Abstract
Canonical Wnt/β-catenin signaling has central roles in development and diseases, and is initiated by the action of the frizzled (Fz) receptor, its coreceptor LDL receptor-related protein 6 (Lrp6), and the cytoplasmic dishevelled (Dvl) protein. The functional relationships among Fz, Lrp6 and Dvl have long been enigmatic. We demonstrated previously that Wnt-induced Lrp6 phosphorylation via glycogen synthase kinase 3 (Gsk3) initiates Wnt/β-catenin signaling. Here we show that both Fz and Dvl functions are critical for Wnt-induced Lrp6 phosphorylation through Fz-Lrp6 interaction. We also show that axin, a key scaffolding protein in the Wnt pathway, is required for Lrp6 phosphorylation via its ability to recruit Gsk3, and inhibition of Gsk3 at the plasma membrane blocks Wnt/β-catenin signaling. Our results suggest a model that upon Wnt-induced Fz-Lrp6 complex formation, Fz recruitment of Dvl in turn recruits the axin-Gsk3 complex, thereby promoting Lrp6 phosphorylation to initiate β-catenin signaling. We discuss the dual roles of the axin-Gsk3 complex and signal amplification by Lrp6-axin interaction during Wnt/β-catenin signaling.
Keywords: Axin, Dishevelled, Frizzled, Gsk3, Lrp6, Wnt
INTRODUCTION
Wnt/β-catenin signaling is essential for animal development and is commonly involved in human diseases (Clevers, 2006; Logan and Nusse, 2004). The mechanism of Wnt/β-catenin signaling, however, remains to be fully defined. In the absence of Wnt stimulation, the β-catenin protein is sequentially phosphorylated by casein kinase 1α (CK1α; also known as CSNK1A1 – Human Gene Nomenclature Database, and Csnk1a1 – Mouse Genome Informatics) and Gsk3 within a protein complex that is assembled by the scaffolding protein axin, and is thereby ubiquitinated and degraded. Upon Wnt stimulation, β-catenin phosphorylation and degradation is inhibited, thereby resulting in elevated β-catenin protein levels and β-catenin-dependent transcriptional activation (Clevers, 2006; Logan and Nusse, 2004). Two types of cell surface receptors, a member of the frizzled (Fz) serpentine receptor family and a single-span transmembrane receptor LDL receptor-related protein 6 (Lrp6) or closely related Lrp5, mediate Wnt signaling at the plasma membrane (He et al., 2004; Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). Biochemical studies indicate that Wnt may induce Fz-Lrp6 complex formation (Semenov et al., 2001; Tamai et al., 2000), and experimentally induced Fz-Lrp6 proximity appears to be sufficient to stimulate Wnt/β-catenin signaling (Cong et al., 2004; Holmen et al., 2005; Liu et al., 2005; Tolwinski et al., 2003).
We previously demonstrated a phosphorylation-dependent mechanism for Lrp6 activation (Tamai et al., 2004). We and others showed that Wnt activates Lrp6 by inducing Gsk3 and CK1-mediated phosphorylation of PPPSPxS motifs within the Lrp6 intracellular domain (Davidson et al., 2005; Zeng et al., 2005). We showed that Gsk3 phosphorylation of the PPPSP motif appears to be the primary Wnt-inducible event, which primes the subsequent phosphorylation of xS in the PPPSPxS motif by CK1 (Wei et al., 2007; Zeng et al., 2005; Davidson et al., 2005). The dually phosphorylated PPPSPxS motifs provide docking sites for axin (Tamai et al., 2004; Zeng et al., 2005). The association of the axin complex with the phosphorylated Lrp6 is believed to lead to (via an as yet unknown mechanism) inhibition of β-catenin phosphorylation and activation of β-catenin signaling (Mao et al., 2001; Tamai et al., 2004; Zeng et al., 2005). The involvement of Gsk3 and CK1 in both Wnt pathway activation (via Lrp6 phosphorylation) and inhibition (via β-catenin phosphorylation) is surprising and implies intricate regulatory mechanisms. In the meantime, the biochemical nature by which the Fz protein and its downstream cytosolic partner dishevelled (Dvl or Dsh) protein exert their obligatory roles in Wnt signal transduction has been obscure. In this study, we demonstrate that the Fz, Dvl and axin proteins are important components that control Wnt-induced Lrp6 phosphorylation by Gsk3.
MATERIALS AND METHODS
Plasmids and lentiviruses
VSVG-tagged full-length human LRP6 (Tamai et al., 2000), Flag-tagged mouse Dvl2 and the deletion mutants (Habas et al., 2001), HA-tagged human GSK3 (Zeng et al., 2005), HA-tagged human Fz5 (Habas et al., 2001), Flag-tagged mouse axin (Liu et al., 2002) in the pCS2+ vector were previously described. The Fz mutants m11-12 (P267L and I353A), m14-15 (deleting the cytoplasmic tail from the fourth amino acid residue adjacent to the seventh transmembrane domain) were generated by PCR-mediated mutagenesis or truncation in the pCS2+Fz5 vector. The fragments containing the mutations replaced the corresponding fragments in the DKK1-Fz5 (Holmen et al., 2005). The Axin L396Q mutant was generated by PCR-mediated mutagenesis. All mutations were confirmed by DNA sequencing. Details of the plasmids are available from the authors upon request. Flag-tagged Shisa (Yamamoto et al., 2005) (also known as TMEM46 – Human Gene Nomenclature Database) was kindly provided by Shinichi Aizawa (Riken, Japan). Myc-tagged DKK1-Fz5 and DKK3-Fz5 (Holmen et al., 2005) were kindly provided by Bart O. Williams (Grand Rapids, MI). The CAAX-motif derived from K-Ras (DGKKKKKSKTKCVIM) was fused C-terminal to the axin-derived GID and the GID-LP mutant (Hedgepeth et al., 1999) to allow myristylation. The lentiviral-based shRNA vectors against mouse Dvl3 and Axin2 were purchased from Sigma (Mission TRC shRNA Target Set).
Antibodies
The polyclonal antibodies against total Lrp6 and the phosphorylated Lrp6 (Ab1490) were used as previously described (Tamai et al., 2004; Zeng et al., 2005). Other antibodies were used according to manufacturer’s instructions: mouse monoclonal antibodies anti-β-catenin (BD Transduction Laboratories), anti-β-actin and Flag (M2, Sigma), anti-Gsk3 (4G-1E, Upstate Biotechnology), anti-Dvl3 (Sc-8027, Santa Cruz Biotechnology), anti-transferin receptor (TfR) (Zymed), rabbit polyclonal anti-Dvl2 (Sc-13974, Santa Cruz), anti-Myc (A14, Santa Cruz), anti-Frizzled 5 (Upstate Biotechnology). The rabbit polyclonal anti-Dvl1 antibody was kindly provided by Lin Mei (Augusta, GA).
Cells, transfection, reporter assay, lentivirus production and infection, Wnt induction and immunoblotting
Dvl1−/−;Dvl2−/− MEFs were derived from a mouse Dvl1 and Dvl2 double knockout embryo (Wang et al., 2006). Stable Dvl1−/−;Dvl2−/− MEFs expressing the mouse Dvl2 and the various mutants were established by puromycin-selection after cotransfection of the Dvl2 expression vectors with pBABE-puro plasmids. Mouse ES cells for Gsk3α−/−, Gsk3β−/− and the double knockout Gsk3α−/−;Gsk3β−/− (Doble et al., 2007) and the mouse Axin−/− (Zeng et al., 1997) have been described previously. HEK293T, L cells and MEFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin-glutamine (PSA; Invitrogen). ES cells were cultured in the Knockout DMEM (Invitrogen) supplemented with 15% FBS, 1% PSA, 103 Units/ml of ESGRO (Chemicon) on a MEF feeder layer for passages. ES cells were separated from the feeder cells by three passages onto gelatin-coated plates for a 0.5-hour incubation to eliminate the attached MEF cells. Fugene6 (Roche) was used for all cell transfections. For induction of Lrp6 phosphorylation, cells were treated for 1 hour with Wnt3a CM (collected from mouse L cell stably expressing Wnt3a; ATCC) as previously described (Tamai et al., 2004; Zeng et al., 2005). The lentiviruses encoding the shRNA against mouse Dvl3 and Axin2 were produced in HEK293T cells by cotransfection with the packaging plasmids. The conditioned media containing the viral particles were collected and filtered through 0.4 μm syringe filters. The MEFs or ES cells were incubated with the virus-containing conditioned media for 6 hours, followed by fresh media for 3 days before Wnt treatment. Luciferase assays were carried out in duplicate in HEK293T cells in 12-well plates as previously described (Tamai et al., 2004; Zeng et al., 2005) and the results were shown as fold induction from multiple experiments, presented as mean ± s.d. Cell extracts for western blotting were collected in a lysis buffer containing 50 mM Hepes (pH 7.4), 1.5 mM EDTA, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, 0.5 mM DTT and a cocktail of protease inhibitors. Cytosolic β-catenin was prepared using hypotonic buffer as described previously (Liu et al., 2002).
Xenopus laevis embryo manipulations and immunofluorescent staining in animal pole explants
Xenopus embryo injection and animal pole explant assays were performed as described previously (Tamai et al., 2000). For immunostaining, in vitro transcribed mRNAs for Flag-axin, human Fz5 and Xenopus Dishevelled (Xdsh), XdshΔN, or Xdsh-GFP were injected into animal pole cells at the 2–4 cells stage. Animal pole explants were dissected at stage 9.5–10 and fixed in 4% paraformaldehyde for 1.5 hours. The explants were washed and blocked with 2% goat serum in PBST (PBS with 0.2% Triton X-100) and were subsequently incubated with anti-Flag M2 (for Axin) and anti-Fz5 primary antibodies overnight at 4°C. Secondary antibodies used in the experiment were goat-anti-rabbit or mouse IgG conjugated with Cy5, Alexa Fluor 568 or Alexa Fluor 488 (Molecular Probes). The explants were mounted and examined by Zeiss LSM510 confocal microscopy.
RESULTS
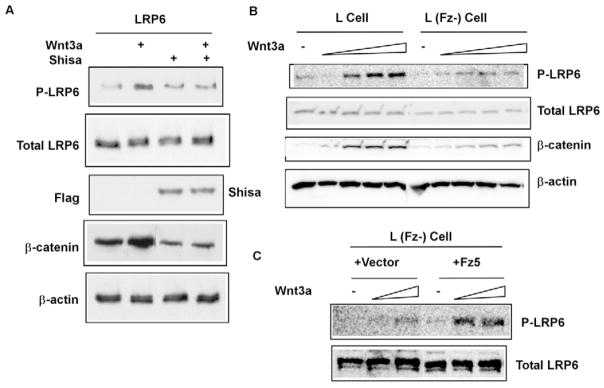
Because Wnt induces Lrp6 phosphorylation (Tamai et al., 2004) and probably Fz-Lrp6 complex formation, we suspect that Fz may function in regulating Lrp6 phosphorylation. Given that there are nine Fz genes in the mammalian genome (Huang and Klein, 2004; Logan and Nusse, 2004), we first employed Shisa, a specific Fz antagonist (Yamamoto et al., 2005). Shisa is an endoplasmic reticulum (ER) resident protein that functions to retain Fz proteins in the ER, thereby preventing Fz from trafficking to the plasma membrane (Yamamoto et al., 2005). We found that Shisa expression in HEK293T cells inhibited Wnt3a-induced LRP6 phosphorylation, and as expected, β-catenin stabilization (Fig. 1A). We further generated a mouse L cell line in which two highly expressed Fz proteins, Fz2 and Fz7, were stably knocked down using shRNAs (J-C.H., unpublished). Wnt3a-induced Lrp6 (endogenous) phosphorylation and β-catenin stabilization was significantly diminished in these cells (Fig. 1B). Expression of human Fz5 (FZ5) in these cells rescued Wnt3a-induced Lrp6 phosphorylation (Fig. 1C). These results suggest that Fz function is required for Wnt-induced Lrp6 phosphorylation.
Fig. 1. Fz function is required for Wnt-induced LRP6 phosphorylation.
(A) Shisa inhibited Wnt3a-induced LRP6 phosphorylation. HEK293T cells co-transfected with LRP6 plus Flag-tagged Shisa (or a control vector) were incubated with Wnt3a conditioned medium (CM) or the control CM for 1 hour. Shisa inhibited Wnt3a-induced β-catenin accumulation in the cytosol. β-actin: a loading control. (B) L cells stably transfected with shRNAs against Fz2 and Fz7 [L (Fz-) cell] showed diminished Wnt-induced LRP6 (endogenous) phosphorylation. The L (Fz-) cells were treated for 1hour with increasing concentrations of Wnt3a CM. These L (Fz-) cells also exhibited attenuated β-catenin stabilization in response to Wnt3a compared to the control L cells. (C) Human Fz5 expression rescued Wnt3a-induced LRP6 (endogenous) phosphorylation in the L (Fz-) cells. The stable clones of L (Fz-) cell transfected with Fz5 (or the control vector) were pooled together and assayed.
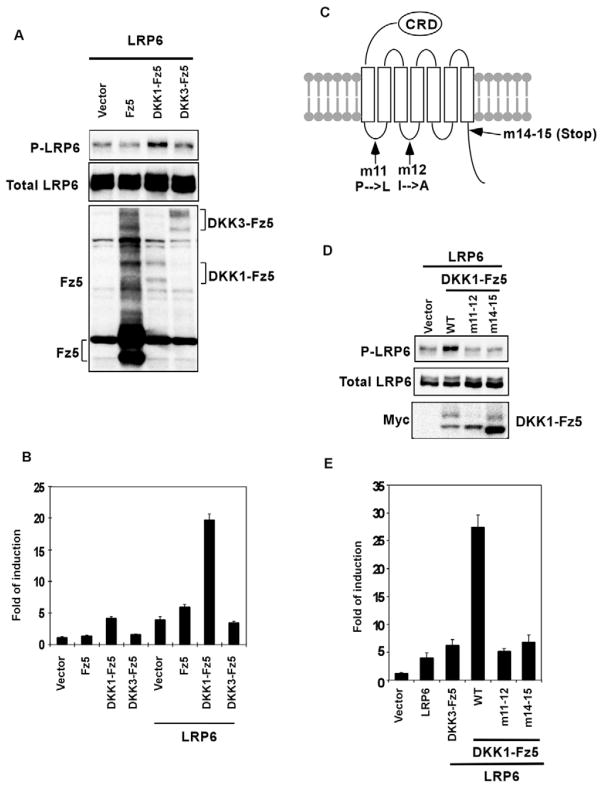
Wnt-induced Fz-Lrp6 complex formation probably underlies the initiation of Wnt signaling (Semenov et al., 2001; Tamai et al., 2000). Indeed, various molecular means to bring Fz and Lrp6 into proximity can activate the β-catenin pathway in the absence of any Wnt stimulation (Cong et al., 2004; Liu et al., 2005; Tolwinski et al., 2003; Holmen et al., 2005). We found that such induced Fz-Lrp6 proximity is sufficient to promote LRP6 phosphorylation (Fig. 2A). Dickkopf-1 (DKK1), but not the related DKK3, is a high affinity antagonistic ligand for LRP6 (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001). A DKK1-Fz5 fusion protein, which fuses DKK1 in frame with the amino terminus of Fz5 thus forcing Fz5 to associate with LRP6 in the absence of the Wnt ligand (Holmen et al., 2005), synergized with LRP6 to activate β-catenin signaling as monitored via the TCF/β-catenin reporter assay (Holmen et al., 2005) (Fig. 2B). By contrast, neither Fz5 nor a DKK3-Fz5 fusion protein exhibited such activity (Holmen et al., 2005) (Fig. 2B). Importantly, we found that DKK1-Fz5, but not Fz5 or DKK3-Fz5, induced LRP6 phosphorylation without any Wnt stimulation (Fig. 2A). These results suggest that the Fz-LRP6 complex formation is sufficient to trigger LRP6 phosphorylation.
Fig. 2. Forced Fz-LRP6 association activates LRP6 phosphorylation.
(A, B) DKK1-Fz5, but neither Fz5 nor DKK3-Fz5, activated LRP6 phosphorylation (A) and TCF/β-catenin reporter expression (B) in HEK293T cells. The expression level of DKK1-Fz5 and DKK3-Fz5 was similar, but was much lower than that of Fz5 (A) as detected by an anti-Fz5 antibody. This was probably due to the presence of the DKK moiety. (C) A diagram showing the position of the amino acid substitutions or the truncation in DKK1-Fz5 mutants. (D) DKK1-Fz5, but neither of the DKK1-Fz5 mutants, enhanced LRP6 phosphorylation. The protein level of Fz5 and DKK-Fz5 fusion proteins was detected via the Myc tag. (E) DKK1-Fz5, but neither of the two DKK1-Fz5 mutants, synergized with LRP6 to stimulate the TCF/β-catenin reporter expression in HEK293T cells.
We further employed the DKK1-Fz5 fusion protein to examine whether the signaling function of Fz is involved in LRP6 phosphorylation. We generated two Fz5 mutants: one contained substitutions in the first and second intracellular loops (m11-12) and the other had a truncation of the carboxyl-terminal region (m14-15; Fig. 2C). Identical or similar mutations in Fz5 and other Fz proteins have been shown to inactivate Fz signaling capability (Cong et al., 2004; Povelones et al., 2005; Umbhauer et al., 2000). The DKK1-Fz5 fusion protein harboring each of these mutations showed diminished ability to induce LRP6 phosphorylation (Fig. 2D) and to synergize with LRP6 to activate β-catenin signaling (Fig. 2E). Therefore Fz signaling is critical for LRP6 phosphorylation.
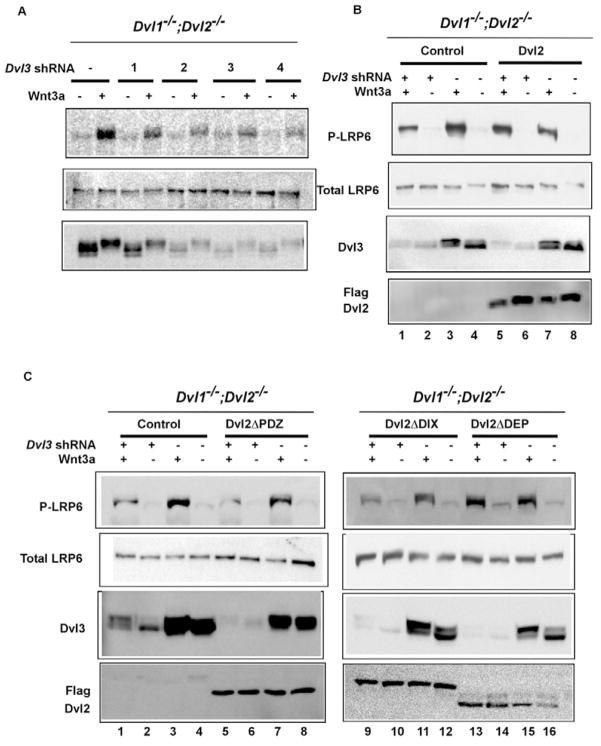
Dvl is an indispensable component downstream of Fz in Wnt signaling (Wallingford and Habas, 2005). Given that the Fz mutants we employed that are incapable of promoting Lrp6 phosphorylation are also defective in binding and/or recruiting Dvl to the plasma membrane (Cong et al., 2004; Wong et al., 2003), we suspect that Dvl may act downstream of Fz in mediating Wnt-induced Lrp6 phosphorylation. Because of functional redundancy of the three mammalian Dvl genes (Wang et al., 2006), we generated primary mouse embryonic fibroblast (MEF) cells that have drastically reduced Dvl function. MEFs derived from the Dvl1 and Dvl2 double knockout mice (Dvl1−/−;Dvl2−/−) (see Fig. S1 in the supplementary material) were infected with lentiviruses expressing shRNAs against Dvl3. The four shRNAs exhibited different efficiency in knocking down the Dvl3 protein level (Fig. 3A). Importantly, Wnt3a-induced Lrp6 phosphorylation was significantly diminished in cells expressing each of the four shRNAs, and the residual Lrp6 phosphorylation was directly proportional to the remaining amount of Dvl3 protein (Fig. 3A). These results show that Dvl function is required for Wnt-induced Lrp6 phosphorylation.
Fig. 3. Dvl is required for Wnt-induced LRP6 phosphorylation and acts via the DIX and PDZ domains.
(A) Reduction of Dvl proteins diminished Wnt3a-induced Lrp6 (endogenous) phosphorylation. MEFs lacking Dvl1 and Dvl2 (Dvl1−/−;Dvl2−/−) (see Fig. S1 in the supplementary material) were infected with each of the four different lentiviral shRNAs against mouse Dvl3. After 3 days, the cells were treated with Wnt3a CM or control CM for 1 hour. Dvl3 protein level were drastically reduced by shRNA2, 3 or 4. Dvl3 protein exhibited typical Wnt-induced mobility shift (due to phosphorylation). shRNA2 and 4 were used in further experiments and yielded identical results. (B) The wild-type Dvl2 rescued LRP6 (endogenous) phosphorylation in Dvl3 knockdown Dvl1−/−;Dvl2−/− MEFs. Dvl1−/−;Dvl2−/− MEFs stably expressing Dvl2 (lanes 5 to 8) or the control vector (lanes 1 to 4) were pooled and infected with the lentiviral Dvl3 shRNA as indicated, then treated with Wnt3a or control CM for 1 hour as indicated. The exogenously expressed Dvl2 was detected by the Flag tag. (C) Dvl2ΔDEP, but neither Dvl2ΔDIX nor Dvl2ΔPDZ, rescued the Wnt3a-induced Lrp6 (endogenous) phosphorylation in Dvl3 knockdown Dvl1−/−;Dvl2−/− MEFs. Dvl1−/−;Dvl2−/− MEFs stably expressing the control vector (lanes 1–4), Dvl2ΔDIX (lanes 9–12), Dvl2ΔPDZ (lanes 5–8) or Dvl2ΔDEP (lanes 13–16) were infected with the lentiviral Dvl3 shRNA as indicated, and treated with Wnt3a CM or control CM for 1 hour as indicated. The expression of Dvl2 mutants was detected by the Flag tag.
We used these MEF cells (Dvl1−/−;Dvl2−/−; Dvl3 knockdown) to examine which domain in Dvl is required for Lrp6 phosphorylation, given that most of the previous investigations on Dvl were based on overexpression studies. Dvl proteins have three conserved domains, the DIX (dishevelled and axin) domain, the PDZ (PSD, discs-large and ZO1) domain, and the DEP (dishevelled, Egl10, and pleckstrin) domain (Wallingford and Habas, 2005). The DIX domain is critical for Wnt/β-catenin signaling, whereas the requirement for the PDZ and DEP domains appears to be somewhat variable from different studies (reviewed by Wallingford and Habas, 2005; Wharton, 2003). We note that these studies employed different Dvl proteins and/or different Dvl deletion mutants, which may account, in part, for the observed differences. Re-expression of the wild-type Dvl2 rescued Wnt-induced Lrp6 phosphorylation (lanes 1–6, Fig. 3B). The Dvl2ΔDEP mutant, which lacks the DEP domain (Habas et al., 2001), was also able to rescue Wnt-induced Lrp6 phosphorylation (compare lanes 1–4 with lanes 13–16, Fig. 3C). However, neither the Dvl2ΔDIX mutant, which lacks the DIX domain (Habas et al., 2001), nor the Dvl2ΔPDZ mutant, which lacks the PDZ domain (Habas et al., 2001), was able to do so (compare lanes 1–4 with lanes 5–8 and lanes 9–12, Fig. 3C). Therefore the Dvl DIX and PDZ domains, but not the DEP domain, appear to be involved in promoting Lrp6 phosphorylation.
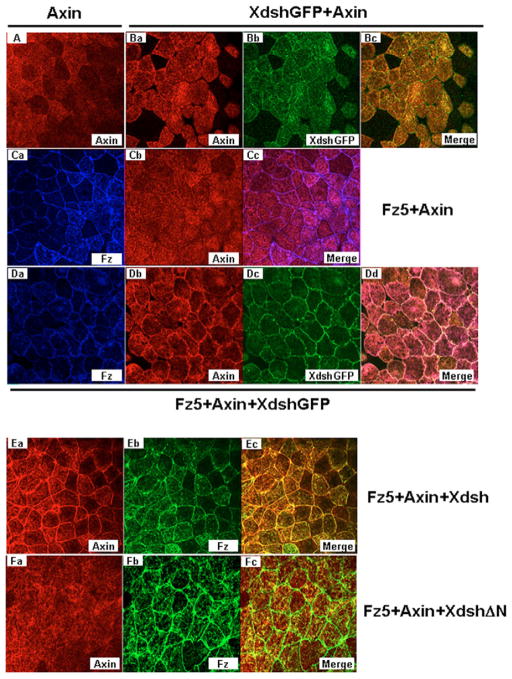
Earlier studies have documented that the Dvl DIX domain is involved in direct binding to axin (Fagotto et al., 1999; Kishida et al., 1999; Li et al., 1999; Salic et al., 2000; Smalley et al., 1999), possibly via the ability of the DIX domain to form dynamic aggregates or polymers (Schwarz-Romond et al., 2007a; Schwarz-Romond et al., 2007b), and that the Dvl PDZ domain may mediate association with Fz (Fujii et al., 2007; Shan et al., 2005; Wong et al., 2003). Thus one possible scenario is that Fz recruits Dvl, which in turn, via its DIX domain, recruits axin to promote Lrp6 phosphorylation. We first examined the validity of such a sequential recruitment model using Xenopus embryonic explants, which have been previously used to demonstrate Fz recruitment of Xenopus Dishevelled (referred to here as Xdsh) (Rothbacher et al., 2000; Umbhauer et al., 2000; Yang-Snyder et al., 1996). In these embryonic cells, axin, when overexpressed alone or together with Xdsh, showed diffuse cytoplasmic staining (Fig. 4A, B). As was shown previously, Xdsh-GFP was recruited to the plasma membrane in the presence of human Fz5 (Fig. 4D, compared with Fig. 4B). Axin remained largely cytoplasmic when human Fz5 was coexpressed (Fig. 4C). In the presence of human Fz5 plus Xdsh-GFP, however, axin became prominently plasma-membrane-bound in a pattern that was indistinguishable from that of Xdsh-GFP (Fig. 4D). Either the wild-type Xdsh or Xdsh-GFP was able to mediate human Fz5 recruitment of axin to the plasma membrane (Fig. 4D, E). By contrast, XdshΔN (Tada and Smith, 2000), which lacks the DIX domain, did not mediate human Fz5 recruitment of axin (Fig. 4F). These data support the notion of the sequential recruitment of axin by Fz through Xdsh (Dvl), and are consistent with previous and recent findings on the role of the DIX domain in Dvl-axin association in mammalian cells (Fagotto et al., 1999; Kishida et al., 1999; Li et al., 1999; Salic et al., 2000; Smalley et al., 1999; Schwarz-Romond et al., 2007b). We note, however, one caveat is that XdshΔN may be recruited less effectively by Fz to the plasma membrane (Rothbacher et al., 2000).
Fig. 4. Fz recruitment of axin to the plasma membrane via Dvl (Xdsh) in Xenopus embryonic explants.
Synthetic mRNAs for axin (0.5 ng), Xdsh (Dvl) or XdshΔN or Xdsh-GFP (1 ng), and human Fz5 (1 ng) were injected alone or in combinations as indicated into the Xenopus embryo. Axin and Fz proteins were detected by anti-Flag and anti-Fz5 antibodies, respectively. (A) Axin alone. (Ba–c) Xdsh-GFP plus axin. (Ca–c) Fz5 plus axin. (Da–d) Fz5 plus axin plus Xdsh-GFP. (Ea–c) Fz5 plus axin plus Xdsh. (Fa–c) Fz5 plus axin plus XdshΔN.
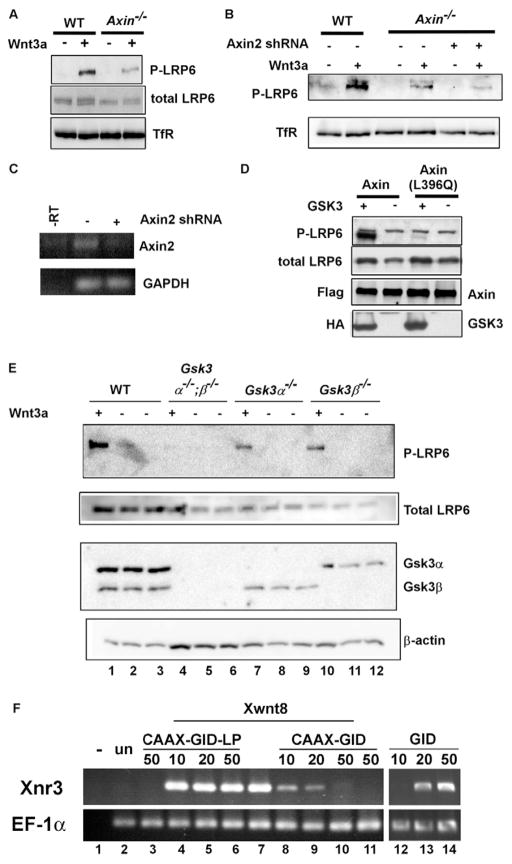
Given the role of the DIX domain in Fz/Dvl recruitment of axin, we therefore asked whether axin is required for Wnt-induced Lrp6 phosphorylation. Indeed axin overexpression can enhance Lrp6 phosphorylation at the PPPSP motif (Yamamoto et al., 2006; Tamai et al., 2004). We found that Wnt3a-induced Lrp6 phosphorylation was significantly reduced in Axin−/− mouse embryonic stem (ES) cells (Fig. 5A). Since these cells also express Axin2 (also known as Axil and conductin) (Behrens et al., 1998; Kikuchi, 1999) (Fig. 5C), which can functionally replace axin and thus shares a redundant function with axin (Chia and Costantini, 2005), we further employed shRNAs to knockdown Axin2 in these Axin−/− cells. Multiple independent shRNAs against Axin2 further reduced Wnt3a-induced Lrp6 phosphorylation (Fig. 5B and see Fig. S2 in the supplementary material), and the diminished Lrp6 phosphorylation level correlated with the reduced amount of Axin2 mRNA expression upon shRNA treatment (Fig. 5C). These results suggest that axin function is required for Wnt-induced Lrp6 phosphorylation. The axin involvement in Lrp6 phosphorylation is likely mediated by its ability to recruit Gsk3, as implied by the overexpression of an axin mutant that lacks the Gsk3-binding domain (Yamamoto et al., 2006). Indeed the wild-type axin was able to enhance LRP6 phosphorylation by GSK3, whereas the axin (L396Q) mutant, which corresponds to the zebrafish masterblind mutation and is incapable of binding to GSK3 [because of the leucine 396 to glutamine mutation (Heisenberg et al., 2001; van de Water et al., 2001)], failed to promote LRP6 phosphorylation by GSK3 (Fig. 5D).
Fig. 5. Axin is required for Lrp6 phosphorylation via its ability to bind Gsk3, and inhibition of Gsk3 at the plasma membrane blocks Wnt/β-catenin signaling.
(A) Axin−/− and the wild-type ES cells were treated with Wnt3a or control CM. Wnt3a-induced Lrp6 (endogenous) phosphorylation was significantly reduced in Axin−/− ES cells. (B, C) Reducing Axin2 expression in Axin−/− ES cells further inhibited Lrp6 (endogenous) phosphorylation (B). Axin−/− ES cells were infected with lentiviral shRNAs against mouse Axin2. TfR, transferin receptor, loading control. The efficiency of Axin2 mRNA knockdown was assessed by RT-PCR analysis. GAPDH, a loading control (C). (D) The wild-type axin, but not the GSK3 binding mutant axin (L396Q) promoted LRP6 phosphorylation by GSK3. Axin and axin (L396Q) were cotransfected with VSVG-tagged LRP6 in the presence or absence of GSK3 in HEK293T cells. Axin and GSK3 were detected by the Flag and HA tags, respectively. (E) Gsk3α and Gsk3β share redundant function in Wnt-induced LRP6 phosphorylation. ES cells null for both Gsk3α and Gsk3β (Gsk3α−/−;β−/−), null for either Gsk3α (Gsk3α−/−) or Gsk3β (Gsk3β−/−) and the control wild-type ES cells were treated with Wnt3a or control CM for 1 hour. The endogenous Lrp6 was examined. β-actin, loading control. (F) A plasma membrane-targeted CAAX-GID blocked wnt8 (Xwnt8) signaling in Xenopus embryo explants. GID induced nr3 (Xnr3) expression; CAAX-GID, but not CAAX-GID-LP, blocked wnt8 (Xwnt8)-induced nr3 expression (each was injected at 10–50 pg mRNA/embryo). wnt8 was injected at 10 pg mRNA/embryo. –, without reverse transcriptase; Un, uninjected control embryo; EF-1α; loading control.
Our results therefore suggest that Gsk3, recruited via axin to the plasma membrane, mediates Lrp6 phosphorylation and activation. Analogous to the redundancy observed among Dvl genes and axin genes, Gsk3α and Gsk3β appear to have redundant roles in Wnt3a-induced Lrp6 phosphorylation, which was not significantly affected in either Gsk3α−/− or Gsk3β−/− ES cells but was completely abolished in Gsk3α−/−;Gsk3β−/− ES cells (Fig. 5E). To demonstrate an activating role of the membrane-associated Gsk3 in Wnt signaling, we previously generated a membrane-tethered Gsk3 whose overexpression activates β-catenin signaling in a manner that depends on the PPPSP motifs in Lrp6 (Zeng et al., 2005). However, genetic studies and pharmacological inhibitors have not been able to reveal this activating role of Gsk3 in Wnt signaling, probably because of Gsk3 phosphorylation of β-catenin that plays a key negative role downstream of the pathway. We therefore attempted to inhibit Gsk3 solely at the plasma membrane. We took advantage of a highly specific Gsk3 inhibitory peptide, GID (Gsk3 interaction domain), which is a 25-amino acid-residue peptide derived from Axin and has been shown to inhibit Gsk3 specifically in vivo (Hedgepeth et al., 1999; Jiang et al., 2005; Zhang et al., 2003). GID expression in Xenopus embryos induced complete axis duplication (Hedgepeth et al., 1999) (and data not shown) and the expression of nr3, a Wnt/β-catenin target gene (lanes 12–14, Fig. 5F), indicating activation of Wnt/β-catenin signaling as expected. Importantly, when GID was targeted to the plasma membrane via the CAAX box from the K-Ras protein, CAAX-GID inhibited wnt8-induced nr3 expression in a dose-dependent fashion (compare lane 7 with lanes 8–10, Fig. 5F), demonstrating that the plasma membrane-tethered GID blocks Wnt signaling. As a specificity control, we generated a membrane tethered GID-LP (CAAX-GID-LP), which harbors a single amino acid residue change (leucine 396 to proline) in the GID peptide and thus neither binds to nor inhibits Gsk3 (Jiang et al., 2005; Zhang et al., 2003). CAAX-GID-LP did not block wnt8 induction of nr3 expression (compare lane 7 with lanes 4–6, Fig. 5F). The protein expression level of CAAX-GID and CAAX-GID-LP was comparable (data not shown). These data suggest that CAAX-GID blocks Wnt signaling by inhibiting Gsk3 at the plasma membrane. The opposite properties of GID and CAAX-GID (activating and inhibiting Wnt/β-catenin signaling; compare lanes 7–10 with lanes 12–14, Fig. 5F) is consistent with the notion that phosphorylation of β-catenin and of Lrp6 by Gsk3/axin occurs in cytosol and at the plasma membrane, respectively, and therefore is spatially separated.
DISCUSSION
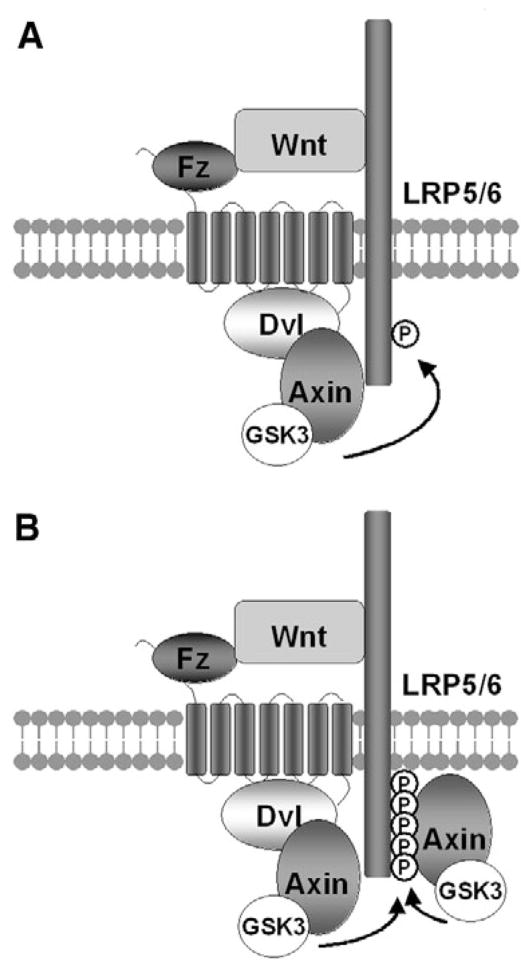
Our study documents compelling evidence that Fz signaling via Dvl and axin is required for Wnt-induced Lrp6 phosphorylation/activation by Gsk3. Given the existing biochemical studies on Fz-Dvl and Dvl-axin interactions (reviewed by Wallingford and Habas, 2005; Cadigan and Liu, 2006) and our demonstration of Fz-Dvl-axin recruitment to the plasma membrane, and that mutations that disrupt Fz-Dvl, Dvl-axin, axin-Gsk3 interactions all perturb or prevent Lrp6 phosphorylation, we suggest a working model that represents important reinterpretations and rewiring of the Wnt signaling diagram, particularly at the receptor initiation stage (Fig. 6). In the Wnt-induced Fz-Lrp6 receptor complex Fz recruits Dvl through the PDZ domain, and Dvl, via its DIX domain and possibly multimerization (Schwarz-Romond et al., 2007a) recruits the axin-Gsk3 complex, thereby promoting Gsk3 phosphorylation of Lrp6 to initiate downstream signaling (Fig. 6). This model is fully compatible with the observation in Drosophila embryos that Dsh is required for axin recruitment to the plasma membrane upon Wingless signaling (Cliffe et al., 2003), and provide clarifications for the long standing enigma and apparently contradicting views regarding the functional relationships among Fz, Lrp6 and Dvl (Li et al., 2002; Schweizer and Varmus, 2003; Tolwinski et al., 2003; Wehrli et al., 2000; Cadigan and Liu, 2006; He et al., 2004).
Fig. 6. A sequential recruitment and amplification model for Wnt-induced Lrp6 phosphorylation.
(A) Initiation. Wnt-induced Fz-Lrp6 complex formation promotes initial Lrp6 phosphorylation via Dvl recruitment of the axin-Gsk3 complex. (B) Amplification. Initial Lrp6 phosphorylation provides docking sites and thereby recruits additional Axin-Gsk3 complex to promote further Lrp6 phosphorylation in cis and possibly in trans if/when Lrp6 multimerizes. See Discussion for details. For clarity, β-catenin, CK1 and other proteins are omitted from the axin complex, the protein composition of which may be different with and without Wnt stimulation.
Our data further reveal that axin is required for Lrp6 phosphorylation and thus activation. This result is consistent with observations that axin overexpression can enhance Lrp6 phosphorylation (Yamamoto et al., 2006; Tamai et al., 2004). Previous genetic studies, analogous to those on Gsk3, have established that axin has a key inhibitory function in Wnt/β-catenin signaling via promoting β-catenin phosphorylation and degradation. This and our previous studies (Zeng et al., 2005) together suggest dual roles of the axin-Gsk3 complex in both activation (via Lrp6 phosphorylation) and inhibition (via β-catenin phosphorylation) of the Wnt pathway. Fz and Dvl appear to control the balance between the positive and negative functions of the axin-Gsk3 complex. Whether the same or distinct axin-Gsk3 complexes carry out these opposing functions remains to be determined. Our results also imply the existence of a positive feedback loop between Lrp6 and axin, given that axin is required for Lrp6 phosphorylation, and Lrp6 phosphorylation at the PPPSPxS motifs in turn provides docking sites for axin (Tamai et al., 2004; Zeng et al., 2005). This in principle could ensure sustained phosphorylation of most or all of the PPPSPxS motifs in Lrp6 in cis, and in trans if/when Lrp6 multimerizes (Baig-Lewis et al., 2007; Bilic et al., 2007; Cong et al., 2004), thereby leading to β-catenin signaling (Fig. 6B). Consistent with this possibility, Lrp6 phosphorylation and β-catenin stabilization, once induced, appear to be long lasting as long as Wnt stimulation exists (Khan et al., 2007; Wei et al., 2007). A recent study in Drosophila, using a fusion protein between Fz and the Lrp6 ortholog Arrow, and an Arrow protein that can dimerize/multimerize, argues that Fz-Arrow initiates whereas multimerized Arrow amplifies Wingless/β-catenin signaling (Baig-Lewis et al., 2007). Our data provide a potential molecular basis for such an initiation amplification phenomenon and further suggest two routes to recruit the axin complex to the Wnt receptor complex. Thus Fz-Dvl recruits axin to initiate Lrp6 phosphorylation (initiation), whereas phosphorylated Lrp6 further recruits axin to sustain and amplify Lrp6 phosphorylation (amplification; Fig. 6).
Our data also help to resolve an important discrepancy in the literature regarding which Lrp6 phosphorylation event is regulated by Wnt. We have demonstrated that PPPSP motif phosphorylation, which is carried out by the redundant function of Gsk3α and Gsk3β (Zeng et al., 2005) (this study), is inducible upon Wnt stimulation (Tamai et al., 2004). This observation has been further supported by others (Binnerts et al., 2007; Bryja et al., 2007; Khan et al., 2007) (J. Reiter, personal communication) and our additional studies (Wei et al., 2007; Zeng et al., 2005) (this study). We further showed that in the extended PPPSPxS motif, PPPSP phosphorylation primes the phosphorylation of xS by CK1, thereby resulting in Wnt induction of phosphorylation by both Gsk3 and CK1 (Zeng et al., 2005). By contrast, one study suggested that PPPSP phosphorylation is constitutive whereas CK1 phosphorylation is the main Wnt-inducible event (Davidson et al., 2005). The origin for this discrepancy remains unclear, but may be because of basal levels of PPPSP phosphorylation variably detected in cell cultures as a result of some autocrine Wnt signaling, and/or to different qualities of the phospho-Lrp6 antibodies. Nonetheless, the data presented here further illustrate Wnt-induced PPPSP phosphorylation is under the control by Fz, Dvl and axin functions, thereby supporting the notion that Gsk3 phosphorylation of Lrp6 is a major regulatory step upon Wnt stimulation. While this manuscript was in preparation, it was reported that Wnt may induce Lrp6 aggregation and phosphorylation at threonine 1479 (T1479) by CK1 in a Dvl-dependent manner, although the mechanism by which CK1 is regulated by Dvl and the involvement of Fz, axin and Gsk3 in the process were not addressed (Bilic et al., 2007). We note that such an ‘aggregation’ model and our sequential recruitment/amplification model may be different but not mutually exclusive. We should note, however, that T1479, unlike the PPPSPxS motif, is not conserved in Drosophila Arrow, and the significance of T1479 phosphorylation in the context of the wild-type Lrp6 function remains to be established.
How Wnt stimulation leads to inhibition of β-catenin phosphorylation remains unclear. Constitutively activated (N-terminal truncated) Lrp5/6, and a single PPPSPxS motif when transferred to a heterologous receptor, are constitutively phosphorylated (Tamai et al., 2004) and are each sufficient to trigger β-catenin signaling, probably in a Dvl-independent manner (Li et al., 2002; Schweizer and Varmus, 2003). These observations suggest that binding of axin to the phosphorylated PPPSPxS motifs in Lrp6 may be sufficient to cause inhibition of β-catenin phosphorylation, and that the only function of Dvl in this pathway may be to promote Lrp6 phosphorylation. However, Dvl overexpression can activate β-catenin signaling in Xenopus egg extracts (Salic et al., 2000) and in an Arrow (Lrp6) mutant background (Wehrli et al., 2000; Kimelman and Xu, 2006), and this signaling capability by overexpressed Dvl seems to correlate with the propensity of Dvl to multimerize (Schwarz-Romond et al., 2007b). Whether Dvl, in addition to mediating Lrp6 phosphorylation, also directly inhibits β-catenin phosphorylation by axin-Gsk3 under physiological conditions remains to be determined. Finally, the observations that the axin-Gsk3 complex plays opposing functions in Lrp6 and β-catenin phosphorylation, and that Dvl interacts with axin in a dynamic manner (Schwarz-Romond et al., 2007a; Schwarz-Romond et al., 2007b) support the emerging view that Wnt/β-catenin signaling is a highly kinetic/dynamic process rather than simple on and off states, the nature of which may require new analytical tools beyond traditional biochemical methodologies to fully capture.
Supplementary Material
Acknowledgments
We thank B. Williams for DKK-Fz5 expression vectors, A. Yamamoto and S. Aizawa for the Shisa expression vector, M. Tada and J. Smith for XdshΔN vector, L. Mei for Dvl1 antisera and J. Reiter for communication. We appreciate constructive suggestions from anonymous reviewers, and comments from B. MacDonald. X.Z. is in part supported by a career development award from the Children’s Hospital Boston. H.H. is supported by a postdoctoral fellowship from CIHR (Canada). This work is in part supported by grants from NIH to X.H., who is a W. M. Keck Foundation Distinguished Young Scholar and a Leukemia and Lymphoma Society Scholar.
Footnotes
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/135/2/367/DC1
References
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Baig-Lewis S, Peterson-Nedry W, Wehrli M. Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Dev Biol. 2007;306:94–111. doi: 10.1016/j.ydbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating axin to the plasma membrane during Wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, et al. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 2007;67:573–579. doi: 10.1158/0008-5472.CAN-06-2726. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Deardorff MA, Rankin K, Klein PS. Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin. Mol Cell Biol. 1999;19:7147–7157. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Robertson SA, Zylstra CR, Williams BO. Wnt-independent activation of beta-catenin mediated by a Dkk1-Fz5 fusion protein. Biochem Biophys Res Commun. 2005;328:533–539. doi: 10.1016/j.bbrc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Khan Z, Vijayakumar S, de la Torre T, Rotolo S, Bafico A. Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor. Mol Cell Biol. 2007;27:7291–7301. doi: 10.1128/MCB.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999;10:255–265. doi: 10.1016/s1359-6101(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mao J, Sun L, Liu W, Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem. 2002;277:5977–5981. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–3482. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Povelones M, Howes R, Fish M, Nusse R. Genetic evidence that Drosophila frizzled controls planar cell polarity and Armadillo signaling by a common mechanism. Genetics. 2005;171:1643–1654. doi: 10.1534/genetics.105.045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007a;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007b;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 1999;18:2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H, Zivkovic D. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877–3888. doi: 10.1242/dev.128.20.3877. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Wharton KA., Jr Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.