Abstract
Pathogenic CD4+ T cells and myeloid cells play critical roles in the pathogenesis of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), an animal model of MS. These immune cells secrete aberrantly high levels of pro-inflammatory cytokines that pathogenically bridge the innate and adaptive immune systems and damage neurons and oligodendrocytes. These cytokines include interleukin-2 (IL-2), IL-6, IL-12, IL-21, IL-23, granulocyte macrophage-colony stimulating factor (GM-CSF), and interferon-γ (IFN-γ). It is, therefore, not surprising that both the dysregulated expression of these cytokines and the subsequent activation of their downstream signaling cascades is a common feature in MS/EAE. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is utilized by numerous cytokines for signal transduction and is essential for the development and regulation of immune responses. Unbridled activation of the JAK/STAT pathway by pro-inflammatory cytokines has been demonstrated to be critically involved in the pathogenesis of MS/EAE. In this review, we discuss recent advancements in our understanding of the involvement of the JAK/STAT signaling pathway in the pathogenesis of MS/EAE, with a particular focus on therapeutic approaches to target the JAK/STAT pathway.
Keywords: Multiple sclerosis, JAK/STAT signaling pathway, Experimental autoimmune encephalomyelitis, JAKinibs, Suppressors Of Cytokine Signaling
I. INTRODUCTION
A. Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the central nervous system (CNS) (brain, spinal cord, optic nerves).1–4 MS affects approximately two million individuals worldwide, and it is the primary cause of nontraumatic neurological disability in young adults in the United States.5 The hallmark features of MS include disruption of the blood-brain barrier (BBB), focal inflammatory infiltrates into the CNS, demyelinating lesions, oligodendrocyte loss, gliosis, and axonal damage.1–4,6 MS is a highly heterogeneous disease with variable clinical symptoms and courses. Symptoms range from numbness in limbs to severe disease including paralysis, loss of vision, and cognitive impairment.1–3,6 According to the presenting clinical characteristics, four subtypes of MS have been defined. Relapsing-remitting MS (RR-MS) is the most common subtype of MS, accounting for approximately 85% of MS patients. RR-MS patients experience periods of disease flares (relapses) followed by periods in which disease symptoms subside (remissions). Over time, approximately 50% of RR-MS patients develop secondary-progressive (SP-MS), where irreversible neurological damage begins to accumulate. The third subtype of MS is primary-progressive MS (PP-MS), which is characterized by steady worsening of neurologic functioning, without any distinct relapses or periods of remission. Progressive-relapsing MS (PR-MS) is the fourth subtype, affecting approximately 5% of patients. PR-MS patients experience steady disease progression with occasional relapses.1
Currently, the etiology of MS remains largely unknown. A combination of immunologic, environmental and genetic factors have been proposed to cause and/or contribute to the development of MS.1,2,6–9 It has become widely acknowledged that MS occurs in genetically predisposed individuals following exposure to an environmental trigger, such as an immune response toward certain pathogens, which may accidentally activate neuroantigen-autoreactive T cells. Once activated, T cells mature and cross the BBB where they are reactivated by CNS resident antigen-presenting cells (APCs). The reactivation of T cells confers their encephalitogenic activity by secreting a panel of cytokines and chemokines which recruit additional immune cells from the periphery (dendritic cells (DCs), macrophages, neutrophils, T cells and B cells) to the CNS. The immune infiltration results in an inflammatory response directed against the myelin sheath and other components of the CNS, ultimately leading to demyelination and axonal damage.1,2,9–16 Interestingly, the nature of the immune infiltrates present in the CNS is associated with disease activity. It has been proposed that T cells are the major pathogenic cell type during periods of relapse, while innate immune cells are involved in progressive disease.15 Given the critical role of immune cells in mediating the pathogenesis of MS, various therapies directed towards modulating the immune system have been developed and approved by the US Food and Drug Administration (FDA), including IFN-β (Avonex, Betaseron, Extavia, Plegridy, and Rebif), glatiramer acetate (GA, Copaxone), mitoxantrone (Novantrone), natalizumab (Tysabri), and, most recently, fingolimod (Gilenya), dimethyl fumarate (Tecfidera), alemtuzumab (Lemtrada), and teriflunomide (Aubagio).17–19 As these agents are only partially effective and do not stop disease progression, there is still a great need for the development of new therapies.14,17,20
B. Experimental Autoimmune Encephalomyelitis (EAE)
Much of our current understanding of disease pathogenesis and potential therapeutic interventions in MS comes directly from animal models.21–25 Experimental autoimmune encephalomyelitis (EAE) is the most widely used experimental model for MS; it resembles many aspects of MS pathology including demyelination, cellular infiltration and axonal loss.21–25 EAE is induced by active immunization with CNS antigens emulsified in adjuvant or by adoptive transfer of neuroantigen-specific CD4+ T cells.21–25 Immunization of C57BL/6 mice results in the development of classical EAE, which is a non-relapsing chronic disease characterized by progressive ascending paralysis with pathology mainly in the spinal cord.21–25 In some instances, immunization of C57BL/6 mice with different genetic modifications (e.g., mice deficient in IFN-γ signaling or mice with specific deletion of suppressor of cytokine signaling 3 (SOCS3) in the myeloid lineage) results in atypical forms of EAE, with pathology predominantly in the brain, rather than spinal cord.26–30 Another frequently used EAE model is PLP139–151-induced RR-EAE in SJL/J mice.31 Initially, RR-EAE develops similarly to classical EAE characterized by ascending paralysis. After the initial inflammatory attack subsides, the disease goes into remission and later, relapses arise.31 As MS represents a heterogeneous group of disorders in many aspects, different models of EAE allow researchers to explore distinct pathogenic pathways and validate therapeutic targets for MS treatment.
Similar to MS, the pathogenesis of EAE is complicated and involves close interplay between cells of the innate and adaptive immune systems.10–15 After immunization, EAE is initiated by the activation of autoantigen-specific CD4+ T cells by APCs in the periphery. Upon recognition of their cognate antigen in the context of MHC II, the auto-reactive CD4+ T cells mature and differentiate into effector phenotypes. IFN-γ–producing T helper 1 (Th1) cells and IL-17–producing Th17 cells are the primary effector T cells in the development of EAE; adoptive transfer of either myelin-specific Th1 cells or Th17 cells can induce EAE in naïve recipients.14,32,33 In addition to the production of their signature cytokines, both Th1 and Th17 cells produce granulocyte macrophage-colony stimulating factor (GM-CSF), a potent pro-inflammatory cytokine that activates DCs, enhances phagocytic activity in monocytes and macrophages, increases MHC II expression and antigen presentation capacity and enhances expression of adhesion molecules in mature myeloid cells.34–37 Alternatively, Th2 cells and regulatory T cells (Tregs) have protective functions in both MS and EAE by producing the anti-inflammatory cytokines IL-4, IL-10, and TGF-β.32,38
An important mechanism in the generation of auto-reactive CD4+ T cells is the checkpoint failures. In homeostatic conditions, self-reactive T cells that have escaped the central tolerance are kept by peripheral tolerance. However, failing of peripheral tolerance, such as loss of the co-inhibitory receptors (such as CTLA-4 and PD-1), contributes significantly to the pathogenesis of MS/EAE. Thus, a number of checkpoints inhibitors have been developed for preventing T-cell–mediated autoimmunity in the CNS. Previous studies have indicated that CTLA-4-Ig is able to block the activation and expansion of pathogenic T cells39 and is effective in preventing the development of EAE.40,41 Interestingly, a recent study pointed out that injection of CTLA-4 Ig at days 7 and 9 after immunization (when myelin-reactive T cells have been primed and start migrating toward the CNS) exacerbated disease signs and resulted in more severe disease.42 These results indicated that CTLA-4-Ig treatment at a particular disease stage might lead to suppression of Tregs, which is associated with a more severe disease.42 In addition, it has been shown that administration of PD-L1 overexpression DCs that were loaded with MHC-MOG peptide inhibited T-cell expansion, reduced cell infiltration into spinal cord, and decreased the severity of MOG peptide-induced EAE,43 indicating targeting the PD-1 pathway might have therapeutic benefit in MS/EAE treatment.
Innate immune cells, including DCs, macrophages/ monocytes, microglia, and neutrophils also have critical roles in both initial and advanced stages of EAE development.10–12,15,44–46 Interestingly, macrophages/monocytes and microglia have been shown to play a dual role in the pathogenesis of MS/EAE as they contribute to lesion formation and axonal damage, but they also support repair mechanisms.44,45 Two distinct phenotypes of macrophages, classically activated macrophages (M1) and alternatively activated macrophages (M2), exist in MS/EAE lesions.44,45 Macrophages are polarized to the M1 phenotype by exposure to Th1 cytokines such as IFN-γ and GM-CSF, or in the presence of bacterial products such as LPS. M2 macrophages are polarized by exposure to Th2 cytokines such as IL-4 and IL-13, as well as macrophage-colony stimulating factor (M-CSF).47 Macrophage polarization is plastic, suggesting that the M1 to M2 switch during the progression of inflammatory responses enables macrophages to both orchestrate the onset of inflammation and subsequently promote healing and repair.47–49 M1 macrophages secrete a plethora of pro-inflammatory mediators, such as cytokines, reactive oxygen species, nitric oxide and glutamate, which are able to induce tissue damage. Depletion of infiltrating macrophages by clodronate liposomes suppressed axonal damage and clinical symptoms of EAE.50–54 Conversely, M2 macrophages have been shown to play a protective role in EAE by producing extracellular matrix molecules and anti-inflammatory cytokines promoting tissue repair. Adoptive transfer of M2 macrophages ameliorates EAE development by inhibiting CD4+ T-cell activation.26,55,56 Therefore, an important therapeutic strategy against MS is to deviate the pathogenic Th1/Th17 and M1 responses toward protective Th2/Treg and M2 responses. Another pathogenic characteristic of MS/EAE is the aberrant production of many pro-inflammatory cytokines and chemokines, including IL-1β, IL-2, IL-6, IL-12, IL-17A, IL-17F, IL-21, IL-23, GM-CSF, TNF-α, IFN-γ, CCL2, and CXCL10,10–15 suggesting that direct targeting of these pro-inflammatory mediators or blocking their signaling pathway(s) may be a promising therapeutic strategy in MS/EAE.
C. The Janus Kinase/Signal Transducers and Activators of Transcription (JAK/ STAT) Pathway
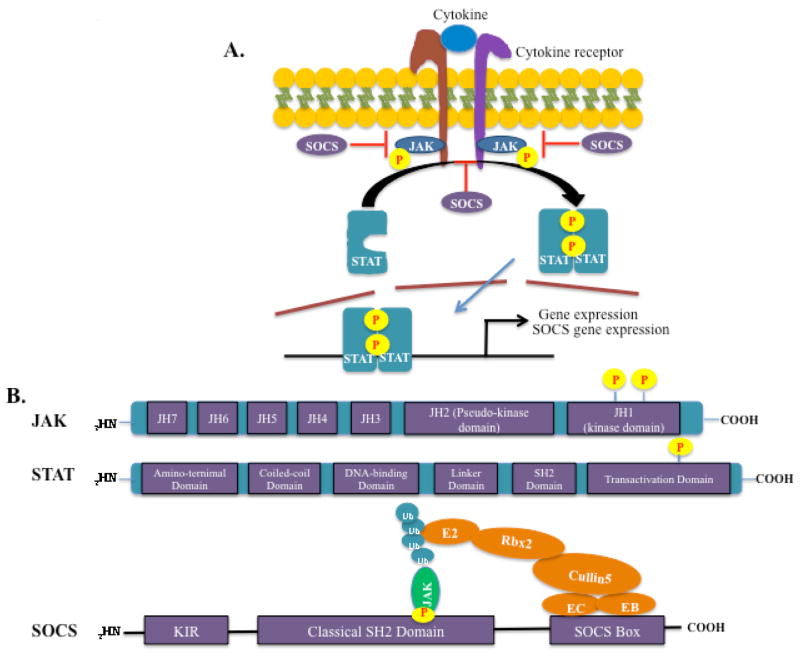
The JAK/STAT signaling pathway is one of the most critical pleiotropic cascades utilized by cells to transduce signals for numerous hormones, growth factors, and cytokines.57 A wide array of cytokines, including IL-2, IL-3, IL-4, IL-6, IL-7, IL-9, IL-12, IL-13, IL-15, IL-21, IL-23, IFN-γ, GM-CSF, and others use the JAK/STAT pathway for their biological activities.57,58 In general, cytokine binding to its cognate receptor trans-activates receptor-associated JAKs, leading to cross-phosphorylation and activation. The JAKs then phosphorylate the intracellular tail of receptors on tyrosine residues, creating docking sites for the recruitment of STATs (Fig. 1A). STATs are recruited to the phosphorylated receptor through the SH2 domain and are tyrosine-phosphorylated by JAKs, resulting in their activation. Activated STATs will subsequently dimerize and translocate to the nucleus, where they bind to specific DNA elements and modulate the expression of numerous target genes (Fig. 1A). 59 In mammals, four members of the JAK family (JAK1, JAK2, JAK3 and TYK2) and seven STATs (STAT 1, 2, 3, 4, 5a, 5b and 6) have been identified. Different JAKs and STATs are recruited based on cell type (or state) specificity and the receptors engaged in the signaling event, and different JAK/STAT combinations result in differential gene expression profiles. JAKs contain a JH1 domain, which is the kinase domain responsible for phosphorylation. STATs contain a coiled-coil domain, DNA-binding domain, SH2 domain, and transactivation domain, which is phosphorylated by JAKs57,58 (Fig. 1B). Appropriate activation of the JAK/STAT pathway is carefully orchestrated by a number of negative regulators to prevent STAT hyperactivation-associated immunopathology.58,60
FIG. 1.
The JAK/STAT pathway and structure of JAK, STAT, and SOCS proteins. (A). Cytokine binding to its cognate receptor transactivates receptor-associated JAKs, lead to cross-phosphorylation and activation of the JAKs. The JAKs then phosphorylate the intracellular tail of the receptors on tyrosine residues, creating docking sites for the recruitment of STATs. STATs are then recruited to the phosphorylated receptor though the SH2 domain and tyrosine-phosphorylated by JAKs, resulting in STAT activation. Activated STATs will subsequently dimerize and translocate to the nucleus, where they bind to specific DNA elements and modulate the expression of target genes. SOCS proteins are not constitutively expressed; they are induced upon STAT activation and function in a negative feedback loop. (B). JAK, STAT, and SOCS protein domain structure. JAK proteins contain 7 JH domains including the pseudo-kinase domain (JH2) and the kinase domain (JH1). Trans- and autophosphorylation of tyrosine residues in the C-terminal kinase domain leads to the recruitment and activation of STATs. STAT proteins contain an aminoterminal domain, a coiled-coil domain, a DNA-binding domain, a linker domain, an SH2 domain, and a transactivation domain. Phosphorylation in the C-terminal transactivation domain by JAKs leads to STAT activation and dimerization. SOCS proteins contain a C-terminal conserved SOCS box, a classical SH2 domain, and an N-terminal variable length and organization region. The SOCS box interacts with components of the ubiquitin ligase machinery (Elongin B, Elongin C, Cullin-5, and Ring-box 2, and an E2 ubiquitin transferase), thereby mediating proteosomal degradation of associated target proteins, such as JAKs. SOCS1 and SOCS3 contain a kinase inhibitory region (KIR), which can directly inhibit JAK activity.
Suppressors of cytokine signaling (SOCS) proteins, which are induced by cytokines, act in a classic negative feedback mechanism for termination of excessive activation of cytokine-induced JAK/ STAT signaling by inhibiting JAK kinase activity (Fig. 1A). Eight members (CIS and SOCS1–7) of the SOCS protein family contain a C-terminal conserved SOCS box, a classical SH2 domain, and an N-terminal variable region (Fig. 1B). The SOCS box interacts with components of the ubiquitin ligase machinery (Elongin B, Elongin C, Cullin-5, and Ring-box 2, and an E2 ubiquitin transferase), thereby mediating proteosomal degradation of associated target proteins, such as JAKs (Fig. 1B). The SH2 domain functions as an adapter, allowing SOCS proteins to bind to activated JAKs and to certain cytokine receptors to suppress further signaling events (Fig. 1A). Two SOCS family members, SOCS1 and SOCS3, have a unique kinase-inhibitory region (KIR), which serves a pseudosubstrate for JAKs, conferring these two proteins with the ability to directly inhibit JAK kinase activity61 (Fig. 1B). A recent study has defined a novel role for SOCS proteins in inhibiting JAK/STAT pathway activation in neighboring cells through the secretion of SOCS proteins in exosomes. Bourdonnay et al. identified SOCS1 and SOCS3-containing exosomes in the extracellular space being delivered from alveolar macrophages to neighboring epithelial cells in the context of lung inflammation.62
D. The Role of the JAK/STAT Pathway in Modulating Immune Responses
The JAK/STAT pathway is critical in influencing the quality and nature of both innate and adaptive immune responses. As mentioned above, macrophages can be polarized to the M1 or M2 phenotype depending on local environmental cues. Generally, IFN-γ mediates M1 polarization through JAK1/2 and STAT1 activation, whereas IL-4 skews macrophages toward the M2 phenotype through JAK1/3 and STAT6 activation. 63,64 In addition, the M1 phenotype can also be achieved in response to GM-CSF stimulation through JAK2 and STAT5 activation.64 GM-CSF has also been shown to induce the in vitro differentiation of mouse and human hematopoietic progenitors or human monocytes into DCs, indicating an essential role of GM-CSF induced JAK2/STAT5 activation in DC development.65 The JAK/STAT pathway is also critical in regulating T-cell differentiation, maturation, and function.66–68 For example, under Th1 differentiation conditions, IL-12 signals through JAK2/TYK2/STAT4 to induce IFN-γ production. Subsequently, IFN-γ signals through JAK1/2 and STAT1 to upregulate expression of T-bet, the master transcription factor for Th1 cells. Under Th17 differentiation conditions, STAT3 activation is essential to induce expression of Th17 master transcription factor RORγt, which is critical to maintaining the stability and function of Th17 cells.69,70 IL-6 signals through JAK1/2/TYK2/STAT3 to induce expression of IL-21 and IL-23R, and IL-6 signaling, together with IL-21 and IL-23 signaling, leads to sustained activation of STAT3.37,71 In addition, IL-6-induced STAT3 activation is critical to overriding Foxp3-mediated repression of RORγt and is capable of redirecting Treg cells toward the Th17 lineage.72 Under Th2 differentiation conditions, IL-4 activates JAK1/3 and STAT6 to induce the expression of GATA3, the master transcription factor of Th2 cells.
As negative regulators of the JAK/STAT pathway, SOCS proteins affect immune responses in many aspects. SOCS1 negatively regulates M1 macrophage polarization by inhibiting IFN-γ-induced JAK2/STAT1 activation, and SOCS1-deficient M1 macrophages exhibit increased levels of IL-6, IL-12, MHC II, and nitric oxide, characteristics of the M1 phenotype.73 Our lab has recently demonstrated that SOCS3-specific deletion in macrophages leads to a “heightened” M1 phenotype associated with aberrant STAT1/3 activation, indicating that SOCS3 is also a negative regulator for the M1 phenotype.74 In T-cell differentiation, loss of SOCS1 in CD4+ T cells augments the generation of Th1 cells75,76 whereas overexpression of SOCS1 suppresses Th1 cell differentiation.70 SOCS3 also inhibits IL-12-induced STAT4 signaling; thus, ectopic expression of SOCS3 favors Th2 cell generation and blocking SOCS3 facilitates the Th1 phenotype.76 Interestingly, SOCS1 and SOCS3 display different roles in Th17 cell differentiation. As SOCS3 inhibits STAT3 signaling, the critical signaling pathway in Th17 cell differentiation, deletion of SOCS3 promotes Th17 cell generation.77,78 Furthermore, leukemia inhibitory factor (LIF), a member of the IL-6 family of cytokines, inhibits IL-6/STAT3-induced Th17 cell differentiation through SOCS3 upregulation in addition to ERK activation.79 In contrast, loss of SOCS1 leads to defective Th17 cell generation due to STAT3 suppression by enhanced expression of SOCS3 through hyper-STAT1 activation.80 Overall, cytokines bridge the innate immune system (IL-6, IL-12, IL-23) and adaptive immune system (GM-CSF, IL-17 and IFN-γ), which makes the JAK/STAT pathway essential in mediating this crosstalk. Therefore, aberrant regulation of activation of the JAK/ STAT pathway plays a central role in pathological processes and inflammatory responses in the context of autoimmune and inflammatory diseases.
II. ROLE OF THE JAK/STAT SIGNALING PATHWAY IN MS/EAE
Detailed mechanisms of the JAK/STAT pathway and how this pathway serves as a fundamental paradigm utilized by cells for sensing environmental signals and interpreting these cues to regulate cell growth and differentiation, as well as the clinical relevance of targeting this pathway for therapeutic interventions, have been elegantly summarized by Villarino et al. and O’Shea et al.58,59 In this review, we focus on the therapeutic potential of targeting this pathway in the potential treatment of neuroinflammatory diseases in order to add new components to those reviews.
The importance of the JAK/STAT pathway in MS/EAE pathogenesis has been highlighted in recent genome-wide association (GWAS) studies. Genetic variations of STAT3, STAT4 and TYK2 have been shown to confer susceptibility to develop MS in a variety of populations.7,81–83 A follow-up study was conducted focusing on the functional impact of the TYK2 variant on human T cells.84,85 The reactivity and cytokine expression profiles of T cells were compared from individuals expressing the protective TYK2 genotype with the disease-associated TYK2 genotype, and it was determined that the protective allele confers decreased TYK2 activity, leading to a shift of T-cell differentiation into the Th2 phenotype. 85 Interestingly, a SOCS1 variant was recently identified as a new genetic risk factor for MS.86,87 This variant modulates SOCS1 expression in DCs, thereby affecting their function by influencing expression of CD86 and HLA-DR.87 In addition to JAK/ STAT pathway components, GWAS studies have also identified many JAK/STAT pathway-associated genes as MS risk factors, including IL-2RA, IL-7, IL-7R, IL-12A, IL-12B, and HLA-DRB1 and HLA-A.7,81–83 Interestingly, CD40 has also been identified as a MS susceptibility gene,88 and we have shown that CD40 is induced by the JAK/STAT pathway. IFN-γ induces CD40 expression in macrophages and microglia and SOCS1 inhibits expression.89
Although no study has assessed the levels of STAT(s) activation in CNS lesions in patients with MS, several cytokines, which signal through the JAK/ STAT pathway, have been identified in the lesions from MS patients.90,91 Li et al. found that the levels of IL-23p19 were elevated in brain lesions from patients with active and chronic MS.91 In addition, increased levels of IL-12 have also been observed in acute MS plaques.90 Overall, both genetic evidence and increased levels of JAK/STAT associated cytokines directly or indirectly demonstrates the prominent involvement of the JAK/STAT pathway in the pathogenesis of MS.
A. Role of the JAK/STAT Signaling Pathway in CD4+ T cells in MS/EAE
CD4+ Th1 and Th17 cells are the primary effector cells in the pathogenesis of MS/EAE. Enhanced expression of Th1 and Th17 cell cytokines are detected in the active CNS lesions in both MS patients and EAE mice.92–97 Enhanced STAT1 and STAT3 activation in T cells has been identified in MS patients during relapse compared with cells from patients in remission, and elevated levels of activated STAT1 and STAT3 strongly correlate with enhanced disease activity in the brain and spinal cord, suggesting an association of increased STAT1 and STAT3 activation and MS relapse.98 In addition, persistent high levels of activated STAT3 in circulating CD4+ T cells from patients with clinically isolated syndrome predicts conversion to clinically defined MS.99
To study the involvement of the JAK/STAT pathway in EAE, mice with targeted deletion of STAT genes have been generated. TYK2 is involved in IL-12– and IL-23–mediated signaling, and TYK2-deficient mice showed complete resistance against EAE due to severely impaired Th1 cell differentiation. 100 Similarly, mice deficient in STAT4 are resistant to the induction of EAE, as naïve CD4+ T cells fail to differentiate into Th1 cells due to disruption of the IL-12/STAT4 signaling axis.101 However, STAT1-deficient mice are susceptible to EAE, indicating that IFN-γ/STAT1 signaling is not essential for EAE development.102 IL-23–deficient mice (IL-23p19−/−)103 and IL-6–deficient mice are resistant to EAE.104,105 As STAT3 is the common transcription factor activated in response to IL-23 and IL-6, these cytokine-deficient mice have reduced STAT3 activation. In accord with these findings, conditional deletion of STAT3 in T cells renders mice resistant to EAE due to defective Th17 differentiation.106 In addition, deficiency of STAT3 in T cells results in decreased levels of activated integrins, including α4 integrin (CD49d) and β1 integrin (CD29), disrupting the trafficking of these T cells into the CNS.106 STAT5 has been shown to promote generation of regulatory T cells (Tregs) and immune suppression.107 Surprisingly, a recent study demonstrated that specific deletion of STAT5 in CD4+ T cells resulted in diminished development of EAE.108 The loss of encephalitogenic ability of STAT5-deficient CD4+ T cells was independent of IFN-γ or IL-17 production, but was due to the impaired expression of GM-CSF, a crucial mediator of T-cell pathogenicity. The authors further demonstrated that IL-7 mediated activation of STAT5 promotes the generation of GM-CSF-producing CD4+ T cells, and they suggested that these cells are more pathogenic than Th1 or Th17 cells.108 Th2 cells are proposed to play a protective role in EAE. The IL-4/ STAT6 axis controls Th2 cell differentiation. Thus, STAT6-deficient mice experience a more severe clinical course of EAE because STAT6-deficient naïve CD4+ T cells predominantly differentiate to the Th1 phenotype.101
SOCS proteins also influence EAE development by regulating CD4+ T-cell differentiation. Mice with specific SOCS1 deletion in CD4+ T cells exhibit less EAE disease severity.80 The protective effect of SOCS1-deficiency in naïve CD4+ T cells is due to T-cell differentiation predominantly into Th1 cells and poorly into Th17 cells.80 The overproduction of IFN-γ by hyper-activated Th1 cells inhibits Th17 differentiation through induction of SOCS3.75 As such, mice with SOCS3 overexpressed in T cells exhibit delayed onset of EAE and restricted Th17 cell differentiation.80 However, loss of SOCS3 in CD4+ T cells protects mice from experimental autoimmune uveitis (EAU), another animal model of neuroinflammation.107 This protective effect was due to enhanced expression of CTLA-4 and expansion of IL-10–producing Tregs, as SOCS3 deletion led to increased levels of Tregs with enhanced suppressive function.107 Interestingly, SOCS3 deletion in CD4+ T cells did not result in enhanced generation of Th17 cells in this study, which differs from other studies.72,73 The reason for this discrepancy in findings is unclear and demonstrates a need for further investigation into the role of SOCS3 in CD4+ T-cell differentiation.
B. Role of the JAK/STAT Signaling Pathway in Myeloid Cells in MS/EAE
As mentioned previously, the JAK/STAT pathway is essential in modulating immune responses in myeloid cells. Given that these cells are critical in MS/EAE pathogenesis, the JAK/STAT signaling pathway contributes to EAE/MS through modulating the function of myeloid cells. In relapsing MS patients, high levels of STAT3 activation and low levels of SOCS3 expression were found in monocytes compared with cells from remitting MS patients.110 Additionally, leptin, one of the pathogenic mediators in EAE,111 induced an up-regulation of STAT3 activation only in monocytes from patients in relapse.110 In EAE models, the number of SOCS1-expressing macrophages at the peak of RR-EAE was significantly higher than in chronic EAE, and this correlated with diminished expression of iNOS.112 Because expression of iNOS is negatively regulated by SOCS1,113 the authors propose that SOCS1 expression by macrophages may promote disease remission in RR-EAE through iNOS inhibition.112 Interestingly, SOCS3 expression in DCs has also been shown to be protective in EAE, as adoptive transfer of SOCS3-transduced DCs reduces the clinical severity of EAE.114 These SOCS3-transduced DCs expressed low levels of MHC II and CD86 and produced high levels of IL-10 but low levels of IL-12, IL-23 and IFN-γ, which resulted in a limited differentiation of Th1 and Th17 cells and a robust induction of Th2 cells.114
To further investigate the role of SOCS3 in myeloid cells in vivo, we generated mice with targeted deletion of SOCS3 in myeloid cells (LysMCre-SOCS3fl/fl mice) to study how SOCS3 in myeloid cells regulates immune responses in the context of EAE. Importantly, we found that LysMCre-SOCS3fl/ fl mice develop an early onset and severe, nonresolving atypical form of EAE, which is associated with lesions in the cerebellum, rather than the spinal cord, and ataxia and tremors.26 LysMCre-SOCS3fl/ fl mice with atypical EAE exhibit hyperactivation of STAT3 and STAT4, and they have elevated numbers of inflammatory cells in the cerebellum and brainstem, including extensive macrophage and neutrophil infiltrates, and a prominent Th1 and Th17 cell infiltrate compared with SOCS3fl/fl mice with classical EAE.26,46 The absence of SOCS3 in macrophages led to a pronounced polarization to the M1 phenotype, which was associated with increased expression of M1 genes, including iNOS, IL-1β, IL-12p40, IL-23p19, IL-6, CCL2, CXCL10, CD40, CD80, CD86, and IRF5, providing the microenvironment to polarize Th1 and Th17 cells and induce neuronal death26,74 Our recent studies demonstrated that the atypical EAE observed in LysMCre-SOCS3fl/ fl mice is characterized by extensive neutrophil infiltration into the cerebellum and brainstem, increased iNOS levels in the cerebellum and brainstem, and prominent axonal damage.46 Importantly, infiltrating SOCS3-deficient neutrophils produce high levels of CXCL2, CCL2, CXCL10, nitric oxide (NO), TNF-α and IL-1β.46 Thus, our data directly demonstrate that SOCS3 expression in myeloid cells provides protection from EAE through deactivation of multiple neuroinflammatory responses.
C. Role of the JAK/STAT Signaling Pathway in Glial Cells in MS/EAE
Glial cells participate in local innate immune responses upon activation by various insults, and activation of glial cells is implicated in the pathogenesis of MS/EAE through the production of a variety of soluble mediators, such as CCL2, CXCL1, CXCL2, CXCL10, GM-CSF, and IL-6.115–118 The JAK/STAT pathway has been shown to regulate glial cell activation, thereby affecting their function during the pathogenesis of MS/EAE. We have shown that IFN-β treatment of astrocytes induces robust expression of chemokines, such as CCL2, CCL3, CCL4, CCL5, and CXCL10 through activation of STAT1.119 IFN-β treatment also induces SOCS1 and SOCS3 as negative feedback regulators to constrain the expression of these chemokines. Specific small interfering RNA (siRNA) targeting of SOCS1 and SOCS3 in astrocytes enhances their proinflammatory response to IFN-β stimulation and promotes chemotaxis of macrophages and CD4+ T cells.119 The accumulation of misfolded proteins and induction of endoplasmic reticulum (ER) stress are associated with MS.120 Our lab has recently shown that ER stress is also present in the CNS concomitant with inflammation and astrogliosis in EAE.121 In addition, we found that ER stress-induced activation of astrocytes through activation of the JAK1/STAT3 axis triggers the production of IL-6, CCL2, and CCL20.121 Furthermore, we demonstrated that ER-stressed astrocytes, via the JAK1/STAT3 pathway, express IL-6 and oncostatin M (OSM) to stimulate microglia activation, which synergizes with ER stress in astrocytes to create a feed-forward loop to drive inflammation in EAE.121 The involvement of reactive astrocytes in disease progression is highly controversial; generally, they are detrimental for neuronal function, but studies also suggest that reactive astrocytes may have beneficial effects and promote neuronal survival.112–114 Interestingly, a recent study showed that the JAK/STAT3 pathway is a common inducer of reactive astrocytes in animal models of Alzheimer’s disease and Huntington’s disease.125 Lentiviral gene transfer of SOCS3 in astrocytes inhibited this pathway, prevented astrocyte reactivity, and decreased microglial activation in models of both diseases.125 In contrast, in a spinal cord injury model, STAT3 activation was shown to be crucial for astrocyte migration and glial-scar formation, which limited inflammatory cell infiltration and protected neurons and oligodendrocytes from cell death.126
Microglia are CNS resident immune cells that participate in pathology associated with MS/EAE. Microglial activation is believed to be an early event in CNS inflammation. Upon activation, microglia produce soluble mediators, including NO and TNF-α, which are toxic to oligodendrocytes and neurons.127,128 It has been reported that gangliosides, which are predominantly found in the CNS, can rapidly activate JAK1/2 and STAT1/3 to induce expression of NO and CCL2 in microglia, and treatment with AG490, a JAK inhibitor, reduces the expression of these pro-inflammatory mediators.129 Thrombin is generated from the precursor prothrombin, which is endogenously expressed in dopaminergic neurons in the substantia nigra. Treatment of microglia with thrombin rapidly activates JAK2 and STAT3, leading to the production of NO and TNF-α and induction of neurodegeneration of dopaminergic neurons in vitro.130 AG490 treatment inhibits thrombin-induced production of TNF-α and NO, which rescues dopaminergic neurons.130 In an ischemia-induced neuronal damage model, transient middle cerebral artery occlusion in adult rats led to increased JAK2 and STAT3 phosphorylation in the ipsilateral cortex and striatum. Fluorescent immunohistochemistry revealed that both pJAK2 and pSTAT3 staining was predominantly localized in macrophages/microglia in the post-ischemic brain.131 Treatment with AG490 or siRNA specific for STAT3 prevented post-ischemic JAK2 and STAT3 activation, significantly decreased the infarct volume and the number of apoptotic cells and improved neurological functions.131 Interestingly, in a model of amyotrophic lateral sclerosis, treatment with a selective JAK2 inhibitor, R723, significantly reduced the number of Ly-6C+ blood monocytes, and suppressed expression of IFN-γ and NO in the spinal cord but did not alter expression of IL-1β, IL-6, TNF-α, and NADPH oxidase 2 (NOX2), progression or survival in mSOD1G93A mice132 These data are particularly interesting because they indicate that JAK inhibitors have different effects in the context of different neurologic disease models.
Oligodendrocytes are the glial cells responsible for the myelination of neurons in the CNS, crucial for proper signal transduction and neuronal survival, and they are therefore the target of immune attacks in lesions of demyelination during MS/EAE.133,134 Apoptosis of oligodendrocytes induced by CNS inflammation is a hallmark in MS/EAE.133 It has been shown that oligodendrocytes express most of the cytokine receptors that signal through the JAK/ STAT pathway, including receptors for IL-4, IL-6, IL-10, IL-12, IL-18 and IFN-γ. IFN-γ activation of STAT1 leads to oligodendrocyte death,135,136 and IFN-γ expression in adult animals after demyelination inhibits remyelination. The deleterious effect of IFN-γ on oligodendrocytes can be corrected by overexpression of SOCS1, resulting in protection of myelinating oligodendrocytes against the harmful effects of inflammation.137 In contrast, LIF-induced STAT3 activation in oligodendrocytes is protective in EAE, as LIF administration reduces oligodendrocyte apoptosis and disease severity.138 LIF induces SOCS3 expression in oligodendrocytes, and mice with specific deletion of SOCS3 in oligodendrocytes display less oligodendrocyte loss in the cuprizone model of demyelination.139
Collectively, these studies implicate the JAK/ STAT pathway in the regulation of glial cell activation and function during demyelinating inflammatory events that contribute to the pathogenesis of MS/EAE.
III. THE JAK/STAT PATHWAY AS A PROMISING THERAPEUTIC TARGET IN EAE
The involvement of the JAK/STAT pathway in the pathogenesis of MS/EAE is intriguing, and further understanding of its multi-faceted role could potentially lead to preclinical and clinical studies with a more targeted focus on this pathway. Interestingly, intravenous administration of MOG35–55 peptide suppresses EAE through inhibiting STAT1 and STAT4 activation in both myeloid cells and T cells,140 strengthening the clinical promise of the JAK/STAT pathway as a therapeutic target for MS/ EAE. Below, we summarize current research on therapeutic interventions in EAE models that are relevant to the JAK/STAT pathway.
A. Natural Compounds
A variety of natural compounds have been found to have therapeutic efficacy in the treatment of EAE. Plumbagin, an herbal compound derived from roots of the medicinal plant Plumbago zeylanica, controls encephalitogenic T-cell responses and amelioration of EAE through down-regulation of the STAT1/ STAT4/T-bet axis and STAT3/RORγt axis.141 Berbamine (BM) is an herbal compound derived from Berberis vulgaris L, and BM treatment suppresses EAE development. Interestingly, BM treatment up-regulated expression of STAT-interacting LIM protein (SLIM), an ubiquitin E3 ligase for STAT4, which promotes STAT4 degradation, resulting in markedly decreased IFN-γ production in CD4+ T cells from EAE mice.142 The same group showed that another natural compound, Berberine (BBR), an isoquinoline alkaloid derived from plants, is able to influence Th1 and Th17 cell differentiation and ameliorate EAE disease by targeting activation of TYK2/ JAK1/2/STAT1/4 in Th1 cells and STAT3 in Th17 cells.143 Studies from the Bright group have shown that three natural compounds, curcumin (a naturally occurring polyphenolic phytochemical isolated from rhizomes of the medicinal plant Curcuma longa),144 quercetin (a flavonoid phytoestrogen),145 and 1,25 dihydroxyvitamin-D3146 can individually inhibit EAE development by suppressing IL-12–induced JAK2/ TYK2/STAT3/4 activation. A recent study by Zhang et al. reported that tripchlorolide (T4), an extract of the natural herb Tripterygium wilfordii Hook F (TWHF), reduces the severity of EAE and inhibits ongoing disease by suppressing the JAK2/STAT1/ STAT3 axis.147 Additionally, another study using an EAE model in rats demonstrated that cornel iridoid glycoside (CIG), the main component extracted from Cornus officinalis, reduced disease severity, incidence, disease onset and ongoing paralysis through blocking the JAK/STAT1/3 axis in the brain148 (Table 1).
TABLE 1.
Therapeutic Interventions in EAE That are Relevant to the JAK/STAT Pathway.
| Drug | Property | Disease(s) | Mechanisms of action relevant to JAK/STAT | Reference(s) |
|---|---|---|---|---|
| Plumbagin | Natural compound | EAE | Target JAK1/2 and STAT1/3/4 | 142 |
| Berbamine | Natural compound | EAE | Up-regulation of SLIM to promote STAT4 degradation | 143 |
| Berberine | Natural compound | EAE | Target TYK2/JAK1/2 and STAT1/3/4 | 144 |
| Curcumin | Natural compound | EAE | Target JAK2/TYK2 and STAT3/4 | 163 |
| Quercetin | Natural compound | EAE | Target JAK2/TYK2 and STAT3/4 | 161 |
| 1,25 Dihydroxyvitamin-D3 | Natural compound | EAE | Target JAK2/TYK2 and STAT3/4 | 147 |
| Tripchlorolide | Natural compound | EAE | Target JAK2 and STAT1/3 | 148 |
| Cornel iridoid glycoside | Natural compound | EAE | Target JAK1/3 and STAT1/3 | 149 |
| Glatiramer acetate | Pharmacologic compound | EAE | Target STAT1 | 150 |
| Glatiramer acetate | Pharmacologic compound | EAE | Target JAK2/TYK2 and STAT3/4 | 153 |
| Fumurates | Pharmacologic compound | MS/EAE | Target STAT1 | 152 |
| Laquinimod | Pharmacologic compound | EAE | Target STAT1 | 151 |
| Statins | HMG-CoA reductase inhibitors | EAE/MS | Induce SOCS1 and SOCS3, Target JAK2/TYK2 and STAT4 | 156, 157, 159 |
| COX-2 inhibitors | Pharmacologic compound | EAE | Target JAK2/TYK2 and STAT3/4 | 161 |
| GSK3 inhibitors | Pharmacologic compound | EAE | Target STAT1 | 162 |
| PPARγ agonists | Pharmacologic compound | EAE | Target JAK2/TYK2 and STAT3/4 | 163 |
| Tyrphostin B42 | JAK2 inhibitor | EAE | Target JAK2 and STAT3 | 167 |
| CEP-701 | JAK2 inhibitor | EAE | Target JAK2 | 168, 169 |
| AZD1480 | JAK1/2 inhibitor | EAE | Target JAK1/2 and STAT1/3/4 | 170 |
| ORLL-NIH001 | STAT3 inhibitor | EAU | Target STAT3 | 174 |
| Tkip | SOCS1 mimetics | EAE | Target JAK2 | 113, 175, 176 |
| SOCS1-KIR | SOCS1/3 mimetics | EAE | Target JAK2/TYK2 and STAT3 | 177, 178 |
B. Pharmacologic Compounds
A number of pharmacologic compounds have been tested in MS/EAE (Table 1). Glatiramer acetate (GA, copolymer-1, copaxone), fumurates and laquinimod, which either have been approved by the FDA for treatment of MS patients or are under evaluation for RR-MS, have been shown to induce protective M2 macrophage and type II DC phenotypes in EAE by interfering with STAT1/3 activation, leading to decreased levels of IL-6, IL-12, and IL-23, and elevated levels of IL-10.26,149–152 In addition, GA has been shown to limit Th1 and Th17 differentiation by targeting the JAK2/TYK2/STAT3/4 axis.152 Other pharmacologic inhibitors have also been shown to affect the JAK/STAT axis, thereby modulating clinical symptoms of EAE. Statins are selective inhibitors of 3-hydroxy-3-methylglutaryl (HMG) coenzyme A (Co-A) reductase, utilized primarily for hypercholesterolemia treatment.153,154 Recent data suggest that simvastatin may have therapeutic benefit in certain clinical subtypes of MS patients.155,156 Mechanistically, it has been shown that lovastatin induced the expression of GATA3 and phosphorylation of STAT6, whereas it inhibited tyrosine phosphorylation of JAK2/TYK2/STAT4, promoting a deviation of Th1 cell differentiation to the protective Th2 phenotype.157 Another study showed that simvastatin induced expression of SOCS1 and SOCS3, which are associated with inhibition of STAT1 and STAT3 activation in monocytes of RR-MS patients, leading to decreased levels of IL-6, IL-21, IL-23, IL-12p70 and inhibition of Th17 cell differentiation.158 In addition, our lab has demonstrated that simvastatin inhibits IFN-γ-induced CD40 gene expression in macrophages and microglia by suppressing STAT1 activation.159 Cyclooxygenase (COX-2) inhibitors160 and glycogen synthase kinase-3 (GSK3) inhibitors161 are reported to alleviate EAE development by blocking Th1 cell differentiation through suppression of IL-12-induced JAK2/TYK2/STAT4 activation and IFN-γ-induced STAT1 activation, respectively. Other rather unconventional therapeutic agents linked to JAK/STAT inhibition in EAE are peroxisome proliferator-activated receptor-γ (PPARγ) agonists. Natarajan et al. showed that treatment of SJL/J mice with PPARγ agonists decreased the duration and clinical severity of active immunization and adoptive transfer models of EAE. This beneficial effect was accomplished by inhibition of IL-12-induced activation of the JAK/STAT signaling pathway and Th1 cell differentiation.162
C. JAK Inhibitors (JAKinibs)
The JAK/STAT pathway has become an attractive therapeutic target in inflammation, autoimmune diseases, solid and liquid tumors, and transplant rejection.58,163–165 The most common type of inhibitors of this pathway are small-molecule JAK inhibitors, also termed JAKinibs. A variety of JAKinibs have been developed and have demonstrated clinical efficacy in rheumatoid arthritis and other inflammatory disorders.58,163,164 Given the breadth of data implicating association of dysregulation of the JAK/STAT pathway and MS/EAE pathogenesis, several studies have examined direct inhibition of the JAK/STAT pathway in EAE using JAKinibs (Table 1). Tyrphostin B42 (AG490), a JAK2 inhibitor, was tested by Bright et al., in the EAE model.165 They found that in vitro treatment of T cells with tyrphostin B42 inhibited IL-12-induced tyrosine phosphorylation and activation of JAK2, resulting in a decreased level of IFN-γ production. In vivo, treatment of mice with tyrphostin B42 reduced the incidence and severity of both active and adoptive transfer EAE, which was associated with decreased proliferation and decreased IFN-γ production in Th1 cells.166 CEP-701, originally developed as a FLT3 inhibitor, has also been shown to function as a JAK2 inhibitor. Whartenby et al. demonstrated that treatment of mice with CEP-701 significantly improved the clinical course of established disease in mice with EAE.167 The same group also tested the effect of JAK2 inhibition in DCs and found that treatment of DCs led to a decrease in secretion of TNF-α, IL-6 and IL-23, as well as a decrease in expression of costimulatory molecules, including CD40 and CD86.168
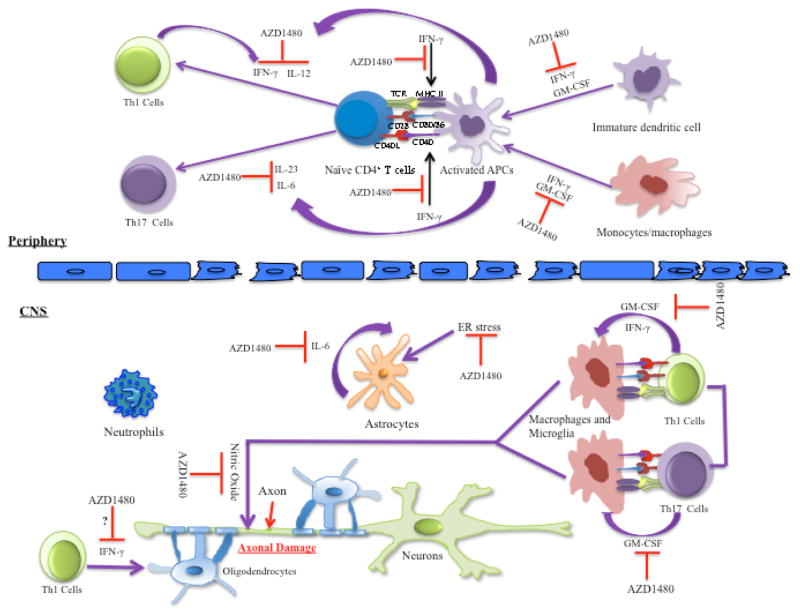
We have recently demonstrated that AZD1480, an ATP competitive inhibitor of JAK1 and JAK2, has striking protective effects in multiple models of EAE169,170 (Fig. 2). Specifically, in vitro AZD1480 treatment inhibits STAT1 and STAT4 activation in T cells, leading to decreased Th1 cell differentiation as assessed by decreased IFN-γ production, and decreased mRNA levels of IFN-γ and T-bet. AZD1480 also reduces differentiation of naive T cells to Th17 cells and inhibits mRNA levels of STAT3 target genes, including IL-17A, RORγt, IL-22 and IL-23R by suppression of STAT3 activation (Fig. 2). AZD1480 also influences APCs by inhibiting IFN-γ–induced STAT1, IFN-γ and IL-6-induced STAT3, and GM-CSF-induced STAT5 activation and expression of genes such as iNOS, MHC Class II and CD40, exerting an inhibitory effect on the M1 phenotype169 (Fig. 2). In vivo, inhibition of the JAK/STAT pathway by AZD1480 suppresses classical and atypical EAE by preventing entry of immune cells into the brain, suppressing differentiation of Th1 and Th17 cells, deactivating myeloid cells, inhibiting STAT activation in the brain and reducing expression of proinflammatory cytokines and chemokines. In addition, AZD1480 treatment exerts a protective effect in RR EAE and Th1 and Th17-induced EAE.169 Mechanistically, AZD1480 treatment impaired both the priming and expansion of T cells, suppressed proliferation of CD4+ T cells and CD11b+ cells and attenuated the antigen presentation functions of myeloid cells.169 Most importantly, AZD1480 was administered in a therapeutic manner after the appearance of clinical symptoms, with potent clinical efficacy (Fig. 2).
FIG. 2.
Potential mechanisms of action for the JAK1/JAK2 inhibitor, AZD1480. In the periphery, AZD1480 inhibits IFN-γ induced expression of MHC II and CD40 in APCs. In addition, AZD1480 inhibits IL-12 induced Th1 cell differentiation and IL-6 and IL-23 induced Th17 cell polarization. In the CNS, AZD1480 inhibits the reactivation of Th1 cells and Th17 cells by CNS APCs, and inhibits the production of nitric oxide by activated macrophages and microglia. Furthermore, AZD1480 suppresses ER stress-induced activation of astrocytes, and inhibits the synergistic pro-inflammatory effect of IL-6 with ER stress. Through inhibition of IFN-γ-induced STAT1 activation, AZD1480 could have potential protective effects on IFN-γ-mediated apoptosis in oligodendrocytes, although the effects of AZD1480 on oligodendrocytes have not been formally tested. Pathogenic Th1 and Th17 cells secrete GM-CSF during EAE, which promotes myeloid cell maturation. AZD1480 treatment suppresses GM-CSF induced STAT5 activation, exerting inhibitory effect on GM-CSF induced myeloid cell maturation. APCs, antigen presenting cells; CNS, central nervous system; ER, endoplasmic reticulum.
D. STAT Inhibitors
There is much interest in directly targeting STATs, particularly STAT3 because STAT3 is a relevant target in numerous autoimmune diseases, including MS, as well as many cancers.26,147,170–172 Although efforts to develop STAT inhibitors has seen limited success due to issues with bioavailability, in vivo efficacy and selectivity,58 a number of STAT3 inhibitors have been described and tested successfully in preclinical models of cancers.173 Of relevance to neuroinflammation, ORLL-NIH001, a STAT3 inhibitor, has been tested in EAU.174 ORLL-NIH001 treatment substantially reduced the frequency of both pathogenic IL-17A+ Th17 cells and IL-17+IFN-γ+ T cells. In vivo, treatment with ORLL-NIH001 attenuated disease severity by interfering with lymphocyte trafficking into the retina through downregulation of α4β1, α4β7, CCR6 and CXCR3.174 These encouraging findings support the therapeutic potential of the future use of STAT3 inhibitors in MS/EAE.
E. SOCS Mimetics
There is also considerable interest in testing SOCS mimetics in autoimmune diseases. As mentioned previously, SOCS1 and SOCS3 contain a KIR domain that binds to tyrosine-phosphorylated JAKs and inhibits their kinase activity. The tyrosine kinase inhibitor peptide (Tkip), a short 12-mer peptide, was developed as a SOCS1 mimetic.175 Tkip is able to bind to the autophosphorylation site of JAK2, resulting in inhibition of its autophosphorylation as well as its phosphorylation of the IFN-γ receptor subunit IFNGR-1.175 In vivo, treatment of New Zealand white mice with Tkip before EAE induction suppressed the development of acute EAE, while Tkip treatment in SJL/J mice blocked the acute and relapse phases of EAE.176 Administration of Tkip also reduced disease severity in chronic EAE in C57BL/6 mice.112 Another small peptide, SOCS1-KIR, which corresponds to the KIR sequence of SOCS1, was developed later by the same group.177 SOCS1-KIR inhibits kinase activity by binding to the activation loop of JAK2 and TYK2.177 SOCS1-KIR also inhibits IL-23 induced STAT3 activation.178 Treatment of SJL/J mice with SOCS1-KIR inhibited severe relapsing paralysis by preventing cellular infiltration into the CNS, inhibiting proliferation and expansion of Th17 cells in EAE and suppressing IL-17, IFN-γ and TNF-α production by CNS inflammatory infiltrates.178
Collectively, these findings indicate that intervention of the JAK/STAT pathway can attenuate neuroinflammatory responses in the CNS and may therefore represent a new therapeutic approach for treatment of MS patients.
IV. CONCLUSIONS
Dysregulation of the JAK/STAT pathway promotes aberrant activation of innate and adaptive arms of the immune system, including activation of pathogenic Th1 and Th17 cells, activation of macrophages, neutrophils and DCs, and excessive production of proinflammatory mediators, all of which drive the development of MS/EAE. Despite our knowledge on the pathogenic role of this pathway in a variety of inflammatory and autoimmune diseases, translation of this knowledge into clinical therapy has lagged behind until recently. During the past several years, much progress has been achieved in developing specific JAK inhibitors. Presently, 25 JAKinibs are currently being assessed as therapeutic agents in clinical trials for myelofibrosis, ulcerative colitis, psoriasis, rheumatoid arthritis, spondyloarthropathy, systemic lupus erythematosus, and various cancers.58 Most excitingly, two JAKinibs have been approved by the FDA: ruxolitinib, a JAK1/JAK2 inhibitor, was approved in 2011 for patients with myelofibrosis and polycythemia, and tofacitinab, a JAK3/ JAK1 inhibitor, was approved in 2012 for treatment of patients with rheumatoid arthritis. In addition, second-generation JAKinibs with more specificity are being developed.58 Undoubtedly, components of this pathway will continue to receive considerable attention as potential therapeutic targets.
Notably, current therapies for MS are partially effective in ameliorating disease symptoms, thus, the use of small molecules that target the JAK/ STAT pathway represents an important addition to the therapeutic options available for MS patients. Given the overwhelming evidence demonstrating the promising therapeutic efficacy of targeting the JAK/STAT pathway in animal models of MS, it will be exciting to follow these studies and hopefully the transition from bench to the bedside in the case of MS patients. Furthermore, other diseases such as Parkinson’s disease, spinal cord injury, and Alzheimer’s disease, which have a prominent neuroinflammatory component, may also benefit from treatment with JAKinibs.
Acknowledgments
This work was supported in part by the National Institutes of Health (grant nos. NS45290 and NS57563 to E.N.B. and H.Q. and grant no. T32 AI007051 to S.A.G.), the National Multiple Sclerosis Society (grant no. CA-1059-A-13 to E.N.B.), and the Michael J. Fox Foundation through a grant to E.N.B. and H.Q.). The authors thank Cheryl Lyles and Kim Sanders for their assistance.
ABBREVIATIONS
- APC
antigen-presenting cell
- BBB
blood–brain barrier
- CNS
central nervous system
- DCs
dendritic cells
- EAE
experimental autoimmune encephalomyelitis
- EAU
experimental autoimmune uveitis
- GM-CSF
granulocyte macrophage-colony stimulating factor
- GWAS
genome-wide association study
- JAK
Janus kinase
- MS
multiple sclerosis
- PP
primary progressive
- PR
progressive relapsing
- RR
relapsing-remitting
- siRNA
small interfering RNA
- SOCS
suppressors of cytokine signaling
- STAT
signal transducers and activators of transcription
- Th
T-helper cell
- Treg
regulatory T cell
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–56. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8(11):602–12. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–58. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 5.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New Engl J Med. 2000;343(13):938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 6.Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10(4):225–38. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- 7.Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, Oturai A. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–60. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–8. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 10.Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev. 2012;248(1):170–87. doi: 10.1111/j.1600-065X.2012.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–21. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64(1):123–32. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan SV. The evidence for a role of B cells in multiple sclerosis. Neurology. 2012;78(11):823–32. doi: 10.1212/WNL.0b013e318249f6f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, Malefyt RW, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16(4):406–12. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol. 2008;255(Suppl 1):3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 16.Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM. Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med. 2015;212(1):23–35. doi: 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis--a multifaceted adversary. Nat Rev Drug Discov. 2008;7(11):909–25. doi: 10.1038/nrd2358. [DOI] [PubMed] [Google Scholar]
- 18.Cross AH, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014;275(4):350–63. doi: 10.1111/joim.12203. [DOI] [PubMed] [Google Scholar]
- 19.English C, Aloi JJ. New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015;37(4):691–715. doi: 10.1016/j.clinthera.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS, Weber MS. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs. 2011;25(5):401–14. doi: 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batoulis H, Recks MS, Addicks K, Kuerten S. Experimental autoimmune encephalomyelitis--achievements and prospective advances. APMIS. 2011;119(12):819–30. doi: 10.1111/j.1600-0463.2011.02794.x. [DOI] [PubMed] [Google Scholar]
- 22.Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: historical facts and future implications. Biochim Biophys Acta. 2011;1812(2):177–83. doi: 10.1016/j.bbadis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiol. 2011;18(1):21–9. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129(Pt 8):1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164(4):1079–106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin H, Yeh W-I, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox THI, Park K, Harrington LE, Raman C, Benveniste EN. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci USA. 2012;109(13):5004–9. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205(11):2633–42. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wensky AK, Furtado GC, Garibaldi Marcondes MC, Chen S, Manfra D, Lira SA, Zagzag D, Lafaille JJ. IFN-γ determines distinct clinical outcomes in autoimmune encephalomyelitis. J Immunol. 2005;174(3):1416–23. doi: 10.4049/jimmunol.174.3.1416. [DOI] [PubMed] [Google Scholar]
- 29.Simmons SB, Liggitt D, Goverman JM. Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis. J Immunol. 2014;193(2):555–63. doi: 10.4049/jimmunol.1400807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoolman JS, Duncker PC, Huber AK, Segal BM. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J Immunol. 2014;193(2):564–70. doi: 10.4049/jimmunol.1400825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206(6):1303–16. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nat Med. 2008;14(3):337–42. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 35.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–67. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 37.Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23(6):707–12. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchroo VK, Ohashi PS, Sartor RB, Vinuesa CG. Dysregulation of immune homeostasis in autoimmune diseases. Nat Med. 2012;18(1):42–7. doi: 10.1038/nm.2621. [DOI] [PubMed] [Google Scholar]
- 39.Cross AH, Girard TJ, Giacoletto KS, Evans RJ, Keeling RM, Lin RF, Karr RW. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. J Clin Invest. 95(6):2783–9. doi: 10.1172/JCI117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 174(4):1888–97. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 41.Khoury SJ, Akalin E, Chandraker A, Turka LA, Linsley PS, Sayegh MH, Hancock WW. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 155(10):4521–4. [PubMed] [Google Scholar]
- 42.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 157:1333–6. [PubMed] [Google Scholar]
- 43.Vogel I, Kasran A, Cremer J, Kim YJ, Boon L, Van Gool SW, Ceuppens JL. CD28/CTLA-4/B7 costimulatory pathway blockade affects regulatory T-cell function in autoimmunity. Eur J Immunol. 45(6):1832–1841. doi: 10.1002/eji.201445190. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160(1):17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):265–74. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Preferential recruitment of neutrophils into the cerebellum and brainstem contributes to the atypical experimental autoimmune encephalomyelitis phenotype. J Immunol. 2015;195(3):841–52. doi: 10.4049/jimmunol.1403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A, Locati M. Orchestration of macrophage polarization. Blood. 2009;114(15):3135–6. doi: 10.1182/blood-2009-07-231795. [DOI] [PubMed] [Google Scholar]
- 49.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–44. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith KJ, Kapoor R, Hall SM, Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 2001;49(4):470–6. [PubMed] [Google Scholar]
- 51.Huitinga I, van Rooijen N, de Groot CJA, Uitdehaag BMJ, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–33. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, amd microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. GLIA. 1995;15:437–46. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- 53.Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Brain Res Rev. 2005;48(2):185–95. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Ruuls SR, Bauer J, Sontrop K, Huitinga I, t Hart BA, Dijkstra CD. Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J Neuroimmunol. 1995;56(2):207–17. doi: 10.1016/0165-5728(94)00154-g. [DOI] [PubMed] [Google Scholar]
- 55.Vaknin I, Kunis G, Miller O, Butovsky O, Bukshpan S, Beers DR, Henkel JS, Yoles E, Appel SH, Schwartz M. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PloS One. 2011;6(11):e26921. doi: 10.1371/journal.pone.0026921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of Multiple Sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- 57.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of JAK/STAT signaling in immunity and disease. J Immunol. 2015;194(1):21–7. doi: 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–11. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 61.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30(8):392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourdonnay E, Zaslona Z, Penke LR, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212(5):729–42. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and T(H)1-T(H)17 responses. Nat Immunol. 2011;12(3):231–38. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 64.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178(8):5245–52. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 65.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383–93. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- 66.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15(12):1104–15. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T-cell lineages. Ann Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 69.Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. 2009;47(3):149–156. doi: 10.1016/j.cyto.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 71.Hirahara K, Ghoreschi K, Laurence A, Yang XP, Kanno Y, O’Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21(6):425–34. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whyte CS, Bishop ET, Ruckerl D, Gaspar-Pereira S, Barker RN, Allen JE, Rees AJ, Wilson HM. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90(5):845–54. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- 74.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–48. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, Inoue H, Nakanishi Y, Kobayashi T, Yoshimura A. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-γ on STAT3 and Smads. J Immunol. 2008;180(6):3746–56. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 76.Eyles JL, Metcalf D, Grusby MJ, Hilton DJ, Starr R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling-1. J Biol Chem. 2002;277(46):43735–40. doi: 10.1074/jbc.M208586200. [DOI] [PubMed] [Google Scholar]
- 77.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincón M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13(6):805–15. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 78.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, Cacalano N, O’Garra A, Oshida T, Saito H, Johnston JA, Yoshimura A, Kubo M. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9(8):1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 79.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of SOCS3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206(10):2067–77. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F, Hong J, Shi Y, Leung S, Dong C, Zhang JZ. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35(2):273–84. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–19. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oksenberg JR, Baranzini SE. Multiple sclerosis genetics--is the glass half full, or half empty? Nat Rev Neurol. 2010;6(8):429–37. doi: 10.1038/nrneurol.2010.91. [DOI] [PubMed] [Google Scholar]
- 84.Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, Purcell S, Koivisto K, Tienari P, Sumelahti ML, Elovaara I, Pirttilä T, Reunanen M, Aromaa A, Oturai AB, Søndergaard HB, Harbo HF, Mero IL, Gabriel SB, Mirel DB, Hauser SL, Kappos L, Polman C, De Jager PL, Hafler DA, Daly MJ, Palotie A, Saarela J, Peltonen L. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Gen. 2010;86(2):285–91. doi: 10.1016/j.ajhg.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ban M, Goris A, Lorentzen AR, Baker A, Mihalova T, Ingram G, Booth DR, Heard RN, Stewart GJ, Bogaert E, Dubois B, Harbo HF, Celius EG, Spurkland A. Replication analysis identifies TYK2 as a multiple sclerosis susceptibility factor. Eur J Hum Genet. 2009;17(10):1309–13. doi: 10.1038/ejhg.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Couturier N, Bucciarelli F, Nurtdinov RN, Debouverie M, Lebrun-Frenay C, Defer G, Moreau T, Confavreux C, Vukusic S, Cournu-Rebeix I, Goertsches RH, Zettl UK, Comabella M. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility. Brain. 2011;134(Pt 3):693–703. doi: 10.1093/brain/awr010. [DOI] [PubMed] [Google Scholar]
- 87.Vandenbroeck K, Alvarez J, Swaminathan B, Alloza I, Matesanz F, Urcelay E, Comabella M, Alcina A, Fedetz M, Ortiz MA, Izquierdo G, Fernandez O, Rodriguez-Ezpeleta N, Matute C, Caillier S, Arroyo R, Montalban X, Oksenberg JR, Antigüedad A, Aransay A. A cytokine gene screen uncovers SOCS1 as genetic risk factor for multiple sclerosis. Genes Immun. 2012;13(1):21–8. doi: 10.1038/gene.2011.44. [DOI] [PubMed] [Google Scholar]
- 88.Lopez de Lapuente A, Pinto-Medel MJ, Astobiza I, Alloza I, Comabella M, Malhotra S, Montalban X, Zettl UK, Rodriguez-Antiguedad A, Fernandez O, Vandenbroeck K. Cell-specific effects in different immune subsets associated with SOCS1 genotypes in multiple sclerosis. Mult Scler. 2015;21(12):1498–512. doi: 10.1177/1352458514566418. [DOI] [PubMed] [Google Scholar]
- 89.Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41(7):824–8. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- 91.Winghagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wesemann D, Dong Y, O’Keefe GM, Nguyen VT, Benveniste EN. Suppressor of cytokine signaling 1 inhibits cytokine induction of CD40 expression in macrophages. J Immunol. 2002;169:2354–60. doi: 10.4049/jimmunol.169.5.2354. [DOI] [PubMed] [Google Scholar]
- 93.Traugott U, Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988;24(2):243–51. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- 94.Winghagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Merrill JE, Kong DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci USA. 1992;89:574–8. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human T(H)17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015;11:134–42. doi: 10.1038/nrneurol.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Segal BM. Stage-specific immune dysregulation in multiple sclerosis. J Interferon Cytokine Res. 2014;34(8):633–40. doi: 10.1089/jir.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frisullo G, Angelucci F, Caggiula M, Nociti V, Iorio R, Patanella AK, Sancricca C, Mirabella M, Tonali PA, Batocchi AP. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J Neurosci Res. 2006;84(5):1027–36. doi: 10.1002/jnr.20995. [DOI] [PubMed] [Google Scholar]
- 100.Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Mirabella M, Tonali PA, Batocchi AP. The persistency of high levels of pSTAT3 expression in circulating CD4+ T cells from CIS patients favors the early conversion to clinically defined multiple sclerosis. J Neuroimmunol. 2008;205(1–2):126–34. doi: 10.1016/j.jneuroim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Oyamada A, Ikebe H, Itsumi M, Saiwai H, Okada S, Shimoda K, Iwakura Y, Nakayama KI, Iwamoto Y, Yoshikai Y, Yamada H. Tyrosine kinase 2 plays critical roles in the pathogenic CD4 T cell responses for the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(11):7539–46. doi: 10.4049/jimmunol.0902740. [DOI] [PubMed] [Google Scholar]
- 102.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108(5):739–47. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okuda Y, Sakoda S, Bernard CCA, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6–deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10(5):703–8. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 106.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161(12):6480–6. [PubMed] [Google Scholar]
- 107.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180(9):6070–6. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheng W, Yang F, Zhou Y, Yang H, Low PY, Kemeny DM, Tan P, Moh A, Kaplan MH, Zhang Y, Fu XY. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24(12):1387–402. doi: 10.1038/cr.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu CR, Kim SH, Mahdi RM, Egwuagu CE. SOCS3 deletion in T lymphocytes suppresses development of chronic ocular inflammation via upregulation of CTLA-4 and expansion of regulatory T cells. J immunol. 2013;191(10):5036–43. doi: 10.4049/jimmunol.1301132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frisullo G, Mirabella M, Angelucci F, Caggiula M, Morosetti R, Sancricca C, Patanella AK, Nociti V, Iorio R, Bianco A, Tomassini V, Pozzilli C, Tonali PA, Matarese G, Batocchi AP. The effect of disease activity on leptin, leptin receptor and suppressor of cytokine signalling-3 expression in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;192(1–2):174–83. doi: 10.1016/j.jneuroim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 112.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2005;102(14):5150–55. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berard JL, Kerr BJ, Johnson HM, David S. Differential expression of SOCS1 in macrophages in relapsing-remitting and chronic EAE and its role in disease severity. Glia. 2010;58(15):1816. doi: 10.1002/glia.21051. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L, Dang RJ, Li H, Li P, Yang YM, Guo XM, Wang XY, Fang NZ, Mao N, Wen N, Jiang XX. SOCS1 regulates the immune modulatory properties of mesenchymal stem cells by inhibiting nitric oxide production. PloS One. 2014;9(5):e97256. doi: 10.1371/journal.pone.0097256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/ DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177(3):1679–88. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 116.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;1(3):374–89. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 117.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112(2):195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 118.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):1338–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 119.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20(10):1147–56. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181(5):3167–76. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12(1):105–18. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 122.Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN. PERK dependent activation of JAK1 and STAT3 contributes to ER stress induced inflammation. Mol Cell Biol. 2014;34(20):3911–25. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–9. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–48. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2013;20(2):160–72. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 126.Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, Aubry F, Auregan G, Guillermier M, Ruiz M, Petit F, Houitte D, Faivre E, Vandesquille M, Aron-Badin R, Dhenain M, Déglon N, Hantraye P, Brouillet E, Bonvento G, Escartin C. The JAK/ STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J Neurosci. 2015;35(6):2817–29. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of STAT3 or SOCS3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829–34. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 128.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24(3):224–9. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 129.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15(5):300–12. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 130.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem. 2002;277(43):40594–601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 131.Huang C, Ma R, Sun S, Wei G, Fang Y, Liu R, Li G. JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J Neuroimmunol. 2008;204(1–2):118–25. doi: 10.1016/j.jneuroim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98(5):1353–68. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- 133.Tada S, Okuno T, Hitoshi Y, Yasui T, Honorat JA, Takata K, Koda T, Shimagami H, Chi-Jing C, Namba A, Sugimoto T, Sakoda S, Mochizuki H, Kikutani H, Nakatsuji Y. Partial suppression of M1 microglia by Janus kinase 2 inhibitor does not protect against neurodegeneration in animal models of amyotrophic lateral sclerosis. J Neuroinflamm. 2014;11:179. doi: 10.1186/s12974-014-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El Waly B, Macchi M, Cayre M, Durbec P. Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci. 2014;8:145. doi: 10.3389/fnins.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 136.Popko B, Baerwald KD. Oligodendroglial response to the immune cytokine interferon gamma. Neurochem Res. 1999;24(2):331–8. doi: 10.1023/a:1022586726510. [DOI] [PubMed] [Google Scholar]
- 137.Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1(7):732–743. [PMC free article] [PubMed] [Google Scholar]
- 138.Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-γ. J Neurosci. 2006;26:5143–52. doi: 10.1523/JNEUROSCI.0737-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Butzkueven H, Zhang J-G, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8(6):613–9. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- 140.Emery B, Cate HS, Marriott M, Merson T, Binder MD, Snell C, Soo PY, Murray S, Croker B, Zhang JG, Alexander WS, Cooper H, Butzkueven H, Kilpatrick TJ. Suppressor of cytokine signaling 3 limits protection of leukemia inhibitory factor receptor signaling against central demyelination. Proc Natl Acad Sci USA. 2006;103(20):7859–64. doi: 10.1073/pnas.0602574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Z, Li H, Fitzgerald DC, Zhang GX, Rostami A. MOG(35–55) i.v suppresses experimental autoimmune encephalomyelitis partially through modulation of Th17 and JAK/STAT pathways. Eur J Immunol. 2009;39(3):789–99. doi: 10.1002/eji.200838427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jia Y, Jing J, Bai Y, Li Z, Liu L, Luo J, Liu M, Chen H. Amelioration of experimental autoimmune encephalomyelitis by plumbagin through down-regulation of JAK-STAT and NF-kappaB signaling pathways. PLoS One. 2011;6(10):e27006. doi: 10.1371/journal.pone.0027006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren Y, Lu L, Guo TB, Qiu J, Yang Y, Liu A, Zhang JZ. Novel immunomodulatory properties of berbamine through selective down-regulation of STAT4 and action of IFN-γ in experimental autoimmune encephalomyelitis. J Immunol. 2008;181(2):1491–8. doi: 10.4049/jimmunol.181.2.1491. [DOI] [PubMed] [Google Scholar]
- 144.Qin X, Guo BT, Wan B, Fang L, Lu L, Wu L, Zang YQ, Zhang JZ. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185(3):1855–63. doi: 10.4049/jimmunol.0903853. [DOI] [PubMed] [Google Scholar]
- 145.Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168(12):6506–13. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- 146.Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24(5):542–52. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- 147.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83(7):1299–309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J, Zeng YQ, Pan XD, Kang DY, Huang TW, Chen XC. Tripchlorolide ameliorates experimental autoimmune encephalomyelitis by down-regulating ERK1/2-NF-kappaB and JAK/STAT signaling pathways. J Neurochem. 2015;133(1):104–12. doi: 10.1111/jnc.13058. [DOI] [PubMed] [Google Scholar]
- 149.Yin L, Chen Y, Qu Z, Zhang L, Wang Q, Zhang Q, Li L. Involvement of JAK/STAT signaling in the effect of cornel iridoid glycoside on experimental autoimmune encephalomyelitis amelioration in rats. J Neuroimmunol. 2014;274(1–2):28–37. doi: 10.1016/j.jneuroim.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 150.Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13(8):935–43. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 151.Schulze-Topphoff U, Shetty A, Varrin-Doyer M, Molnarfi N, Sagan SA, Sobel RA, Nelson PA, Zamvil SS. Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PLoS One. 2012;7(3):1–10. doi: 10.1371/journal.pone.0033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ghoreschi K, Bruck J, Kellerer C, Deng C, Peng H, Rothfuss O, Hussain RZ, Gocke AR, Respa A, Glocova I, Valtcheva N, Alexander E, Feil S, Feil R, Schulze-Osthoff K, Rupec RA, Lovett-Racke AE, Dringen R, Racke MK, Röcken M. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen C, Liu X, Wan B, Zhang JZ. Regulatory properties of copolymer I in Th17 differentiation by altering STAT3 phosphorylation. J Immunol. 2009;183(1):246–53. doi: 10.4049/jimmunol.0900193. [DOI] [PubMed] [Google Scholar]
- 154.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23(10):482–6. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 155.Stuve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol. 2003;16(3):393–401. doi: 10.1097/01.wco.0000073942.19076.d1. [DOI] [PubMed] [Google Scholar]
- 156.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363(9421):1607–8. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 157.Chataway J, Schuerer N, Alsanousi A, Chan D, MacManus D, Hunter K, Anderson V, Bangham CR, Clegg S, Nielsen C, Fox NC, Wilkie D, Nicholas JM, Calder VL, Greenwood J, Frost C, Nicholas R. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383(9936):2213–21. doi: 10.1016/S0140-6736(13)62242-4. [DOI] [PubMed] [Google Scholar]
- 158.Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172(2):1273–86. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- 159.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180(10):6988–96. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 160.Lee SJ, Qin H, Benveniste EN. Simvastatin inhibits IFN-γ-induced CD40 gene expression by suppressing STAT-1α. J Leukoc Biol. 2007;82(2):436–47. doi: 10.1189/jlb.1206739. [DOI] [PubMed] [Google Scholar]
- 161.Muthian G, Raikwar HP, Johnson C, Rajasingh J, Kalgutkar A, Marnett LJ, Bright JJ. COX-2 inhibitors modulate IL-12 signaling through JAK-STAT pathway leading to Th1 response in experimental allergic encephalomyelitis. J Clin Immunol. 2006;26(1):73–85. doi: 10.1007/s10875-006-8787-y. [DOI] [PubMed] [Google Scholar]
- 162.Beurel E, Kaidanovich-Beilin O, Yeh WI, Song L, Palomo V, Michalek SM, Woodgett JR, Harrington LE, Eldar-Finkelman H, Martinez A, Jope RS. Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3. J Immunol. 2013;190(10):5000–11. doi: 10.4049/jimmunol.1203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3(2):59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 164.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus Kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–15. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Eng J Med. 2013;368(2):161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bright JJ, Du C, Sriram S. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999;162(10):6255–62. [PubMed] [Google Scholar]
- 168.Whartenby KA, Calabresi PA, McCadden E, Nguyen B, Kardian D, Wang T, Mosse C, Pardoll DM, Small D. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci USA. 2005;102(46):16741–6. doi: 10.1073/pnas.0506088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skarica M, Wang T, McCadden E, Kardian D, Calabresi PA, Small D, Whartenby KA. Signal transduction inhibition of APCs diminishes th17 and Th1 responses in experimental autoimmune encephalomyelitis. J Immunol. 2009;182(7):4192–9. doi: 10.4049/jimmunol.0803631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Y, Holdbrooks AT, De Sarno P, Rowse AL, Yanagisawa LL, McFarland BC, Harrington LE, Raman C, Sabbaj S, Benveniste EN, Qin H. Therapeutic efficacy of suppressing the JAK/ STAT pathway in multiple models of experimental autoimmune encephalomyelitis. J Immunol. 2014;192:59–72. doi: 10.4049/jimmunol.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Benveniste EN, Liu Y, McFarland BC, Qin H. Involvement of the Janus kinase/signal transducer and activator of transcription signaling pathway in multiple sclerosis and the animal model of experimental autoimmune encephalomyelitis. J Interferon Cytokine Res. 2014;34(8):577–88. doi: 10.1089/jir.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fan Y, Mao R, Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4(3):176–85. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: Have all roads been explored? JAKSTAT. 2013;2(1):e22882. doi: 10.4161/jkst.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA. 2012;109(24):9623–28. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PLoS One. 2012;7(1):e29742. doi: 10.1371/journal.pone.0029742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Flowers LO, Johnson HM, Mujtaba MG, Ellis MR, Haider SM, Subramaniam PS. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J Immunol. 2004;172(12):7510–8. doi: 10.4049/jimmunol.172.12.7510. [DOI] [PubMed] [Google Scholar]
- 177.Mujtaba MG, Flowers LO, Patel CB, Patel RA, Haider MI, Johnson HM. Treatment of mice with the suppressor of cytokine signaling-1 mimetic Peptide, tyrosine kinase inhibitor Peptide, prevents development of the acute form of experimental allergic encephalomyelitis and induces stable remission in the chronic relapsing/remitting form. J Immunol. 2005;175:5077–86. doi: 10.4049/jimmunol.175.8.5077. [DOI] [PubMed] [Google Scholar]
- 178.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, Johnson HM. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol. 2007;178(8):5058–68. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]