Abstract
Objective
A prior longitudinal study demonstrates that free-water diffusion magnetic resonance imaging (dMRI) tracks progression in the substantia nigra (Ofori et al., 2015b). Here, we test the acute effects of antiparkinsonian medication on this established imaging progression marker for the first time.
Methods
Fifteen PD patients underwent dMRI OFF and ON-medication one day apart. ON-medication, patients were tested approximately 45 min after their usual dose of antiparkinsonian medication. OFF-medication, patients were tested after not taking antiparkinsonian medication for > 12 h. OFF and ON-medication was counter-balanced across subjects. For dMRI, we computed free-water and free-water corrected fractional anisotropy (FAt) within the following regions: caudate, putamen, substantia nigra, and subthalamic nucleus.
Results
ON-medication significantly reduced parkinsonian motor symptoms compared with OFF-medication (p < 0.001). dMRI measures (free-water and FAt) were not different between the OFF and ON-medication conditions.
Conclusions
Administration of an acute does of anti-parkinsonian medication in PD does not affect free-water and FAt in key nigrostriatal structures. Free-water and FAt biomarkers reflect the chronic state of the nigrostriatal circuit, and therefore are potential viable biomarkers for disease-modifying therapeutic studies in PD.
Keywords: dMRI, Parkinson's disease, Antiparkinsonian medication, Free-water, Free-water corrected fractional anisotropy
Highlights
-
•
Effects of dopaminergic medication on PD progression markers
-
•
Investigate dMRI biomarkers in early PD
-
•
Acute PD medication does not alter the progression markers.
1. Introduction
Parkinson's disease (PD) is the second most common age-related neurodegenerative disorder and is characterized by the presence of tremor, rigidity, bradykinesia, and impaired posture and gait. Histologically, PD results in a significant reduction of dopaminergic cells in the substantia nigra pars compacta (SNc) (Fearnley and Lees, 1991, Stocchi and Olanow, 2003) and a dopamine deficiency in specific nuclei of the basal ganglia. Along with this substantial neuronal loss, the main pathological hallmark of PD is the presence of intracellular α-synuclein-positive inclusions, known as Lewy bodies and Lewy neurites, throughout subcortical and cortical brain regions (Braak et al., 2003, Stocchi and Olanow, 2003). Developing non-invasive methods to assay features reflecting this degeneration is a critical area of ongoing research.
Numerous studies using diffusion magnetic resonance imaging (dMRI) have detected altered diffusion in the substantia nigra of PD compared with control individuals (Cochrane and Ebmeier, 2013, Du et al., 2012, Langley et al., 2016, Ofori et al., 2015a). Using free-water analysis of dMRI signals revealed that free-water in the substantia nigra increased longitudinally over one year in PD with no changes in a control group (Ofori et al., 2015b). This dMRI progression biomarker of the substantia nigra holds promise for being useful in clinical trials testing disease-modifying therapies for PD.
A key unanswered question is whether free-water in the substantia nigra is influenced by antiparkinsonian medication. This is important because most disease modifying clinical trials will include PD patients on levodopa and it is necessary to determine if administration of levodopa alters this biomarker of progression. If administering antiparkinsonian medication affects dMRI measurements, then they likely would represent faster changes in the basal ganglia than would be useful for a biomarker of disease progression, whereas if they are not influenced by acute antiparkinsonian medication then they may be well suited as progression biomarkers that would reflect the chronic state of basal ganglia microstructure. Here, we evaluated for the first time whether administration of antiparkinsonian medication was associated with changes in diffusion MRI free-water and free-water corrected fractional anisotropy in the substantia nigra and other basal ganglia structures.
2. Materials and methods
2.1. Participants
A total of 15 PD patients were diagnosed by a movement disorder specialist using established criteria (Hughes et al., 2001), and recruited from University of Florida Center for Movement Disorders and Neurorestoration. The study was approved by the Institutional Review Board and all participants provided informed consent prior to participating in the study (Table 1).
Table 1.
Demographics and clinical data.
| Demographics | Clinical Data | OFF-medication | ON-medication | p-Value | pFDR |
|---|---|---|---|---|
| Sample size | 15 | |||
| Age, yrs | 62.00 (10.51) | |||
| Gender (M | F) | 9 | 6 | |||
| Disease duration, yrs | 5.17 (4.78) | |||
| Hoehn and Yahr stage (OFF-med) | 1.67 (0.62) | |||
| Total LEDD | 674.00 (364.59) | |||
| MDS-UPDRS-III – Total | 16.80 (8.51) | 13.13 (6.98) | < 0.001 | < 0.001 |
| MoCA | 27.20 (2.18) | 27.67 (1.54) | 0.301 | 0.597 |
Data are count or mean (± SD) (n = 15). Disease duration is defined as time since diagnosis. p-Values are uncorrected. pFDR values are FDR corrected. Abbreviations: F = females; LEDD = levodopa equivalent daily dose; M = males; MoCA = Montreal Cognitive Assessment; MDS-UPDRS-III = the motor section of the Movement Disorder Society Unified Parkinson's Disease Rating Scale.
2.2. Procedures
The experiments for the patients were performed on two consecutive days; (i) OFF-medication; and (ii) ON-medication. The order of testing day was counter-balanced across subjects. PD patients were tested in the practically defined off state (Langston et al., 1992), with testing following a 12-hour withdrawal from anti-parkinsonian medication. Testing ON-medication occurred approximately 45 min corresponding to the time required for levodopa plasma level to reach its peak (Brooks, 2008) after taking the patient-specific dose of antiparkinsonian medication (Table 2). Each patient underwent dMRI evaluation, and assessment of motor symptoms and cognitive status on two consecutive days. Motor symptoms and cognitive status were assessed using part III of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III) and the Montreal Cognitive Assessment (MoCA), respectively (Goetz et al., 2008, Nasreddine et al., 2005). The total levodopa equivalent daily dose (LEDD) and the single dose LEDD before dMRI scan were calculated according to previously published recommendations (Tomlinson et al., 2010) (Table 1).
Table 2.
Patient antiparkinsonian medications.
| Subject | Medication | Total LEDD (mg)/a day | Single dose LEDD (mg) |
|
|---|---|---|---|---|
| LEDD (mg) | % of Total LEDD | |||
| PD 1 | Carbidopa/Levodopa (Atamet, Sinemet) Pramipexole | 1100 | 300 | 27.27 |
| PD 2 | Carbidopa/Levodopa (Atamet, Sinemet) Amantadine (Symmetrel) | 1450 | 350 | 24.14 |
| PD 3 | Carbidopa/Levodopa (Atamet, Sinemet) Pramipexole ER (Mirapex) | 1050 | 650 | 61.90 |
| PD 4 | Rotigotine Transdermal Patch (Neupro) Carbidopa/Levodopa/Entacapone (Stalevo) | 1260 | 396 | 31.43 |
| PD 5 | Carbidopa/Levodopa (Atamet, Sinemet) Rasagiline (Azilect) | 500 | 200 | 40.00 |
| PD 6 | Carbidopa/Levodopa (Atamet, Sinemet) | 600 | 300 | 50.00 |
| Rasagiline (Azilect) | ||||
| Pramipexole | ||||
| PD 7 | Carbidopa/Levodopa (Atamet, Sinemet) Rasagiline (Azilect) | 500 | 200 | 40.00 |
| PD 8 | Carbidopa/Levodopa (Atamet, Sinemet) Selegiline (Eldepryl) | 500 | 150 | 30.00 |
| PD 9 | Carbidopa/Levodopa (Atamet, Sinemet) Rasagiline (Azilect) | 400 | 200 | 50.00 |
| PD 10 | Carbidopa/Levodopa (Atamet, Sinemet) Rasagiline (Azilect) | 500 | 200 | 40.00 |
| PD 11 | Carbidopa/Levodopa (Atamet, Sinemet) | 500 | 100 | 20.00 |
| PD 12 | Carbidopa/Levodopa Sustained Release (Sinemet CR) | 300 | 150 | 50.00 |
| PD 13 | Carbidopa/Levodopa Sustained Release (Sinemet CR) Selegiline (Eldepryl) | 750 | 150 | 20.00 |
| PD 14 | Carbidopa/Levodopa (Atamet, Sinemet) Rasagiline (Azilect) | 400 | 200 | 50.00 |
| PD 15 | Carbidopa/Levodopa (Atamet, Sinemet) | 300 | 100 | 33.33 |
Abbreviations: LEDD = total levodopa equivalent daily dose; PD = Parkinson's disease.
2.3. MRI data acquisition protocol
MRI was performed on a 3T system (Philips Achieva) equipped with a 32-channel quadrature volume head coil. Head movement was minimized by foam padding within the coil and scanner noise was attenuated using a combination of earplugs and circumaural headphones. Whole brain diffusion imaging data was acquired using a single-shot spin echo EPI sequence: repetition time = 7748 ms, echo time = 86 ms, flip angle = 90°, field of view = 224 × 224 mm, voxel size = 2 mm isotropic with no gap between slices (n = 60), diffusion gradient (monopolar) directions = 64, diffusion gradient timing DELTA/delta = 42.4/10 ms, b-values: 0, 1000 s/mm2, fat suppression using SPIR, in-plane, SENSE factor = 2.
2.4. Diffusion MRI data analysis
FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl/) and custom UNIX shell scripts were used to preprocess the data (Ofori et al., 2015b). Diffusion MRI data were not flipped. Each diffusion scan was eddy current and head motion corrected. Diffusion gradients were compensated for rotations, and non-brain tissue was removed. Free-water and free-water-corrected fractional anisotropy (FAt) maps were calculated from the corrected volumes using a custom code written in MATLAB R2013a (The Mathworks, Natick, MA) (Pasternak et al., 2009). To create the free-water map, a minimization procedure was used that fit a bi-tensor model to each voxel to quantify its fractional volume of free-water. The free-water component was then eliminated from each voxel to generate the FAt maps. The b0 images were normalized to a MNI-T2 template (2 × 2 × 2 mm) by an affine transformation with 12 degrees of freedom and trilinear interpolation using FLIRT (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). The spatial transformation parameters obtained from this step were applied to the free-water and FAt maps. Since FLIRT-transformed images are not sufficient for accurate registration, we used regions of interest hand-drawn on the b0 image of each subject in MNI space. The regions were drawn by an experienced rater who was blinded to the free-water image and medication state, and then used to extract values from the corresponding free-water and FAt maps. The size of each region of interest was chosen to fit within the brain structure across all subjects. Bilateral regions of interest (ROI) were drawn in the following areas (number of voxels per hemisphere, n): anterior substantia nigra (n = 8), posterior substantia nigra (n = 8), putamen (n = 88), caudate nucleus (n = 68), and subthalamic nucleus (n = 8) (Burciu et al., 2016, Ofori et al., 2015b, Planetta et al., 2015, Prodoehl et al., 2013).
2.5. Statistical analysis
Statistical analyses were performed in SPSS (version 24.0; IBM Corp). To examine the effect of antiparkinsonian medication, we compared each dependent variable OFF-medication with the ON-medication dependent variable using two-tailed paired t-test. The significance level was set at p < 0.05. All p-values reported in the Results section of the manuscript were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) method (Benjamini and Hochberg, 1995). Uncorrected p-values are also reported. Power analysis was performed in G*Power (version 3.1.9.2) (Faul et al., 2007). We report required sample size at 80% power for future studies that would attempt to detect an effect of acute antiparkinsonian medication on free-water and FAt measures in the five ROIs (Table 4). The required sample size was calculated based on mean differences in free-water and FAt between the OFF and ON-medication conditions at three levels of α error (0.05, 0.01, and 0.005).
Table 4.
Sample size estimation.
| Diffusion MRI (FW | FAt) | Δ Mean (OFF-ON) | Δ SD (OFF-ON) | Effect size | Sample size (Power 80%) |
||
|---|---|---|---|---|---|---|
| 0.05 | 0.01 | 0.005 | ||||
| FW - Caudate | − 0.0027 | 0.0177 | − 0.1503 | 350 | 521 | 594 |
| FW - Putamen | 0.0018 | 0.0075 | 0.2457 | 132 | 197 | 225 |
| FW - STN | − 0.0018 | 0.0210 | − 0.0878 | 1,021 | 1,519 | 1,732 |
| FW - SN anterior | − 0.0090 | 0.0307 | − 0.2940 | 93 | 139 | 158 |
| FW - SN posterior | 0.0077 | 0.0337 | 0.2292 | 152 | 226 | 258 |
| FAt - Caudate | − 0.0002 | 0.0148 | − 0.0128 | 47,690 | 70,963 | 80,890 |
| FAt - Putamen | − 0.0001 | 0.0121 | − 0.0091 | 94,974 | 141,319 | 161,088 |
| FAt - STN | 0.0024 | 0.0295 | 0.0799 | 1,230 | 1,831 | 2,087 |
| FAt - SN anterior | − 0.0037 | 0.0387 | − 0.0958 | 857 | 1,275 | 1,454 |
| FAt - SN posterior | 0.0068 | 0.0402 | 0.1691 | 277 | 412 | 470 |
Power analysis for diffusion MRI measurements (FW and FAt) (n = 15). OFF and ON-medication paired differences Mean and SD. Sample size is calculated by 80% power at each α error (0.05, 0.01, and 0.005). Abbreviations: FAt = free-water corrected fractional anisotropy; FW = free-water; SN = substantia nigra; STN = subthalamic nucleus; Δ = differences.
3. Results
3.1. Clinical and force data
Table 1 shows the demographic and clinical results. ON-medication significantly reduced parkinsonian motor symptoms compared with OFF-medication (pFDR < 0.001). There was no effect of medication for the MoCA.
3.2. Diffusion MRI data
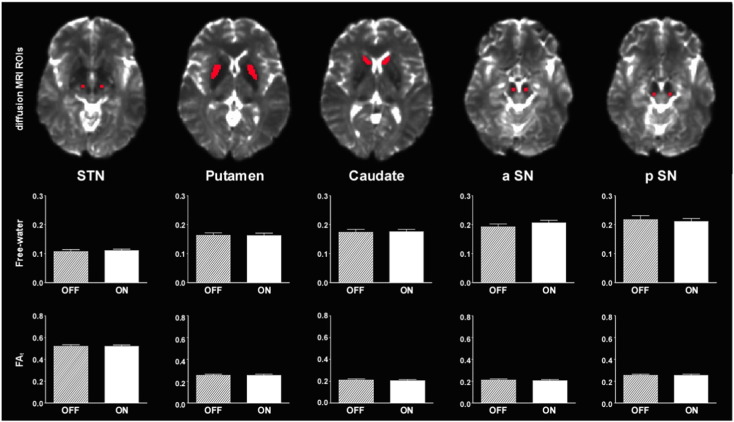
Fig. 1 and Table 3 show the results of free-water and FAt in the 5 ROIs of diffusion MRI. The results of free-water showed no significant effect of antiparkinsonian medication in the caudate, putamen, subthalamic nucleus, anterior substantia nigra, and posterior substantia nigra. Also, the results of FAt showed no significant effect of antiparkinsonian medication in the caudate, putamen, subthalamic nucleus, anterior substantia nigra, and posterior substantia nigra (Table 3). The uncorrected p-values did not approach significance.
Fig. 1.
dMRI measurements (Free-water and FAt) differences between OFF and ON-medication. Mean (± SE) dMRI measurements in the 5 dMRI-ROIs respectively. Abbreviation: a = anterior; FAt = free-water corrected fractional anisotropy; p = posterior; SN = substantia nigra; STN = subthalamic nucleus.
Table 3.
Diffusion MRI.
| Diffusion MRI (FW | FAt) | OFF-medication | ON-medication | p-Value | pFDR |
|---|---|---|---|---|
| FW - Caudate | 0.176 (0.038) | 0.179 (0.032) | 0.569 | 0.951 |
| FW - Putamen | 0.164 (0.035) | 0.162 (0.033) | 0.358 | 0.951 |
| FW - STN | 0.106 (0.024) | 0.108 (0.019) | 0.739 | 0.951 |
| FW - SN anterior | 0.191 (0.038) | 0.201 (0.032) | 0.274 | 0.951 |
| FW - SN posterior | 0.229 (0.070) | 0.221 (0.054) | 0.390 | 0.951 |
| FAt - Caudate | 0.209 (0.038) | 0.210 (0.036) | 0.961 | 0.972 |
| FAt - Putamen | 0.246 (0.036) | 0.246 (0.033) | 0.972 | 0.972 |
| FAt - STN | 0.526 (0.039) | 0.524 (0.022) | 0.761 | 0.951 |
| FAt - SN anterior | 0.627(0.084) | 0.631 (0.068) | 0.716 | 0.951 |
| FAt - SN posterior | 0.640 (0.056) | 0.633 (0.063) | 0.523 | 0.951 |
OFF and ON-medication mean (± SD) diffusion MRI evaluations (n = 15). p-Values are uncorrected. pFDR values are FDR corrected. Abbreviations: FAt = free-water corrected fractional anisotropy; FW = free-water; SN = substantia nigra; STN = subthalamic nucleus.
The results of the power analysis based on mean differences in free-water and FAt between the OFF and ON-medication conditions are listed in Table 4. To detect a significant effect of antiparkinsonian medication on free-water in the posterior substantia nigra at 80% power and α error = 0.005, the required sample size would be 258 patients. For the same α error = 0.005, 470 patients would be needed to detect significant effects of antiparkinsonian medication on FAt in the posterior substantia nigra. These power analyses assume the same mean difference and standard deviation as found in the current study.
4. Discussion
The current study examined if an acute administration of dopaminergic medication affects free-water and FAt in the basal ganglia. Although dopaminergic medication reduced the MDS-UPDRS III score, there was no evidence that taking dopaminergic medication affected the dMRI regional measures previously shown to progress with PD. These findings are discussed in the context of the recent literature advancing these measures as potential biomarkers of progression in PD.
Prior studies using dMRI have shown significant differences in either fractional anisotropy (FA) or mean diffusivity in the substantia nigra in PD compared to healthy controls (Du et al., 2012, Langley et al., 2016, Schwarz et al., 2013). A Cochrane meta-analysis concluded that diffusion imaging abnormalities are common findings across multiple studies and suggests dMRI could detect parkinsonian syndromes and monitor disease progression (Cochrane and Ebmeier, 2013). The novel contribution of this study is that an acute dose of dopaminergic medication failed to alter the free-water and FAt measures from the basal ganglia regions. In a longitudinal study, free-water in the posterior substantia nigra increased over one year in PD, but not in a control group (Ofori et al., 2015b). When using a single tensor diffusion tensor model, progression effects have also been observed in the substantia nigra (Loane et al., 2016). In a prior study examining a retrospective cohort of PD treated with rasagiline compared with PD not treated with rasagiline (Burciu et al., 2016), it was found that PD patients who had not taken rasagiline had significantly higher free-water in the posterior substantia nigra than PD patients who had taken rasagiline and healthy controls. Further, PD patients who had taken rasagiline had significantly higher free-water in the posterior substantia nigra than healthy controls (Burciu et al., 2016). The main differences between the prior study and the current one, is that patients were chronically taking rasagiline, whereas the current study examined a single dose of carbidopa/levodopa and other medications. Collectively, these results suggest that dMRI measurements in the basal ganglia most likely reflect the chronic state of microstructural integrity and do not seem to be altered by short-term, dopaminergic medication.
There were some caveats in the present study. The current study focused on the effects of acute dopaminergic medication on changes of dMRI. It is possible that longer-term use of dopaminergic medication could influence dMRI measurements. An example of this experimental paradigm from studies in rodents found that 4 weeks of dopaminergic treatment affected 11C-dihydrotetrabenazine (DTBZ, VMAT2 marker) and 11C-methylphenidate (MP, DAT marker) (Sossi et al., 2010). It is also the case that DTBZ, VMAT2, MP, and DAT are functional biomarkers from positron emission tomography, whereas the current biomarker is structural in nature and does not use a contrast agent. In addition, although we found significant MDS-UPDRS-III changes between ON and OFF-medication conditions, the mean changes were on the order of a few points because the patients were mild, and it is possible that moderate stage patients with a stronger drug response could lead to a different result. Studies with drug-naïve subjects and controlled doses of medication, as well as patients in various stages of the PD disease are needed to confirm the null changes of dMRI by dopaminergic medication.
In summary, we have shown for the first time that free-water and FAt in the basal ganglia are not influenced by administration of acute antiparkinsonian medication. These findings provide evidence that free-water, a proposed progression marker (Ofori et al., 2015b) is not affected by a patient-specific acute dose of antiparkinsonian medication, suggesting that this marker is measuring the chronic state of the basal ganglia. Collectively, these neuroimaging measurements such as dMRI free-water and FAt, are well-suited disease progression biomarkers to reflect the chronic state of basal ganglia in PD. These biomarkers could be useful in clinical trials testing disease-modifying therapies for PD.
Acknowledgements
This work was supported by NINDS R01 NS075012 and R01 NS052318.
References
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Braak H., Tredici K. Del, Rüb U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brooks D.J. Optimizing levodopa therapy for Parkinson's disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr. Dis. Treat. 2008;4:39–47. doi: 10.2147/ndt.s1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burciu R.G., Ofori E., Shukla P., Pasternak O., Chung J.W., McFarland N.R., Okun M.S., Vaillancourt D.E. Free-water and BOLD imaging changes in Parkinson's disease patients chronically treated with a MAO-B inhibitor. Hum. Brain Mapp. 2016;37:2894–2903. doi: 10.1002/hbm.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C.J., Ebmeier K.P. Diffusion tensor imaging in parkinsonian syndromes. Neurology. 2013;80:857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Sen S., Wang J., Shaffer M.L., Styner M., Yang Q.X., Huang X. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2012;27:1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fearnley J.M., Lees A.J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Hughes A.J.F., Ben-Shlomo Y.M., Daniel S.E.Mrcp, Lees A.J.F. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 2001;57 [PubMed] [Google Scholar]
- Langley J., Huddleston D.E., Merritt M., Chen X., McMurray R., Silver M., Factor S.A., Hu X. Diffusion tensor imaging of the substantia nigra in Parkinson's disease revisited. Hum. Brain Mapp. 2016 doi: 10.1002/hbm.23192. 10.1002/hbm.23192 (n/a-n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J.W., Widner H., Goetz C.G., Brooks D., Fahn S., Freeman T., Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov. Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Loane C., Politis M., Kefalopoulou Z., Valle-Guzman N., Paul G., Widner H., Foltynie T., Barker R.A., Piccini P. Aberrant nigral diffusion in Parkinson's disease: a longitudinal diffusion tensor imaging study. Mov. Disord. 2016;31:1020–1026. doi: 10.1002/mds.26606. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Ofori E., Pasternak O., Planetta P.J., Burciu R., Snyder A., Febo M., Golde T.E., Okun M.S., Vaillancourt D.E. Increased free water in the substantia nigra of Parkinson's disease: a single-site and multi-site study. Neurobiol. Aging. 2015;36:1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori E., Pasternak O., Planetta P.J., Li H., Burciu R.G., Snyder A.F., Lai S., Okun M.S., Vaillancourt D.E. Longitudinal changes in free-water within the substantia nigra of Parkinson's disease. Brain J. Neurol. 2015;138:2322–2331. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O., Sochen N., Gur Y., Intrator N., Assaf Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Planetta P.J., Kurani A.S., Shukla P., Prodoehl J., Corcos D.M., Comella C.L., McFarland N.R., Okun M.S., Vaillancourt D.E. Distinct functional and macrostructural brain changes in Parkinson's disease and multiple system atrophy. Hum. Brain Mapp. 2015;36:1165–1179. doi: 10.1002/hbm.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J., Li H., Planetta P.J., Goetz C.G., Shannon K.M., Tangonan R., Comella C.L., Simuni T., Zhou X.J., Leurgans S., Corcos D.M., Vaillancourt D.E. Diffusion tensor imaging of Parkinson's disease, atypical parkinsonism, and essential tremor. Mov. Disord. 2013;28:1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S.T., Abaei M., Gontu V., Morgan P.S., Bajaj N., Auer D.P. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clin. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V., Dinelle K., Schulzer M., Mak E., Doudet D.J., de la Fuente-Fernández R. Levodopa and pramipexole effects on presynaptic dopamine PET markers and estimated dopamine release. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:2364–2370. doi: 10.1007/s00259-010-1581-3. [DOI] [PubMed] [Google Scholar]
- Stocchi F., Olanow C.W. Neuroprotection in Parkinson's disease: clinical trials. Ann. Neurol. 2003;53:S87–S99. doi: 10.1002/ana.10488. [DOI] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]