Abstract
Background
An association between serum calcium level and risk of metabolic syndrome (MetS) has been suggested in cross-sectional studies. This study aimed to evaluate the association between baseline serum calcium level and risk of incident MetS in a longitudinal study.
Methods
We conducted a retrospective longitudinal study of 12,706 participants without MetS who participated in a health screening program, had normal range serum calcium level at baseline (mean age, 51 years), and were followed up for 4.3 years (18,925 person-years). The risk of developing MetS was analyzed according to the baseline serum calcium levels.
Results
A total of 3,448 incident cases (27.1%) of MetS developed during the follow-up period. The hazard ratio (HR) for incident MetS did not increase with increasing tertile of serum calcium level in an age- and sex-matched model (P for trend=0.915). The HRs (95% confidence interval [CI]) for incident MetS comparing the second and the third tertiles to the first tertile of baseline serum calcium level were 0.91 (95% CI, 0.84 to 0.99) and 0.85 (95% CI, 0.78 to 0.92) in a fully adjusted model, respectively (P for trend=0.001). A decreased risk of incident MetS in higher tertiles of serum calcium level was observed in subjects with central obesity and/or a metabolically unhealthy state at baseline.
Conclusion
There was no positive correlation between baseline serum calcium levels and incident risk of MetS in this longitudinal study. There was an association between higher serum calcium levels and decreased incident MetS in individuals with central obesity or two components of MetS at baseline.
Keywords: Calcium; Longitudinal studies; Metabolic syndrome; Obesity, abdominal
INTRODUCTION
Metabolic syndrome (MetS) is characterized by a cluster of risk factors such as central obesity, dyslipidemia, elevated blood pressure, insulin resistance, and glucose intolerance, leading MetS to be associated with a poor cardiovascular prognosis and increased mortality [1]. Although the prevalence of MetS varies according to age, sex, geographic location, and ethnicity [2,3,4], it has increased over the past decades [5,6] to become a global pandemic. Abdominal obesity is the most prevalent manifestation of MetS [7], and obese patients with MetS are at higher risk of cardiovascular disease than non-obese patients with MetS [8]. Estimates from the National Health and Nutrition Examination Survey from 1999 to 2010 demonstrated that the prevalence of abdominal obesity has increased [4].
Intracellular calcium plays an important role in the regulation of lipid metabolism [9] and insulin sensitivity [10]. Serum calcium homeostasis is regulated within a narrow range, and is under tight hormonal control. Several epidemiological studies have demonstrated that a high level of serum calcium is associated with increased risk for type 2 diabetes mellitus (T2DM) [11], overweight or obesity [12], elevated blood pressure, hypercholesterolemia [13], as well as MetS [14]. Serum calcium concentrations were also positively correlated with increasing numbers of conventional MetS components [15]. Finally, an increased serum calcium concentration can predict increased all-cause and cardiovascular disease mortality in men [16].
A longitudinal study that evaluated the effect of serum calcium on the development of T2DM among the non-diabetes mellitus general population (n=863) demonstrated that higher serum calcium levels are associated with an increased risk of developing T2DM [11]. On the other hand, Becerra-Tomas et al. [17] reported that an increased risk of T2DM was associated with change in serum calcium concentration, but not with baseline serum calcium levels among individuals at high cardiovascular risk (n=707). As shown in previous studies, the association between serum calcium and incident metabolic diseases, including T2DM and MetS, is affected by several factors, including study design, patient population, and baseline metabolic health status. To our knowledge, there have been no longitudinal studies to date investigating the association between normal range of serum calcium level and risk of incident MetS. Therefore, the aim of this study was to investigate the association between serum calcium within the normal range and risk of incident MetS in a longitudinal study.
METHODS
Subjects
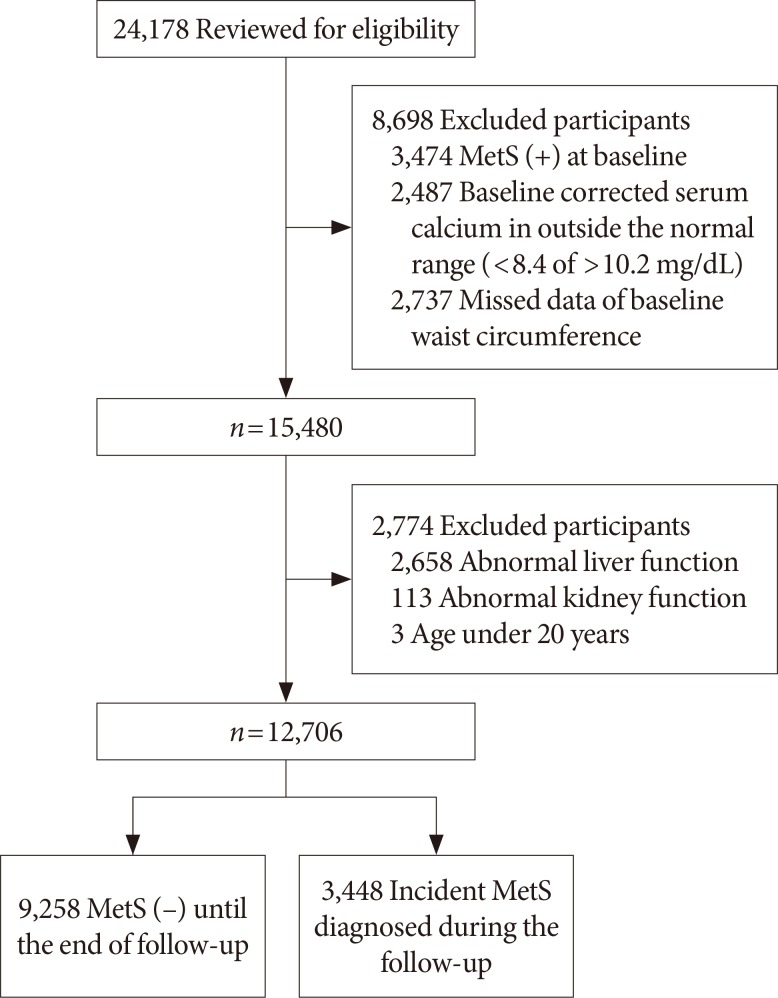
We conducted a retrospective longitudinal study in subjects who visited a health care center at the Samsung Medical Center for an annual or biannual comprehensive medical check-up. Of the 24,178 people who underwent at least four medical check-ups within 7 years (from January 2006 to December 2012), 12,706 individuals were included for analysis. Medical check-up records from the first visit served as baseline data. We excluded 11,472 people based on the following criteria: initial visit records showed that they had already met the criteria for MetS (n=3,474); baseline albumin-corrected serum calcium levels were outside the normal range (8.4 to 10.2 mg/dL, n=2,487); baseline waist circumference (WC) data were unavailable (n=2,737); total bilirubin or liver enzymes were elevated to greater than twice the upper limit of normal (n=2,658); estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was <60 mL/min/1.73 m2 (n=113); or they were under 20 years of age (n=3) (Fig. 1). The Institutional Review Board of Samsung Medical Center approved this study, and waived the informed consent requirement for this study, as the researcher accessed the database solely for analytic purposes, and did not access any personal information.
Fig. 1. Subject inclusion. MetS, metabolic syndrome.
Definition of MetS
MetS was defined according to the modified criteria proposed by the Adult Treatment Program III of the National Cholesterol Education Program (NCEP-ATP III) [18]. People with three or more of the following criteria were classified as having MetS: (1) WC ≥90 cm in men or ≥80 cm in women; (2) triglycerides (TG) ≥150 mg/dL; (3) high density lipoprotein cholesterol (HDL-C) <40 mg/dL in men or <50 mg/dL in women; (4) elevated blood pressure (systolic blood pressure [SBP] ≥130 mm Hg and/or diastolic blood pressure [DBP] ≥85 mm Hg) or being on anti-hypertensive medications; or (5) fasting serum glucose (FPG) ≥100 mg/dL or being treated for diabetes [19].
Clinical evaluation
During the clinical visits, we collected lifestyle behavior information and medical histories through a standardized self-reported questionnaire. We also assessed cigarette smoking and previously or newly diagnosed disease (hypercholesterolemia, diabetes, and hypertension).
Blood pressure was measured in the seated position after 5 minutes of rest, and was taken by trained nurses using a mercury sphygmomanometer in accordance with the Hypertension Detection and Follow-up Program protocol [20]. We measured patient height and body weight while wearing light clothing and no shoes. WC was measured at the narrowest point between the costal margin and the iliac crest.
Laboratory methods
We obtained blood samples by venipuncture after an overnight fast, and samples were analyzed at the same certified laboratory at the Samsung Medical Center. Serum calcium concentrations (normal range, 8.4 to 10.2 mg/dL) were determined by the o-cresolphthalein complexone method (intra-assay coefficient of variation <2%). Serum albumin levels (normal range, 3.8 to 5.3 g/dL) were measured by bromocresol green using a Roche modular DP analyzer (Roche Diagnostics, Basel, Switzerland). Albumin-corrected serum calcium (mg/dL) was calculated as [serum calcium+0.8×(4–albumin)]. Serum TG and HDL-C levels were determined by an enzymatic colorimetric method using the Roche modular DP analyzer. Serum glucose levels were determined by the hexokinase method using a GLU kit (Roche Diagnostics) on the Roche modular DP analyzer. Glycosylated hemoglobin (HbA1c) measurements were taken by HPLC using the G8 Elution buffer kits (Tosoh, Yokkaichi, Japan) on HLC-723G8 (Tosoh). The HbA1c results were standardized to the reference method suggested by the Diabetes Control and Complication Trial, and met the National Glycohemoglobin Standardization Program standards. The homeostasis model assessment 2 of insulin resistance (HOMA2-IR) calculation was based on model-derived estimates (rather than linear approximations) using the HOMA2 calculator version 2.2.3 (Diabetes Trials Unit, University of Oxford, Oxford, UK; http://www.dtu.ox.ac.uk/homacalculator/download.php) [21].
Statistical analysis
Baseline characteristics were presented as the mean±standard deviation for continuous variables and the number and percentage for categorical variables. One-way analysis of covariance was used for continuous variables, and chi-square tests were used for categorical variables in order to assess the baseline characteristics according to the albumin-corrected serum calcium tertile. Pearson correlation coefficients were used to examine the strength of the relationship between serum calcium and the MetS conditions, as well as the HOMA2-IR.
We used multivariate Cox proportional regression models to evaluate the relative risk of incident MetS according to the albumin-corrected serum calcium tertiles, and as a continuous variable per 1 mg/dL increase of albumin-corrected serum calcium at baseline. Collinearity tests for variables used in multivariate Cox proportional regression analysis were performed using linear modeling of the outcome variables with calculation of the variance inflation factor (VIF) of the independent predictors. A VIF <5 was considered optimal for stability. Two different Cox regression models were used to adjust for potential confounding factors. The first model (model 1) was adjusted for sex and age, and the second model (model 2) was adjusted for age, sex, smoking status (current, ex-smoker, non-smoker), WC, SBP, DBP, TG level, HDL-C, FPG, and treatment for medical conditions, including hypertension, diabetes, and hypercholesterolemia.
The analysis was further stratified by presence of central obesity and presence of two components of MetS at baseline. Tests of linearity were conducted by identifying the mean value of the albumin-corrected serum calcium in each tertile as a continuous variable. A separate subgroup analysis for incident MetS in the metabolic risk group, such as the presence of central obesity or presence of two components of MetS at baseline, was also performed. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and P≤0.05 (two-tailed) were considered statistically significant. Meanwhile, P≤0.025 (two-tailed) were considered statistically significant after correcting for multiple comparisons in the subgroup multivariate Cox regression analysis, according to central obesity and presence of two components of MetS.
RESULTS
Albumin-corrected serum calcium levels and metabolic conditions at baseline
Of all subjects (n=12,706), the mean age was 51 years and 6,873 (54.1%) were men. Baseline characteristics of the anthropometric and biochemical parameters according to the albumin-corrected serum calcium at baseline are shown in Table 1. With increasing serum calcium tertiles, there was a significant increase in age, SBP, DBP, FPG, HbA1c, and TG levels (all P<0.001). In our simple linear regression analyses, there were also positive correlations between baseline albumin-corrected serum calcium levels and age, SBP, DBP, HDL-C, FPG, and HbA1c. Meanwhile, HOMA2-IR was inversely correlated with albumin-corrected serum calcium levels (r=–0.026, P=0.021) (Table 2).
Table 1. Baseline characteristics according to albumin-corrected serum calcium tertile.
| Characteristic | Corrected serum calcium tertile, mg/dL | Total | P valuea | ||
|---|---|---|---|---|---|
| Tertile 1 (<8.70) |
Tertile 2 (8.70–8.94) |
Tertile 3 (>8.94) |
|||
| Number | 4,174 | 4,334 | 4,198 | 12,706 | |
| Incident case | 1,263 (28.0) | 1,095 (27.4) | 1,090 (26.0) | 3,448 (27.1) | 0.089 |
| Male sex | 2,319 (55.6) | 2,395 (55.3) | 2,159 (51.4) | 6,873 (54.1) | <0.001b |
| Smoker | 0.045b | ||||
| Never | 2,352 (56.3) | 2,431 (56.1) | 2,474 (58.9) | 7,257 (57.1) | |
| Current | 697 (16.7) | 734 (16.9) | 689 (16.4) | 2,120 (16.7) | |
| Past | 1,125 (27.0) | 1,169 (27.0) | 1,035 (24.7) | 3,329 (26.2) | |
| Age, yr | 50.5±8.1 | 51.0±8.3 | 51.3±8.2 | 51.0±8.2 | <0.001 |
| WC, cm | 81.1±8.7 | 81.4±8.7 | 80.9±8.6 | 81.1±8.7 | 0.029 |
| BMI, kg/m2 | 23.3±2.6 | 23.4±2.6 | 23.3±2.6 | 23.3±2.6 | 0.170 |
| SBP, mm Hg | 110±14 | 112±14 | 113±15 | 112±14 | <0.001 |
| DBP, mm Hg | 68±10 | 69±10 | 70±10 | 69±10 | <0.001 |
| FPG, mg/dL | 88±13 | 89±13 | 91±15 | 89±14 | <0.001 |
| HbA1c, % | 5.4±0.5 | 5.4±0.5 | 5.5±0.6 | 5.4±0.6 | <0.001 |
| Triglyceride, mg/dL | 111±58 | 115±61 | 117±68 | 114±62 | <0.001 |
| HDL-C, mg/dL | 59±14 | 59±13 | 60±14 | 59±14 | 0.001 |
| Corrected calcium, mg/dL | 8.56±0.08 | 8.82±0.07 | 9.17±0.18 | 8.85±0.28 | - |
| Diabetes | 170 (4.1) | 174 (4.0) | 201 (4.8) | 545 (4.3) | 0.148b |
| Hypertension | 404 (9.7) | 495 (11.4) | 512 (12.2) | 1,411 (11.1) | 0.001b |
| Hypercholesterolemia | 78 (1.9) | 119 (2.7) | 133 (3.2) | 330 (2.6) | 0.001b |
| Presence of two components of MetS | 938 (22.5) | 1,107 (25.5) | 1,123 (26.8) | 3,168 (24.9) | <0.001b |
| HOMA2-IR (n=7,945) | 1.16 (0.49) | 1.16 (0.48) | 1.13 (0.45) | 1.15 (0.47) | 0.066 |
Values are presented as number (%) or mean±standard deviation.
WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; MetS, metabolic syndrome; HOMA2-IR, homeostasis model assessment index 2 for insulin resistance.
aOne-way analysis of variance for continuous variables, bChi-square test.
Table 2. Simple correlation coefficient for corrected serum calcium and metabolic syndrome condition at baseline (n= 12,704).
| Variable | B | P value |
|---|---|---|
| Age, yr | 0.041 | <0.001 |
| BMI, kg/m2 | 0.282 | <0.001 |
| SBP, mm Hg | 0.081 | <0.001 |
| DBP, mm Hg | 0.091 | <0.001 |
| TG, mg/dL | 0.042 | <0.001 |
| HDL-C, mg/dL | 0.033 | <0.001 |
| FPG, mg/dL | 0.095 | <0.001 |
| HbA1c, % | 0.100 | <0.001 |
| HOMA2-IR (n=7,945) | –0.026 | 0.021 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL-C, high density lipoprotein; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HOMA2-IR, homeostasis model assessment index 2 for insulin resistance.
After a mean follow-up period of 4.3 years, a total of 3,448 incident cases (27.1%) of MetS developed. In crude and age- and sex-matched models, the hazard ratio (HR) for incident MetS comparing the second and third quartiles to the first quartile of change in serum calcium level did not increase significantly. However, the HR for incident MetS comparing the second and third quartiles to the first quartile of change in serum calcium level significantly decreased in the fully adjusted model (P for trend=0.001) (Table 3).
Table 3. Risk of developing MetS based on corrected serum calcium tertile at baseline.
| Variable | Corrected serum calcium tertile, mg/dL | P for trenda | Continuous variables HR (95% CI) | ||
|---|---|---|---|---|---|
| Tertile 1 (<8.70) |
Tertile 2 (8.70–8.94) |
Tertile 3 (>8.94) |
|||
| Number | 4,174 | 4,334 | 4,198 | 0.482b | |
| Incident cases, n (%) | 1,164 (27.9) | 1,194 (27.5) | 1,090 (26.0) | ||
| Person-years of follow-up | 18,925 | 18,974 | 17,320 | ||
| Incident cases of MetS/1,000 person-year | 61.5 | 62.9 | 62.9 | ||
| Adjusted HR (95% CI)a | |||||
| Crude model | 1 (reference) | 1.02 (0.94–1.11) | 1.02 (0.94–1.11) | 0.849 | 1.05 (0.93–1.19) |
| Model 1 | 1 (reference) | 1.01 (0.93–1.10) | 1.02 (0.94–1.11) | 0.915 | 1.06 (0.94–1.20) |
| Model 2 | 1 (reference) | 0.91 (0.84–0.99) | 0.85 (0.78–0.92) | 0.001 | 0.80 (0.71–0.91) |
Model 1: adjusted for age and sex; Model 2: adjusted for model 1+systolic blood pressure, diastolic blood pressure, triglyceride, high density lipoprotein, fasting plasma glucose, smoking status, and conditions under medical treatment, including hypertension, diabetes, and hypercholesterolemia.
MetS, metabolic syndrome; HR, hazard ratio; CI, confidence interval.
aCox proportional hazard model, bChi-square test for linear by linear association.
Albumin-corrected serum calcium and the risk of incident MetS stratified by presence of central obesity at baseline
When the analysis was stratified by presence of central obesity, incident cases of MetS developed more frequently in those with central obesity (48.6% vs. 19.9%, P<0.001). With regard to baseline central obesity, the association between the albumin-corrected serum calcium and the risk for incident MetS showed different trends between the central obesity group and the non-central obesity group (Table 4). In the subjects with central obesity (n=3,223), the HR of incident MetS comparing the second and third quartiles to the first quartile of change in serum calcium level significantly decreased in the crude (P for trend=0.030), age- and sex-matched (P for trend=0.090), and fully adjusted models (P for trend=0.009). However, the decrease in HR was not significant in subjects without central obesity (Table 4).
Table 4. Risk of developing metabolic syndrome by baseline albumin-corrected serum calcium tertile, stratified by the presence of central obesity.
| Variable | Corrected calcium tertile, mg/dL | P for trenda | Continuous variables HR (95% CI) | ||
|---|---|---|---|---|---|
| Tertile 1 (<8.70) |
Tertile 2 (8.70–8.94) |
Tertile 3 (>8.94) |
|||
| Subjects with central obesity (n=3,223) | 1,032 | 1,121 | 1,070 | ||
| No. of subjects incident cases, n (%) | 547 (53.0) | 545 (48.6) | 473 (44.2) | <0.001b | |
| Adjusted HR (95% CI)a | |||||
| Crude model | 1 (reference) | 0.92 (0.81–1.03) | 0.85 (0.75–0.96) | 0.030 | 0.84 (0.70–1.00) |
| Model 1 | 1 (reference) | 0.92 (0.82–1.04) | 0.87 (0.77–0.99) | 0.090 | 0.88 (0.73–1.05) |
| Model 2 | 1 (reference) | 0.87 (0.77–0.98) | 0.83 (0.74–0.94) | 0.009 | 0.82 (0.68–0.98) |
| Subjects without central obesity (n=9,483) | 3,142 | 3,213 | 3,128 | ||
| No. of subjects incident cases, n (%) | 617 (19.6) | 649 (20.2) | 617 (19.7) | 0.833b | |
| Adjusted HR (95% CI)a | |||||
| Crude model | 1 (reference) | 1.08 (0.97–1.20) | 1.13 (1.01–1.27) | 0.087 | 1.19 (1.01–1.40) |
| Model 1 | 1 (reference) | 1.07 (0.95–1.19) | 1.13 (1.01–1.26) | 0.117 | 1.20 (1.02–1.41) |
| Model 2 | 1 (reference) | 0.99 (0.88–1.10) | 0.91 (0.81–1.02) | 0.183 | 0.83 (0.70–0.98) |
Model 1: adjusted for age and sex; Model 2: adjusted for model 1+systolic blood pressure, diastolic blood pressure, triglyceride, high density lipoprotein, fasting plasma glucose, smoking status, and conditions under medical treatment, including hypertension, diabetes, and hypercholesterolemia.
HR, hazard ratio; CI, confidence interval;
aCox proportional hazard model (P<0.025 considered statistically significant after correction for multiple comparisons), bChi-square test for linear by linear association.
Albumin-corrected serum calcium and the risk of incident MetS stratified by presence of two components of MetS
When the analysis was stratified by the presence of two components of MetS based on the NCEP-ATP III criteria [19], incident cases of MetS developed more frequently in those with two components of MetS at baseline than those with fewer than two components (57.7% vs. 17.0%, P<0.001). In the subjects having two components of MetS at baseline (n=3,168), the HR for incident MetS comparing the second and the third quartiles to the first quartile of change in serum calcium levels significantly decreased in the crude (P for trend=0.016), age- and sex-matched (P for trend=0.024), and fully adjusted model (P for trend=0.002). However, the decrease in HR was not significant in subjects with fewer than two components of MetS at baseline (Table 5).
Table 5. Risk of developing MetS by baseline albumin-corrected serum calcium tertile, stratified by the presence of two components of MetS.
| Variable | Corrected calcium tertile, mg/dL | P for trenda | Continuous variables HR (95% CI) | ||
|---|---|---|---|---|---|
| Tertile 1 (<8.70) |
Tertile 2 (8.70–8.94) |
Tertile 3 (>8.94) |
|||
| Subjects with two components of MetS (n=3,168) | 1,027 | 1,101 | 1,040 | ||
| No. of subjects incident cases, n (%) | 643 (62.6) | 630 (57.2) | 554 (53.3) | <0.001b | |
| Adjusted HR (95% CI)a | |||||
| Crude model | 1 (reference) | 0.89 (0.80–1.00) | 0.85 (0.76–0.95) | 0.016 | 0.76 (0.64–0.89) |
| Model 1 | 1 (reference) | 0.89 (0.80–1.00) | 0.86 (0.77–0.96) | 0.024 | 0.77 (0.65–0.90) |
| Model 2 | 1 (reference) | 0.87 (0.78–0.97) | 0.82 (0.73–0.92) | 0.002 | 0.71 (0.60–0.84) |
| Subjects with fewer than two components of MetS (n=9,538) | 3,236 | 3,227 | 3,075 | ||
| No. of subjects incident cases, n (%) | 566 (17.5) | 565 (17.5) | 490 (15.9) | 0.103b | |
| Adjusted HR (95% CI)a | |||||
| Crude model | 1 (reference) | 1.05 (0.93–1.18) | 1.03 (0.91–1.16) | 0.724 | 1.08 (0.90–1.29) |
| Model 1 | 1 (reference) | 1.05 (0.93–1.17) | 1.03 (0.91–1.17) | 0.751 | 1.10 (0.92–1.32) |
| Model 2 | 1 (reference) | 0.94 (0.83–1.05) | 0.89 (0.79–1.01) | 0.173 | 0.89 (0.74–1.08) |
Model 1: adjusted for age, sex; Model 2: adjusted for model 1+waist circumference, systolic blood pressure, diastolic blood pressure, triglyceride, high density lipoprotein, fasting plasma glucose, smoking status, and conditions under medical treatment, including hypertension, diabetes, and hypercholesterolemia.
MetS, metabolic syndrome; HR, hazard ratio; CI, confidence interval.
aCox proportional hazard model (P<0.025 considered statistically significant after correction for multiple comparisons), bChi-square test for linear by linear association.
DISCUSSION
In this retrospective longitudinal cohort study, the positive correlation between serum calcium and MetS observed in previous cross-sectional studies was not found. Unexpectedly, risk of MetS was inversely associated with serum calcium level in individuals with central obesity or two components of MetS.
The association between serum calcium level and risk factors for MetS has been well documented in the cross-sectional study design [14,15,22,23,24]. Previous studies demonstrated that serum calcium concentrations were associated with MetS components [14] and with increased MetS risk scores [23], as well as non-conventional cardio-metabolic risk factors (markers of oxidative stress, including uric acid, homocysteine, and γ-glutamyltransferase) [15]. We confirmed positive correlations between serum calcium and MetS components at baseline, including blood pressure, BMI, TGs, HDL-C, FPG, and HbA1c. Impaired insulin secretion [22] and insulin sensitivity [25], or increased insulin resistance [26], may explain the association between increased baseline serum calcium levels and the risk of T2DM or MetS. Furthermore, increased serum calcium levels have been shown to cause calcium influx into arterial smooth muscle, which induces muscle contraction, resulting in blood pressure elevation and increased peripheral vascular resistance [27].
To the best of our knowledge, the present study is the first to evaluate the association between baseline serum calcium and the development of MetS in a longitudinal study. In the present study, the positive correlation between serum calcium and MetS observed in previous cross-sectional studies [14,15,22,23,24] was not found. Moreover, the fully adjusted model showed an unexpectedly decreased risk of incident MetS during the follow-up period, in contrast with the results in the crude and age- and sex-matched models. For this reason, we stratified the analysis by presence of central obesity and two components of MetS at baseline. The results of the stratified analysis consistently showed decreased risk of MetS associated with increasing serum calcium level in subjects with central obesity and/or two components of MetS at baseline. Although it is difficult to explain the mechanisms, these findings suggest that it is important to accurately adjust for metabolic risk factors and to optimize subject selection to those bearing similar metabolic risk factors in assessing the modulating effect of calcium on the risk of incident MetS, because serum calcium level is closely associated with each component of MetS. Alternatively, an increase in serum calcium level might possibly be a defense mechanism for increased insulin resistance. If a cause-and-effect relationship of the findings in this study is confirmed, further studies are warranted to understand the mechanisms involved between alterations in serum calcium levels and insulin resistance.
There are several limitations to our study. First, we did not collect serum levels of parathyroid hormone (PTH) or 25-hydroxy vitamin D, and did not gather information on dietary and supplemental calcium or vitamin D intake, although serum PTH is associated with several factors involved in MetS [28,29]. The strength of our study lies in its longitudinal design with a large sample size, which allowed us to evaluate the association between basal serum calcium and the incidence of MetS after adjusting for multiple metabolic risk factors and/or stratification.
In contrast to the results of the cross-sectional analysis in the previous studies, there was no positive correlation between serum calcium levels and incident risk of MetS in this retrospective longitudinal cohort study. The unexpected association between higher serum calcium levels and decreased incident MetS, which was shown in individuals with central obesity or two components of MetS, is hypothesis-generating. Further prospective studies designed to specifically address the hypothesis could explain the discrepancy.
ACKNOWLEDGMENTS
We acknowledge the efforts of the health screening group at the Samsung Medical Center in Seoul, Korea. A Samsung Biomedical Research Institute Grant supported this work. J.H.K. is the guarantor of this work and, as such, had full access to the data in the study, and takes responsibility for the integrity of the data and the accuracy of data analyses.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 2.Vernay M, Salanave B, de Peretti C, Druet C, Malon A, Deschamps V, Hercberg S, Castetbon K. Metabolic syndrome and socioeconomic status in France: The French Nutrition and Health Survey (ENNS, 2006-2007) Int J Public Health. 2013;58:855–864. doi: 10.1007/s00038-013-0501-2. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 9.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–1138. [PubMed] [Google Scholar]
- 10.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+) Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. Calcium and phosphate concentrations and future development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetologia. 2014;57:1366–1374. doi: 10.1007/s00125-014-3241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren XH, Yao YS, He LP, Jin YL, Chang WW, Li J, Chen Y, Song XL, Tang H, Ding LL, Guo DX, Li CP. Overweight and obesity associated with increased total serum calcium level: comparison of cross-sectional data in the health screening for teaching faculty. Biol Trace Elem Res. 2013;156:74–78. doi: 10.1007/s12011-013-9856-8. [DOI] [PubMed] [Google Scholar]
- 13.Lind L, Jakobsson S, Lithell H, Wengle B, Ljunghall S. Relation of serum calcium concentration to metabolic risk factors for cardiovascular disease. BMJ. 1988;297:960–963. doi: 10.1136/bmj.297.6654.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saltevo J, Niskanen L, Kautiainen H, Teittinen J, Oksa H, Korpi-Hyovalti E, Sundvall J, Mannisto S, Peltonen M, Mantyselka P, Vanhala M. Serum calcium level is associated with metabolic syndrome in the general population: FIN-D2D study. Eur J Endocrinol. 2011;165:429–434. doi: 10.1530/EJE-11-0066. [DOI] [PubMed] [Google Scholar]
- 15.Guessous I, Bonny O, Paccaud F, Mooser V, Waeber G, Vollenweider P, Bochud M. Serum calcium levels are associated with novel cardiometabolic risk factors in the population-based CoLaus study. PLoS One. 2011;6:e18865. doi: 10.1371/journal.pone.0018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorde R, Sundsfjord J, Bonaa KH. Determinants of serum calcium in men and women. The Tromso Study. Eur J Epidemiol. 2001;17:1117–1123. doi: 10.1023/a:1021272831251. [DOI] [PubMed] [Google Scholar]
- 17.Becerra-Tomas N, Estruch R, Bullo M, Casas R, Diaz-Lopez A, Basora J, Fito M, Serra-Majem L, Salas-Salvado J. Increased serum calcium levels and risk of type 2 diabetes in individuals at high cardiovascular risk. Diabetes Care. 2014;37:3084–3091. doi: 10.2337/dc14-0898. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Hypertension Detection and Follow-up Program Cooperative Group 1979. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1997;277:157–166. [PubMed] [Google Scholar]
- 21.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 22.Kim MK, Kim G, Jang EH, Kwon HS, Baek KH, Oh KW, Lee JH, Yoon KH, Lee WC, Lee KW, Son HY, Kang MI. Altered calcium homeostasis is correlated with the presence of metabolic syndrome and diabetes in middle-aged and elderly Korean subjects: the Chungju Metabolic Disease Cohort study (CMC study) Atherosclerosis. 2010;212:674–681. doi: 10.1016/j.atherosclerosis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Kim SK, Bae YJ. Relationship between serum calcium and magnesium concentrations and metabolic syndrome diagnostic components in middle-aged Korean men. Biol Trace Elem Res. 2012;146:35–41. doi: 10.1007/s12011-011-9224-5. [DOI] [PubMed] [Google Scholar]
- 24.Cho GJ, Shin JH, Yi KW, Park HT, Kim T, Hur JY, Kim SH. Serum calcium level is associated with metabolic syndrome in elderly women. Maturitas. 2011;68:382–386. doi: 10.1016/j.maturitas.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Hagstrom E, Hellman P, Lundgren E, Lind L, Arnlov J. Serum calcium is independently associated with insulin sensitivity measured with euglycaemic-hyperinsulinaemic clamp in a community-based cohort. Diabetologia. 2007;50:317–324. doi: 10.1007/s00125-006-0532-9. [DOI] [PubMed] [Google Scholar]
- 26.Sun G, Vasdev S, Martin GR, Gadag V, Zhang H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes. 2005;54:3336–3339. doi: 10.2337/diabetes.54.11.3336. [DOI] [PubMed] [Google Scholar]
- 27.Aoki K, Miyagawa K. Correlation of increased serum calcium with elevated blood pressure and vascular resistance during calcium infusion in normotensive man. J Hypertens. 1990;8:579–583. doi: 10.1097/00004872-199006000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Ahlstrom T, Hagstrom E, Larsson A, Rudberg C, Lind L, Hellman P. Correlation between plasma calcium, parathyroid hormone (PTH) and the metabolic syndrome (MetS) in a community-based cohort of men and women. Clin Endocrinol (Oxf) 2009;71:673–678. doi: 10.1111/j.1365-2265.2009.03558.x. [DOI] [PubMed] [Google Scholar]
- 29.Hjelmesaeth J, Hofso D, Aasheim ET, Jenssen T, Moan J, Hager H, Roislien J, Bollerslev J. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol. 2009;8:7. doi: 10.1186/1475-2840-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]