Abstract
Clear cell adenocarcinoma of the cervix (CCAC) is a rare type of gynecological cancer. The risk factors and pathogenesis have yet to be clearly determined. This is a case report of a 19-year-old woman, who was never exposed to DES from her mother, who had gone for an examination for vaginal bleeding. A polypoid mass measured at 3 cm presenting in her cervix was discovered. Histological diagnosis following cervical biopsy proved the tumor to be a clear cell carcinoma. The patient was then referred to our hospital. A loop electrosurgical excision procedure (LEEP) was done and the pathological diagnosis was confirmed for clear cell carcinoma with a positive endocervical margin. Radical hysterectomy, pelvic lymphadenectomy and incidental appendectomy were achieved without any complications. The microscopic findings had revealed no residual tumor. The patient did not require adjuvant treatment. No sign of recurrence has been detected throughout 6 months of follow-up. We have performed the literature review on the clinical presentation, pathogenesis, pathology, diagnosis, treatment, and prognosis of this unusual tumor.
Keywords: Cervical cancer, Clear cell adenocarcinoma, Diethylstilbestrol, Young woman
Highlight
-
•
CCAC in adolescents continue to occur even no exposed to DES.
-
•
Risk factors and pathogenesis are still unclear.
-
•
More data are needed to conclude the association between HPV infection and CCAC.
-
•
The presenting symptom is often misdiagnosed as dysfunctional uterine bleeding.
-
•
The fertility-preserving treatment appears to be safe in selected case.
1. Introduction
Clear cell adenocarcinoma of the cervix (CCAC) is rare. The etiology and pathogenesis of the tumors are, presently, unclear. It may occur either in young women, associated with diethylstilbestrol (DES) exposure in utero from maternal use, or older women. The tumor may arise in both the ectocervix (typically, associated with DES) or the endocervix. We report on a case of CCAC in an adolescent without prior, maternal DES exposure.
2. Case report
A 19-year-old woman, without coexisting disease, reported post-coital bleeding and vaginal discharge for approximately 1 month. She has reported to be monogamous and she had first experienced sexual intercourse was at the age of 15. She had neither family history of cancer nor DES exposure. A 3-centimeter polypoid mass at her cervix was detected during a pelvic examination. A cervical biopsy and a conventional PAP smear were obtained. The cytological specimen was negative for intraepithelial lesions or malignancy (NILM). The histopathological specimen had shown cervical tissue involved with malignant infiltration consisting of large round cells with clear cytoplasm and glandular formation. These findings were suggestive of a clear cell carcinoma tumor. She was referred to the gynecological oncology unit of our hospital in March 2016. We were unable to locate a cervical mass. However, a small ulcer at upper lip of cervix was discovered. On pelvic and rectal examinations, both parametriums were smooth. The high-risk human papilloma virus (hrHPV) test resulted positive for human papilloma virus (HPV) type 51. A colposcopy was performed and revealed a transformation zone type 1 with an ulcerative lesion positioned at 10–2 o'clock with coarse punctuation and a mosaic pattern at the 5 o'clock position. A loop electrosurgical excision procedure (LEEP) was performed and the histopathological typing was consistent with a clear cell carcinoma (Fig. 1, Fig. 2). Nonetheless, the endocervical margins were not free. All parameters from blood chemistry analysis were within normal ranges. Chest X-ray and Anti-HIV antibody tests were negative. The patient was diagnosed with CCAC; stage Ib1, in accordance to the International Federation of Obstetricians and Gynecologists (FIGO) classification. She was then admitted as an inpatient on May 2016. A radical abdominal hysterectomy, bilateral pelvic lymphadenectomy, bilateral salpingectomy and an incidental appendectomy were performed. During the operation, there was a small accumulation of serous fluid in the pelvic cavity. No sign of extra-uterine spread of neoplastic disease was observed. The postoperative period was without complications and unremarkable. The final specimen had revealed no residual clear cell tumor. Cervical intraepithelial neoplasia 3 (CIN3) was observed in all quadrants. Free vaginal resection margins from the CIN3 in all quadrants were shown. Both sides of the parametrium were unremarkable. No evidence of metastatic carcinoma in any lymph nodes was indicated. After the surgery, the plan was to undergo follow-up without adjuvant treatment. Up to the time of writing this case report and it has been 6 months post operatively, she remains free of disease.
Fig. 1.
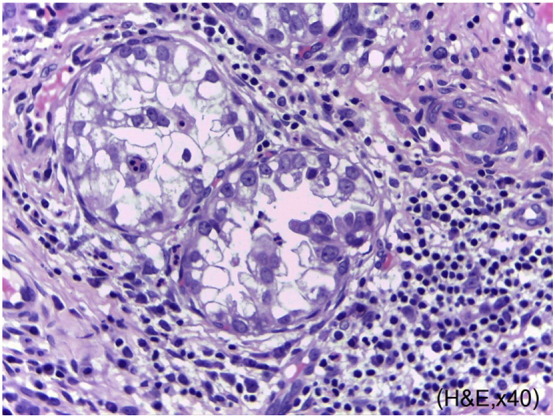
Tumor cells are composed of a tubulocystic pattern of cells with abundant clear cytoplasm and large irregular nuclei. (Hematoxyline and eosin staining, original magnification 40 ×.)
Fig. 2.
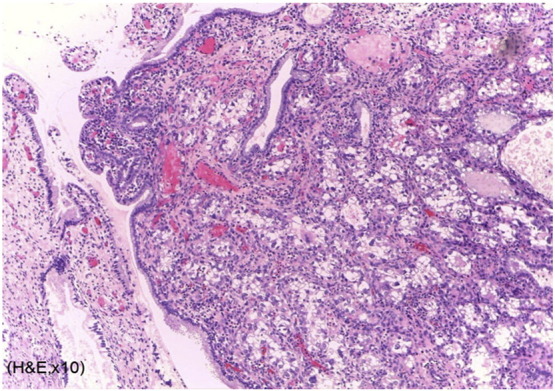
Microscopically, the tumor cells invade endocervical tissue without definite lymphatic invasion. (Hematoxyline and eosin staining, original magnification 10 ×.)
3. Discussion
In the post-DES era, the prevalence of CCAC was found to be approximately 4–9% of the cases of adenocarcinoma of the cervix (Reich et al., 2000). There are clearly separated peaks of incidence in young and advanced aged women who have been exposed to DES in utero. The first peak occurs in women who are 17 to 37 years of age (mean age, 26 years), while the second peak arises in women who are 44 to 88 years of age (mean age, 71 years) (Jiang et al., 2014). In contrast, Thomas et al., and Seki et al., suggest that the mean ages of non-DES exposed women was at 53 and 50.8 years old, respectively (Thomas et al., 2008, Seki et al., 2003). The etiology and pathogenesis of CCAC remain unclear. Risk factors of CCAC are not associated with risk factors of squamous cell carcinoma (SCC). These factors include; advanced age, epidemiological risk, multiple sexual partners, smoking, socioeconomic status, contraceptive use and infection by human papillomavirus (HPV) (Thomas et al., 2008, Pirog et al., 2014). HPV DNA was not detected in any of the non-mucinous adenocarcinomas including clear cell, serous, and mesonephric carcinomas (Pirog et al., 2014). Only a few studies have explored the association between hrHPV and CCAC. Most of the HPV-positive CCAC cases have been confirmed with HPV 18 and 31 DNA sequences (Garg & Arora, 2012). In this study, the hrHPV test result was positive for type 51 which has a high oncogenic potential. It may be either a cofactor in the development of CCAC or a coincidental finding. Therefore, more data is needed to confirm the association between HPV infection and CCAC. One study has found that maternal DES exposure, incident of a previous maternal miscarriage, birth during the autumn months, and premature births are risk factors for CCAC in young women (Baykara et al., 2014). As in clear cell ovarian cancer, there have been case reports that have suggested an association between CCAC and cervical endometriosis, however, the evidence is far from being confirmed (Hiromura et al., 2009).
The microscopic patterns of CCAC are arranged as; solid, tubulocystic, papillary patterns or a combination of features (Hasegawa et al., 2014). The tumor in a solid pattern displays sheets of cells containing abundant glycogen-rich clear cytoplasm, an atypical nuclei, and mitoses. The tubulocystic pattern contains tubules and cystic spaces outlined by oxyphilic, hobnail, or clear cells. The papillary pattern is the least common variant and often coexists with the solid or tubulocystic areas. The most favorable outcome is associated with the tubulocystic pattern, followed by papillary and solid patterns, respectively.(Hasegawa et al., 2014). The patient in this study presented with vaginal bleeding, which is the most common initial symptom. In young patients, the condition is often misdiagnosed as dysfunctional uterine bleeding or a vaginal tumor (Thomas et al., 2008). During the pelvic examination, 81% of the cervix appeared to be abnormal (Jiang et al., 2014). It was found that 80% of the cervix was involved with endophytic growth with a high possibility of deep cervical infiltration (Reich et al., 2000). Cytology has been found to be less efficient in diagnosing cervical adenocarcinomas (Jiang et al., 2014, Thomas et al., 2008). Thomas and colleagues reported that only 6 of 31 CCAC patients (18%) had an abnormal Pap test (Thomas et al., 2008). Cytology was of no benefit in the diagnosis in this case.
Patients with CCAC were reported to have a worse 5-year survival rate (67%) than those with SCC (80%) and non-clear cell carcinomas (77%) but without statistical significance (Thomas et al., 2008). A study had previously reported that the prognosis of patients with early-stage CCAC was not inferior to other types of cervical adenocarcinomas (Jiang et al., 2014). In comparison with SCC of the cervix, CCAC has a higher risk of lymph node metastasis and more frequently extends to the uterine corpus and parametrium (Reich et al., 2000, Thomas et al., 2008). The clear cell histology, by itself, does not appear to portend a poor prognosis. Most CCAC patients were diagnosed in the early stages (I/II) (Jiang et al., 2014, Hasegawa et al., 2014). The overall survival rate of patients with stages I/II of CCAC is excellent (81.5–91%) (Jiang et al., 2014, Thomas et al., 2008).
Surgery is the definitive treatment of CCAC in the early stages (Thomas et al., 2008, Seki et al., 2003). Important negative prognostic factors are; a tumor size more than 4 cm, a presence of positive lymph node metastasis, and carcinoma in advanced stages (Reich et al., 2000, Jiang et al., 2014, Thomas et al., 2008). These factors are similar to what has been found in patients with SCC. Additionally, when considering the pathological findings, the unfavorable prognosis is also associated with a presence of nuclear atypia, a high mitotic activity, and a solid or mixed growth pattern. The most common extra-pelvic sites of recurrence are the lungs, liver, and skeletal system (Reich et al., 2000). Adjuvant radiotherapy appears to be effective in the local control and should be administered using similar indications as is used in the high-risk, early stage SCC (Thomas et al., 2008). The median time for tumor recurrence in stages I and II, CCAC, was at 12 months (Reich et al., 2000, Thomas et al., 2008). Vaginal recurrence commonly occurred at 3.5 to 4 years (Thomas et al., 2008).
In conclusion, this case study exhibits a CCAC arising in a young adult who presented with vaginal bleeding. Differential diagnosis of carcinoma of cervix and vagina should be considered even without a history of exposure to DES. According to the literature review, tumor cells in this case, were arranged in a tubulocystic pattern and there was no residual disease in the pathology in the specimen obtained from the radical hysterectomy. This is indicative of a favorable prognosis. We also reviewed the literature of CCAC in the young women without DES exposure from the period of 2000–2016 (Table 1). Nearly all of the cases occurred in women of Asian descent and it may be one epidemiological risk of CCAC in the young women. New cases of CCAC of cervix continue to occur although there has been no exposure to DES. Moreover, most of the cases were diagnosed in the early stages and had a favorable prognosis. In selected young patients who present with favorable prognostic factors such as a small tumor size and is well differentiated, a fertility-preserving treatment appears to be safe and should be considered as a viable option. However, in one case, the patient developed extra pelvic metastasis to the lungs and brain despite having received adjuvant chemotherapy. This may be explained by the negative prognostic factors obtained from the microscopic findings. In the surveillance program; pelvic examination, cytology, and imaging techniques (CXR, IVP and CT) should be considered.
Table 1.
Reported cases of clear cell carcinoma in young women without DES exposure; literature search during 2000–2016 period.
| Age (years old) | FIGO stage | Tumor size (cm) | Treatment | Follow-up period (months) | Outcome | |
|---|---|---|---|---|---|---|
| Seki et al. (2003) | 18 | Ib2 | 6 | NAC (mitomycin etoposide carboplatin) RH, BPLND | 48 | Free of disease |
| Ding et al. (2004) | 19 | Ib2 with pelvic node metastasis | 8 | Conization, BPLND, BOT Adjuvant RT | 24 | Free of disease |
| Ahrens et al. (2005) | 6 | Ib1 | 2 | Partial trachelectomy | 12 | Free of disease |
| Yabushita et al. (2008) | 17 | Ib1 | 1.5 | RH, BPLND | 24 | Free of disease |
| Baykara et al. (2014) | 16 | Ib1 | 3 | RH, BPLND, adjuvant CT (carboplatin, paclitaxel) | 24 | Free of disease |
| 14 | Ib2 | 6 | TAH BSO BPLND | 24 | Free of disease | |
| Choi (2013) | 15 | IIA pelvic LN metastasis | 7 | RH BPND BOT adjuvant CT (carboplatin, paclitaxel) | 6 | Free of disease |
| Ashton et al. (2013) | 18 pregnant 33 week | Ib1 | 0.6 | Cesarean section at 34 weeks then RH BPND BSO | 36 | Free of disease |
| Andi Asri et al. (2016) | 10 | Ib2 | unknown (pelvic mass 14 cm) | RH, BPLND adjuvant CT (carboplatin + paclitaxel) | 3 | Recurrent in lung and brain |
DES, diethylstilbestrol; TAH, total abdominal hysterectomy; RH, radical hysterectomy; BSO, bilateral salpingoophorectomy; BPLND, bilateral pelvic node dissection; BOT; bilateral ovarian transposition; NAC, neoadjuvant chemotherapy; CT, chemotherapy; RT, radiotherapy.
Consent
Written, informed consent was obtained from the patient for publication of this case report and accompanying images.
Potential conflicts of interest
None.
Acknowledgement
The authors are grateful to the patient, her family, and the medical records audit team at the HRH Princess Maha Chakri Sirindhorn Medical Center for all their support.
Footnotes
Funding sources: None.
References
- Reich O., Tamussino K., Lahousen M., Pickel H., Haas J., Winter R. Clear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage IB-IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol. Oncol. 2000;76(3):331–335. doi: 10.1006/gyno.1999.5700. [DOI] [PubMed] [Google Scholar]
- Jiang X., Jin Y., Li Y., Huang H.F., Wu M., Shen K. Clear cell carcinoma of the uterine cervix: clinical characteristics and feasibility of fertility-preserving treatment. Onco. Targets Ther. 2014;7:111–116. doi: 10.2147/OTT.S53204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.B., Wright J.D., Leiser A.L., Chi D.S., Mutch D.G., Podratz K.C. Clear cell carcinoma of the cervix: a multi-institutional review in the post-DES era. Gynecol. Oncol. 2008;109(3):335–339. doi: 10.1016/j.ygyno.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H., Takada T., Sodemoto T., Hoshino H., Saitoh K., Uekusa T. A young woman with clear cell adenocarcinoma of the uterine cervix. Int. J. Clin. Oncol. 2003;8(6):399–404. doi: 10.1007/s10147-003-0358-0. [DOI] [PubMed] [Google Scholar]
- Pirog E.C., Lloveras B., Molijn A., Tous S., Guimera N., Alejo M. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod. Pathol. 2014;27(12):1559–1567. doi: 10.1038/modpathol.2014.55. [DOI] [PubMed] [Google Scholar]
- Garg M.M., Arora V.K. Clear cell adenosquamous carcinoma of the cervix: a case report with discussion of the differential diagnosis. Int. J. Gynecol. Pathol. 2012;31(3):294–296. doi: 10.1097/PGP.0b013e31823b6f37. [DOI] [PubMed] [Google Scholar]
- Baykara M., Benekli M., Erdem O., Taskiran C., Demirci U., Vargol E. Clear cell adenocarcinoma of the uterine cervix: a case report and review of the literature. J. Pediatr. Hematol. Oncol. 2014;36(2):e131–e133. doi: 10.1097/MPH.0b013e318290cb1b. [DOI] [PubMed] [Google Scholar]
- Hiromura T., Tanaka Y.O., Nishioka T., Satoh M., Tomita K. Clear cell adenocarcinoma of the uterine cervix arising from a background of cervical endometriosis. Br. J. Radiol. 2009;82(973):e20–e22. doi: 10.1259/bjr/75304693. [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Nagao S., Yasuda M., Millan D., Viswanathan A.N., Glasspool R.M. Gynecologic cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the uterine corpus and cervix. Int. J. Gynecol. Cancer. 2014;24(9 Suppl 3):S90–S95. doi: 10.1097/IGC.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Andi Asri A.A., Lim B.K., Lim Y.K., Latiff L.A. Clear cell adenocarcinoma of the cervix in a ten-year-old girl without prenatal diethylstilbestrol exposure. Singap. Med. J. 2016;57(8):470. doi: 10.11622/smedj.2016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D.C., Chang F.W., Yu M.H. Huge clear cell carcinoma of the cervix in teenager not associated with diethylstilbestrol: a brief case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;117(1):115–116. doi: 10.1016/j.ejogrb.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Ahrens W.A., Barron-Rodriguez L.P., McKee M., Rivkees S., Reyes-Mugica M. Clear cell adenocarcinoma of the cervix in a child without in utero exposure to diethylstilbestrol: a case report and review of the literature. Pediatr. Dev. Pathol. 2005;8(6):690–695. doi: 10.1007/s10024-005-0047-2. [DOI] [PubMed] [Google Scholar]
- Yabushita H., Kanyama K., Sekiya R., Noguchi M., Wakatsuki A. Clear-cell adenocarcinoma of the uterine cervix in a 17-year-old adolescent. Int. J. Clin. Oncol. 2008;13(6):552–554. doi: 10.1007/s10147-008-0781-3. [DOI] [PubMed] [Google Scholar]
- Choi S.J. Clear cell adenocarcinoma of the uterine cervix in a 15-year-old girl: a case report. J. Korean Soc. Radiol. 2013;69(4):321–325. [Google Scholar]
- Ashton E., Brown A., Hoffman J., Khutti S. Clear cell adenocarcinoma of the uterine cervix in an 18 year-old pregnant female. Gynecol. Oncol. Case Rep. 2013;5:49–51. doi: 10.1016/j.gynor.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


