Abstract
Background
The aim of this study was to investigate the association between regional body fat distribution, especially leg fat mass, and the prevalence of diabetes mellitus (DM) in adult populations.
Methods
A total of 3,181 men and 3,827 postmenopausal women aged 50 years or older were analyzed based on Korea National Health and Nutrition Examination Surveys (2008 to 2010). Body compositions including muscle mass and regional fat mass were measured using dual-energy X-ray absorptiometry.
Results
The odds ratios (ORs) for DM was higher with increasing truncal fat mass and arm fat mass, while it was lower with increasing leg fat mass. In a partial correlation analysis adjusted for age, leg fat mass was negatively associated with glycosylated hemoglobin in both sexes and fasting glucose in women. Leg fat mass was positively correlated with appendicular skeletal muscle mass and homeostasis model assessment of β cell. In addition, after adjusting for confounding factors, the OR for DM decreased gradually with increasing leg fat mass quartiles in both genders. When we subdivided the participants into four groups based on the median values of leg fat mass and leg muscle mass, higher leg fat mass significantly lowered the risk of DM even though they have smaller leg muscle mass in both genders (P<0.001).
Conclusion
The relationship between fat mass and the prevalence of DM is different according to regional body fat distribution. Higher leg fat mass was associated with a lower risk of DM in Korean populations. Maintaining leg fat mass may be important in preventing impaired glucose tolerance.
Keywords: Absorptiometry, photon; Body composition; Body fat distribution; Diabetes mellitus
INTRODUCTION
As the life span of individuals increases, metabolic disorder, especially type 2 diabetes mellitus (DM), has also been increasing rapidly worldwide [1]. Accordingly, DM has become a major health care problem regarding reduced life span, increased morbidity, and significant financial burden [2]. To date, it has been known that higher body mass, which is generally represented as increased weight or body mass index (BMI), correlates with insulin resistance and the risk of diabetes [3,4]. However, there is growing evidence that subjects with higher BMI may have a lower mortality and a better outcome in several chronic diseases; in fact, this phenomenon is described as the ‘obesity paradox’ [5]. This finding may indicate that BMI is a crude anthropometric index that does not accurately reflect an individual's fat mass/muscle mass status, nutritional status, and body fat distribution.
Several recent studies have suggested that adipose tissue stored in various body locations differentially impact metabolic health. Hu et al. [6] reported adverse risk for metabolic disorder with high trunk adiposity, but high leg adiposity was associated with a decreased risk of having two or more cardiometabolic risk factors in both African American and white adults. In addition, Snijder et al. [7] showed that larger leg fat mass was associated with lower fasting and postload glucose levels from a 75-g oral glucose tolerance test.
Sarcopenia, characterized by low muscle mass, has been considered to be associated with insulin resistance and type 2 diabetes [8,9]. Because skeletal muscle is the primary site of insulin-stimulated glucose disposal at euglycemia [10], low muscle mass may contribute to the development of diabetes. In that sense, larger thigh circumference, which reflects both larger leg muscle mass and larger leg fat mass, might have protective effects against metabolic disorders. In fact, a recent study reported that larger thigh circumference is associated with a lower risk of DM, independent of BMI, age, and waist circumference, whereas a larger waist circumference is associated with a higher risk of DM [11].
Although adipose tissue deposited in different body locations may differentially impact glucose tolerance, few studies have examined the association between regional body fat distribution and the prevalence of DM. In addition, while some studies have already suggested that both higher leg fat mass and higher leg muscle mass have beneficial effects on metabolic health, there are no studies regarding which parameter—leg fat mass or muscle mass—may be more important for diabetes in adult populations. The aim of our study was to determine whether the association between fat mass and the prevalence of DM is influenced by site-specific adipose tissue accumulation in relatively healthy Korean adult populations. We also evaluated the relative contributions of leg fat mass and leg muscle mass to DM prevalence according to sex.
METHODS
Study design and population
We recruited participants from the 2008 to 2010 Korea National Health and Nutrition Examination Surveys (KNHANES). The KNHANES has been performed periodically since 1998 by the Division of Chronic Disease Surveillance of the Korean Centers for Disease Control and Prevention in order to assess the health and nutritional status of the civilian, non-institutionalized population of Korea. It is a cross-sectional and nationally representative survey, composed of a health interview survey, a nutrition survey, and a health examination survey. Data were collected by household interviews and by direct, standardized physical examinations conducted in mobile examination centers. Daily total energy intake and medical history were evaluated using a 24-hour recall method. Regular exercise was indicated as “yes” if the subject exercised for more than 20 minutes at a time and more than three times per week. Women were also asked whether their menstruation had stopped and whether they had been treated with hormone replacement therapy. Postmenopausal status was defined as the self-reported cessation of menstruation for more than 1 year only and we excluded women who had undergone a hysterectomy (n=941). Considering that a rapid decline of sex hormones occurs at around the age of 50 in most adults, we included participants aged 50 years or older. Subjects with malignancy (n=88), thyroid disease (n=193), chronic liver disease (n=12), and chronic renal disease (n=21) or subjects taking medications, such as corticosteroids and statins (n=436) known to alter glucose level were excluded from the analysis. Finally, a total of 6,675 participants (3,027 men and 3,548 postmenopausal women) aged 50 years or older were recruited. Because the KNHANES survey data are publicly available, ethical approval was not required for this study. The data used from the KNHANES database were fully anonymized.
Data collection and measurements
Body weight and height were obtained using standard protocols. Waist circumference was measured at the narrowest point between the lower borders of the rib cage and the uppermost borders of the iliac crest at the end of normal expiration. Well-trained observers manually measured blood pressure with a mercury sphygmomanometer (Baumanometer; WA Baum Co., Copiague, NY, USA). Body composition, including truncal/peripheral fat mass and appendicular skeletal muscle mass (ASM), were measured by dual-energy X-ray absorptiometry (DXA; QDR 4500A; Hologic Inc., Waltham, MA, USA). Collected blood samples were immediately refrigerated, transported to the Central Testing Institute in Seoul, Korea, and analyzed within 24 hours. Fasting plasma glucose, total cholesterol, triglycerides (TG), and high density lipoprotein cholesterol (HDL-C) levels were measured with a Hitachi 700–110 chemistry analyzer (Hitachi, Tokyo, Japan). Glycosylated hemoglobin (HbA1c) levels were analyzed by high performance liquid chromatography using HLC-723G7 (Tosoh, Tokyo, Japan) in subjects with DM. Serum 25-hydroxyvitamin D3 level was measured by radioimmunoassay (DiaSorin Inc., Stillwater, MN, USA) using a γ-counter (1470 Wizard; PerkinElmer, Turku, Finland). The homeostasis model assessment of β-cell function (HOMA-β) was calculated using the following formula: [fasting plasma insulin (µIU/mL)×20]/[fasting glucose (mmol/L)–3.5] [12].
Definition of DM
We defined DM as the presence of 1 or more of the following components: (1) fasting plasma glucose 126 mg/dL (7.0 mmol/L) or higher; (2) a medical diagnosis of DM by a trained medical professional; and (3) treatment with oral hypoglycemic agents or insulin injections.
Statistical analyses
Statistical analyses were conducted using PASW Statistics version 20 (IBM Co., Armonk, NY, USA). A comparison between the groups was performed using the t-test for continuous variables. For categorical variables, a chi-square test was used to compare frequencies among the groups. To assess the association between leg fat mass and parameters, we performed a partial correlation analysis after adjustment for age. A multiple logistic regression analysis was used to examine the association between leg fat mass and DM by evaluating the odds ratio (OR) after adjusting for confounding factors. Last, to investigate the relative contributions of leg fat mass and leg muscle mass to DM in different combinations, we additionally subdivided the participants into four groups according to the median values of leg fat mass and leg muscle mass. These groups were high fat-low muscle (HF-LM), high fat-high muscle (HF-HM), low fat-low muscle (LF-LM), and low fat-high muscle (LF-HM).
RESULTS
Baseline characteristics of participants
Table 1 summarizes the demographic and clinical characteristics of participants according to the presence of diabetes. The mean age of the participants was 63.76±8.96 years for men and 64.67±9.17 years for women (50 to 93 years of age). The overall prevalence of DM was 16.95% in men and 13.22% in postmenopausal women. As expected, obesity indices such as body weight, BMI, waist circumference, total body fat (%), and truncal/arm fat mass were higher in the DM group compared with those of the non-DM group. However, leg fat mass was lower in postmenopausal women with diabetes but this trend was not shown in men. Skeletal muscle mass index (ASM/weight) was lower in the DM group in both sexes. People with diabetes were more likely to have higher total cholesterol and TG and lower HDL-C levels in both sexes.
Table 1. Characteristics of the study population according to the presence of DM.
| Characteristic | Men | Women | ||||
|---|---|---|---|---|---|---|
| Non-DM (n=2,514) | DM (n=513) | P value | Non-DM (n=3,079) | DM (n=469) | P value | |
| Age, yr | 63.24±8.85 | 64.29±8.38 | 0.011 | 63.68±8.88 | 67.04±7.97 | <0.001 |
| Weight, kg | 65.08±9.60 | 67.84±9.66 | <0.001 | 56.06±8.56 | 58.91±9.07 | <0.001 |
| BMI, kg/m2 | 23.44±2.91 | 24.43±2.90 | <0.001 | 23.92±3.16 | 25.24±3.36 | <0.001 |
| Waist circumference, cm | 84.38±8.50 | 88.32±8.67 | <0.001 | 81.49±9.15 | 87.04±9.23 | <0.001 |
| Current smoking | 847 (33.8) | 164 (32.0) | 0.424 | 140 (4.6) | 16 (23.4) | 0.272 |
| Regular exercise | 471 (18.8) | 83 (16.2) | 0.169 | 392 (12.8) | 43 (9.2) | 0.031 |
| SBP, mm Hg | 124.13±17.16 | 125.67±16.33 | 0.061 | 125.53±17.87 | 131.44±18.24 | <0.001 |
| DBP, mm Hg | 77.03±10.45 | 75.35±10.26 | <0.001 | 76.43±10.05 | 75.70±10.15 | 0.144 |
| Fasting glucose, mmol/L | 5.4±0.6 | 8.1±2.6 | <0.001 | 5.84±1.54 | 5.84±1.28 | <0.001 |
| HOMA-β | 104.24±52.73 | 61.42±55.92 | <0.001 | 117.37±53.01 | 75.72±66.83 | <0.001 |
| Total cholesterol, mmol/L | 4.85±0.92 | 4.69±0.99 | 0.025 | 5.28±0.91 | 5.17±1.01 | 0.025 |
| LDL-C, mmol/L | 2.80±0.89 | 2.57±0.96 | <0.001 | 3.22±0.83 | 3.05±0.91 | <0.001 |
| HDL-C, mmol/L | 1.18±0.29 | 1.08±0.26 | <0.001 | 1.25±0.28 | 1.16±0.27 | <0.001 |
| Triglyceride, mmol/L | 3.68±2.23 | 4.25±2.40 | <0.001 | 3.46±1.95 | 4.17±2.26 | <0.001 |
| Total body fat, % | 21.98±5.23 | 23.44±4.91 | <0.001 | 33.85±5.59 | 34.85±5.05 | <0.001 |
| Truncal fat mass, kg | 8.06±3.12 | 9.29±2.99 | <0.001 | 10.15±3.28 | 11.73±3.35 | <0.001 |
| Arm fat mass, kg | 1.50±0.52 | 1.65±0.51 | <0.001 | 2.32±0.73 | 2.55±0.78 | <0.001 |
| Leg fat mass, kg | 3.89±1.31 | 3.98±1.29 | 0.141 | 5.77±1.68 | 5.44±1.70 | <0.001 |
| ASM/weight, % | 31.77±2.71 | 30.49±2.49 | 0.036 | 25.02±2.65 | 24.17±2.46 | <0.001 |
| Leg muscle mass, kg | 15.26±2.23 | 15.33±2.38 | 0.510 | 10.62±1.56 | 10.76±1.62 | 0.090 |
| Glucose lowering drug use | ||||||
| No medication | - | 124 (24.2) | - | - | 52 (10.1) | - |
| Insulin | - | 34 (6.6) | - | - | 40 (8.5) | - |
| Oral anti-hypoglycemic agent | - | 355 (69.2) | - | - | 377 (80.4) | - |
| Current smoking | 847 (338) | 164 (32.0) | 0.424 | 140 (4.6) | 16 (3.4) | 0.273 |
| Regular exercise | 471 (18.8) | 83 (16.2) | 0.169 | 393 (12.8) | 43 9 (9.25) | 0.030 |
| Total energy intake, kcal | 2,022.1±792.9 | 2,112.1±772.1 | 0.023 | 1,480.5±698.2 | 1,563.1±593.5 | 0.008 |
| Hormone replacement (women) | - | - | - | 478 (16.3) | 53 (12%) | 0.019 |
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-β, homeostasis model assessment β-cell; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; ASM, appendicular skeletal mass.
Prevalence of DM according to body fat distribution
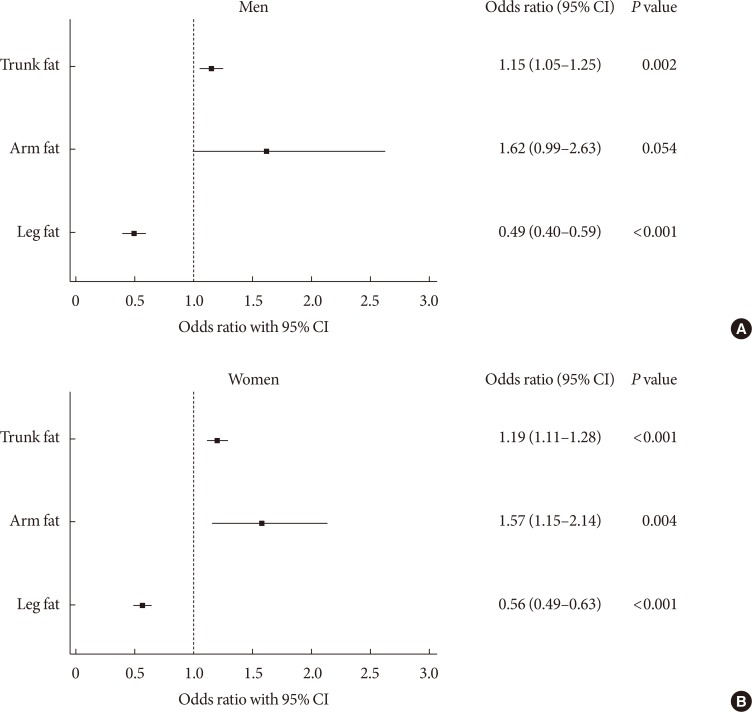
To investigate the independent contribution of trunk and extremities adiposity to DM, we calculated the adjusted OR of each kilogram increase in trunk and upper/lower extremities fat mass for DM using a multiple logistic regression analysis (Fig. 1). A 1-kg increase in trunk fat mass was associated with a 15% increase in the presence of DM in men and a 19% increase in women after adjustment for BMI, ASM, current smoking status, regular exercise, total cholesterol, TG, systolic blood pressure, daily total energy intake, and hormone replacement therapy status (women). Similarly, a 1-kg increase in arm fat mass also tended to be associated with an increased risk of DM in both sexes. Conversely, each kilogram increase in leg fat mass was significantly associated with a 51% reduction of DM in men and a 44% reduction in women after adjustment for confounding factors.
Fig. 1. Adjusted odds ratios with 95% confidence interval (CI) for diabetes mellitus for each kilogram increase in trunk fat, arm fat, and leg fat in (A) men and (B) women.
Correlation between leg fat mass and metabolic parameters
In a partial correlation analysis adjusted for age, leg fat mass was positively associated with BMI, trunk fat mass, and arm fat mass in both sexes (Table 2). There was a significant positive association between leg fat mass and ASM in both sexes (men: Rp=0.33, P<0.001; women: Rp=0.35, P<0.001). Leg fat mass was inversely associated with HbA1c in both sexes and fasting glucose in women. There was a significant positive relationship between leg fat mass and HOMA-β in both sexes. With respect to lipid profile, leg fat mass was positively associated with total cholesterol and TG, and negatively associated with HDL-C only in men.
Table 2. Partial correlations between leg fat mass and parameters.
| Variable | Leg fat mass, kg | |
|---|---|---|
| Men | Women | |
| Body composition | ||
| BMI, kg/m2 | 0.66a | 0.72a |
| Truncal fat mass, kg | 0.83a | 0.68a |
| Arm fat mass, kg | 0.85a | 0.78a |
| Appendicular skeletal muscle, kg | 0.33a | 0.35a |
| Leg muscle mass, kg | 0.36a | 0.37a |
| Metabolic parameter | ||
| HbA1c, % | −0.10b | −0.14c |
| Fasting glucose, mg/dL | −0.07 | −0.09b |
| Total cholesterol, mg/dL | 0.09b | 0.01 |
| HDL-C, mg/dL | −0.15c | 0.01 |
| Triglyceride, mg/dL | 0.11b | −0.01 |
| HOMA-β | 0.22a | 0.13a |
Values are partial correlation coefficients between variables and leg fat mass adjusted for age.
BMI, body mass index; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; HOMA-β, homeostasis model assessment β-cell.
aP<0.001, bP<0.05, cP<0.01.
Prevalence of DM according to leg fat mass and leg muscle mass
When the participants were classified into four groups according to sex-specific leg fat mass quartiles, the OR for the presence of DM significantly decreased gradually as leg fat mass increased in both sexes after adjustment for potential confounding factors (Table 3). To define the relative importance of leg fat mass and leg muscle mass on DM, we categorized subjects into four groups according to the sex-specific median value of leg fat mass and leg muscle mass: HF-LM, HF-HM, LF-LM, and LF-HM. Compared with the LF-LM group which is at the highest risk of metabolic disorder, higher leg fat mass groups (HF-HM and HF-LM) were associated with a decreased risk of DM in both sexes (Table 4). However, the lower leg fat mass groups, even though they have a large leg muscle mass, were associated with an increased risk of DM especially in women.
Table 3. Adjusted odds ratios with 95% confidence interval for diabetes mellitus according to sex-specific leg fat mass quartiles.
| Sex | Leg fat mass | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend | |
| Mena | Reference | 0.45 (0.34–0.61) | 0.36 (0.26–0.49) | 0.16 (0.11–0.25) | <0.001 |
| Womenb | Reference | 0.44 (0.32–0.59) | 0.35 (0.25–0.49) | 0.16 (0.11–0.25) | <0.001 |
Quartile 1: ≤2.97 kg in men and ≤4.54 kg in women; Quartile 2: 2.98 to 3.81 kg in men and 4.55 to 5.57 kg in women; Quartile 3: 3.82 to 4.66 kg in men and 5.58 to 6.71 kg in women; Quartile 4: ≥4.67 kg in men and ≥6.72 kg in women.
aData adjusted for age, body mass index, leg muscle mass, current smoking status, regular exercise, total cholesterol, triglyceride, systolic blood pressure, and daily total energy intake, bData adjusted for age, body mass index, leg muscle mass, current smoking status, regular exercise, total cholesterol, triglyceride, systolic blood pressure, daily total energy intake, and hormone replacement therapy status.
Table 4. Adjusted odds ratios with 95% confidence interval for diabetes mellitus according to body composition group.
| Sex | Body composition group | ||||
|---|---|---|---|---|---|
| LF-LM | LF-HM | HF-LM | HF-HM | P for trend | |
| Mena | Reference | 1.31 (0.88–1.96) | 0.69 (0.49–0.96) | 0.60 (0.39–0.90) | <0.001 |
| Womenb | Reference | 1.38 (1.02–1.87) | 0.47 (0.32–0.69) | 0.57 (0.40–0.81) | <0.001 |
We subdivided men and women in each age group into four groups according to leg fat mass and leg muscle mass, which were halved into low or high values by the median value.
LF-LM, low fat-low muscle group; LF-HM, low fat-high muscle group; HF-LM, high fat-low muscle group; HF-HM, high fat-high muscle group.
aData adjusted for age, body mass index, current smoking status, regular exercise, total cholesterol, triglyceride, systolic blood pressure, and daily total energy intake, bData adjusted for age, body mass index, current smoking status, regular exercise, total cholesterol, triglyceride, systolic blood pressure, daily total energy intake, and hormone replacement therapy status.
DISCUSSION
Our study found that higher leg fat mass was independently associated with a lower risk of DM in adult populations. We also demonstrated that adipose tissue, deposited in different body locations, may differentially impact the risk of DM. In addition, we observed that subjects with higher leg fat mass have a lower risk of DM even though they have a low leg muscle mass; but subjects with lower leg fat mass have a higher risk of DM even though they have large leg muscle mass. To our knowledge, this is the first population-based study of the association between body compositions and DM considering body fat distribution.
Adiposity is a well-known risk factor for DM and cardiovascular disease [13]. Abdominal adiposity and specifically visceral fat are considered to be more closely associated with metabolic abnormalities and cardiovascular disease [14,15,16]. However, there is growing evidence that lower-extremity adiposity might be protective against adverse metabolic disease risk [17,18]. Therefore, recent studies have suggested that upper body versus lower body obesity, represented by the waist-hip ratio, is more closely associated with metabolic disorders [19]. Finally, these facts suggest a discrepancy between regional fat depots and their relation to the risk of disease, such that lower-body adiposity appears more protective and upper body or trunk adiposity appears more harmful for metabolic disorder. From this perspective, we speculated that the increased leg fat mass, reflecting increased subcutaneous fat, may have a protective role in diabetes after controlling for the highly detrimental effects of abdominal visceral adiposity. Because there is a paucity of systemic data for the association between leg fat mass and the prevalence of DM, we investigated this association in community-dwelling adult Korean populations.
In our study, in contrast to trunk fat mass and arm fat mass, there was a negative association between leg fat mass and DM prevalence. This result is consistent with a recent study showing that leg fat mass was inversely associated with glucose levels and homeostatic model assessment of insulin resistance from an oral glucose tolerance test [7]. The possible protective role of leg fat mass and the differential role of adiposity according to location in DM can be explained by the following mechanisms. The first possible mechanism is that adipocytes in lower extremities are less lipolytic [20] and counter free fatty acid release from upper-body fat regions [21]. Consequently, the decrease in free fatty acids, as they are taken up in the adipose tissue of the lower extremities, may protect pancreatic β-cells from lipotoxicity. In contrast to lower body adiposity, upper body adiposity is more sensitive to lipolysis and secretes a higher amount of inflammatory cytokines [22]. Second, differential secretion of adipokines by different fat depots may also be involved. Adiponectin, predominantly produced by adipocytes, has been considered to have insulin-sensitizing, anti-inflammatory, and anti-atherosclerotic properties [23,24]. It is known to enhance glucose uptake and lipid oxidation through activation of adenosine monophosphate-activated protein kinases [25]. Recent studies reported that lower body fat was positively associated with adiponectin levels, while trunk fat was negatively associated with adiponectin levels [26]. Furthermore, Kovacova et al. [27] reported that visceral adipose tissue has lower gene expression of total adiponectin than subcutaneous depots. The above mechanisms can explain the potential beneficial impact of leg fat on pancreatic β-cell function and glucose tolerance. Our study demonstrated that leg fat mass was positively associated with HOMA-β, which is a well-known index of pancreatic β-cell function. This result suggests that leg fat may positively influence glucose tolerance by protecting pancreatic β-cells from lipotoxicity and inflammatory cytokines.
Recently, the interaction between appendicular muscle mass and glucose tolerance has been a subject of interest. Because skeletal muscle is responsible for insulin-mediated glucose disposal, low muscle mass can have a negative impact on glucose tolerance. Therefore, previous studies have shown that larger hip or thigh circumference is associated with decreased diabetes risk [11,28,29]. However, these studies did not completely analyze which component of lower extremities has a more dominant effect on DM. In our study, we observed that leg fat mass may contribute more to DM than leg muscle mass. In addition, we found that higher leg fat mass was associated with higher ASM. From these findings, we speculate that anti-inflammatory properties of leg fat may protect against age-related loss of skeletal muscle mass and consequently may favorably influence glucose metabolism. This fact may partly explain the dominant effect of leg fat over leg muscle on DM.
In addition, although in both genders there was a significant inverse relationship between leg fat mass and DM after adjustment for confounding factors, including BMI, we found that the association between leg fat mass and DM was more prominent in postmenopausal women than in men based on an independent t-test and a partial correlation analysis. In addition, we also found that there was a significant positive relationship between leg fat mass and TG in men but there was no association between leg fat mass and TG in women. Similarly, Van Pelt et al. [30] reported favorable correlation between lower-body adiposity with markers of insulin resistance and dyslipidemia in postmenopausal women after adjusting for upper-body adiposity. These gender differences in the association of lower-body adiposity and DM can be explained by the fact that women have an increased propensity to store fat in lower extremity adipose tissue and away from abdominal visceral tissue depots. In line with this fact, our data also showed that leg fat mass was more strongly associated with upper body adiposity in men than in women (Table 2). This result suggests that the benefit of leg fat on glucose tolerance and lipid metabolism is countered by the greater upper body adiposity in men.
The major strength of our study is that we analyzed representative data collected from a nationwide survey in Korea, including large numbers of participants of both sexes. In addition, we compared significant associations between body compositions and DM according to gender for the first time. However, there were some limitations in this study. First, as the present study was a cross-sectional study, a causal relationship between leg fat mass and diabetes could not be definitively established. Second, because DXA is unable to distinguish between subcutaneous and intramuscular fat in the lower and upper extremities, we could not conclude whether the beneficial effects of leg fat on glucose tolerance were due to subcutaneous or intramuscular fat. Third, although the association between leg fat and glucose tolerance was presumed to result from adipokines and inflammatory factors, we could not further prove it due to lack of data. Finally, as we did not examine the associations between other ethnic groups with different body compositions, we were not able to extend our results to other ethnic groups.
In conclusion, our study demonstrates that, in contrast to trunk and arm adiposity, there is a favorable association of leg adiposity with DM in Korean adults aged 50 years or older. We also found that the contributory effects of lower extremity fat on DM are more dominant than those of the lower extremity muscle. Our findings support the notion that subcutaneous fat and glucose metabolism are intimately interlinked. Further prospective studies are needed to confirm the causal interactions between leg fat mass and the development of diabetes.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94:322–332. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 5.Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu G, Bouchard C, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Katzmarzyk PT. Trunk versus extremity adiposity and cardiometabolic risk factors in white and African American adults. Diabetes Care. 2011;34:1415–1418. doi: 10.2337/dc10-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC Hoorn study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 11.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 16.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 17.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 19.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness: a critical review. Int J Obes Relat Metab Disord. 1998;22:719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 20.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol. 2007;292:H1655–H1663. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 26.Snijder MB, Flyvbjerg A, Stehouwer CD, Frystyk J, Henry RM, Seidell JC, Heine RJ, Dekker JM. Relationship of adiposity with arterial stiffness as mediated by adiponectin in older men and women: the Hoorn Study. Eur J Endocrinol. 2009;160:387–395. doi: 10.1530/EJE-08-0817. [DOI] [PubMed] [Google Scholar]
- 27.Kovacova Z, Tencerova M, Roussel B, Wedellova Z, Rossmeislova L, Langin D, Polak J, Stich V. The impact of obesity on secretion of adiponectin multimeric isoforms differs in visceral and subcutaneous adipose tissue. Int J Obes (Lond) 2012;36:1360–1365. doi: 10.1038/ijo.2011.223. [DOI] [PubMed] [Google Scholar]
- 28.Jung KJ, Kimm H, Yun JE, Jee SH. Thigh circumference and diabetes: obesity as a potential effect modifier. J Epidemiol. 2013;23:329–336. doi: 10.2188/jea.JE20120174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway B, Xiang YB, Villegas R, Zhang X, Li H, Wu X, Yang G, Gao YT, Zhang W, Shu XO. Hip circumference and the risk of type 2 diabetes in middle-aged and elderly men and women: the Shanghai women and Shanghai men's health studies. Ann Epidemiol. 2011;21:358–366. doi: 10.1016/j.annepidem.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab. 2002;282:E1023–E1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]