Abstract
Background
Development of periodontal disease (PD) may be affected by socioeconomic status. This study examined the relationship between occupational status and PD in a 5-year prospective cohort of Japanese workers.
Methods
In total, 19,633 participants had initial examinations at the Aichi Health Promotion Foundation, of whom 8210 participants aged 20 years or older did not have PD. Follow-up examinations were conducted for 3757 participants, accounting for 45.8% of baseline participants. Ultimately, 3390 participants were analyzed according to the criterion of job classification at baseline, which was based on the International Standard Classification of Occupations, 1987. Oral examinations were performed using the Community Periodontal Index (CPI). The CPI scores were coded as follows: healthy (score of 0); bleeding after probing (1); dental calculus (2); shallow pockets (3); and deep pockets (4). Participants with one or more sextants with a score >2 were diagnosed with PD. Poisson regression analysis was performed to adjust for age and other potential confounders.
Results
Overall, 31.6% of men and 23.8% of women had developed PD (CPI scores of 3 or 4). The adjusted relative risk (RR) for PD (CPI scores of 3 or 4) in men was not significant. On the other hand, the adjusted RRs for PD (CPI score of 4) in men were 2.52-, 2.39-, and 2.74-fold higher for skilled workers, sales persons, and drivers, respectively, than for professionals. In contrast, we found no gradient in women.
Conclusions
We found a gradient related to the risk of developing PD according to occupational status among men in a Japanese worker population.
Keywords: Periodontitis, Cohort study, Occupation, Lifestyle factors, Behavior
1. Introduction
Periodontal disease, marked by inflammation of the gingival tissue caused by bacterial plaque, is one of the most widespread inflammatory chronic diseases.1 Systemic inflammation induced by periodontal disease may play a significant role in the pathogenesis of atherosclerosis or diabetes progression.2,3 Moreover, people who are unable to fully masticate due to severe periodontal disease and/or tooth loss have insufficient daily nutrient intake and could be more vulnerable to non-communicable disease.4
Biological and lifestyle factors, including smoking, alcohol consumption, and psychological stress, are well-known risk factors for periodontal disease.2–10 Recently, however, some studies have suggested that socioeconomic status (SES) is a determinant of oral health or periodontal disease.11–15 For example, poorer oral health was observed among individuals with a lower poverty-income ratio and education level.16 In addition, a marked difference in prevalence of periodontal disease was found among five social groups classified according to income in both in Australia and Vietnam. Furthermore, within-country social variation in periodontal disease was quite similar between the two countries.17 These results indicate that SES may explain a large portion of individual variation in periodontal disease risk.
A recent study indicated differences in periodontal status according to job classification in Japan.18 However, our current understanding of occupational status as a risk factor in periodontal health is mainly based on a few cross-sectional studies with small sample sizes,18–21 although numerous studies have demonstrated associations between occupational status and other health outcomes.22–24 Thus, there is still demand for a long-term follow-up study in a large population to investigate occupational status as a possible independent risk factor of periodontal disease. We therefore examined the relationship between occupational status and incidence of periodontal disease in a 5-year prospective cohort study in Japanese workers.
2. Methods
2.1. Study design and participants
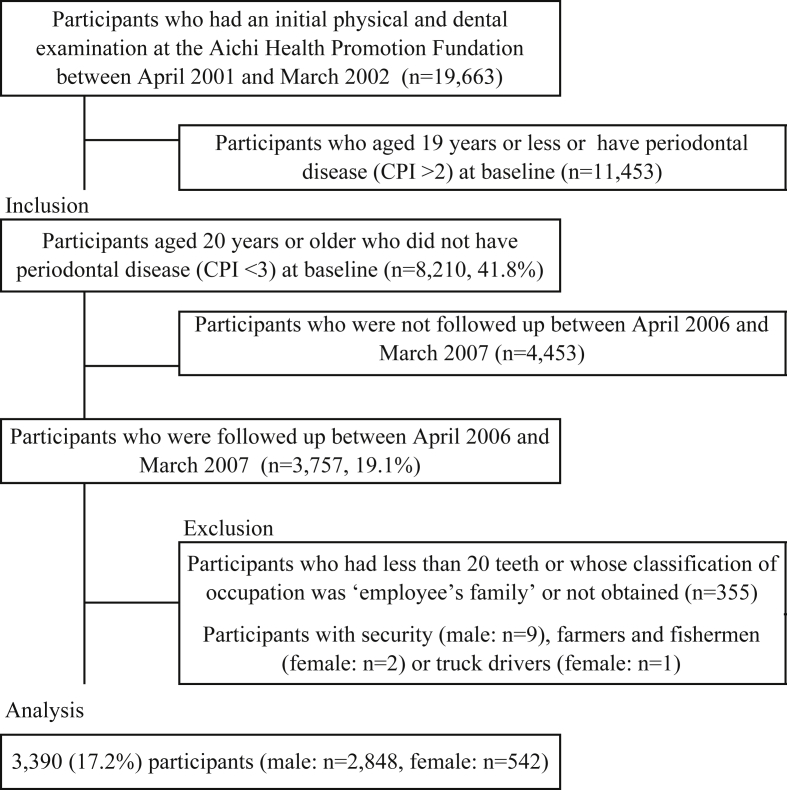
We conducted a prospective cohort study to investigate the relationship between occupation and periodontal disease. Subjects were those who participated in the annual health checks that are recommended for all employees by the Ministry of Health, Labour and Welfare of Japan. Subjects in this study worked in and around Nagoya City, which is the largest city in Aichi Prefecture, with a population of approximately 2.3 million. A total of 19,633 participants had an initial physical and dental examination at the Aichi Health Promotion Foundation between April 2001 and March 2002. Inclusion in the present study was restricted to participants aged 20 years or older who did not have periodontal disease (Community Periodontal Index [CPI] score <3) at baseline (n = 8210).25 Follow-up examinations were completed between April 2006 and March 2007 for 3757 participants, accounting for 45.8% of all baseline participants. Participants who had less than 20 teeth were excluded to avoid under- or over-estimation of the prevalence of periodontal disease, which can occur when examining the periodontal status of patients with fewer teeth in partial-mouth assessments.26 Participants whose classification of occupation was ‘employee's family’ or not obtained at baseline were also excluded. Moreover, we excluded participants with the following occupations due to the small sample size by gender: security (men only; n = 9), farmers and fishermen (women only; n = 2), and truck drivers (women only; n = 1). After applying these eligibility criteria, a total of 3390 participants were entered into the analysis. The study was reviewed and approved by the Ethics Committee of Aichi Gakuin University.
2.2. Classification of occupation
Occupational status of participants was classified according to the criteria of the Ministry of Health, Labour and Welfare of Japan, which was based on the International Standard Classification of Occupations, 1987.27 The criteria classify the following nine major job groups: 1) professional (e.g., professionals and specialists); 2) managers; 3) office workers (e.g., computer operators, clerks, and secretaries); 4) skilled workers (e.g., factory workers and construction workers); 5) salespersons (e.g., shop assistants); 6) service occupations (e.g., superintendents, cleaners, and car park attendants); 7) security (e.g., guards); 8) farmers and fishermen; and 9) transport and telecommunication workers (e.g., truck drivers). A self-administered questionnaire was used to assess participants' classification of occupation, and the dental examiners were blinded to the results.
2.3. Diagnosis of periodontal disease
Seven dentists with calibrated inter-examiner kappa index values of 0.7–0.9 examined the participants under a reflected light using a mouth mirror and compressed air. Periodontal status was assessed using the standard World health Organization (WHO) criteria for CPI.25 The oral cavity of the participants was divided into six sextants, which delineated four groups of teeth each containing the molars and premolars of one side of one jaw, and the two groups of teeth each containing canines and incisors of one jaw. According to the WHO criteria, 10 teeth were selected for periodontal examination: 2 M in each posterior sextant, and the upper right and lower left central incisors. Measurements were made using a CPI probe (YDM Co., Tokyo, Japan) at six sites (mesio-buccal, mid-buccal, disto-buccal, disto-lingual, mid-lingual, and mesio-lingual) of each tooth.25 The CPI scores were coded as follows: healthy (score 0), bleeding after probing (score 1), dental calculus detected by probing (score 2), 4–5-mm shallow pockets (score 3), and ≥6-mm deep pockets (score 4). Participants with one or more sextants with a score >2 were diagnosed with periodontal disease.25 As scores of 4 (pockets ≥6-mm deep) were considered to indicate irreversible damage due to the destruction of periodontal tissue,28 it was deemed be reasonable to analyze the data for such participants separately from those with scores >2, to observe progression in periodontal disease.
2.4. Covariates
A health examination included height and weight measurement and blood tests. Body mass index (BMI) was defined as weight in kilograms divided by the square of height in meters. The value for HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP)-equivalent value, which was calculated using the formula A1C (%) = A1C (Japan Diabetes Society [JDS]) (%) + 0.4%, in consideration of the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and A1C (NGSP).29 Participants were considered diabetic if they met at least one of the following parameters: fasting blood glucose level ≥126 mg/dL (≥7.0 mmol/L), random plasma glucose level ≥200 mg/dL (≥11.1 mmol/L), or HbA1c ≥ 6.5% (HbA1c ≥ 6.1% according to JDS). Diabetes was diagnosed if the blood sample was confirmed to be a diabetic type according to both plasma glucose level and HbA1c at the same time.
A self-administered questionnaire was also used to assess medical history and lifestyle variables, including smoking habits (never, former, or current) and drinking habits (never, sometimes, or every day). In previous studies, BMI, diabetes, and smoking and drinking habits were considered to be independent risk factors for periodontal disease; therefore, these were entered into a multivariate analysis as potential confounding factors.2,3
2.5. Statistical analysis
All analyses were stratified by gender because career decisions and work environments of participants often differ by gender, which could therefore influence the effect on incidence of periodontal disease. To adjust for demographics and possible confounding factors and to estimate the relative risk (RR) of periodontal disease according to baseline occupation, Poisson regression analysis was performed with classification of occupation as an independent variable.30,31 For the endpoint, sensitivity analysis was conducted in the following two ways: having one or more sextants with CPI score 3 or 4 (shallow or deep pockets ≥4 mm), or having one or more sextants with CPI score of 4 (deep pockets ≥6 mm; most severe periodontal disease and loss of tooth function).25 The RR of periodontal disease and 95% confidence interval (CI) were estimated in three models: crude, age-adjusted, and fully adjusted models. In the fully adjusted model, the following variables were entered into the model as possible confounding factors: age (per 10 years), BMI (<18.5, 18.5–22.9, 23.0–26.9, or ≥27.5),32 presence of diabetes (no or yes), smoking status (never, former, or current), and drinking status (never, sometimes, or every day). In the multivariate analysis, participants with professional occupations were used as the reference group, in accordance with previous studies.18,20 To establish the cutoff point for number of teeth, sensitivity analysis was also conducted in the participants with 10 or more teeth (see eTable 1 and eTable 2). To account for missing data, multiple imputation analysis was performed in addition to available-case analysis, assuming that the missing data were missing at random. For the multiple imputations, we used the fully conditional specification (FCS) method, which assumes a separate conditional distribution for each imputed variable, because we imputed a variable that only took on specific values, similar to the binary outcomes of a logistic models. The FCS method can produce estimates that are comparable to those obtained with the multivariate normal distribution method.33 Each of the 10 complete datasets was analyzed, and the parameter estimates obtained from each analyzed dataset were then combined for inference. All P-values were two sided, with a significance level of 5%. All statistical analyses were performed using the SAS statistical software (SAS Institute, Cary, NC, USA).
3. Results
Fig. 1 shows a flowchart of participants. We followed up 3757 participants from baseline (follow-up rate: 45.8%). After applying the eligibility criteria, a total of 3390 participants were entered into the analysis. The sample comprised 2848 (84.0%) men and 542 (16.0%) women. The mean age of men was 41.0 (standard deviation [SD], 9.77) years, and the mean age of women was 41.6 (SD, 10.46) years (Table 1). The prevalence of obesity (BMI ≥27.5) was 8.2% in men and 4.1% in women, and prevalence of diabetes mellitus was 1.9% and 0.2%, respectively, at baseline. Forty-three percent of men and 8.6% of women were current smokers. Women were more likely to be classified as ‘never smokers’ and ‘never alcohol drinkers’ than men. Women were also more likely to be office workers.
Fig. 1. A flowchart of participants. Percentages shown in the figure are a number of eligible participants divided by the total number of target population.
Table 1. Baseline characteristics of participants.
| Baseline characteristics | Men (n = 2848) | Women (n = 542) | Missing value |
|---|---|---|---|
| Mean (SD) age, years | 41.0 (9.77) | 41.6 (10.46) | 0 |
| BMI, kg/m2 | 0 | ||
| <18.5 | 128 (4.5) | 86 (15.9) | |
| 18.5–22.9 | 1304 (45.8) | 344 (63.5) | |
| 23.0–27.5 | 1182 (41.5) | 90 (16.6) | |
| ≥27.5 | 234 (8.2) | 22 (4.1) | |
| Diabetes | 51 (1.9) | 1 (0.2) | 122 |
| Smoking status | 18 | ||
| Never smoker | 894 (31.5) | 469 (87.3) | |
| Former smoker | 734 (25.9) | 22 (4.1) | |
| Current smoker | 1207 (42.6) | 46 (8.6) | |
| Alcohol drinking | 23 | ||
| Never | 712 (25.2) | 316 (58.7) | |
| Sometimes (=1) | 1315 (46.5) | 165 (30.7) | |
| Everyday | 802 (28.4) | 57 (10.6) | |
| Community periodontal index | 0 | ||
| 0 | 136 (4.8) | 45 (8.3) | |
| 1 | 313 (11.0) | 109 (20.1) | |
| 2 | 2399 (84.2) | 388 (71.6) | |
| Classification of occupation | 0 | ||
| Professional | 787 (27.6) | 54 (10.0) | |
| Managers | 455 (16.0) | 26 (4.8) | |
| Office workers | 443 (15.6) | 321 (59.2) | |
| Skilled workers | 512 (18.0) | 99 (18.3) | |
| Sales persons | 368 (12.9) | 25 (4.6) | |
| Service occupations | 88 (3.1) | 17 (3.1) | |
| Driver | 195 (6.9) | 0a |
Data are presented as n (%), unless otherwise noted.
Valid percentages for available data are shown.
1 woman was removed due to the small sample size.
After the 5-year follow up, 31.6% of men (899 of 2848) and 23.8% of women (129 of 542) had developed periodontal disease (CPI scores of 3 or 4) (Table 2). Poisson regression analysis showed that the crude RRs of periodontal disease (CPI scores of 3 or 4) in men were 1.65 (95% CI, 1.36–2.00), 1.25 (95% CI, 1.01–1.54), 1.50 (95% CI, 1.05–2.14), and 1.49 (95% CI, 1.15–1.93) for managers, office workers, service occupations, and drivers compared with professionals, respectively. The RRs were not significant after adjusting for age and other potential confounders. For periodontal disease (CPI score of 4), managers, skilled workers, sales persons, and drivers had significant RRs for periodontal disease compared to professionals (Table 2). After fully adjusting for potential confounders, the adjusted RRs of male skilled workers, sales persons, and drivers were 2.52 (95% CI, 1.15–5.54), 2.39 (95% CI, 1.04–5.48), and 2.74 (95% CI, 1.10–6.79) compared with professionals, although managers' risk of periodontal disease was not significantly different from that of professionals. In the multiple imputation analysis, the adjusted RR of sales persons did not remain significant. In contrast, there was a lower level of periodontal disease among women office workers compared to professionals (CPI score of 4) (RR 0.17; 95% CI, 0.03–0.89) (Table 3). We additionally performed sensitivity analysis for participants with 10 or more teeth, but the results were not different from those for participants who had 20 or more teeth (eTable 1 and eTable 2).
Table 2. Relative risk of classification of occupation for periodontal disease in male with 20 teeth or more.
| Classification of occupation | Cases (n) | Relative risk (95% confidence interval) for periodontal disease | |||
|---|---|---|---|---|---|
| Crude | Age-adjusted | Fully-adjusteda | Fully-adjustedb | ||
| CPI = 3 or 4 | |||||
| Professional | 209 (787) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Managers | 199 (455) | 1.65 (1.35, 2.00) | 1.14 (0.93, 1.41) | 1.06 (0.86, 1.31) | 1.07 (0.87, 1.32) |
| Office workers | 147 (443) | 1.25 (1.01, 1.54) | 1.12 (0.91, 1.39) | 1.07 (0.86, 1.32) | 1.09 (0.88, 1.35) |
| Skilled workers | 124 (512) | 0.91 (0.73, 1.14) | 0.94 (0.75, 1.17) | 0.92 (0.74, 1.16) | 0.93 (0.74, 1.16) |
| Sales persons | 108 (368) | 1.11 (0.88, 1.39) | 1.09 (0.87, 1.38) | 1.08 (0.85, 1.37) | 1.03 (0.82, 1.30) |
| Service occupations | 35 (88) | 1.50 (1.05, 2.14) | 1.34 (0.93, 1.91) | 1.23 (0.86, 1.76) | 1.24 (0.87, 1.78) |
| Driver | 77 (195) | 1.49 (1.15, 1.93) | 1.45 (1.12, 1.89) | 1.27 (0.97, 1.65) | 1.29 (0.99, 1.68) |
| CPI = 4 | |||||
| Professional | 11 (787) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Managers | 19 (455) | 2.99 (1.42, 6.28) | 1.68 (0.77, 3.72) | 1.54 (0.69, 3.45) | 1.44 (0.66, 3.16) |
| Office workers | 12 (443) | 1.94 (0.86, 4.39) | 1.63 (0.72, 3.71) | 1.74 (0.75, 4.05) | 1.58 (0.69, 3.60) |
| Skilled workers | 17 (512) | 2.38 (1.11, 5.07) | 2.37 (1.11, 5.07) | 2.52 (1.15, 5.54) | 2.27 (1.06, 4.89) |
| Sales persons | 13 (368) | 2.53 (1.13, 5.64) | 2.49 (1.12, 5.56) | 2.39 (1.04, 5.48) | 2.11 (0.94, 4.73) |
| Service occupations | 1 (88) | 0.81 (0.10, 6.30) | 0.68 (0.088, 5.29) | 0.67 (0.085, 5.24) | 0.63 (0.080, 4.87) |
| Driver | 9 (195) | 3.30 (1.37, 7.97) | 3.16 (1.31, 7.62) | 2.74 (1.10, 6.79) | 2.58 (1.06, 6.29) |
CPI, community periodontal index.
Bold font shows a statistically significant odds ratio for periodontal disease.
Adjusted for age (+10), diabetes (yes or no), smoking (current, former, or never), drinking (everyday, sometimes, or never), and BMI (<18.5, 18.5–22.9, 23.0–27.5, or ≥27.5).
Multiple imputation for missing data.
Table 3. Relative risk of classification of occupation for periodontal disease in female with 20 teeth or more.
| Classification of occupation | Cases (n) | Relative risk (95% confidence interval) for periodontal disease | |||
|---|---|---|---|---|---|
| Crude | Age-adjusted | Fully-adjusteda | Fully-adjustedb | ||
| CPI = 3 or 4 | |||||
| Professional | 17 (54) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Managers | 9 (26) | 1.10 (0.49, 2.47) | 0.73 (0.32, 1.66) | 0.90 (0.38, 2.10) | 0.78 (0.34, 1.82) |
| Office workers | 62 (321) | 0.61 (0.36, 1.05) | 0.64 (0.37, 1.09) | 0.65 (0.37, 1.14) | 0.65 (0.38, 1.12) |
| Skilled workers | 26 (99) | 0.83 (0.45, 1.54) | 0.72 (0.39, 1.32) | 0.79 (0.42, 1.48) | 0.77 (0.41, 1.43) |
| Sales persons | 9 (25) | 1.14 (0.51, 2.57) | 1.17 (0.52, 2.63) | 1.33 (0.58, 3.06) | 1.17 (0.51, 2.65) |
| Service occupations | 6 (17) | 1.12 (0.44, 2.84) | 0.87 (0.34, 2.21) | 0.89 (0.34, 2.30) | 0.88 (0.34, 2.25) |
| CPI = 4 | |||||
| Professional | 3 (54) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Managers | 1 (26) | 0.69 (0.072, 6.66) | 0.37 (0.035, 3.84) | 0.35 (0.030, 3.98) | 0.34 (0.029, 3.88) |
| Office workers | 4 (321) | 0.22 (0.050, 1.00) | 0.23 (0.052, 1.03) | 0.17 (0.033, 0.88) | 0.17 (0.034, 0.89) |
| Skilled workers | 2 (99) | 0.36 (0.061, 2.18) | 0.29 (0.048, 1.75) | 0.30 (0.048, 1.92) | 0.31 (0.049, 1.95) |
| Sales persons | 1 (25) | 0.72 (0.075, 6.92) | 0.73 (0.075, 6.98) | 0.68 (0.061, 7.60) | 0.68 (0.063, 7.39) |
| Service occupations | 0 (17) | 0 | 0 | 0 | 0 |
CPI, community periodontal index.
Bold font shows a statistically significant relative risk for periodontal disease.
Adjusted for age (+10), diabetes (yes or no), smoking (current, former, or never), drinking (everyday, sometimes, or never), and BMI (<18.5, 18.5–22.9, 23.0–27.5, or ≥27.5).
Multiple imputation for missing data.
4. Discussion
We found a significant association between occupational status and developing periodontal disease over 5 years among workers in Japan. The RR for periodontal disease (i.e., a CPI score of 4) in men was 2.52, 2.39, and 2.74 times higher for skilled workers, sales persons, and drivers compared with professionals, even after adjusting for age and other potential confounders. In contrast, we found that female office workers had lower levels of periodontal disease than professionals. This is the first large cohort study to evaluate the relationship between occupational status and periodontal disease in workers.
The findings of the present study are similar to those of previous cross-sectional studies.18–21 Asawa et al.21 reported that fishermen (skilled workers) had more severe periodontal disease than non-fishermen in an Indian community. Similarly, other studies by Craig et al.19,20 reported greater severity of periodontal pockets and attachment level in unskilled and skilled workers compared to professionals among participants recruited in New York. Moreover, the odds ratio in a cross-sectional study for periodontal disease was significantly higher among those in service occupations, sales persons, managers and drivers than among professionals,18 although we found no association between developing periodontal disease and occupational status in service or management occupations, after adjusting for potential confounders. These studies strongly support our findings.
In this study, we used two cutoff points, namely CPI score of 3 or 4 (indicating moderate/severe periodontal disease) and a CPI score of 4 (indicating severe periodontal disease) because the relationship between occupational status and severe periodontal disease was unclear. A CPI score of 4 is considered to indicate the most severe condition of periodontal disease and loss of tooth function.25 Therefore, the odds ratio for periodontal disease using a CPI score of 4 predicts the severity of periodontal disease better than a CPI score of 3 or 4 does.
The possible mechanism underlying the higher incidence of periodontal disease in skilled workers, sales persons, and drivers at baseline may be related to their social circumstances and psychosocial factors, such as work-related mental demand and stress.11–13 People in Japan work longer hours than those in other developing countries of the Organization for Economic Co-operation and Development.34 In particular, skilled workers and drivers tend to work overtime, have low quality of sleep or rest,35 and may have higher levels of stress.36,37 Moreover, many types of shift work schedules, such as night, irregular, or rotating shifts, are considered detrimental to workers' health,38,39 and most shift workers are found among sales persons.34 Furthermore, lack of flexibility in peoples' daily lives decreases tooth-cleaning frequencies and makes the cleaning less effective.6,18 These reports may support our argument.
Another possible explanation is that behavioral factors influenced by occupational status could affect the incidence of periodontal disease. A nationally representative cross-sectional survey in Japan showed that men in lower-status occupations, such as the service, transport, and labor sectors, were significantly more likely to exhibit health-risk behaviors, including smoking, alcohol drinking, or physical inactivity, which are known risk factors of periodontal disease,2–6 than were professionals.40 Similarly, our results of difference in the RR between adjusted models indicated that health behaviors partially explained the association between occupational status and periodontal disease. According to the framework by Brunner and Marmot,41 periodontal disease is also affected by contextual factors, such as social environment and work, individual health behaviors, and psychological resistance (e.g., personality or coping) and vulnerability (e.g., life events or chronic stressors). While psychological resistance and vulnerability have an indirect impact on pathophysiological changes of periodontal tissues via immune response, oral and general health behaviors directly lead to such changes.17 Thus, upstream contextual factors may play an important role in the development of periodontal disease thorough the life course, in connection with each individual's early life and cultural and genetic factors.
We identified a gender difference in the effect of occupation on periodontal disease. In particular, female office workers had lowers levels of periodontal disease at baseline than did professional women. Some studies have reported gender differences as well as social and psychological impact on oral health.42,43 In addition, the occupational disadvantage of women is poorly reflected in current measures of social position.44 Moreover, SES has a greater impact on mortality, morbidity, and health behaviors in men than in women.45 These results indicate that the effects of occupational status on periodontal disease differed by gender, as it does for other health outcomes.
To prevent and control periodontal disease through consideration of the social environment, a public health approach is required.46 Thomson et al. reported that a population strategy aimed at altering life practices and promoting self-care behaviors (particularly, effective oral hygiene practices) reduced plaque levels and tobacco use in the community.17 The success of such approaches depends on identifying individuals at particular risk of developing future disease at an early stage.17 Combined with our results, these data indicate that inquiring about the work environment, alongside the population approach, may be useful for predicting and preventing periodontal disease in subjects visiting the hospital for the first time or having a health check-up.
Several limitations of this study warrant mention. First, there is a possibility that residual confounders might have distorted the associations reported. Second, we did not measure working hours in the cohort of workers, which would influence their mental health and may have led to overestimation of our results. Third, we also did not survey oral hygiene practice, which are an unmeasured confounder in the association between occupation and periodontal disease. Fourth, we were unable to ascertain participants' education, income level, and job position, which are important determinants of health conditions. Further studies should investigate the impact of occupational status on the incidence of periodontal disease, taking into consideration oral hygiene practice, SES, and work environment. Finally, we measured the periodontal status of the population using CPI; however, this might not fully reflect individuals' severity of periodontal disease.
In conclusion, we revealed a difference in the risk of developing periodontal disease according to occupational status among men in a cohort of Japanese workers in a 5-year follow-up study. After adjustment for potential confounders, skilled workers, sale persons, and drivers had higher risks of periodontal disease than professionals. The present study and previous results indicate that occupation level and shift work might be associated with developing periodontal disease. These are potentially modifiable factors, and this study provides important new information for dentists concerned with prevention of periodontal disease.
Conflicts of interest
None declared.
Acknowledgements
This work was supported by the 8020 Research Grant for fiscal from the 8020 Promotion Foundation. Adopted number: 14–4–11.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.je.2016.09.002.
Footnotes
Peer review under responsibility of the Japan Epidemiological Association.
References
- 1.Pink C, Kocher T, Meisel P, et al. Longitudinal effects of systemic inflammation markers on periodontitis. J Clin Periodontol. 2015;42:988–997. [DOI] [PubMed] [Google Scholar]
- 2.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol. 2000;1997(14):9–11. [DOI] [PubMed] [Google Scholar]
- 3.Akinkugbe AA, Saraiya VM, Preisser JS, Offenbacher S, Beck JD. Bias in estimating the cross-sectional smoking, alcohol, obesity and diabetes associations with moderate-severe periodontitis in the Atherosclerosis Risk in Communities study: comparison of full versus partial-mouth estimates. J Clin Periodontol. 2015;42:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004;7:201–226. [DOI] [PubMed] [Google Scholar]
- 5.Marcenes WS, Sheiham A. The relationship between work stress and oral health status. Soc Sci Med. 1992;35:1511–1520. [DOI] [PubMed] [Google Scholar]
- 6.Abegg C, Croucher R, Marcenes WS, Sheiham A. How do routines of daily activities and flexibility of daily activities affect tooth-cleaning behavior? J Public Health Dent. 2000;60:154–158. [DOI] [PubMed] [Google Scholar]
- 7.Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70:711–723. [DOI] [PubMed] [Google Scholar]
- 8.Pistorius A, Krahwinkel T, Willershausen B, Boekstegen C. Relationship between stress factors and periodontal disease. Eur J Med Res. 2002;7:393–398. [PubMed] [Google Scholar]
- 9.Akhter R, Hannan MA, Okhubo R, Morita M. Relationship between stress factor and periodontal disease in a rural area population in Japan. Eur J Med Res. 2005;10:352–357. [PubMed] [Google Scholar]
- 10.Ng SK, Keung Leung W. A community study on the relationship between stress, coping, affectivedispositions and periodontal attachment loss. Community Dent Oral Epidemiol. 2006;34:252–266. [DOI] [PubMed] [Google Scholar]
- 11.Sheiham A, Nicolau B. Evaluation of social and psychological factors in periodontitis. Periodontol. 2000;2005(39):118–131. [DOI] [PubMed] [Google Scholar]
- 12.Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the dental atherosclerosis risk in communities study. Am J Public Health. 2006;96:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchwald S, Kocher T, Biffar R, Harb A, Holtfreter B, Meisel P. Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J Clin Periodontol. 2013;40:203–211. [DOI] [PubMed] [Google Scholar]
- 14.Linden GJ, Mullally BH, Freeman R. Stress and the progression of periodontal disease. J Clin Periodontol. 1996;23:675–680. [DOI] [PubMed] [Google Scholar]
- 15.Boillot A, El Halabi B, Batty GD, Range H, Czernichow S, Bouchard P. Education as a predictor of chronic periodontitis: a systematic review with meta-analysis population-based studies. PLoS One. 2011;6:e21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabbah W, Tsakos G, Chandola T, Sheiham A, Watt RG. Social gradients in oral and general health. J Dent Res. 2007;86:992–996. [DOI] [PubMed] [Google Scholar]
- 17.Thomson WM, Sheiham A, Spencer AJ. Sociobehavioral aspects of periodontal disease. Periodontol. 2000;2012(60):54–63. [DOI] [PubMed] [Google Scholar]
- 18.Morita I, Nakagaki H, Yoshii S, et al. Gradients in periodontal status in Japanese employed males. J Clin Periodontol. 2007;34:952–956. [DOI] [PubMed] [Google Scholar]
- 19.Craig RG, Boylan R, Yip J, et al. Prevalence and risk indicators for destructive periodontal diseases in 3 urban American minority populations. J Clin Periodontol. 2001;28:524–535. [DOI] [PubMed] [Google Scholar]
- 20.Craig RG, Yip JK, Mijares DQ, LeGeros RZ, Socransky SS, Haffajee AD. Progression of destructive periodontal diseases in three urban minority populations: role of clinical and demographic factors. J Clin Periodontol. 2003;30:1075–1083. [DOI] [PubMed] [Google Scholar]
- 21.Asawa K, Pujara P, Tak M, et al. Oral health status of fishermen and non-fishermen community of Kutch district, Gujarat, India: a comparative study. Int Marit Health. 2014;65:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Kagamimori S, Gaina A, Nasermoaddeli A. Socioeconomic status and health in the Japanese population. Soc Sci Med. 2009;68:2152–2160. [DOI] [PubMed] [Google Scholar]
- 23.Nomura K, Nakao M, Tsurugano S, et al. Job stress and healthy behavior among male Japanese office workers. Am J Ind Med. 2010;53:1128–1134. [DOI] [PubMed] [Google Scholar]
- 24.Otoshi K, Takegami M, Sekiguchi M, et al. Chronic hyperglycemia increases the risk of lateral epicondylitis: the locomotive syndrome and health outcome in Aizu cohort study (LOHAS). Springerplus. 2015;4:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Oral Health Surveys, Basic Methods. fourth ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 26.Leroy R, Eaton KA, Savage A. Methodological issues in epidemiological studies of periodontitis – how can it be improved? BMC Oral Health. 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Labor Organization International Standard Classification of Occupations (ISCO-88); 2008. http://laborsta.ilo.org/applv8/data/isco88e.html.
- 28.Katagiri S, Nitta H, Nagasawa T, et al. High prevalence of periodontitis in non-elderly obese Japanese adults. Obes Res Clin Pract. 2010;4:e247–342. [DOI] [PubMed] [Google Scholar]
- 29.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 31.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. [DOI] [PubMed] [Google Scholar]
- 32.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- 33.Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171:624–632. [DOI] [PubMed] [Google Scholar]
- 34.Bannai A, Ukawa S, Tamakoshi A. Long working hours and psychological distress among school teachers in Japan. J Occup Health. 2015;57:20–27. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki T, Iwasaki K, Mori I, Hisanaga N, Shibata E. Overtime, job stressors, sleep/rest, and fatigue of Japanese workers in a company. Ind Health. 2007;45:237–246. [DOI] [PubMed] [Google Scholar]
- 36.Kawaharada M, Saijo Y, Yoshioka E, Sato T, Sato H, Kishi R. Relations of occupational stress to occupational class in Japanese civil servants–analysis by two occupational stress models. Ind Health. 2001;45:247–255. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko SY, Maeda T, Sasaki A, et al. Effect of shift work on mental state of factory workers. Fukushima J Med Sci. 2004;50:1–9. [DOI] [PubMed] [Google Scholar]
- 38.Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559. [DOI] [PubMed] [Google Scholar]
- 39.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa Y, Tabata M, Kido T, Koyama Y. Occupational class inequalities in behavioral and biological risk factors for cardiovascular disease among workers in medium- and small-scale enterprises. Ind Health. 2012;50:529–539. [DOI] [PubMed] [Google Scholar]
- 41.Bruner E, Marmot M. Social organization, stress and health. In: Marmot M, Wilkinson RG, eds. Social Determinants of Health Oxford: Oxford University Press; 1999:17–43. [Google Scholar]
- 42.McGrath C, Bedi R. Measuring the impact of oral health on quality of life in Britain using OHQoL-UK(W). J Public Health Dent. 2003;63:73–77. [DOI] [PubMed] [Google Scholar]
- 43.Mundt T, Polzer I, Samietz S, et al. Gender-dependent associations between socioeconomic status and tooth loss in working age people in the Study of Health in Pomerania (SHIP). Ger Community Dent Oral Epidemiol. 2011;39:398–408. [DOI] [PubMed] [Google Scholar]
- 44.Gregorio DI, Walsh SJ, Paturzo D. The effects of occupation-based social position on mortality in a large American cohort. Am J Public Health. 1997;87:1472–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Bennett J, Krause N, et al. Old age mortality in Japan: does the socioeconomic gradient interact with gender and age? J Gerontol B Psychol Sci Soc Sci. 2002;57:S294–S307. [DOI] [PubMed] [Google Scholar]
- 46.Watt RG, Petersen PE. Periodontal health through public health-the case for oral health promotion. Periodontol. 2000;2012(60):147–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.je.2016.09.002.