Abstract
In a previous study, we demonstrated the feasibility of retaining poly(N-isopropylacrylamide) (pNIPAAm) on hydroxylated surfaces by spin-coating a blend of pNIPAAm with a small amount of 3-aminopropyltriethoxysilane (APTES), an organosilane, followed by thermal annealing. In this study, we detail the conditions for retaining pNIPAAm films by APTES. Our results show that the difference in surface energy between pNIPAAm and APTES in the blended film resulted in the segregation of APTES molecules to the film/substrate interface, as verified by XPS, during annealing, and the segregated APTES molecules cross-linked to form the APTES network, thus entrapping pNIPAAm. The retained pNIPAAm films (25–35 nm) exhibited thermo-responsive behavior, determined by water contact angles and film thickness in water at temperatures above and below the lower critical solution temperature of pNIPAAm, as well as good cell attachment and rapid detachment (<10 minutes). The gained insights would allow a better design of these thermo-responsive surfaces for cell sheet engineering and other relevant applications.
Keywords: organosilane, segregation, thermo-responsive thin films
Graphical Abstract
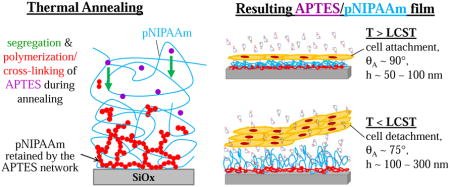
Introduction
Cell sheet engineering without scaffolds has attracted significant attention in tissue engineering in recent years, mainly owing to its advantage in maintaining cell-cell interactions and in mimicking the microarchitecture of native tissue. One common approach for generating cell sheets is utilizing thermo-responsive polymers (TRPs), such as poly(N-isopropylacrylamide, pNIPAAm). These polymers exhibit hydrophobic/hydrophilic transition with a change in temperature. For pNIPAAm, the transition temperature occurs at ~ 32°C. Above 32°C, the pNIPAAm chains are in the collapsed form and are hydrophobic, allowing cell attachment and proliferation. They become hydrophilic when the temperature is below 32°C, and the polymer chains hydrate, promoting cell detachment. The use of pNIPAAm to harvest cells offers an advantage over using trypsin, which is known to cause some damage to cell membrane proteins and is reported to affect cell metabolism and differentiation[1–3]
There are two common approaches for generating pNIPAAm layers. The first is a grafting based approach to generate pNIPAAm brushes. The chains are grafted to tissue culture polystyrene dishes and other substrates via electron beam[4], plasma[5–7] or UV irradiation[8]. The equipment used in these grafting approaches might not be accessible for most researchers working in the cell sheet engineering field. Also, the grafting density and thickness of pNIPAAm brushes need to be optimized for cell adhesion and cell detachment[9]. One hindering issue of the pNIPAAm brushes is that these surfaces could take a long time for the cell sheet to detach in cold medium, which could damage the cells [10]. The second approach is to simply cast the pNIPAAm layer from a solution[11]. The critical issue with this approach is the poor cell adhesion, and the co-polymers or proteins incorporated for enhancing cell adhesion could leach out and cause potential contamination or diseases[11].
We have recently reported a simpler and more easily adoptable method[10] on fabricating thermo-responsive pNIPAAm surfaces via spin-coating followed by thermal annealing, which eliminates the need for using sophisticated equipment. In this earlier study, we demonstrated the feasibility of the approach for enhanced cell attachment and rapid cell sheet detachment. Briefly, we blended pNIPAAm with a small amount of adhesion promoter, 3-aminopropyltriethoxysilane (APTES), the solution of the blend was spin-coated onto a glass slide, and then the sample was annealed in a vacuum oven at 160°C to allow the APTES molecules to cross-link and form a network that would entrap the pNIPAAm chains and retain a layer of pNIPAAm on the surface. Cell attachment and proliferation on our surfaces were observed, and the confluent cell sheets detached within minutes from our surfaces when a cold medium (4°C initially) was added. However, the results reported in the previous study were not fully understood and the details of the entrapment of pNIPAAm by APTES network need to be elucidated. The gained insights would allow a better design of these thermo-responsive surfaces for cell sheet engineering.
In this current study, we examined the blend thin films of APTES/pNIPAAm in more detail. A gradient distribution of the APTES molecules along the thickness direction was observed for the cured APTES/pNIPAAm films, with the highest concentration at the film/substrate interface, while the presence of APTES was minimal at the film/air interface. The blend films without curing exhibited a relative uniform distribution of the APTES along the thickness of the film. Based on the observations, we rationalized that the formation of the APTES network at and near the substrate was the result of segregation of APTES molecules to the film/substrate interface and the cross-linking of these molecules when they reached each other’s vicinities during the annealing process, hence, entrapping pNIPAAm. The total mass per unit area of APTES in the cured blend films was roughly the same when spin-coated from solutions having the same solute content (e.g. ~ 1.5 wt.%), but different APTES/pNIPAAm ratios. All films showed thermo-responsive behavior determined using advancing contact angles and thickness measurements in water above and below the LCST of pNIPAAm. Cell sheet detachment from all the blend films (from an 80/20 to 10/90 APTES/pNIPAAm ratio) was also observed, mostly within 10 minutes. Additionally, there was no clear correlation between the detachment time of mouse embryonic fibroblast cell sheet and the retained film thickness (6 nm to 120 nm) we investigated.
Materials and Methods
Materials and equipment
Poly(N-isopropylacrylamide) (pNIPAAm) with a number average molecular weight, Mw, of 20,000 – 40,000 g/mol and 99% (3-Aminopropyl)triethoxysilane (APTES, Mw = 221 g/mol or 137 g/mol when fully hydrolyzed) were from Sigma-Aldrich. Butyltrimethoxysilane (BTMS, Mw = 179 g/mol or 136 g/mol when fully hydrolyzed) was purchased from Gelest. 200 proof ethanol was from EMD, and DI water was purified in house with a conductivity of ~ 0.5 μS/cm. Other chemicals used included 30 % hydrogen peroxide from BDH, 98% concentrated sulfuric acid and concentrated acetic acid from VWR. The cell culture medium used was MEME (minimum essential medium eagle) +10% FBS (fetal bovine serum) + 1% of antibiotic antimycotic solution (100x). Unless otherwise mentioned, all reagents, such as 1x trypsin/EDTA, PBS, were purchased from Sigma-Aldrich. STO cells (a type of mouse embryonic fibroblast) was a gift from Drs. William Chilian and Liya Yin at Northeast Ohio Medical School (NEOMED). Argon gas with a purity of 99.999% was from Praxair. Other supplies included microscope glass slides (from VWR), silicon wafer (P type P<100> from Silicon specialist), treated 24-well plates and 35 mm culture dishes, both from greiner bio-one,
Basic equipment used for this study involved a spin coater (p-6000 Spin Coater, Specialty Coating System Inc., Indianapolis, IN), a plasma chamber (Harrick Plasma PDC-32G), a UV/ozone cleaner (model 42, Jelight), analytical balances with an accuracy of 0.1 mg, a vacuum oven (VWR) and its pump (Welch), a humid CO2 cell incubator, a contact angle goniometer (Ramé-Hart Instrument Co., Netcong, NJ with a CCD camera attached), an ellipsometer (Rudolph Instruments, Inc., Fairfield, NJ equipped with a λ = 632.8 nm laser), a microscope heating stage (TP-110R, TOKAI HIT), a digital camera, an optical microscope (OM) with an eye-piece digital camera, a humidifier, and XPS (PHI VersaProbe II Scanning XPS microprobe) with an Al Kα excitation source (X-ray setting: 100 μm, 25 W and 15kV). A take-off angle of 45° was used.
Preparation of pNIPAAm, APTES/pNIPAAm and BTMS/pNIPAAm films
Glass slides and Si-wafers were cut into ~ 1 cm × 1 cm pieces, and then cleaned using a freshly prepared piranha solution1 followed with copious DI water rinsing. The slide or wafer was dried with a stream of dry air, and then oxidized for 8 minutes in the UV/Ozone chamber.
1.5 wt.% APTES and 1.5 wt.% pNIPAAm in 200 proof ethanol were prepared separately, and then they were mixed to form solutions with a total solute of 1.5 wt.% and APTES/pNIPAAm mass ratios of 80/20 (i.e., 1.2 wt.% APTES and 0.3 wt.% pNIPAAm), 60/40, 50/50 (i.e., 0.75 wt.% APTES and 0.75 wt.% pNIPAAm), 40/60, 30/70, 20/80, and 10/90. In the main-text, these films are described as 80/20 blend, 60/40 blend, 50/50 blend, 40/60 blend, 30/70 blend, 20/80 blend, and 10/90 blend. 1.5 wt.% BTMS was also prepared in ethanol, and mixed with the equal amount of 1.5 wt.% of pNIPAAm solution to make the 50/50 BTMS/pNIPAAm solution. Each prepared solution was spin-coated (~50 μl solution flooding the sample surface), within 1 – 2 hrs, on a freshly cleaned and oxidized glass slide or Si-wafer at a spin-speed of ~ 2000 rpm for 30 seconds. For investigating the solution age effects on the resulting blend films, the prepared 1.5 wt.% 50/50 blend solution and 0.75 wt.% of APTES or BTMS solution was stored inside the tightly sealed vials for a predetermined period of time before spin-coating them.
The spin-coated slides and wafers were placed inside glass petri-dishes for at least ~ 30 minutes under ambient conditions, and then placed inside the vacuum oven to be cured for 3 days at 160°C. The cured samples, after removing from the oven and cooled, were individually placed into the wells of 24-well plates for storage. The non-cured samples were also placed inside the wells of 24-well plates under ambient conditions.
Characterization of APTES/pNIPAAm and BTMS/pNIPAAm films
The films on Si-wafer were mainly used for thickness measurements and chemical composition analysis via XPS. They were occasionally used to verify the water contact angles measured on the films prepared on glass slides. The film thickness for each sample was measured after the sample was removed from the oven, after it was thoroughly rinsed with DI water, and after it was soaked in DI water for 3 days in ambient conditions. The film thickness was measured via an ellipsometer with a λ = 632.8 nm laser; and the values were used to estimate the amount of APTES or BTMS in the blended films and to assess the retention of the films on the surface. For pNIPAAm films and all silane/pNIPAAm blend films, a refractive index of 1.47 [12, 13] was used to estimate the film thickness in air. For the thickness of APTES, BTMS and silicon oxide in air, the refractive indices of 1.423, 1.40 and 1.462 were used, respectively. The thickness of the thoroughly rinsed or 3 days soaked APTES/pNIPAAm blend films in water was also measured at 40°C and 25°C using a liquid cell and a heating/cooling stage. The liquid cell, filled with DI water, was placed on the heating/cooling stage until a set temperature was reached, and then the sample was submerged in the water. A heating/cooling cycle was first applied to observe the thermo-responsiveness of the blend film, and a transition occurred when the temperature reached 31 – 32°C. Then the sample was submerged in water at either 25°C or 40°C to equilibrate for ~10 minutes before measurement. A longer equilibrating time was also used to ensure that the film thickness was no longer changing. To interpret the data, at 25°C, a refractive index of 1.35 to 1.37, estimated based on the refractive indices of pure pNIPAAm brushes (n = 1.35) [14] and APTES in water at 25C and the % of APTES retained in the films, was used for the pNIPAAm/APTES blend film, and a value of 1.33 was used for the medium (water). At 40°C in water, the refractive indices of 1.40 [14] for the blend film and 1.33 for water were used to estimate the thickness.
The water contact angle on the film was measured using a contact angle goniometer equipped with a heating/cooling stage. For the thoroughly rinsed APTES/pNIPAAm blend films, advancing and static water contact angles were measured at 40°C and 25°C by placing the sample on the heating stage. After the heating stage reached to the set temperature (40°C or 25°C), the sample was placed on the stage and allowed to equilibrate for ~ 5 minutes. Then, a water drop (~ 10 μl) was placed on the sample, with the needle in the drop, and more water was slowly added until the drop was ready to advance, at which point the image was captured. The time from adding the drop to taking the image was ~ 1 minute. After two advancing measurements, the needle was withdrawn, and the drop (~ 20 μl) was allowed to sit on the sample for ~ 1 minute before the image was taken for the static contact angle. The contact angles were measured from the captured images using ImageJ software.
The 50/50 blend films were used to profile the chemical compositions at different depths of the films using XPS. After rinsing off the loose (i.e., un-entrapped pNIPAAm) top layer, the film was either un-treated or further etched by Ar plasma to obtain a thickness of ~ 25 nm, 10 – 20 nm, and 5 – 10 nm. These films, along with pure APTES and pNIPAAm, both cured for 3 days under vacuum at 160°C, were de-gassed in the vacuum oven overnight prior to XPS scanning. A separate set of un-cured 50/50 APTES/pNIPAAm films with a thickness of ~ 25 nm, 10 – 20 nm, and 5 – 10 nm, was also used for the comparison study. Survey scans and the high resolution scans of C1s, O1s, N1s and Si2p were obtained for all the samples.
Cellular behaviors on APTES/pNIPAAm films
~ 150K cells/ml were seeded in a 35 mm dish containing an APTES/pNIPAAm coated glass slide after the slide was thoroughly rinsed with cold DI water and allowed to incubate at 37°C (> LCST of pNIPAAm or 32°C) for 2–3 days until the cells reached confluence. The dish was placed on the microscope stage and the warm medium (> 32°C) was replaced with ~ 3 mL of 4°C cold medium. A thermo-couple was placed inside the medium to monitor the temperature, and in most cases the temperature reached 15 – 20°C (< LCST of pNIPAAm). The cell detachment process was followed via a microscope-video system and using a 4X or a 10X phase objective. A sequence of images were captured with a preset time interval (5 s, 10 s, 20 s or 30 s) until the entire sheet was detached or the attached cells were detached.
Results and Discussion
pNIPAAm film retention by APTES and thermal curing
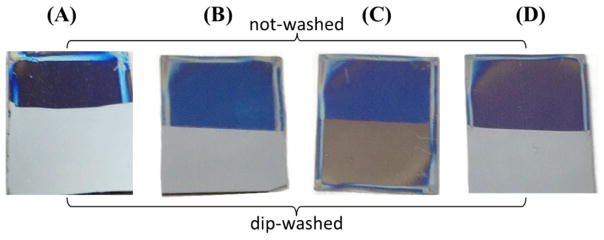
Without APTES, the spin-coated pNIPAAm films, with or without thermal curing, were easily removed by rinsing the sample with room temperature DI water (Fig. 1 and Table 1). For the un-cured samples, the films were almost completely removed after rinsing with DI water. Additionally, when a drop of water was placed on the rinsed surface at both room temperature (< LCST of pNIPAAm, i.e., hydrophilic state) and 40°C (> LCST of pNIPAAm, i.e., hydrophobic state), it spread and led to a water contact angle of < 10°. For the pNIPAAm film cured for 3 days at 160°C, the film was also mostly removed when rinsed with room temperature DI water, resulting in a ~ 4 nm adsorbed pNIPAAm residual layer on the oxidized Si-wafer. A static water contact angle of ~ 58° was observed on this surface when measured at 40°C, which is close to those observed on pNIPAAm at this temperature (> LCST). We further examined this rinsed sample using XPS, and noticed it had both C and N peaks (Fig. S1) with a C/N ratio of ~ 6, the same ratio for pure pNIPAAm. This thin pNIPAAm layer, however, did not show a noticeable thermo-responsive behavior based on the water contact angle measurements.
Fig. 1.
Digital images of cured pNIPAAm only (A), 50/50 APTES/pNIPAAm non-cured (B) and cured films (C), and 50/50 BTMS/pNIPAAm cured film (D) before and after dip-washing in ~ 23°C DI water. The pNIPAAm film was spin-coated on silicon wafer (1 cm × 1.2 cm) from 1.5 wt.% solution in ethanol, and the blend films were spin-coated from ~ 3 wt.%, instead of 1.5 wt.%, of total solute to show a better color contrast for imaging.
Table 1.
Film thickness and water contact angles of some pNIPAAm, 50/50 APTES/pNIPAAm and 50/50 BTMS/pNIPAAm films. The average and standard derivation reported were from measurements of three to six films.
| Films | film thickness (nm)* after rinsing | water contact angle (°) after rinsing, at 40°C |
|---|---|---|
| non cured pNIPAAm | 0.4 ± 0.2 | 7.4 ± 0.5 |
| cured pNIPAAm | 3.8 ± 0.6 | 58.5 ± 2.1 |
| non-cured APTES layer | 1.2 ± 0.2 | 38.3 ± 2.1 |
| non-cured 50/50 APTES/pNIPAAm | 0.8 ± 0.3 | 28.7 ± 0.8 |
| cured 50/50 APTES/pNIPAAm | 25 – 35** | 68.9 ± 2.2 |
| cured 50/50 BTMS/pNIPAAm | 3.2 ± 0.3 | 57.8 ± 0.4 |
The average silicon oxide layer of 2.5 nm, measured for our silicon wafer, has been subtracted from all the thickness values reported in this table. This slightly thicker (2.5 nm) than usual (~ 1.5 nm) SiOx layer could be the result of the growth of SiOx from the UV/Ozone oxidization process applied for cleaning the wafer.
The film thickness of the cured 50/50 APTES/pNIPAAm blend films depended on the age of solution used for spin-coating, so the value of the rinsed film varied in this range.
By blending with APTES, it was found that pNIPAAm could only be trapped after thermal curing. The 50/50 blend films without thermal curing but stored under ambient conditions for 3 days were easily removed when rinsed with cold DI water (~ 23°C), leaving behind a thin layer of ~ 1 nm, which had a water contact angle of ~ 29° at 40°C. This contact angle is too hydrophilic to suggest the presence of a sufficient amount of pNIPAAm. The 50/50 blend films thermally cured at 160°C for 3 days had a thickness of 25 – 35 nm after cold water rinsing with a water contact angle of ~ 69° measured at ~ 40°C, which is close to the contact angle measured on pure pNIPAAm. The contact angle is higher than the values reported by others (45°–51°) [11]. However, the reported angles were on films that were not cured at such high temperatures. In general, with sufficient mobility, polymer chains rearrange to minimize surface free energy[15]. In our case, pNIPAAm was cured at a temperature above its glass transition temperature, allowing pNIPAAm chains to re-arrange to minimize the surface free energy. To verify that the high contact angle was a result of curing, water contact angles were measured on non-cured pNIPAAm films (at 40 °C) and were found to be ~45°, very close to values reported for uncured spin-coated pNIPAAm films [11]. More importantly, the cured blend films exhibited thermo-responsive behavior (TRB) as shown in Table 2. These results demonstrate that the presence of APTES as well as curing are both needed to obtain sufficiently thick pNIPAAm films that exhibit TRB.
Table 2.
The advancing water contact angles (qA) above and below the transition temperature (LCST) of APTES/pNIPAAm blend films cured for 3 days in vacuum at 160°C and rinsed by cold DI water. (A) various APTES/pNIPAAm ratios, (B) 50/50 APTES/pNIPAAm from solutions of different ages.
| (A)
|
(B)
|
||||
|---|---|---|---|---|---|
| APTES/pNIPAAm | θA (°) at 40°C > LCST | θA (°) at 25°C < LCST | solution age (days) | θA (°) at 40°C > LCST | θA (°) at 25°C < LCST |
|
|
|
||||
| 0/100* | 65.4 ± 5.7** | dissolved | 0 | 89.2 ± 1.6 | 78.4 ± 2.5 |
| 0/100 | 90.4 ± 4.7** | dissolved | |||
| 10/90 | 91.1 ± 3.6 | 53.1 ± 3.8 | 3 | 90.2 ± 0.4 | 78.6 ± 1.8 |
| 20/80 | 94.2 ± 4.1 | 82.3 ± 3.1 | 7 | 89.1 ± 1.8 | 77.5 ± 3.0 |
| 30/70 | 99.0 ± 3.4 | 79.7 ± 6.0 | |||
| 40/60 | 89.9 ± 3.2 | 78.8 ± 5.6 | 14 | 89.2 ± 1.7 | 80.8 ± 5.6 |
| 50/50 | 95.3 ± 1.7 | 75.2 ± 2.2 | |||
| 60/40 | 105.3 ± 1.3 | 77.3 ± 4.7 | 21 | 86.4 ± 2.8 | 75.2 ± 1.1 |
| 80/20 | 92.5 ± 2.8 | 74.1 ± 4.1 | 42 | 89.1 ± 1.8 | 78.6 ±2.5 |
| 100/0 | 88.1 ± 3.3 | 88.2 ± 2.5 | |||
|
|
|
||||
un-cured pNIPAAm film;
measured without rinsing the films; the average and standard derivation were the results of four to eight measurements.
Effects of curing time and APTES content on retaining pNIPAAm film
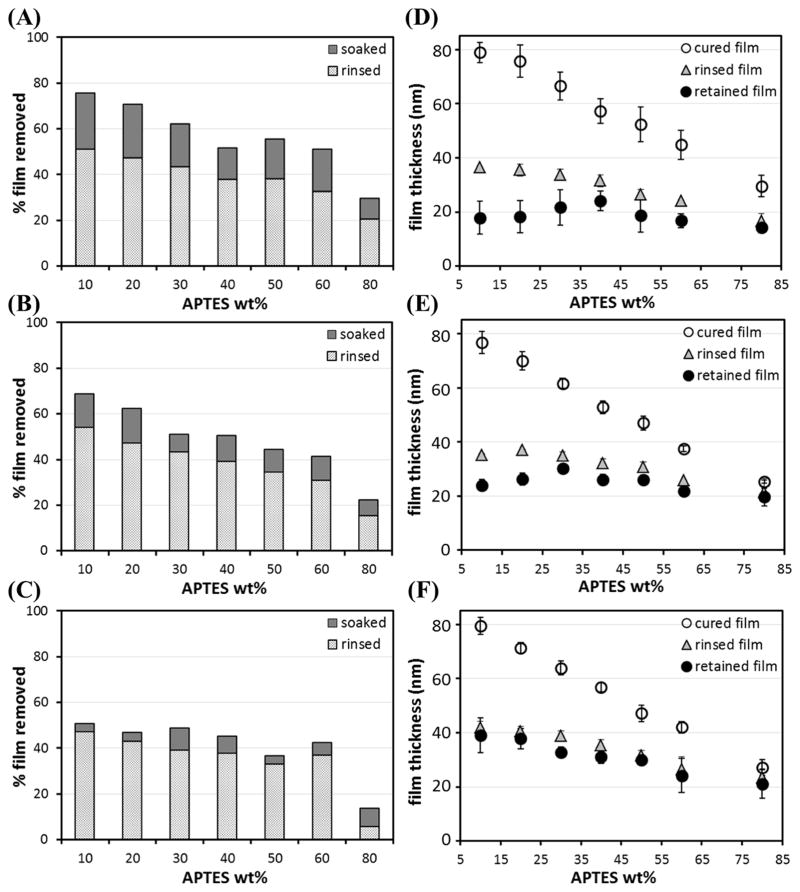
Fig. 2 illustrates the effects of curing time and APTES content on retaining pNIPAAm. On average, cold water rinsing removed ~ 40% of film for all three curing durations. In term of thickness, the cured film from a blend containing less APTES (i.e., more pNIPAAm) was thicker before rinsing, and more film was removed by rinsing. The thickness of the rinsed films for 1 day, 2 days and 3 days of curing appeared to be similar; mostly within 20 – 40 nm, and decreased slightly with the increase of APTES content. For the 80/20 blend, rinsing removed ~ 20%, ~ 15% and ~ 6%, respectively, for films cured for 1 day, 2 days and 3 days, less than those of other blends, but still resulted in the thinnest films (15 – 20 nm) due to the lowest pNIPAAm content in the cured films.
Fig. 2.
Spin-coated films, from 1.5 wt.% total APTES+pNIPAAm in ethanol, containing different wt.% of APTES in the blend cured in the vacuum oven at 160°C for 1 day (A & D), 2 days (B & E) and 3 days (C & F). The amounts of films removed after rinsing and then soaking in ~ 23°C DI water for 3 days and the film thickness before and after rinsing/soaking are summarized. The error bars are the standard deviations of at least three sets of measurements.
The amount of APTES in the cured films was roughly estimated using their thickness, and the values, i.e., wt.% and mass/area of APTES, are summarized in Table 3. In all the cases, the wt.% of APTES in the cured films was lower than that in the total solute in the spin-coating solution, indicating more APTES, as compare to pNIPAAm, were removed by spin-coating followed by thermal curing (Fig. 5(A) & (B)). The wt.% APTES in the cured films increased from ~ 8.5% to ~ 21% as the APTES/pNIPAAm ratio increased from 10/90 to 60/40. For the 80/20 blend film, the wt.% APTES was ~ 38%, about half of the ratio present in its spin-coating solution. The mass/area of APTES retained in the cured films was 0.7 – 0.8 μg/cm2 for all the blends except 80/20, which had a mass/area of ~ 0.93 μg/cm2.
Table 3.
Estimated wt.% silane (APTES or BTMS) presented in the 3 days cured but non-rinsed APTES/pNIPAAm and BTMS/pNIPAAm films.
| ratio of APTES/pNIPAAm in the 1.5 wt.% solution in ethanol used for spin-coating
|
50/50 BTMS/pNIPAAm | |||||||
|---|---|---|---|---|---|---|---|---|
| 10/90 | 20/80 | 30/70 | 40/60 | 50/50 | 60/40 | 80/20 | ||
| wt. % silane in the blend film | 8.5 ± 2.7 | 10.9 ± 4.8 | 12.5 ± 5.0 | 14.4 ± 4.9 | 15.7 ± 4.8 | 20.7 ± 7.2 | 38.0 ± 10.0 | ~0.6 |
| mass of silane (μg/cm2) in the blend film | 0.71 ± 0.22 | 0.79 ± 0.35 | 0.80 ± 0.32 | 0.80 ± 0.32 | 0.73 ± 0.22 | 0.82 ± 0.29 | 0.93 ± 0.24 | ~0.04 |
wt% and mass/area of silane in the blend films were estimated based on the cured film thickness of at least three sets of films.
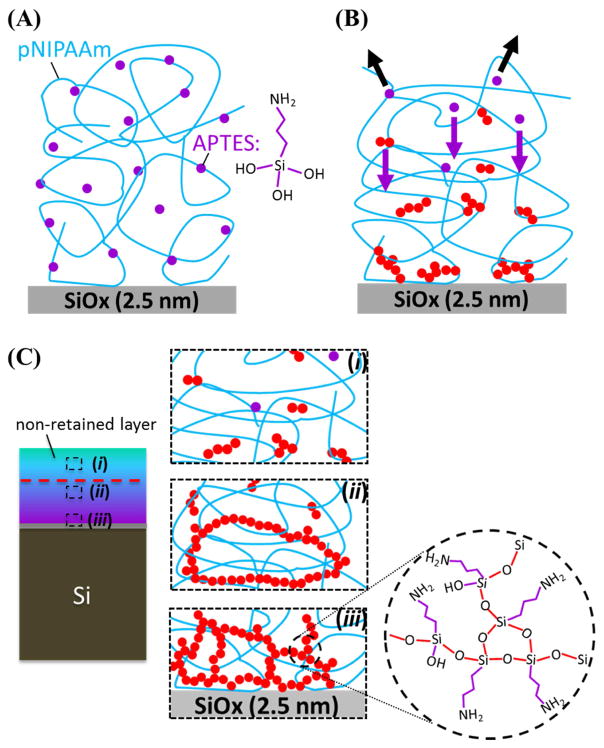
Fig. 5.
A potential explanation of how pNIPAAm chains are retained is presented: (A) some APTES molecules are retained during spin coating (the molecular structure of fully hydrolyzed APTES, having three -SiOH groups, is sketched to the right), (B) evaporation (black arrows) and segregation (purple arrows) of APTES during annealing under vacuum to result in a gradient distribution of the APTES within the film, and (C) formation of the APTES network and APTES oligomers within the film during curing. The red circles represent the polymerized APTES, forming siloxane bonds by condensing the –SiOH groups between APTES molecules. When all three of the –SiOH groups within one APTES molecules are all condensed, cross-linking would result (see the enlarged circle of (iii)).
Since the APTES molecules are relatively small, they could be easily removed during processing. The retention of APTES in the cured film during processing could be the result of the –NH2 and –OH groups in APTES interacting with the amide group in pNIPAAm via hydrogen bonding. While a few of the retained APTES would chemically graft to the substrate, a majority of the retained APTES molecules, each of them contains three –SiOH groups when fully hydrolyzed, would polymerize with each other by condensing the –SiOH groups to form intermolecular siloxane bonds. When all three –SiOH groups are condensed, cross-linking of polymerized APTES into a complex network would result [10, 16–19]. A higher APTES content in the 80/20 blend films would form a tighter APTES network, thus making the entrapped pNIPAAm harder to be removed upon rinsing.
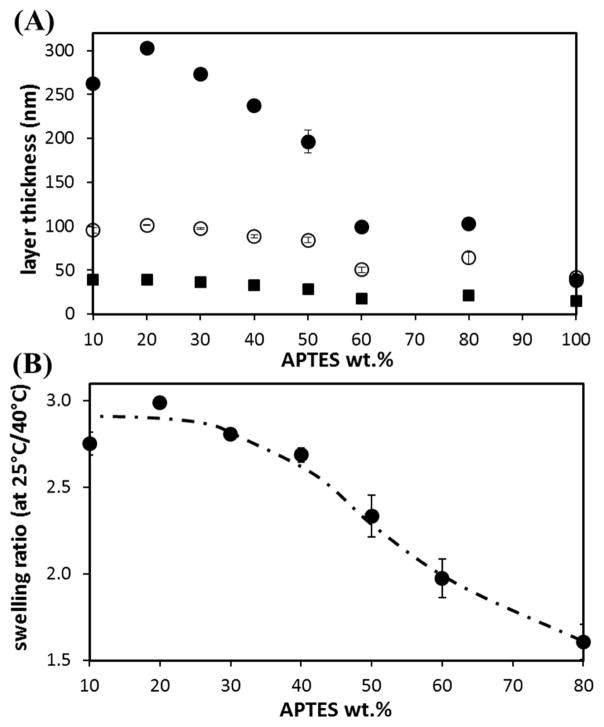
The rinsed APTES/pNIPAAm films were further soaked in cold DI water (~ 23°C) for 3 days to assess the stability of the films. 3 days soaking was chosen based on previous experiments (data not shown) that showed no further reduction in film thickness beyond 2–3 days of soaking. Soaking resulted in an additional removal of all the films, with the greatest amount (~21% in average) removed with 1 day curing. 2 days and 3 days of curing led to ~ 12% and ~ 6%, respectively, of additional film removal. Due to the hygroscopic nature of amines[20], soaking in water could cause the network to swell and/or the pNIPAAm chains to re-arrange, making them easier to be pulled out, especially for those that were loosely trapped. Separate experiments of soaking thermally cured APTES films showed that soaking did indeed swell the network, with a 50 % increase in thickness, hence volume, when films were dried by a stream of air and measured in air (Fig. S1) or 160-18-% increase in thickness when measured in water (Fig. 3(A) – data point at 100%). For cured 10/90 to 60/40 APTES/pNIPAAm blend films, containing ≤ 20 wt.% of APTES or an APTES mass/area of 0.7 – 0.8 μg/cm2, the APTES networks formed to retain pNIPAAm could be similar, resulting in similar additional removal of pNIPAAm by soaking these films.
Fig. 3.
(A) The film thickness, in air at 25°C (■), in water at 40°C (○) and in water at 25°C (●), of various APTES/pNIPAAm blend films cured in a vacuum oven at 160°C for 3 days and then rinsed by cold DI water. (B) The swelling, or thickness, ratio of these films in water at 25°C to 40°C. The error bars are the standard deviations of three measurements (on two samples of each type of film).
In terms of curing time, for 1 day curing, the retained films, for the various APTES/pNIPAAm blends, essentially had the same thickness (14 – 24 nm, average ~ 20 nm). The 80/20 blend films showed the least removal of the film (Fig. 2D), from ~ 17 nm to ~ 14 nm; while the 10/90 blend films had the most removal (~ 61 nm). For 2 days of curing at 160°C, the retained films had an average thickness of ~ 26 nm. Again, the 80/20 blend barely decreased (< 6 nm) in thickness, while the 10/90 blend had ~ 53 nm film removed. For APTES/pNIPAAm films cured for 3 days, soaking only led to a small removal (~ 6 % in average) of films, and the retained films (~ 32 nm on average) were slightly thicker than those films cured for 1 day and 2 days. The retained film, in this case, decreased slightly in thickness as the APTES content increased (Fig. 2F). The above results indicate that the APTES network forms within 1 day of curing to retain pNIPAAm, although additional days of curing leads to more retention of pNIPAAm. With 3 days of curing at 160°C, the APTES network reached a sufficient degree of cross-linking that greatly hampers additional pNIPAAm removal upon soaking in cold water. One possible reason is that with a longer curing time, a tighter APTES network forms with more APTES molecules segregated to the film/substrate interface; however, the exact value of degree of crosslinking is challenging to be quantified as elucidated in a later section.
Distribution of APTES in the APTES/pNIPAAm blend films
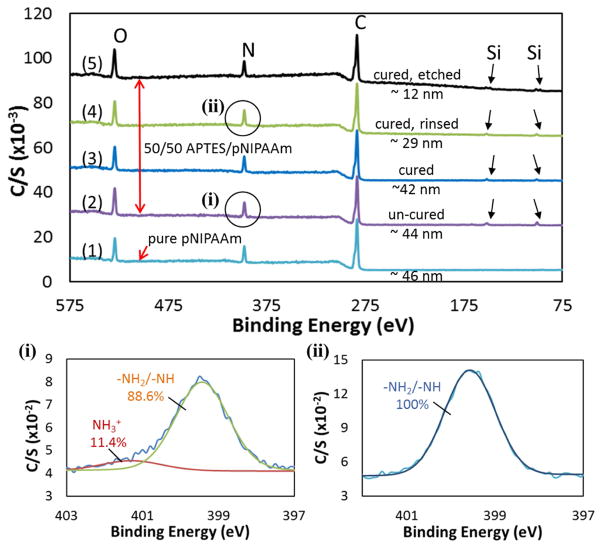
From the results above (Fig. 2), it is clear that the top layer (~ 40% of the films resulted from 1.5 wt.% of total solute used for spin-coating) of the blend films could be easily removed by rinsing. One possibility is that, during thermal curing, the APTES molecules segregated to the film/substrate interface, and cross-linked at and near the film/substrate interface to form a network for entrapping pNIPAAm. The top portion of the film, however, had insufficient amount of APTES to form a network for retaining pNIPAAm. To verify this possibility, XPS scans of spin-coated 50/50 blend of APTES/pNIPAAm films, with and without curing, were obtained. Some films were etched by Argon plasma to different thicknesses (10 – 20 nm, and < 10 nm) for depth profiling of the two components at different locations within the film.
Relevant XPS scans are presented in Fig. 4, with the elemental atomic % summarized in Table 4. For the 3 days cured pure pNIPAAm film (~ 46 nm), three elements: C, N and O, were detected, with an atomic % of 76.6, 11.8 and 11.6, respectively. These values are very close to the expected values for pNIPAAm, i.e., 75%, 12.5%, and 12.5% for C, N, and O, respectively. For all the APTES/pNIPAAm blend films, all four elements: C, N, O and Si, were detected, and the C/N ratio is close to the value of 6 for pNIPAAm. This indicates that the top layer (5–7 nm) of the retained film is mainly pNIPAAm. The O content in the blend films is slightly higher than that of pure pNIPAAm (i.e., O/C = 1/6), which is attributed to the presence of APTES that has an O/C ratio of 1.
Fig. 4.
XPS survey scans of different ((1) pure pNIPAAm, (2) non-cured, (3) cured, and (4) & (5) etched 50/50 APTES/pNIPAAm) films are shown. The high resolution N1s peaks (circled) for the un-cured (i) and cured + rinsed (ii) 50/50 APTES/pNIPAAm films are also presented. The small peaks (pointed out by the arrows) of Si2p are observed for all the blended films.
Table 4.
Atomic % of C, O, N and Si, obtained from the XPS survey scans of the top layer of films presented in Figs. 3 & 5.
| element atomic % | pNIPAAm cured (~ 46 nm) | 50/50 APTES/pNIPAAm
|
50/50 BTMS/pNIPAAm cured (~ 46 nm) | ||||
|---|---|---|---|---|---|---|---|
| non-cured (~ 44 nm) | cured, not rinsed (~ 42 nm) | cured rinsed (~ 29 nm) | cured, rinsed, etched & rinsed (~ 12 nm) | cured, rinsed, etched & rinsed (~ 3.5 nm)* | |||
| C | 76.6 | 71.8 | 74.8 | 74 | 70.9 | 39.6 | 74.7 |
| N | 11.8 | 10.8 | 11.4 | 11 | 10.9 | 7.1 | 12.6 |
| O | 11.6 | 14.8 | 12.7 | 13.6 | 15.9 | 31.1 | 12.3 |
| Si | --- | 2.6 | 1.0 | 1.4 | 2.3 | 22.2 | 0.4 |
|
| |||||||
| silane mol.% (top ~ 6 nm)** | 0 | ~ 20.8 | ~ 8 | ~ 11 | ~ 18.4 | ~ 65 | 3.2 |
This spectrum was presented in Fig. S1;
The average mole percent of APTES in the bulk 50/50 APTES/pNIPAAm film is 13.4, and in the solution used for spin-coating is 45.2, suggesting that ~ 32 mol.% of APTES molecules were lost during the film preparation (i.e., spin-coating and thermal annealing) process.
In addition to the increased O content, the more obvious evidence indicating the presence of APTES in the blend films is the detection of Si (spectra (2) – (5) in Fig. 4). For the un-cured 50/50 APTES/pNIPAAm film, the Si atomic % is 2.6, corresponding to ~ 20 mole % of APTES. The estimated wt.% of APTES in the film, based on the cured film thickness, is ~ 18, corresponding to ~ 15 mole %. The slightly lower APTES content in the cured film as compared to the un-cured film is due to the evaporation of APTES molecules during thermal curing under vacuum, as illustrated in Fig. 5(B). This is also reflected by the reduction (~ 10 nm) in the blend film thickness after curing.
The thermally cured 50/50 APTES/pNIPAAm films before rinsing, however, showed a lower Si content (1 atomic %) as compared to its un-cured counterpart. According to the manufacturer website, the normal probing depth of XPS is approximately ~5 nm, although values 3 to 10 nm have been reported, hence a probing depth of 6 nm was chosen in our analysis. The 1 atomic % of Si corresponds to ~ 8 mole % of APTES in this top ~ 6 nm of the blend film. The Si for the top ~ 6 nm layer of the rinsed film increased to 1.4 atomic %, i.e., ~ 11 mole% of APTES. Removal of the rinsed films by Ar plasma etching to a thickness of ~12 nm resulted in a Si atomic % of 2.3, or ~ 18 mole% of APTES. Further etching of the cured blend film to a thickness of 3.5 nm resulted in a Si atomic % of ~ 22%, partially contributed by Si from the silicon substrate. For this film, ~ 58% of the high resolution Si2p peak was found to be associated with SiO3– at a binding energy of 102.8 eV and ~ 42% to be associated with SiO4– from the silica layer at a binding energy of 103.6 eV. Since the SiOx layer on the silicon wafer was ~ 2.5 nm for our study, the peaks associated with binding energy agreed well with the thickness % of the APTES/pNIPAAm film (i.e., 3.5 nm/6 nm ~ 58%) and the underneath silica layer (i.e., 2.5 nm/6 nm ~ 42%) by using a X-ray penetration depth of 6 nm. As a result, the mole % of APTES for the 3.5 nm blend film was found to be 45 – 85% depending on the values of x, which ranges from 1.2 – 1.6 as reported by others[20]. The profiling of the blend film at different depths by XPS clearly indicates that the cured 50/50 APTES/pNIPAAm blend films had a gradient distribution of APTES, from ~ 8 mole% at the air/film interface to an average of ~ 65 mole% at the film/substrate interface.
Segregation as a cause for APTES network formation to entrap pNIPAAm
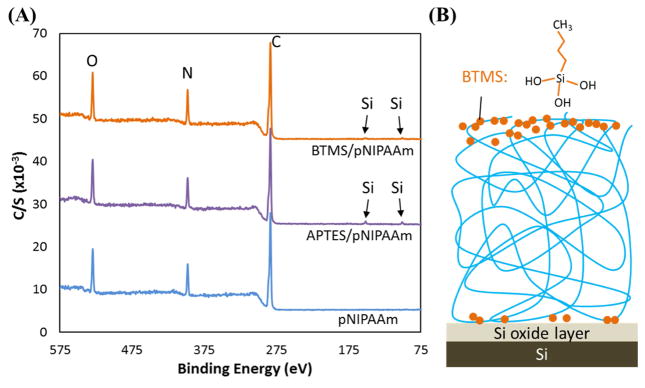
One reason for such a gradient distribution is the segregation of APTES molecules during thermal annealing [Fig. 5(B)]. The segregation of small molecules through polymer melts is common[22–25], especially when the molecules have a surface energy that is different from that of the polymer. In the case of APTES/pNIPAAm blends, APTES has a surface energy ~ 40 mJ/m2, while pNIPAAm has a surface energy of ~ 36 mJ/m2, hence APTES molecules segregate to the film/substrate interface. The small size of the APTES molecules would facilitate the segregation process. To further confirm the segregation is indeed driven by the surface energy difference between the two components in the silane/pNIPAAm blend, in a separate experiment, n-Butyltrimethoxysilane (BTMS), a molecule with a similar size and structure as APTES and having a surface energy of 29 mJ/m2, was used to make the 50/50 blend film with pNIPAAm and cured for 3 days. By using XPS (Fig. 6(A)), it was found that BTMS molecules were mostly concentrated in the top surface layer. The total amount of BTMS in the cured film, estimated from its thickness, is ~ 0.6 wt.% (Table 3) or 0.5 mol.%; whereas the amount present in the top surface layer based on Si atomic % obtained from XPS is ~ 3.2 mol.% (Table 4). This result shows that approximately 75% of the BTMS molecules in the film segregated to the film/air interface, as a result, an insufficient amount of BTMS is present in the bottom portion of the film to form a network that can retain pNIPAAm (Fig. 6(B)). The cured BTMS/pNIPAAm blend film was mostly removed by cold water rinsing (Fig. 1 and Table 1).
Fig. 6.
(A) The XPS survey scan of 3 days cured 50/50 BTMS/pNIPAAm blend film suggesting the enrichment of BTMS at the film/air interface, and (B) the potential illustration of the distribution of BTMS molecules, with a fully hydrolyzed molecule sketched, in the blend film after thermal curing.
The segregation would also depend on the annealing temperature. In an earlier study[10], we evaluated the curing temperature effects on entrapping of pNIPAAm in the APTES network. It was found that curing at 145°C or lower was insufficient to immobilize pNIPAAm, although APTES would form a network by curing at 80°C or above for 4 hours[17,26]. This is likely due to the glass transition temperature of pNIPAAm being round 120°C to 142°C [27], and as a result, curing at 145°C might not provide sufficient mobility to allow APTES molecules to segregate in the pNIPAAm/APTES film; whereas curing at 160°C, which is above the Tg of pNIPAAm, allows for easier segregation.
The segregation of APTES within the APTES/pNIPAAm appears to occur in a short period of time. The XPS scans of 1 day cured and 3 days cured 50/50 blend, after rinsing, show a similar Si content. Thus, the difference in the amount of pNIPAAm retained for films cured for different amounts of time (1 day to 3 days) could be due to the extent of cross-linking or how tight the APTES network is formed. Cross-linking occurs when a sufficient amount of APTES molecules are present in a location. The estimated APTES mass in the cured 50/50 blend film is 0.7 – 0.8 μg/cm2. If APTES were uniformly distributed over a thickness of ~ 42 nm, it would average to ~ 15 mole% within the film or one APTES molecule per 6 NIPAAm units, then the probability of APTES to cross-link and form a network would be low. With the APTES molecules segregated to the film/substrate interface, as confirmed by the XPS scans, there is an average of ~ 65 mol.% of APTES in the bottom 3.5 nm, or ~ 2 APTES molecules per NIPAAm unit, which should be abundant to cross-link and form a tight network (Fig. 5(C – ii & iii)). For the ~ 8 nm layer right above this highly segregated APTES bottom layer, the average mole% is ~ 25%, or ~ 0.25 APTES molecule per NIPAAm unit, which might be enough to cross-link and form a looser network than that of the bottom layer. For the layer (~ 11 nm) further above, the average APTES is ~ 15 mole%, corresponding to ~ 1 APTES molecule per 6 NIPAAm units. As indicated above, the possibility of cross-linking APTES with such a low content is low. However, the pNIPAAm in this layer could be trapped by the APTES network in the layer beneath, these pNIPAAm chains could entangle additional pNIPAAm chains present in this layer or above to retain them (Fig. 5(C– ii)).
As demonstrated when a pNIPAAm film is placed on top of an APTES layer, either cured or non-cured, and thermally cured for 3 days, a pNIPAAm film of 4 – 12 nm (Fig. S2(C)) is retained on the APTES layer. Also, APTES within this layer might form oligomers that link to the APTES network (Fig. 5(C– ii)), and the interactions between pNIPAAm and APTES could lead to the retention of some of the pNIPAAm in this layer. With a longer curing time (i.e., 3 days), a slightly more cross-linking of APTES could occur to form a relatively tighter network as compare to those cured for 1 day and 2 days. The tighter network swells less and makes it harder for the pNIPAAm chains to be pulled out during soaking. However, the exact degree of cross-linking of APTES could not be easily quantified for the entire thickness of our retained films using either the XPS probing or swelling measurements. The XPS probing depth (~ 6 nm) is shorter than the thickness (20 – 40 nm) of our films retained, and the presence of pNIPAAm in the film makes it difficult to determine the degree of swelling of APTES only, since both compounds are swell-able by most solvents. The less film loss by soaking for 3 days (Fig. 2F) of the 3 days cured films as compared to those cured for 1 day and 2 days (Fig. 2D & E) indicates that the APTES network reached a sufficient degree of cross-linking with 3 days of curing.
In addition to survey scans, high resolution scans of N1s for these films were also obtained to verify the cross-linking of the APTES molecules. For the cured films after rinsing and etching, the N1s scans only showed one peak at a binding energy of ~ 400 eV (Fig. 4(ii) and Fig. S2(c)), which is associated with –NH2/–NH present in APTES and pNIPAAm. No hydrogen-bonded/protonated N, which shifts the binding energy to ~ 402 eV, was noticed. On the contrary, for the un-cured blend film, the hydrogen bonded NH2, showing as NH3+ (~ 402 eV) in the high resolution spectrum (Fig. 4(i)) was observed. The hydrogen-bonded/protonated amine groups are attributed to the presence of un-crosslinked Si-OH groups[19]. The disappearance of the NH3+ peak after 3 days curing indicates the lack of free Si-OH groups, which cross-link to form siloxane bonds.
Effect of APTES and BTMS oligomerization on retaining pNIPAAm
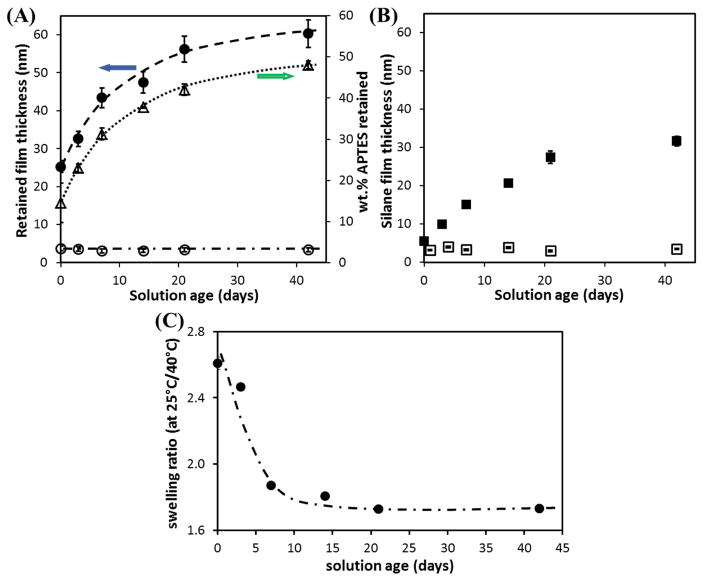
While the pNIPAAm entrapment approach using organosilanes is relatively simple compared to other approaches, one of the main factors that could influence the reproducibility of the films is the age of the blend solution. In the case of APTES, solutions prepared days before spin-coating result in thicker films compared to freshly prepared solutions, as shown in Fig. 7. The retained film thickness prepared using 1.5wt.% 50/50 APTES/pNIPAAm solution increased from ~25 nm (using freshly prepared solution) to ~ 60 nm (using 42 days old solution). The increase in thickness is attributed to the oligomerization of APTES, as confirmed by the increase in APTES layer thickness, for the APTES layer prepared with a longer solution age (Fig. 7(B)). A greater APTES oligomerization results in higher APTES retention in the film and subsequently, increase in film thickness. The total APTES (wt.%) retained in the film appeared to approach the concentration in the initial blend solution (50 wt.%) with time. On the other hand, for BTMS, solution age did not appear to influence the retained film thickness, even though the time is sufficient to promote oligomerization. This result further demonstrates that BTMS is unable to form the network required to retain pNIPAAm.
Fig. 7.
(A) The retained silane/pNIPAAm blend film thickness (● and ○ symbolize APTES/pNIPAAm and BTMS/pNIPAAm, respectively) of the 3 days cured films, after cold water rinsing + 3 days soaking; and the wt.% of APTES (△) in the 3 days cured films, prepared from aged 50/50 silane/pNIPAAm solution mixtures with a total solute of 1.5 wt.%. (B) The corresponding 3 days cured silane only layer thickness (■ and □ represent APTES and BTMS, respectively) prepared from 0.75 wt.% solution. (C) the ratio of film thickness in water (or swelling ratio) at 25°C to 40°C of APTES/pNIPAAm films prepared from aged solutions. The error bar for each data point is the standard deviation of at least three measurements.
Thermo-responsive behaviors of APTES/pNIPAAm blend films
The thermo-responsive behavior of the pNIPAAm/APTES blend films under study were first assessed by measuring the water contact angles on these films above (40°C) and below (25°C) the LCST of pNIPAAm, which is ~ 32°C. The advancing (θA) and static (θ) water contact angles on various blend films are summarized in Table 2 and Table S1, respectively.
All blend films showed a drop in water contact angle (ΔθA > 0 and Δθ > 0), or a hydrophobic to hydrophilic transition, from 40°C to 25°C. The advancing angle was suggested to be more sensitive in showing water-polymer interactions [11]. For most blend films, ΔθA was > 10°, and the largest ΔθA was ~ 37°. There were no clear trends on the contact angle values as the APTES/pNIPAAm ratio varied, likely due to the local heterogeneity of the films. During thermal curing, as the APTES molecules segregated to the film/substrate interface, the pNIPAAm chains would likely rearrange to exposure the low energy portion to the surface and leading to some local surface heterogeneity. As a result, the measured ΔθA for the different ratio films, after rinsing by cold water, were ≥ 90° at 40°C. It is important to note that in literature [11, 28], contact angles on pNIPAAm brushes and coated films were reported to have discrepancy. More importantly, pNIPAAm films exhibited a stick-slip behavior above the LCST where water contact angles reached to a value as high as 111° [28] and reduced to 92° after few stick-slip cycles. The authors attributed this reduction to the adsorption of water into the film even at temperatures higher than the LCST. This behavior was also observed in our case based on contact angle and thickness (Fig. 3A) measurements.
The 50/50 APTES/pNIPAAm films prepared from solutions of different ages also exhibited hydrophobic to hydrophilic transition from 40°C to 25°C, with a ΔθA of ~ 10°. Most values of θA at 40°C were very close at 89 – 90°, and less stick-slip of water drop was noticed during advancing measurements for films prepared from a solution aged longer (≥14 days) which might indicate that the surface became more homogenous.
In addition to water contact angles that probe the surface wettability of the pNIPAAm film, the thermo-responsive behavior of the bulk pNIPAAm/APTES films is better described by film thickness changes in water (above and below LCST). The film thickness of 3 days cured films was measured in water at temperatures above and below LCST of pNIPAAm. When following the laser light intensity of the ellipsometer during the water heating/cooling cycles, a sharp change in intensity was noticed as the water temperature reached 31 – 32°C, suggesting a transition occurred. The results summarized in Fig. 3(A) show that for every film, the thickness in water, at both temperatures, was higher than that in air. Water uptake by pNIPAAm films has been reported previously [28, 29], even at 40°C when the pNIPAAm chain is in its collapsed hydrophobic state. For pure APTES layer, swelling of the network is also noticed, leading to a 160 – 180% increase in thickness, at both temperatures, as compared to that in air. But, the APTES film thickness at 25°C was slightly less than that at 40°C.
It is apparent that, for the APTES/pNIPAAm films, the thickness greatly increases when the water temperature is at 25°C (< LCST of pNIPAAm), indicating a large extension/swelling of the pNIPAAm chains at its hydrophilic state. When the ratio of the thickness at 25°C to that at 40°C is plotted against wt.% of APTES in solute used for spin-coating, a higher ratio (~ 3) at a lower APTES content is observed, and the ratio decreases to ~ 2 for the 60/40 blend, and to ~ 1.6 for the 80/20 blend. A swelling ratio of greater than 3 for pNIPAAm in water has been reported by others [13, 30], and for grafted pNIPAAm film, a ratio of 2× has been observed [12, 31]. For the 50/50 blend from solutions of different ages, the film thickness transition in water is also observed for all the films. The swelling ratio (Fig. 7(C)) is higher (~ 2.6) for a less aged solution, and decreases to ~ 1.8 with a longer aging time (≥ 14 days). Therefore, the thermo-responsive behavior of these APTES/pNIPAAm blend films is confirmed by the thickness change at temperatures above and below the LCST of pNIPAAm.
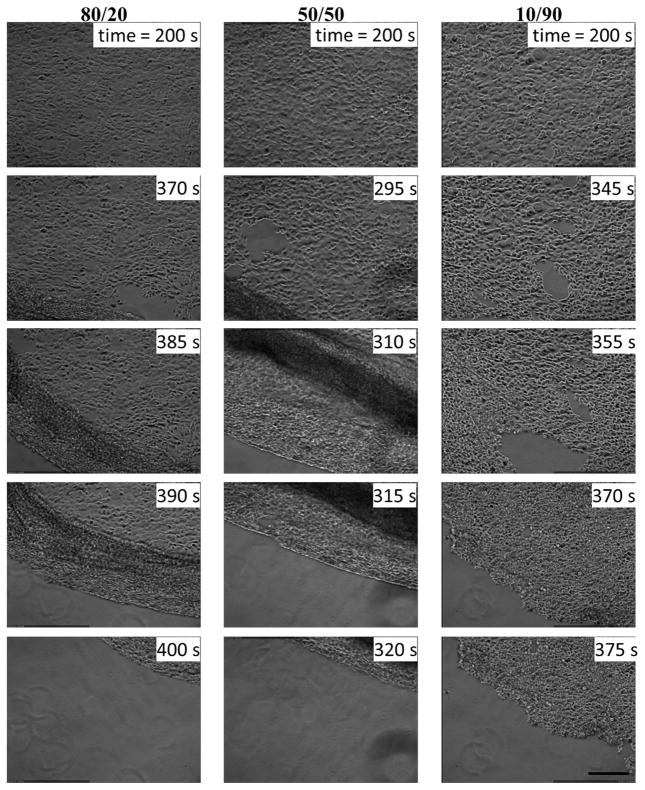
To verify whether the thermo-responsive films can be used for cell/cell sheet detachment, mouse embryonic fibroblast cells (STO) were seeded on those films and incubated at 37°C, above the LCST of pNIPAAm when the film is hydrophobic, to form a confluent layer of cell sheet, which were then allowed to detach by replacing the medium with a cold medium (~ 4°C). After the cold medium was added, there was a short period of response time (3–4 minutes), during which the medium temperature had risen to ~ 20°C, which is still lower than the LCST of pNIPAAm and the film would be hydrophilic if it exhibits the TRB, before the cell sheets started to detach. As shown in Fig. 7, cell sheets detached from most of the films within 6–8 minutes, and cell sheets detached by rolling from one edge or sliding. In a separate experiment, a quicker cell sheet detachment (< 5 minutes) is observed by simply cooling down the medium to room temperature under ambient condition. Fig. S3 summarizes the detachment times for all the APTES/pNIPAAm blend films we have investigated, including some with higher solute contents in the solutions used for spin-coating. The data presented in this figure shows that all the blend films allowed cell/cell sheet detachment in < 10 minutes, and the detachment showed no clear correlation with the film thickness in the range of 10 – 125 nm.
Conclusion
In this study, we detailed the conditions for entrapping pNIPAAm by APTES, an organosilane, to retain a stable layer of pNIPAAm on silica surfaces. The pNIPAAm was blended with APTES in ethanol and spin-coated, and then thermally annealed at 160°C for 1 to 3 days. The retention of the cured film was assessed by rinsing followed with soaking using cold water (~ 23°C). On average, ~ 40% of the blend films was removed by rinsing regardless of curing time, soaking removed additional films and a longer curing time led to higher film retention. The pNIPAAm is retained by the APTES molecules, which polymerize and cross-link to form a network during thermal curing. XPS scans were obtained to probe the distribution of APTES molecules within the film, and the results showed segregation of APTES, having a surface energy higher than that of pNIPAAm, towards the film/substrate interface. When BTMS, a similar sized organosilane that has a lower surface energy than that of pNIPAAm, was used in the place of APTES, BTMS segregated towards the air/film interface resulting in no pNIPAAm entrapment. In addition to curing time and APTES content, a greater oligomerization of APTES in the solution would result in more pNIPAAm being retained on the substrate. The resulting APTES/pNIPAAm films exhibited thermo-responsive behavior, verified by contact angle and thickness measurements, as well as good cell attachment and rapid cell sheet detachment. The gained insights from this study would allow a better design of these thermo-responsive surfaces for various applications including cell sheet engineering.
Supplementary Material
Fig. 8.
The time sequent cell sheet detachment images from 3 days cured films consisting of different APTES/pNIPAAm ratios spin-coated from 1.5 wt.% total solute in ethanol. All images are the same size and the scale bar is 500 μm.
Highlights.
APTES/pNIPAAm blend film was prepared by spin-coating followed by thermal annealing
APTES molecules segregated to the film-substrate interface during annealing
Segregated APTES formed a network to entrap pNIPAAm and retain it on the substrate
Retained pNIPAAm films showed thermo-responsive behavior
Retained pNIPAAm films showed excellent cell attachment and rapid cell detachment
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R15GM097626-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Alghunaim acknowledges the King Abdullah Scholarships Program support for his graduate study from the Ministry of Education in Saudi Arabia. We would like to thank Dr. Zhorro Nikolov for assistance with XPS measurements and analysis, Dr. Bryan D. Vogt and Mr. Clinton Wiener for the help of using their spectroscopic ellipsometer, and Mr. Gregg Butala Jr. for assisting with film thickness measurements.
Footnotes
WARNING: Piranha solutions are VERY DANGEROUS and can be explosive! Always wear protective gloves (rubber gloves as nitrile gloves do not provide sufficient protection against piranha solutions), apron and goggles. Work should be done in a fume hood in the presence of other lab personnel. Piranha solutions are highly reactive and generate a lot of heat upon preparation (possibly more than 100°C) especially in the presence of organic matter. Therefore, only glass containers should be used and the substrates to be cleaned should have no remaining detergents or organic solvents such as acetone or alcohol. Piranha waste should be collected in a vented glass container after the solution cools down to prevent gas buildup and explosion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nash ME, Healy D, Carroll WM, Elvira C, Rochev Ya. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. J Mater Chem. 2012;22:19376. doi: 10.1039/c2jm31748f. [DOI] [Google Scholar]
- 2.Nash ME, Fan X, Carroll WM, Gorelov AV, Barry FP, Shaw G, et al. Thermoresponsive Substrates used for the Expansion of Human Mesenchymal Stem Cells and the Preservation of Immunophenotype. Stem Cell Rev Reports. 2013;9:148–157. doi: 10.1007/s12015-013-9428-5. [DOI] [PubMed] [Google Scholar]
- 3.Fan X, Nash ME, Gorelov AV, Barry FP, Shaw G, Rochev YA. Thermoresponsive Substrates Used for the Growth and Controlled Differentiation of Human Mesenchymal Stem Cells. Macromol Rapid Commun. 2015;36:1897–1901. doi: 10.1002/marc.201500234. [DOI] [PubMed] [Google Scholar]
- 4.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol Chemie, Rapid Commun. 1990;11:571–576. doi: 10.1002/marc.1990.030111109. [DOI] [Google Scholar]
- 5.Lucero AE, Reed JA, Wu X, Canavan HE. Fabrication and Characterization of Thermoresponsive Films Deposited by an RF Plasma Reactor, Plasma Process. Polym. 2010;7:992–1000. doi: 10.1002/ppap.201000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagase K, Kobayashi J, Okano T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J R Soc Interface. 2009;6:S293–309. doi: 10.1098/rsif.2008.0499.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayatpisheh S, Li P, Chan-Park MB. Argon-Plasma-Induced Ultrathin Thermal Grafting of Thermoresponsive pNIPAm Coating for Contractile Patterned Human SMC Sheet Engineering. Macromol Biosci. 2012;12:937–945. doi: 10.1002/mabi.201100477. [DOI] [PubMed] [Google Scholar]
- 9.Patel NG, Zhang G. Responsive systems for cell sheet detachment. Organogenesis. 2013;9:93–100. doi: 10.4161/org.25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel NG, Cavicchia JP, Zhang G, Newby BMZ. Rapid cell sheet detachment using spin-coated pNIPAAm films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012;8:2559–2567. doi: 10.1016/j.actbio.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Nash ME, Carroll WM, Nikoloskya N, Yang R, Connell CO, Gorelov AV, et al. Straightforward, one-step fabrication of ultrathin thermoresponsive films from commercially available pNIPAm for cell culture and recovery. ACS Appl Mater Interfaces. 2011;3:1980–1990. doi: 10.1021/am200204j. [DOI] [PubMed] [Google Scholar]
- 12.Plunkett KN, Zhu X, Moore JS, Leckband DE. PNIPAM Chain Collapse Depends on the Molecular Weight and Grafting Density. Langmuir. 2006;22:4259–4266. doi: 10.1021/la0531502. [DOI] [PubMed] [Google Scholar]
- 13.Bittrich E, Burkert S, Müller M, Eichhorn K, Stamm M, Uhlmann P. Temperature-Sensitive Swelling of Poly( N -isopropylacrylamide) Brushes with Low Molecular Weight and Grafting Density. Langmuir. 2012;28:3439–3448. doi: 10.1021/la204230a. [DOI] [PubMed] [Google Scholar]
- 14.Okada F, Akiyama Y, Kobayashi J, Ninomiya H, Kanazawa H, Yamato M, Okano T. Measurement of the dynamic behavior of thin poly(N-isopropylacrylamide) hydrogels and their phase transition temperatures measured using reflectometric interference spectroscopy. J Nanoparticle Res. 2015;17:148. doi: 10.1007/s11051-015-2951-3. [DOI] [Google Scholar]
- 15.Prathab B, Subramanian V, Aminabhavi TM. Computation of surface energy and surface segregation phenomena of perfluorinated copolymers and blends - A molecular modeling approach. Polymer. 2007;48:417–424. doi: 10.1016/j.polymer.2006.10.043. [DOI] [Google Scholar]
- 16.Vandenberg ET, Bertilsson L, Liedberg B, Uvdal K, Erlandsson R, Elwing H, Lundström I. Structure of 3-aminopropyl triethoxy silane on silicon oxide. J Colloid Interface Sci. 1991;147:103–118. doi: 10.1016/0021-9797(91)90139-Y. [DOI] [Google Scholar]
- 17.Choi SH, Zhang Newby BM. Suppress polystyrene thin film dewetting by modifying substrate surface with aminopropyltriethoxysilane. Surf Sci. 2006;600:1391–1404. doi: 10.1016/j.susc.2006.01.050. [DOI] [Google Scholar]
- 18.Kim J, Seidler P, Wan LS, Fill C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J Colloid Interface Sci. 2009;329:114–119. doi: 10.1016/j.jcis.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, Lerum MZ, Chen W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir. 2012;28:416–423. doi: 10.1021/la203638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acres RG, Ellis AV, Alvino J, Lenahan CE, Khodakov DA, Metha GF, et al. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J Phys Chem C. 2012;116:6289–6297. doi: 10.1021/jp212056s. [DOI] [Google Scholar]
- 21.Dan L, Wu XL. Optical emission from SiO x (x=1.2–1.6) nanoparticles irradiated by ultraviolet ozone. J Appl Phys. 2003;94:7288–7291. doi: 10.1063/1.1626797. [DOI] [Google Scholar]
- 22.Sivaniah E, Jones RAL, Higgins D. Small molecule segregation at polymer interfaces. Macromolecules. 2009;42:8844–8850. doi: 10.1021/ma9017394. [DOI] [Google Scholar]
- 23.Clark MD, Jespersen ML, Patel RJ, Leever BJ. Predicting Vertical Phase Segregation in Polymer-Fullerene Bulk Heterojunction Solar Cells by Free Energy Analysis. ACS Appl Mater Interfaces. 2013;5:4799–4807. doi: 10.1021/am4003777. [DOI] [PubMed] [Google Scholar]
- 24.Kang J, Shin N, Jang DY, Prabhu VM, Yoon DY. Structure and Properties of Small Molecule - Polymer Blend Semiconductors. J Am Chem Soc. 2008;130:12273–12275. doi: 10.1021/ja804013n. [DOI] [PubMed] [Google Scholar]
- 25.Belaroui F, Grohens Y, Boyer H, Holl Y. Depth profiling of small molecules in dry latex films by confocal Raman spectroscopy. Polymer (Guildf) 2000;41:7641–7645. doi: 10.1016/S0032-3861(00)00145-2. [DOI] [Google Scholar]
- 26.Choi S-H, Cai Y, Newby B-MZ. Stability Enhancement Of Polystyrene Thin Films On Aminopropyltriethoxysilane Ultrathin Layer Modified Surfaces. In: Mittal KL, editor. Silanes and Other Coupling Agents. Vol. 4. CRC press; 2007. pp. 179–197. [DOI] [Google Scholar]
- 27.Biswas CS, Patel VK, Vishwakarma NK, Tiwari VK, Maiti B, Maiti P, et al. Effects of Tacticity and Molecular Weight of Poly( N -isopropylacrylamide) on Its Glass Transition Temperature. Macromolecules. 2011;44:5822–5824. doi: 10.1021/ma200735k. [DOI] [Google Scholar]
- 28.Wan L, Meng X, Yang Y, Tian J, Xu Z. Thermo-responsive stick-slip behavior of advancing water contact angle on the surfaces of poly(N-isopropylacrylamide)-grafted polypropylene membranes. Sci China Chem. 2010;53:183–189. doi: 10.1007/s11426-010-0004-4. [DOI] [Google Scholar]
- 29.Cordeiro AL, Zimmermann R, Gramm S, Nitschke M, Janke A, Schäfer N, Grundke K, Werner C. Temperature dependent physicochemical properties of poly(N-isopropylacrylamide-co-N-(1-phenylethyl) acrylamide) thin films. Soft Matter. 2009;5:1367. doi: 10.1039/b816911j. [DOI] [Google Scholar]
- 30.Kooij ES, Sui X, Hempenius MA, Zandvliet HJW, Vancso GJ. Probing the Thermal Collapse of Poly( N -isopropylacrylamide) Grafts by Quantitative in Situ Ellipsometry. J Phys Chem B. 2012;116:9261–9268. doi: 10.1021/jp304364m. [DOI] [PubMed] [Google Scholar]
- 31.Kidoaki S, Ohya S, Nakayama Y, Matsuda T. Thermoresponsive Structural Change of a Poly( N- isopropylacrylamide) Graft Layer Measured with an Atomic Force Microscope. Langmuir. 2001;17:2402–2407. doi: 10.1021/la001522v. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.