Abstract
In the three decades since pluripotent mouse embryonic stem (ES) cells were first described1,2 they have been derived and maintained by using various empirical combinations of feeder cells, conditioned media, cytokines, growth factors, hormones, fetal calf serum, and serum extracts1–7. Consequently ES-cell self-renewal is generally considered to be dependent on multifactorial stimulation of dedicated transcriptional circuitries, pre-eminent among which is the activation of STAT3 by cytokines (ref. 8). Here we show, however, that extrinsic stimuli are dispensable for the derivation, propagation and pluripotency of ES cells. Self-renewal is enabled by the elimination of differentiation-inducing signalling from mitogen-activated protein kinase. Additional inhibition of glycogen synthase kinase 3 consolidates biosynthetic capacity and suppresses residual differentiation. Complete bypass of cytokine signalling is confirmed by isolating ES cells genetically devoid of STAT3. These findings reveal that ES cells have an innate programme for self-replication that does not require extrinsic instruction. This property may account for their latent tumorigenicity. The delineation of minimal requirements for self-renewal now provides a defined platform for the precise description and dissection of the pluripotent state.
Mouse ES cells exist in the artificial milieu of cell culture. They are derived and maintained by using a combination of the cytokine leukaemia inhibitory factor (LIF) to activate STAT3 and either serum or bone morphogenetic protein (BMP) to induce inhibitor-of-differentiation proteins5. Their differentiation involves autoinductive stimulation of the mitogen-activated protein kinase (ERK1/2) pathway by fibroblast growth factor-4 (FGF4)9,10. However, neither LIF nor serum/BMP block the activation of ERK (Supplementary Information and ref. 5). We proposed that the LIF and serum/BMP signals act downstream of phospho-ERK to block ES-cell commitment. To test this idea we used selective small-molecule inhibitors SU5402 (ref. 11) and PD184352 (ref. 12) to inhibit FGF receptor tyrosine kinases and the ERK cascade, respectively.
We found that, in combination with LIF, either inhibitor replaces the requirement for serum/BMP and supports robust long-term ES-cell propagation (Supplementary Information). Lineage commitment does not occur despite a reduced expression of inhibitor-of-differentiation proteins. In contrast, ES cells plated without LIF in either PD184352 or SU5402 progressively degenerate and cannot be maintained even though differentiation is suppressed. To reduce off-target side effects we tried low doses of PD184352 and SU5402 together (PS). In PS we find that undifferentiated ES cells expand through multiple passages (Fig. 1a, b). Differentiation is constrained, although occasional neural rosettes emerge. This result, observed with several independent ES cell lines, suggests that the minimal requirements for ES-cell self-renewal may be to deflect commitment signals emanating from FGF receptor and ERK signalling. However, apoptosis is relatively high in PS, especially immediately after passage, and cells survive poorly at clonal density, which is indicative of collateral compromise to cell growth and viability.
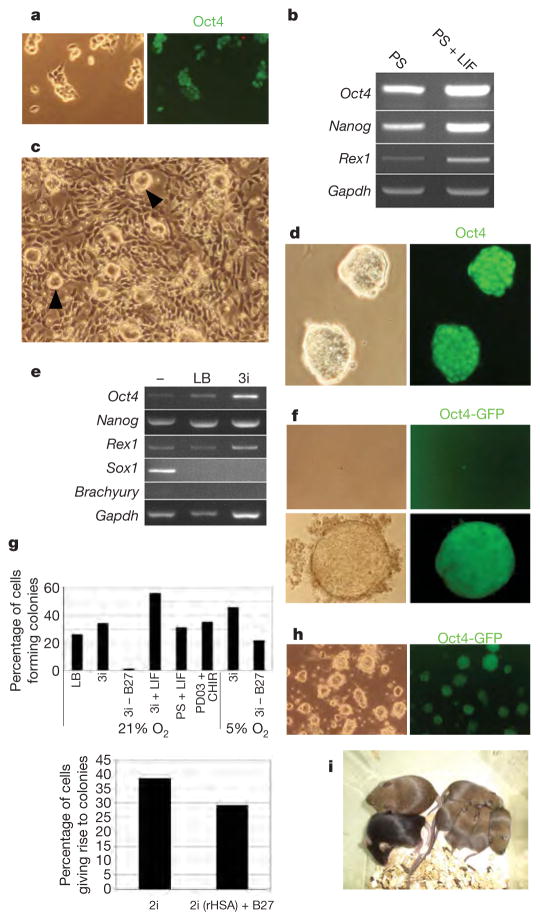
Figure 1. Three inhibitors (3i) support robust self-renewal and de novo derivation of pluripotent ES cells.
a, Immunostaining of E14Tg2a ES cells with Oct4 after four passages in N2B27 plus PD184352 and SU5402 (PS). b, RT–PCR analysis of marker expression in ES cells in N2B27 containing PS with or without LIF. Gapdh, gene encoding glyceraldehyde-3-phosphate dehydrogenase. c, Low-magnification phase-contrast image of ES cells passaged in N2B27 plus CHIR99021 showing a mixture of differentiated cells with compact undifferentiated colonies (arrowheads). d, Immunostaining with Oct4 after several passages in N2B27 plus 3i, showing compact colony morphology. e, RT–PCR analysis of marker expression in ES cells cultured in N2B27 alone (−) or with LIF and BMP4 (LB) or 3i. f, Phase and fluorescence images of expansion from a single Oct4GiP ES cell in 3i. g, Cloning efficiencies of E14Tg2a ES cells after single-cell deposition in the indicated conditions (top), and in CHIR99021 plus PD0325901 (2i, see Fig. 2) with or without B27, or with the replacement of serum albumin with recombinant albumin (rHSA) (bottom; experiment performed in 5% O2). h, Oct4GIP ES cells cultured for five passages (total 28 days) in basal medium supplemented with transferrin and BSA only plus 3 μM CHIR99021, 0.5 μM PD184352 and 1 μM SU5402. i, Chimaera and germline offspring produced from CBA ES cells derived in 3i. Chimaera showing extensive contribution of CBA (agouti coat colour) ES cells mated with C57BL/6 (black) produced agouti pups, indicating the transmission of the CBA genome.
ES-cell propagation has been reported to be enhanced by an indirubin entity, 6-bromo-indirubin-3′-oxime (BIO), that inhibits glycogen synthase kinase-3 (GSK3)4. However, indirubins are not highly selective and cross-react with cyclin-dependent kinases and other kinases13,14. We found reduced viability of ES cells in BIO with or without PS. Nevertheless we speculated that relief of GSK3-mediated negative regulation of biosynthetic pathways might restore growth to ES cells cultured in PS. We therefore used a more selective inhibitor, CHIR99021 (ref. 14,15). Alone, CHIR99021 enhances survival at low cell density but also induces non-neural differentiation. At higher densities some colonies remain morphologically undifferentiated but are progressively overcome by differentiation on passaging (Fig. 1c). Single blockade of GSK3 therefore has pleiotropic effects, promoting non-neural differentiation, suppressing neural differentiation and enhancing growth capacity. Crucially, however, in a combination of all three inhibitors (3i) the differentiation blocking effect of PS is dominant, resulting in a highly efficient expansion of undifferentiated colonies, even at a low cell density. Multiple ES-cell lines tested all expand continuously for many weeks in 3i. They express Oct4, Nanog and Rex1 with minimal levels of lineage commitment markers, Sox1 or brachyury (Fig. 1d, e). In 3i, ES cells expand with a doubling rate comparable to that in LIF plus serum/BMP (Supplementary Information) with the proportion of Oct4–green fluorescent protein (GFP)-positive undifferentiated cells remaining over 90%. As a rigorous test of the sufficiency of 3i to sustain ES-cell self-renewal, we examined the clonogenicity of isolated cells. After single-cell deposition, undifferentiated Oct4-positive colonies develop at higher frequency than in LIF and serum or BMP (Fig. 1f, g).
The B27 supplement used in serum-free culture contains defined additives, in particular antioxidants and free-radical scavengers. We found that ES cells could be propagated in bulk culture in 3i medium prepared with N2 supplement only, but they did not survive at clonal density. However, in physiological oxygen (5% O2) clonal propagation was obtained without B27 (Fig. 1g). This excludes an instructive contribution from B27 components to ES-cell self-renewal, while highlighting the damage potential of non-physiological oxygen levels. When insulin was omitted we found ES cells to be more sensitive to FGF receptor (FGFR) and MAP kinase/ERK kinase (MEK) inhibitors. We therefore decreased their concentrations. In these conditions, with only transferrin and albumin additives, ES cells expanded, even from single cells. They remained mostly undifferentiated over four weeks of continuous culture (Fig. 1h), although after the first passage the propagation rate declined steadily. We conclude that insulin promotes long-term growth capability but does not dictate the fate choice between self-renewal and lineage commitment. Finally, we used recombinant albumin to eliminate serum-derived contaminants. In combination with transferrin and insulin, this supported both bulk passaging and clonal propagation (Fig. 1g).
To eliminate the possibility that self-renewal in 3i might reflect pre-adaptation to specific culture conditions in our laboratory, we investigated the derivation of ES cells from mouse embryos. ES cells were readily derived from blastocysts of the permissive 129 strain plated directly into 3i on gelatin-coated plastic. Expanded lines injected into blastocysts gave chimaeras and germline transmission (Supplementary Information). ES cell lines were also established from the CBA strain, which is refractory to ES-cell production under standard conditions16. Two of these lines were injected into morulae and both yielded high-grade chimaeras and germline transmission (Fig. 1i).
Taken together, the above findings demonstrate that 3i liberates ES cells from requirements for exogenous factors without compromise to developmental potency.
To confirm that blockade of FGF signalling is the critical target of SU5402 we substituted an alternative inhibitor, PD173074 (ref. 17). We found that this could substitute for SU5402 in 3i at 40-fold lower concentrations, which is consistent with its higher affinity for the FGF receptor (Fig. 2a). We then examined fgf4-null ES cells18 and determined that they can expand continuously in CHIR99021 alone (Fig. 2b), providing genetic validation of the significance of autoinductive FGF4.
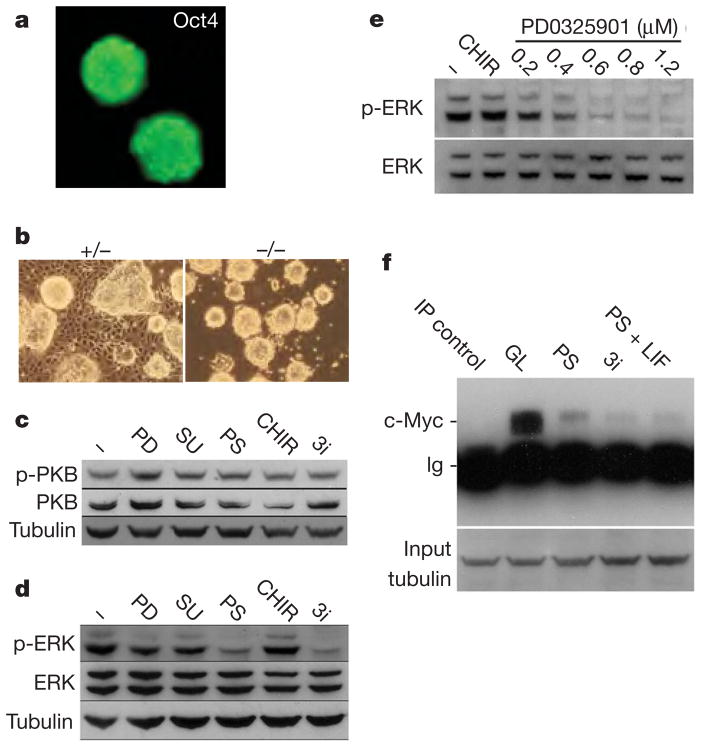
Figure 2. Effects of 3i components on intracellular signalling cascades.
a, E14Tg2a ES cells remain undifferentiated and Oct4-positive in alternative 3i with SU5402 replaced by PD173074. b, fgf4-null ES cells expand without differentiation in N2B27 plus CHIR99021 only, without a requirement for FGFR/MEK inhibition. c, d, Immunoblot analyses of steady-state levels of phospho(Ser 473)-PKB (p-PKB) (c) and phospho(Thr 202, Tyr 204)-ERK (p-ERK) (d) in ES cells after 24 h in N2B27 alone (−), plus 0.8 μM PD184352 (PD), 2 μM SU5402 (SU), 3 μM CHIR99021, PS or 3i. e, Immunoblot analyses of phospho(Thr 202, Tyr 204)-ERK levels in ES cells after 24 h in N2B27 alone (−), plus 3 μM CHIR99021 (CHIR) or 3 μM CHIR99021 plus PD0325901 at the indicated concentrations. f, c-Myc protein in ES cells assayed by sequential immunoprecipitation (IP) and immunoblotting after 24 h in serum plus LIF (GL), PS, 3i, or PS plus LIF. IP control is the GL sample immunoprecipitated with anti-tubulin. Input samples were subjected to SDS PAGE and blotted for tubulin to control for loading.
FGF4 activates the phosphatidylinositol-3-OH kinase/protein kinase B (PKB) and the Ras–MEK–ERK intracellular signalling cascades (Fig. 2c, d). Phosphorylation and activation of PKB is not appreciably altered by the 3i inhibitors. PD184352 or SU5402 applied alone at the low doses used in 3i cause only modest decreases in steady-state phospho-ERK. However, the combination of both inhibitors greatly decreases phospho-ERK levels. CHIR99021 does not modulate phospho-ERK (Fig. 2e). We tested erk2-null ES cells19 and found that these can be maintained at high density with CHIR99021 only, although optimal propagation requires supplementation with PD184352; this is consistent with maintained activity of phospho-ERK1 in these mutants. The central role of the ERK cascade was confirmed by using a structurally related, more potent but equally selective MEK inhibitor, PD0325901 (ref. 15), to achieve greater suppression of ERK activation without side effects. This is sufficient to sustain efficient ES-cell self-renewal in combination with CHIR99021 only (Figs 1g and 2e).
An unwarranted side effect of suppressing phospho-ERK is to depress myc messenger RNA and Myc protein levels (Fig. 2f and Supplementary Information). Upregulation of c-Myc has been suggested to mediate ES-cell self-renewal downstream of LIF and of BIO20. However, the low c-Myc levels in cultures in PS are not increased by CHIR99021 or LIF (Fig. 2f). Therefore elevated c-Myc is not necessary for ES-cell propagation, although some requirement for basal Myc activity is not excluded.
STAT3 signalling is central to previous models of ES-cell self-renewal8,21 and has also been implicated in effects of BIO20,22,23. In 3i, however, we do not detect activation of STAT3 or induction of its target SOCS3 (Supplementary Information). To test definitively whether STAT3 is dispensable for ES-cell self-renewal, embryos from intercrosses of Stat3 heterozygous mice were cultured in 3i. Homozygous mutant ES cells were established (Fig. 3a). Stat3-null cells are morphologically indistinguishable from wild-type ES cells. They express Oct4 and Nanog, and initiate multilineage commitment in embryoid bodies (Fig. 3b–d). They show no induction of SOCS3 when stimulated with LIF (Fig. 3e). When transferred to LIF and serum, stat3−/− cells differentiate rapidly, confirming their incompetence to respond to LIF (Fig. 3f). We conclude that the otherwise absolute requirement for STAT3 in the derivation and self-renewal of mouse ES cells is rendered dispensable by 3i.
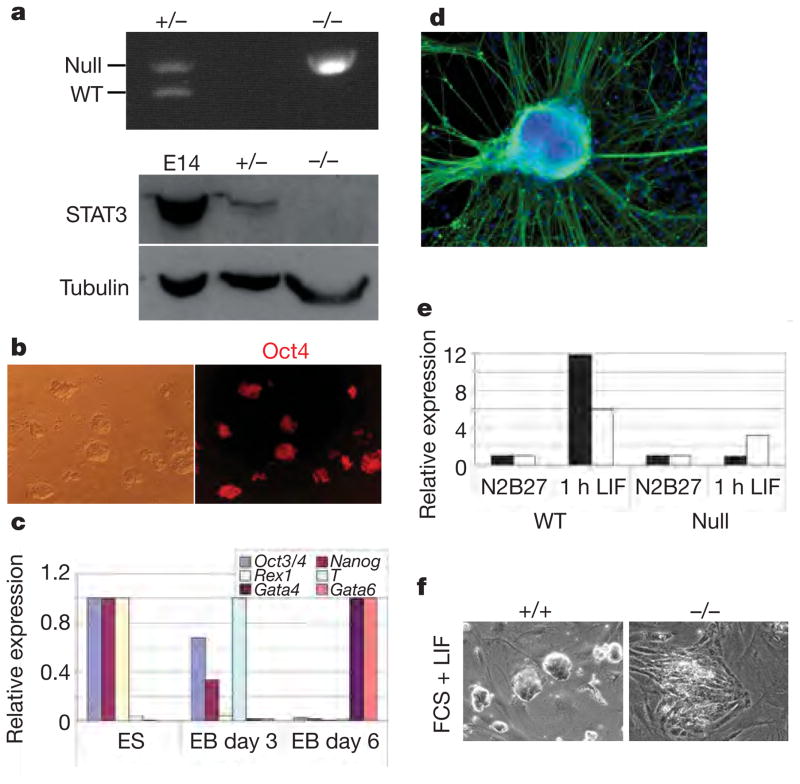
Figure 3. ES-cell propagation in 3i does not involve STAT3.
a, Top: genomic PCR for null and wild-type (WT) Stat3 alleles in heterozygous and Stat3 homozygous null ES cells. Bottom: immunoblot analysis of heterozygous and Stat3 homozygous null ES cells. E14, E74Tg2a ES cells. b, Oct4 immunostaining of Stat3-null ES cells. c, Quantitative RT–PCR analysis of undifferentiated Stat3-null ES cells and derivative embryoid bodies (EB) at days 3 and 6. d, Stat3-null ES cells generate morphologically differentiated cells expressing the neuronal marker βIII-tubulin (TuJ1 immunoreactive). e, Quantitative RT–PCR analysis of Socs3 (Stat3 target gene; filled columns) and Egr1 (ERK target gene; open columns) expression in undifferentiated Stat3 wild-type and null ES cells grown in N2B27 alone or stimulated with LIF for 1 h. f, Stat3-null MF1 ES cells differentiate in the presence of LIF and feeders, in contrast with wild-type MF1 ES cells, which remain undifferentiated.
CHIR99021 induces a decrease in phosphorylation of β-catenin (Supplementary Information) and activation of the T-cell factor (TCF)-responsive TOPFlash reporter (Fig. 4a), simulating canonical Wnt signalling. We investigated whether Wnt could replicate the effect of CHIR99021. Recombinant Wnt3a alone induced non-neural differentiation, as seen with CHIR99021 only. This effect was suppressed by PS and at high concentrations (100 ng ml−1) Wnt3a seemed to eliminate residual neural differentiation and thereby improved ES-cell propagation. However, expansion in PS plus Wnt3a did not match that obtained in 3i. We introduced into ES cells dominant-negative ΔNhLef1, which lacks the β-catenin-binding domain and suppresses TCF-mediated transcriptional activation. As expected, ES cells stably expressing ΔNhLEF1 showed reduced TOPFlash activity (Fig. 4a). Nonetheless they readily formed undifferentiated colonies in 3i. A competitive self-renewal assay was performed after treatment with Cre to excise the floxed ΔNhLEF1 and simultaneously activate GFP. Equivalent numbers of ΔNhLEF1-expressing and revertant GFP-expressing cells were propagated as mixed cultures for four passages. In serum plus LIF the GFP-positive and GFP-negative populations remained equivalent. In 3i the GFP-negative ΔNhLEF1-expressing cells became marginally predominant (Fig. 4b). Decreasing TCF activation therefore does not impede ES-cell self-renewal. Increased β-catenin levels might also enhance cell adhesion. However, E-cadherin-null ES cells that lack adhesion junctions remain undifferentiated and proliferate as rapidly in 3i as in LIF plus serum (Supplementary Information).
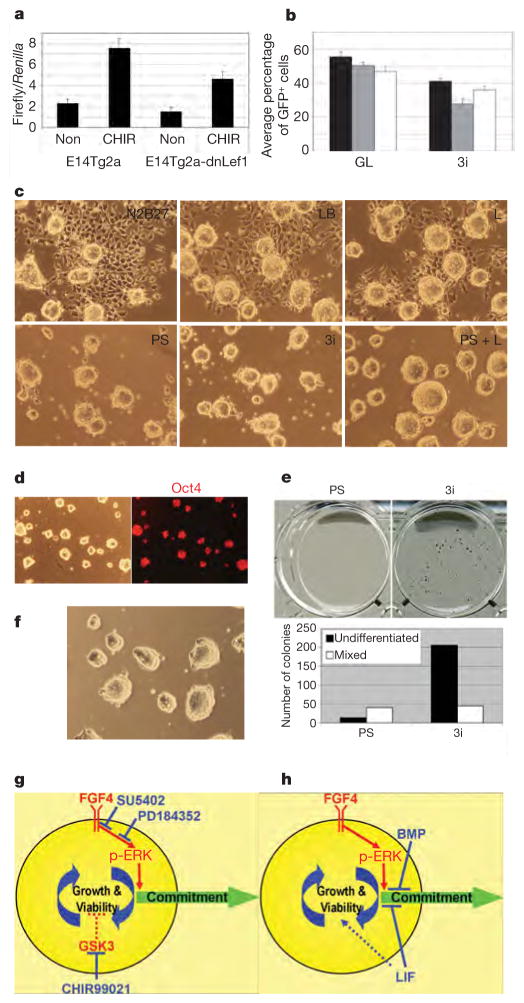
Figure 4. CHIR99021 acts via inhibition of GSK3 to enhance ES-cell growth capacity and viability.
a, TOPFlash assay in ΔNLef1 stable transfectants. Results are shown as means and s.d. for three biological replicates. Non, N2B27 alone; CHIR, CHIR99021. b, Competitive growth assay for three clones of ΔNLef1 stable transfectants and Cre revertants (GFP+) in serum and LIF or in 3i. Results are shown as means and s.d. for four biological replicates. c, GSK3α/β-deficient DKO cells cultured in the indicated conditions for two passages. L, LIF. d, Phase-contrast and Oct4 immunostaining of DKO cells after eight passages in 1 μM PD0325901. Parallel cultures with addition of CHIR99021 were indistinguishable. e, ES cells constitutively expressing Nanog respond to CHIR99021 by enhanced self-renewal at low density in 3i compared with PS. f, ES cells self-renew in 2 μM PD0325901 plus CHIR99021. g, h, Diagrams of self-replication of the pluripotent state when inductive phospho-ERK signalling is either inhibited upstream by chemical antagonists (g) or counteracted downstream by LIF and BMP (h). Inhibition of GSK3 serves a key function in augmenting self-renewal when phospho-ERK (pERK) is suppressed by maintaining cellular growth capacity and additionally reinforcing suppression of neural commitment.
To confirm that the effect of CHIR99021 is mediated through the inhibition of GSK3, we interrogated ES cells in which both GSK3α and GSK3β had been deleted24. These DKO cells are profoundly deficient in neural differentiation. They can be passaged two or three times in non-supplemented medium but succumb to progressive non-neural differentiation. This short-lived propagation is similar to that of wild-type ES cells cultured in CHIR99021 only (compare Fig. 4c with Fig. 2a). Addition of PS or PD0325901 eliminates differentiation and allows continuous passaging (Fig. 4c). However, expansion is slower than in wild-type cells in 3i. LIF restores normal population doubling (Fig. 4c), but CHIR99021 has no beneficial effect. This confirms that the effect of CHIR99021 is mediated through GSK3 and that LIF operates through a parallel STAT3 (refs 8, 21) pathway independent of GSK3 inhibition. DKO cells show constitutive TOPFlash activation24, 50-fold higher than CHIR99021-treated wild-type cells (Supplementary Information). This tonic β-catenin/TCF activity, with upregulation of targets such as brachyury and cdx1 (ref. 24), probably underlies their compromised propagation.
ES cells constitutively expressing elevated levels of Nanog are capable of sustained self-renewal in N2B27 alone but expand poorly at clonal density unless LIF is also added5. They form only a few small colonies at low density in PS but generate abundant undifferentiated colonies in 3i (Fig. 4e). The key effect of CHIR99021 therefore does not involve the induction of Nanog. Because Nanog-overexpressing ES cells are independently blocked in differentiation, this result further suggests that the contribution of GSK3 inhibition extends beyond limiting differentiation. To probe this further, we evaluated whether CHIR99021 could rescue ES cells subjected to a more profound blockade of phospho-ERK. A higher dose of PD0325901 (2 or 3 μM) almost entirely eliminates phospho-ERK and causes growth arrest and cell death. The addition of CHIR99021 restores viability and allows efficient expansion of undifferentiated ES cells in the near absence of ERK signalling (Fig. 4f). We surmise that as phospho-ERK is diminished, downmodulation of GSK3 becomes increasingly crucial to maintain metabolic activity, biosynthetic capacity and overall viability.
This study reveals that the pathways required to sustain undifferentiated ES cells are dictated by the construction of the culture milieu. In a neutralized environment, ES cells can be efficiently derived and maintained without a requirement for growth factors or cytokines (Fig. 4g). We infer that BMP/Smad/Id and LIF/STAT3 signalling do not instruct self-renewal but act in unrefined culture conditions to shield the pluripotent state from induced phospho-ERK (Fig. 4h). Earlier studies have pointed to a positive effect of inhibiting the ERK cascade on ES-cell propagation in the context of additional signals25,26. However, upregulation of c-Myc, Stat3 or anti-apoptotic factors, previously invoked as key effectors of self-renewal, is not relevant in 3i. Our data do not exclude a contribution of stabilized β-catenin through TCF-independent mechanism(s), possibly acting as a noise filter27. Wnt3a does enhance neural suppression in PS cultures, but it gives significantly less benefit for overall propagation than CHIR99021 does. We infer that the pivotal contribution of GSK3 inhibition is to restore full growth and viability. This may be achieved by balancing the loss of ERK input into basic cellular processes. We detected no induction of anti-apoptotic factors (Supplementary Information), indicating that reduced GSK3 activity may exert a global modulation of the ES-cell metabolomic and bio-synthetic capacity rather than having a direct anti-apoptotic action. Furthermore, restoration of the biosynthetic capacity of ES cells might itself increase the threshold for commitment. This possibility is suggested by the effect of feedback in mitogen-activated protein kinase signalling circuitry on the mating switch decision in yeast28.
Previous empirical configurations of the culture environment have obscured the critical requirements for maintaining ES-cell pluripotency. We propose that ES cells are a basal cell state that is intrinsically self-maintaining if shielded effectively from inductive differentiation stimuli including autocrine FGF4. This feature may underlie the well-known predisposition of ES cells to generate terato-carcinomas29,30. They can dispense with an elementary cell signalling pathway, ERK, and do not seem to require any intercellular stimulation. They have not developed G1 cyclin checkpoint control of cell cycle progression and replicate constitutively29. ES cells thus display a self-sufficiency more akin to that of unicellular organisms than the interdependence generally exhibited by metazoan cells.
METHODS
Details of RT–PCR conditions and antibodies are provided in Supplementary Information.
ES-cell culture
N2B27 medium was prepared as described31,32 or with preformulated NDiff N2B27 base medium (catalogue no. SCS-SF-NB-02; Stem Cell Sciences Ltd.) Where specified, recombinant human albumin (Cellastim; Invitria) was substituted for bovine serum albumin. Cells were routinely propagated by trypsinization and replating every three days, with a split ratio of 1 in 10.
ES-cell derivation
Whole diapause blastocysts (strain 129) or isolated epiblasts (CBA) were plated in pre-equilibrated N2B27 3i medium. After several days, cell masses were disaggregated into small clumps of cells with trypsin and replated. Emerging ES-cell colonies were expanded by replating into successively larger wells. Wild-type MF1 ES cells were derived from whole blastocysts as for strain 129. These cells showed decreased substrate attachment, probably as a result of the outbred MF1 genetic background. They can be passaged on laminin-coated plastic but are more readily maintained on murine embryo fibroblast feeders. Accordingly, for Stat3 mutant derivations we employed feeders for part of the process. Eight-cell embryos from intercrosses of Stat3+/− outbred MF1 mice33 were cultured in KSOM medium containing 3i until the formation of expanded blastocysts. Trophectoderm layers were removed by immunosurgery and analysed by PCR for genotype determination34. Four isolated inner-cell masses (three Stat3+/−, one Stat3−/−) were plated individually into four-well plates on feeders in pre-equilibrated N2B27 3i. After five days, cell masses were dis-aggregated with trypsin and plated into fresh four-well plates. ES cells developed in all four cultures and were expanded in laminin-coated wells without feeders.
Transfections
ΔNhLef1 (ref. 35) was inserted between loxP sites in pPyFloxedMTIPgfp36. This construct was transfected by electroporation into E14Tg2a ES cells for stable integration, or into E14T ES cells36 for episomal propagation. Dual luciferase reporter assays were performed 56 h after lipofection with TOPFlash or FOPFlash constructs.
Competitive self-renewal assay
Three clones of E14Tg2a stable transfectants were transiently transfected with a Cre expression vector to excise ΔNhLef1 and simultaneously activate GFP expression. After 24 h, GFP-positive and GFP-negative cells were fractionated by fluorescence-activated cell sorting (FACS), recombined in equal numbers and plated in six-well plates at 105 cells per well in N2B27 with 3i. Cells were expanded for four passages and then analysed by FACS to establish the proportion of GFP+ (ΔNhLef1 excised) and GFP− (ΔNhLef1-expressing) cells.
Supplementary Material
Acknowledgments
We thank D. Alessi for discussion and advice on GSK3 signalling; A. Rizzino, S. Meloche and R. Kemler for Fgf4, Erk2 and Ecadherin targeted ES cells, respectively; F. Watt for the ΔNhLef1 construct; N. Shpiro and R. Marquez for synthesizing PD184352, CHIR99021 and PD0325901; B. Amati and G. Faga for advice on Myc immunoblotting; J. Vrana for fluorescence-activated cell sorting support; and C. Manson, K. Savill and colleagues for mouse husbandry. This research was funded by the Medical Research Council and the Biotechnology and Biological Sciences Research Council of the UK, the Canadian Institutes of Health Research, and by the European Commission Framework VI project EuroStemCell. P.C. is a Royal Society Research Professor, and A.S. is a Medical Research Council Professor.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
Q.L.Y. and A.S. conceived the study; Q.L.Y., J.W. and J.N. designed, executed and interpreted experiments; L.B.M. generated CBA ES cells; B.D. and J.W. generated and provided GSK3 mutant ES cells; P.C. contributed expert advice and inhibitors; and A.S. wrote the paper.
References
- 1.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Rathjen J, et al. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112:601–612. doi: 10.1242/jcs.112.5.601. [DOI] [PubMed] [Google Scholar]
- 4.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 5.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, et al. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech Dev. 1994;45:163–171. doi: 10.1016/0925-4773(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa K, Matsui H, Ohtsuka S, Niwa H. A novel mechanism for regulating clonal propagation of mouse ES cells. Genes Cells. 2004;9:471–477. doi: 10.1111/j.1356-9597.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 8.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunath T, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 10.Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi M, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 12.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhen Y, Sorensen V, Jin Y, Suo Z, Wiedlocha A. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 2007;26:6372–6385. doi: 10.1038/sj.onc.1210473. [DOI] [PubMed] [Google Scholar]
- 14.Murray JT, et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bain J, et al. The selectivity of protein kinase inhibitors; a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buehr M, Smith A. Genesis of embryonic stem cells. Phil Trans R Soc B. 2003;358:1397–1402. doi: 10.1098/rstb.2003.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadi M, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilder PJ, et al. Inactivation of the FGF-4 gene in embryonic stem cells alters the growth and/or the survival of their early differentiated progeny. Dev Biol. 1997;192:614–629. doi: 10.1006/dbio.1997.8777. [DOI] [PubMed] [Google Scholar]
- 19.Saba-El-Leil MK, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright P, et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 23.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci USA. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Arias A, Hayward P. Filtering transcriptional noise during development: concepts and mechanisms. Nature Rev Genet. 2006;7:34–44. doi: 10.1038/nrg1750. [DOI] [PubMed] [Google Scholar]
- 28.Colman-Lerner A, et al. Regulated cell-to-cell variation in a cell-fate decision system. Nature. 2005;437:699–706. doi: 10.1038/nature03998. [DOI] [PubMed] [Google Scholar]
- 29.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 30.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 31.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 32.Ying QL, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 35.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 36.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.