. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1962. n/a–n/a. This study is of major importance because it demonstrated that postnatal dental epithelial and mesenchymal tissues isolated from unerupted porcine wisdom teeth can be recombined to form bioengineered tooth tissues suggesting that the odontogenic potential was maintained within the tissues after extraction. This work supports the possibility of using human dental tissues from unerupted wisdom teeth to generate functional whole replacement teeth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Galler KM, D’Souza RN, Hartgerink JD. Biomaterials and their potential applications for dental tissue engineering. J Mater Chem. 2010;20:8730–8746. [Google Scholar]
- 47.Oshima M, Tsuji T. Whole Tooth Regeneration as a Future Dental Treatment. In: Bertassoni EL, Coelho GP, editors. Engineering Mineralized and Load Bearing Tissues. Cham: Springer International Publishing; 2015. pp. 255–269. [Google Scholar]
- 48.Monteiro N, Yelick PC. Advances and perspectives in tooth tissue engineering. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2134. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue Engineering of Complex Tooth Structures on Biodegradable Polymer Scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 50.Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, Troulis MJ, Yelick PC. Reconstructing Mandibular Defects Using Autologous Tissue-Engineered Tooth and Bone Constructs. J Oral Maxillofac Surg. 2009;67:335–347. doi: 10.1016/j.joms.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Abukawa H, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue engineered hybrid tooth–bone constructs. Methods. 2009;47:122–128. doi: 10.1016/j.ymeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A. In Vivo Generation of Dental Pulp-like Tissue by Using Dental Pulp Stem Cells, a Collagen Scaffold, and Dentin Matrix Protein 1 after Subcutaneous Transplantation in Mice. J Endod. 2008;34:421–426. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, Ahluwalia IP, Literman R, Kaplan DL, Yelick PC. Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol (HFIP) based silk scaffolds. J Biomed Mater Res A. 2011;97:414–422. doi: 10.1002/jbm.a.33062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. Accurately Shaped Tooth Bud Cell–Derived Mineralized Tissue Formation on Silk Scaffolds. Tissue Eng Part A. 2008;14:549–557. doi: 10.1089/tea.2007.0227. [DOI] [PubMed] [Google Scholar]
- 55.Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol. 2014;98:159–161. doi: 10.1136/bjophthalmol-2013-304446. [DOI] [PubMed] [Google Scholar]
- 56.Ventola CL. Medical Applications for 3D Printing: Current and Projected Uses. Pharmacy and Therapeutics. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 57.Cui X, Boland T, D’Lima DD, Lotz MK. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent patents on drug delivery & formulation. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozbolat IT, Yu Y. Bioprinting Toward Organ Fabrication: Challenges and Future Trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 59.Fuellhase C, Soler R, Andersson KE, Atala A, Yoo JJ. 264 GENERATION OF ORGANIZED BLADDER TISUE CONSTRUCTS USING A NOVEL HYBRID PRINTING SYSTEM. European Urology Supplements. 8:186. [Google Scholar]
- 60.Bartlett S. Printing organs on demand. The Lancet Respiratory Medicine. 2013;1:684. doi: 10.1016/S2213-2600(13)70239-X. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 62.D’Souza RN, Happonen RP, Ritter NM, Butler WT. Temporal and spatial patterns of transforming growth factor-β1 expression in developing rat molars. Arch Oral Biol. 1990;35:957–965. doi: 10.1016/0003-9969(90)90015-3. [DOI] [PubMed] [Google Scholar]
- 63.Thesleff I, Mikkola M. Int Rev Cytol. Vol. 217. Academic Press; 2002. The role of growth factors in tooth development; pp. 93–135. [DOI] [PubMed] [Google Scholar]
- 64.Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 65.Jheon AH, Seidel K, Biehs B, Klein OD. From molecules to mastication: the development and evolution of teeth. Wiley Interdisciplinary Reviews: Developmental Biology. 2013;2:165–182. doi: 10.1002/wdev.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jernvall J, Aberg T, Kettunen P, Keranen S, Thesleff I. The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125:161–169. doi: 10.1242/dev.125.2.161. [DOI] [PubMed] [Google Scholar]
- 67.Vaahtokari A, Åberg T, Jernvall J, Keränen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 68.Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin Regulates Enamel Patterning in Mouse Incisors by Asymmetrically Inhibiting BMP Signaling and Ameloblast Differentiation. Dev Cell. 2004;7:719–730. doi: 10.1016/j.devcel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Hosoya A, Kim JY, Cho SW, Jung HS. BMP4 signaling regulates formation of Hertwig’s epithelial root sheath during tooth root development. Cell Tissue Res. 2008;333:503–509. doi: 10.1007/s00441-008-0655-z. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci U S A. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Stock DW, Jackman WR, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133:3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Lü X, Sun X, Bai S, Li S, Shi J. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch Oral Biol. 2011;56:1221–1229. doi: 10.1016/j.archoralbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Dobie KSG, Sloan AJ, Smith AJ. Effects of aliginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Research. 2002;43:387–390. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 75.Unda FJ, Martín A, Hernandez C, Pérez-Nanclares G, Hilario E, Aréchaga J. FGFs-1 and -2, and TGFβ 1 as Inductive Signals Modulating in vitro Odontoblast Differentiation. Adv Dental Res. 2001;15:34–38. doi: 10.1177/08959374010150010801. [DOI] [PubMed] [Google Scholar]
- 76.He H, Yu J, Liu Y, Lu S, Liu H, Shi J, Jin Y. Effects of FGF2 and TGFβ1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int. 2008;32:827–834. doi: 10.1016/j.cellbi.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 77.Štembírek J, Kyllar M, Putnová I, Stehlík L, Buchtová M. The pig as an experimental model for clinical craniofacial research. Lab Anim. 2012;46:269–279. doi: 10.1258/la.2012.012062. [DOI] [PubMed] [Google Scholar]
- 78.Štembírek J, Buchtová M, Král T, Matalová E, Lozanoff S, Míšek I. Early morphogenesis of heterodont dentition in minipigs. Eur J Oral Sci. 2010;118:547–558. doi: 10.1111/j.1600-0722.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 79.Nait Lechguer A, Kuchler-Bopp S, Hu B, Haïkel Y, Lesot H. Vascularization of Engineered Teeth. J Dent Res. 2008;87:1138–1143. doi: 10.1177/154405910808701216. [DOI] [PubMed] [Google Scholar]
- 80.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Meth. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proceedings of the National Academy of Sciences. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, Isobe T, Sugawara A, Ogawa M, Tanaka C, Saito M, et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep. 2014;4:6044. doi: 10.1038/srep06044. This investigation is of importance because it demonstrates that a bio-hybrid design can be used as a possible alternative to whole tooth bioengineering. This bio-hybrid tooth root approach resulted in an implant that was supported by regenerated periodontal tissues formation and function. [DOI] [PMC free article] [PubMed] [Google Scholar]