Abstract
The aim of this study was to examine the impact of well‐controlled uncomplicated type 2 diabetes (T2D) on exercise performance. Ten obese sedentary men with T2D and nine control participants without diabetes matched for age, sex, and body mass index were recruited. Anthropometric characteristics, blood samples, resting cardiac, and pulmonary functions and maximal oxygen uptake (VO2max) and ventilatory threshold were measured on a first visit. On the four subsequent visits, participants (diabetics: n = 6; controls: n = 7) performed step transitions (6 min) of moderate‐intensity exercise on an upright cycle ergometer from unloaded pedaling to 80% of ventilatory threshold. VO2 (τVO2) and HR (τ HR) kinetics were characterized with a mono‐exponential model. VO2max (27.0 ± 3.4 vs. 26.7 ± 5.0 mL kg−1 min−1; P = 0.85), τVO2 (43 ± 6 vs. 43 ± 10 sec; P = 0.73), and τ HR (42 ± 17 vs. 43 ± 13 sec; P = 0.94) were similar between diabetics and controls respectively. The remaining variables were also similar between groups, with the exception of lower maximal systolic blood pressure in diabetics (P = 0.047). These results suggest that well‐controlled T2D is not associated with a reduction in VO2max or slower τVO2 and τ HR.
Keywords: Heart rate kinetics, maximal oxygen uptake, oxygen uptake kinetics, type 2 diabetes
Introduction
The presence of type 2 diabetes, with or without cardiovascular complications, is often associated with reduced maximal oxygen uptake (VO2max) (Schneider et al. 1984; Kjaer et al. 1990; Regensteiner et al. 1995, 1998, 2009; Estacio et al. 1998; Baldi et al. 2003; Fang et al. 2005; Gusso et al. 2008; Lalande et al. 2008; Nadeau et al. 2009; MacAnaney et al. 2011; Wilkerson et al. 2011; O'Connor et al. 2012). Type 2 diabetes also seems to affect submaximal VO2 response, however, results are equivocal. A study conducted in older men (65 ± 5 years) with well‐controlled type 2 diabetes and long disease duration (>5 years) (Wilkerson et al. 2011) reported no difference in VO2 kinetics compared to control participants without diabetes, while others reported a slower VO2 kinetics in pre‐ and postmenopausal women with type 2 diabetes (Regensteiner et al. 1998; MacAnaney et al. 2011; O'Connor et al. 2012), adolescents with type 2 diabetes (Nadeau et al. 2009) and middle‐aged men with type 2 diabetes (O'Connor et al. 2012, 2015).
The regulation of systemic oxygen delivery at the onset of exercise partly rely on changes in heart rate (HR). The adjustment of HR at the onset of exercise (e.g. HR kinetics), thus provides additional insights regarding the influence of central blood flow adjustment and O2 delivery (Jones and Poole 2005), on exercise capacity. To our knowledge, only a few studies have evaluated HR kinetics in men with type 2 diabetes (Wilkerson et al. 2011; O'Connor et al. 2012). Recently, O'Connor et al. (2015) have demonstrated slowed HR kinetics in older men with type 2 diabetes.
Taken together, these findings support the fact that this metabolic disorder eventually affects negatively numerous human body functions depending on patients' glycemic control, disease duration as well as the presence of comorbidities due to diabetes. Whether abnormal exercise capacity is the result of type 2 diabetes per se or a consequence of its associated comorbidities is not clear, as most of the studies on men have been conducted in patients with type 2 diabetes without optimal glycemic control (Wilkerson et al. 2011; O'Connor et al. 2012). Evidence of subclinical abnormalities in autonomic (Maser and Lenhard 2005) and cardiac function (Poirier et al. 2000; Baldi et al. 2006; Brassard et al. 2006; Poanta et al. 2011) have been reported in patients with diabetes. Reduced heart rate variability (Roy et al. 1989), left ventricular diastolic dysfunction (LVDD) (Poirier et al. 2003) and lower lung capacity (Kitahara et al. 2010) have all been associated with reduced maximal exercise capacity in patients with type 2 diabetes. Still, the influence of type 2 diabetes per se on VO2 and HR kinetics in uncomplicated well‐controlled middle‐aged men remains unknown.
The aim of this study was to evaluate the impact of well‐controlled, uncomplicated type 2 diabetes on VO2max as well as VO2 and HR kinetics in obese sedentary middle‐aged type 2 diabetes men compared to control participants matched for sex, age and body mass index. A secondary objective of this study was to provide an integrative view of the impact of type 2 diabetes, including metabolic control, lipid profile, cardiopulmonary function, cardiac structures, and heart rate variability, in relationship to exercise performance. We hypothesized that the presence of well‐controlled, uncomplicated type 2 diabetes would be associated with a reduction in VO2max but similar VO2 and HR kinetics compared to participants without diabetes.
Material and Methods
Participants
Ten sedentary men with type 2 diabetes and nine control participants without diabetes matched for age, sex, and body mass index were recruited for this study. This control group, which represents a real life situation considering the impacts of an inactive lifestyle, age and overweight/obesity on human body systems and exercise performance (Booth et al. 2002; Poirier et al. 2006; Prasad et al. 2007), strengthen the study design. Type 2 diabetes was diagnosed according to American Diabetes Association criteria (American Diabetes Association, 2007). All subjects with diabetes were managed with diet, except two who were treated with metformin alone. No participants were using insulin or any cardiovascular drug regimen for the treatment of other diseases/comorbidities. Exclusion criteria for both groups were the documented presence of cardiovascular disease, a documented office blood pressure above 140/90 mmHg and clinically significant end‐organ complications related to diabetes, i.e. renal failure (creatinine above normal upper limit), macroalbuminuria, proliferative retinopathy, clinically significant sensitive, motor, or autonomic neuropathies as well as the participation to a structured exercise training program. The local ethics committee approved the study, in accordance with the Helsinki declaration, and all participants gave signed informed consent.
Experimental design
Participants visited our laboratory on five different occasions. Blood profile and resting echocardiographic variables, pulmonary function, heart rate variability, and VO2max were evaluated on the first visit (V1). Then, participants performed square‐wave transitions from unloaded pedaling to moderate‐intensity exercise on four different visits separated by 48 h (V2–V5) to determine VO2 and HR kinetics.
Methodology
Blood samples
Upon participant arrival at the laboratory (V1), a 20‐gauge polyethylene catheter was inserted into a forearm vein for blood sampling. Blood samples were drawn at rest after an overnight fasting. Fasting blood glucose was measured using the hexokinase method (Roche Diagnosis, Indianapolis, Indiana). Glycated hemoglobin was assayed using the ion‐exchange high‐performance liquid chromatography method (Bio‐Rad, Hercules, California). Serum cholesterol, triglycerides and high‐density lipoprotein‐cholesterol were analyzed as previously described (Poirier et al. 2000, 2001). Low‐density lipoprotein‐cholesterol was calculated using Friedewald's formula (Friedewald et al. 1972). The cholesterol/high‐density lipoprotein ratio was also calculated.
Echocardiography
Standard parasternal, short‐ and long‐axis and apical views were performed in accordance with the recommendations of the American Society of Echocardiography (Rakowski et al. 1996) with the same observer obtaining all recordings and measurements (Sonos 5500; Hewlet Packard, Andover, Massachusetts) (Poirier et al. 2001). Left atrial volume (LAV) and left ventricular (LV) systolic and diastolic volumes were calculated using the modified Simpson's method. Left ventricular mass (LVM) and wall thickness were evaluated by M‐mode Doppler. LVM was calculated using the following formula (Canadian Cardiovascular Society and the Canadian Hypertension Society, 1995): LVM (g) = 0.8 × 1.04 [(LVEDD + IVST + PWT)3 − (LVEDD)3] + 0.6, where LVEDD is the LV end diastolic dimension, IVST the interventricular septal thickness, and PWT the posterior wall thickness. LAV and LVM were indexed for body surface area (Canadian Cardiovascular Society and the Canadian Hypertension Society, 1995). Ejection fraction was evaluated using the Simpson's method.
Resting LVDD was evaluated using standardized criteria (Dumesnil et al. 1991; Poirier et al. 2001). First, transmitral pulsed Doppler recordings were obtained to measure the following parameters: peak E velocity in cm sec−1 (peak early transmitral filling velocity during early diastole), peak A velocity in cm sec−1 (peak transmitral atrial filling velocity during late diastole), deceleration time in msec (time elapsed between peak E velocity and the point where the extrapolation of the deceleration slope of the E velocity crosses the zero baseline) and E/A ratio (peak E wave velocity divided by peak A wave velocity). In order to reduce the high filling pressures encountered in the pseudonormalized pattern of LV filling, the same measurements were repeated during phase II of the Valsalva maneuver (Poirier et al. 2001). Tissue Doppler velocities were measured at the mitral annular level of the LV septum, to provide additional information about filling pressure. Early (Ea) and late (Aa) velocities were measured and E/Ea ratio was calculated (Dumesnil et al. 2002). LVDD was characterized as normal, abnormal relaxation, pseudonormal pattern and restrictive pattern. Subjects had a normal LV diastolic function if a deceleration time between 140 and 240 msec, an E/A ratio between 1 and 2 and an E/Ea ratio <8 were present. LVDD was characterized as abnormal relaxation if subjects had a deceleration time >240 msec and an E/A ratio <1. LVDD was characterized as a pseudonormal filling pattern if subjects had a deceleration time between 150 and 240 msec, an E/A ratio between 1 and 2 and an E/Ea ratio >15 (Ommen et al. 2000), or a reduction in the E/A ratio >0.5 following the Valsalva maneuver. Finally, LVDD was characterized as a restrictive filling pattern if subjects had a deceleration time <140 msec and E/A ratio >2.
Pulmonary function tests
Standard pulmonary function tests, including body plethysmography, spirometry and single‐breath diffusing capacity of the lung for carbon monoxide [DLCO] were performed in participants (American Thoracic Society, 1995).
Heart rate variability
Heart rate variability was derived from a 24‐h Holter monitoring system (Marquette Electronics, Milwaukee, Wisconsin) in all participants during normal daily life activity. Heart rate variability derived from 24‐h ambulatory monitoring is reproducible and free of placebo effect (European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Within the 24‐h evaluation, three periods were arbitrarily assessed: (1) 24 h; (2) daytime period defined as 8:00 am to 8:00 pm and; (3) night‐time period defined as 12:00 am to 6:00 am (Poirier et al. 2003). In the frequency domain, power in the low‐frequency (0.04–0.15 Hz), and high‐frequency (0.15–0.4 Hz) ranges were calculated. The low frequency/high frequency ratio, considered to be a marker of the ratio of sympathetic to parasympathetic balance, was also determined (Bogaty et al. 2003). Using time domain analysis, the standard deviation (SD) of the RR intervals (SDNN), the square root of the mean squared differences of successive RR intervals (rMSSD), and the SD of the average RR intervals were calculated over 5‐min periods (SDANN) and the average of the SD of RR intervals for all 5‐min periods (ASDNN) were determined. pNN50 is the proportion of interval differences of successive NN intervals >50 msec. rMSSD and pNN50 are indices of parasympathetic modulation. NN intervals are the normal‐to‐normal intervals that include all intervals between adjacent QRS complexes resulting from sinus node depolarizations in the entire 24‐h electrocardiogram recording. The complete signal was carefully edited using visual checks and manual corrections of individual RR intervals and QRS complex classifications.
Blood pressure and heart rate
Following 15 min of quiet rest in a supine position, resting arterial blood pressure and heart rate were measured with the participants seated using an automated sphygmomanometer with headphone circuit option (Model 412, Quinton Instrument Co., Bothell, Washington) and 12‐lead ECG monitoring, respectively, before the maximal exercise protocol.
Maximal exercise protocol
VO2max was evaluated using a ramp incremental exercise protocol of 20 watts min−1 following a warm‐up period of 1 min of unloaded pedaling, performed on an electromagnetically braked upright cycle ergometer (Lode Corival, Lode, Groningen, Netherlands) at a pedaling rate of 50–70 rpm. Expired air was continuously collected for the determination of pulmonary VO2, carbon dioxide production (VCO2), pulmonary ventilation (VE) and the respiratory exchange ratio (RER) (VCO2/VO2), on a breath‐by‐breath basis (Medgraphics, CPX Ultima, St Paul, Minnesota). Heart rate was measured using ECG monitoring during the test. Participants exercised until volitional exhaustion. VO2max was defined as the mean VO2 recorded in the last 15 sec of the ramp protocol concurrent with a RER ≥ 1.15. The ventilatory threshold was evaluated with the V‐slope method (Beaver et al. 1986). The exercise protocol was always performed at the same time of the day at a room temperature of 19°C.
Square‐wave exercise protocol
Each participant performed four square‐wave exercise protocols, from unloaded pedaling to 80% ventilatory threshold, on four different visits separated by at least 48 h. Pulmonary gas exchange and HR were continuously monitored during exercise.
Data analysis and statistics
VO2 and HR kinetics analysis
Breath‐by‐breath data were filtered and linearly interpolated to provide second‐by‐second values, then time‐aligned to the onset of exercise and ensemble‐averaged into 5‐sec bins. The phase 1 response (approximately 20 sec) was omitted (Murias et al. 2011) and a mono‐exponential equation was used to fit the data (Origin software, OriginLab, Northampton, Massachusetts):
where VO2(t) represents VO2 as a function of time t; VO2baseline represents the mean VO2 in the baseline period (unloaded pedaling); Amp is the amplitude or the difference between the baseline and steady‐state VO2, TD is the time delay before the onset of exercise and τVO2, the phase 2 time constant, representing the time required to reach 63% of the steady‐state response.
The same equation was used for the HR kinetics analysis, starting at time 0. Accordingly, there was not time delay incorporated into the model employed to describe HR kinetics.
Statistical analysis
After confirmation of distribution normality using Shapiro‐Wilk normality tests, Student's unpaired t‐tests were used to compare variables between groups. All data is presented as mean ± standard deviation unless otherwise specified. A P value <0.05 was considered statistically significant. A sample size calculation was based on previous work that studied VO2 kinetics (Regensteiner et al. 1998; Brandenburg et al. 1999) in women with type 2 diabetes. Accordingly, eight participants per group were necessary in order to report a statistically significant difference of 15 ± 10 sec in τVO2 between our two groups with a power of 80% and a P < 0.05.
Results
Fasting blood glucose was higher in diabetics versus controls (P = 0.04). Although systolic blood pressure tended to be reduced in diabetics at rest (P = 0.06), the remaining baseline characteristics, resting hemodynamics, metabolic profile (Table 1), cardiac structures, baseline LV systolic function, heart rate variability (Table 2), and pulmonary function (Table 3) were all similar between groups. As for the LV diastolic function, lower septal Ea (P < 0.05) and higher LV filling pressure (measured by the septal E/Ea ratio; P = 0.04) were observed in diabetics versus controls. Five diabetics and five control participants had LVDD.
Table 1.
Baseline characteristics, blood profile and resting hemodynamics in participants with type 2 diabetes compared to control participants
| Diabetes group | Control group | P | |
|---|---|---|---|
| N | 10 | 9 | – |
| Age (years) | 55 ± 8 | 55 ± 9 | 0.95 |
| Height (m) | 1.75 ± 0.06 | 1.75 ± 0.05 | 0.94 |
| Body weight (kg) | 92 ± 16 | 92 ± 18 | 0.97 |
| BMI (kg m−2) | 30 ± 4 | 30 ± 5 | 0.99 |
| Diabetes duration (months)a | 13 (4–138) | – | – |
| FBG (mmol L−1) | 6.5 ± 1.7 | 5.2 ± 0.5 | 0.04 |
| HbA1c (%) | 6.1 ± 0.6 | 5.7 ± 0.3 | 0.12 |
| HR (bpm) | 68 ± 8 | 68 ± 9 | 0.94 |
| SBP (mmHg) | 134 ± 11 | 143 ± 9 | 0.06 |
| DBP (mmHg) | 83 ± 8 | 88 ± 7 | 0.24 |
| Cholesterol (mmol L−1) | 5.1 ± 1.0 | 5.2 ± 0.9 | 0.68 |
| Triglycerides (mmol L−1) | 1.9 ± 0.9 | 1.6 ± 0.8 | 0.54 |
| HDL‐C (mmol l−1) | 1.3 ± 0.2 | 1.2 ± 0.3 | 0.84 |
| LDL‐C (mmol l−1) | 3.0 ± 0.8 | 3.3 ± 0.8 | 0.41 |
| Total‐Cholesterol/HDL | 4.2 ± 1.1 | 4.6 ± 1.4 | 0.56 |
Values are means (SD).
BMI, Body mass index; HR, Heart rate; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FBG, Fasting blood glucose; HbA1c, Glycated hemoglobin; HDL‐C, High‐density lipoprotein cholesterol; LDL‐C, Low‐density lipoprotein cholesterol.
Data presented as median (minimum‐maximum).
Table 2.
Cardiac structures and functions and heart rate variability in participants with type 2 diabetes compared to control participants
| Diabetes group | Control group | P | |
|---|---|---|---|
| Baseline | |||
| Septum (mm) | 10.4 ± 1.4 | 10.2 ± 1.1 | 0.77 |
| LV diameter diastolic (mm) | 51.4 ± 4.4 | 48.8 ± 4.4 | 0.43 |
| LV diameter systolic (mm) | 28.4 ± 8.2 | 31.6 ± 6.3 | 0.37 |
| Posterior wall (mm) | 11.3 ± 6.7 | 9.2 ± 1.2 | 0.37 |
| LV mass index (g m−2) | 87.6 ± 9.0 | 85.2 ± 15.3 | 0.68 |
| LVOT diameter (mm) | 22.6 ± 1.4 | 22.2 ± 2.2 | 0.66 |
| LA volume (mm) | 24.4 ± 5.5 | 21.4 ± 5.6 | 0.26 |
| E wave (cm sec−1) | 67.7 ± 10.6 | 70.7 ± 11.2 | 0.56 |
| A wave (cm sec−1) | 53.2 ± 10.4 | 58.8 ± 13.8 | 0.33 |
| E/A | 1.3 ± 0.3 | 1.3 ± 0.4 | 0.79 |
| DT (msec) | 252 ± 50 | 224 ± 40 | 0.21 |
| EF (%) | 58 ± 5 | 59 ± 7 | 0.49 |
| Valsalva | |||
| E wave (cm sec−1) | 50.9 ± 6.4 | 50.5 ± 12.0 | 0.95 |
| A wave (cm sec−1) | 48.6 ± 14.9 | 47.4 ± 13.6 | 0.87 |
| E/A | 1.2 ± 0.4 | 1.2 ± 0.6 | 0.76 |
| DT (msec) | 292 ± 76 | 263 ± 83 | 0.51 |
| Tissue Doppler | |||
| Ea septal (cm sec−1) | 7.8 ± 2.2 | 10.7 ± 2.6 | 0.02 |
| Ea lateral (cm sec−1) | 11.3 ± 2.8 | 11.9 ± 2.3 | 0.61 |
| Aa septal (cm sec−1) | 11.2 ± 0.9 | 11.5 ± 1.7 | 0.74 |
| Aa lateral (cm sec−1) | 11.0 ± 2.9 | 12.0 ± 3.0 | 0.49 |
| E/Ea septal | 9.0 ± 2.8 | 6.7 ± 1.6 | 0.04 |
| E/Ea lateral | 6.4 ± 1.9 | 6.1 ± 1.2 | 0.72 |
| Heart rate variability | |||
| Time domain | |||
| Average of all NN (n) | 791 ± 73 | 770 ± 106 | 0.63 |
| SDNN (msec) | 143 ± 45 | 148 ± 36 | 0.78 |
| SDANN (msec) | 128 ± 47 | 137 ± 32 | 0.65 |
| ASDNN (msec) | 55 ± 15 | 48 ± 15 | 0.33 |
| rMSSD (msec) | 24 ± 6 | 24 ± 11 | 0.83 |
| pNN50 (%) | 4.6 ± 3.0 | 6.0 ± 7.0 | 0.57 |
| Frequency domain | |||
| VLFln (msec2) | 6.8 ± 0.6 | 6.4 ± 0.6 | 0.23 |
| LFln (msec2) | 6.1 ± 0.7 | 5.7 ± 0.6 | 0.19 |
| HFln (msec2) | 4.4 ± 0.7 | 4.4 ± 0.9 | 0.85 |
| LF/HF ratio | 5.6 ± 2.3 | 4.4 ± 2.3 | 0.27 |
Values are means (SD).
LV, Left ventricular; LA; Left atrial; LVOT, Left ventricular outflow track; E, Mitral early diastolic velocity; A, Mitral late diastolic velocity; DT, Deceleration time; Ea, Mitral annulus early diastolic velocity; Aa, Mitral annulus late diastolic velocity; NN, Normal to normal interval; SDNN, Standard deviation of all NN intervals; SDANN, SD of the average NN intervals for all 5‐min segments; ASDNN, average of the standard deviation of NN intervals for all 5‐min segments; rMSSD, Square root of the mean of the squared differences between adjacent NN intervals; pNN50, NN50 count divided by the total number of all NN intervals; VLF, Very low frequency; LF, Low frequency; HF, High frequency; LF/HF ratio, Low frequency to high frequency ratio.
Table 3.
Pulmonary function at rest and maximal exercise parameters in participants with diabetes and control participants
| Diabetes group | Control group | P | |
|---|---|---|---|
| Pulmonary function | |||
| Total lung capacity (L) | 6.8 ± 0.7 | 7.0 ± 1.1 | 0.56 |
| Vital capacity (L) | 5.1 ± 0.5 | 5.2 ± 0.9 | 0.75 |
| Forced vital capacity (L) | 5.0 ± 0.5 | 5.0 ± 1.0 | 1.0 |
| FEV1 (L) | 3.8 ± 0.4 | 3.7 ± 0.7 | 0.96 |
| Residual volume (L) | 1.7 ± 0.3 | 1.9 ± 0.6 | 0.53 |
| Inspiratory capacity (L) | 3.6 ± 0.8 | 3.7 ± 0.7 | 0.82 |
| DLCO (mL mmHg−1 min−1) | 29.0 ± 5.6 | 27.4 ± 2.9 | 0.46 |
| Exercise capacity | |||
| VO2max (L min−1) | 2.47 ± 0.46 | 2.53 ± 0.51 | 0.77 |
| VO2max (mL kg−1 min−1) | 27.0 ± 3.4 | 26.7 ± 5.0 | 0.85 |
| Work rate at VO2max (W) | 203 ± 33 | 210 ± 40 | 0.69 |
| Ventilatory threshold (L min−1) | 1.5 ± 0.3 | 1.3 ± 0.3 | 0.24 |
| Maximal HR (bpm) | 158 ± 15 | 163 ± 17 | 0.56 |
| Maximal SBP (mmHg) | 212 ± 15 | 229 ± 20 | 0.047 |
| Maximal DBP (mmHg) | 82 ± 10 | 85 ± 9 | 0.53 |
| Maximal VE (L min−1) | 105 ± 34 | 104 ± 25 | 0.96 |
| RER | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.06 |
Values are means (SD).
FEV1, Forced expiratory volume‐second; DLCO, Diffusion capacity to carbon monoxide; VO2, Oxygen uptake; HR, Heart rate SBP, Systolic blood pressure; DBP, Diastolic blood pressure; VE, Minute‐ventilation; RER, Respiratory exchange ratio.
While maximal systolic blood pressure was reduced in diabetics versus controls (P = 0.047), VO2max and the remaining cardiopulmonary responses at maximal exercise were similar between groups (Table 3). Both τVO2 (n = 6; P = 0.73; Fig. 1) and τHR (n = 7; P = 0.94; Fig. 2) were similar between diabetics and control participants. Heart rate amplitude was greater in diabetics (P < 0.05; Table 4). However, the other VO2 and HR kinetics variables were similar between groups (Table 4).
Figure 1.

Maximal oxygen uptake (VO2) kinetic response of a representative subject with type 2 diabetes versus control. Full circles represent single breath by control subjects and overall response is characterized by the grey line. Empty circles represent single breath by subjects with type 2 diabetes while the overall response is characterized by the black line. No significant difference between groups in the phase 2 VO2 kinetic response.
Figure 2.
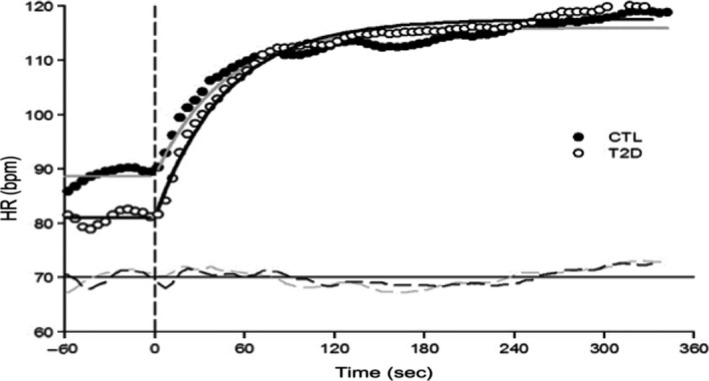
Heart rate (HR) kinetic response of a representative subject with type 2 diabetes versus control. Full circles represent single breath by control subjects and overall response is characterized by the gray line. Empty circles represent single breath by subjects with type 2 diabetes while the overall response is characterized by the black line. No significant difference between groups in the phase 2 HR kinetic response.
Table 4.
VO2 and heart rate kinetics during exercise in participants with type 2 diabetes compared to control participants
| Diabetes group | Control group | P | |
|---|---|---|---|
| Work rate at 80% VT (W) | 73 ± 17 | 64 ± 15 | 0.4 |
| Baseline VO2 (ml min−1) | 548 ± 63 | 595 ± 71 | 0.3 |
| τVO2 (sec) | 43 ± 6 | 43 ± 10 | 0.7 |
| VO2 time delay (sec) | 17 ± 2 | 16 ± 3 | 0.6 |
| VO2 amplitude (ml min−1) | 671 ± 16 | 613 ± 194 | 0.7 |
| End‐exercise VO2 (ml min−1) | 1219 ± 193 | 1208 ± 252 | 0.9 |
| Baseline HR (bpm) | 83 ± 12 | 82 ± 8 | 0.9 |
| τHR (sec) | 42 ± 17 | 43 ± 13 | 0.9 |
| HR amplitude (bpm) | 27 ± 5 | 21 ± 4 | 0.04 |
| End‐exercise HR (bpm) | 110 ± 11 | 102 ± 11 | 0.2 |
Values are means (SD).
HR, Heart rate; VO2, Oxygen uptake; VT, Ventilatory threshold.
Discussion
The results of this study suggest that obese sedentary men with well‐controlled type 2 diabetes without clinical complications or comorbidities do not show a reduction in VO2max or slower VO2 and HR kinetics compared to participants without diabetes carefully matched for sex, age and body mass index. In addition, diabetics and control participants had, for almost all variables studied, comparable baseline characteristics and resting cardiorespiratory function, which may explain the similar submaximal and maximal exercise capacity responses reported in this study.
Type 2 diabetes has been associated with reduced maximal exercise performance (Regensteiner et al. 1995, 1998, 2009; Fang et al. 2005; Gusso et al. 2008; Lalande et al. 2008; Nadeau et al. 2009; MacAnaney et al. 2011; Wilkerson et al. 2011; O'Connor et al. 2012). However, the exact underlying pathophysiological mechanisms are still ambiguous. In our study including well‐matched participants, no difference in VO2max was observed between groups, in contrast with previous reports. It is noteworthy that in most of the studies that reported a reduction in VO2max in patients with diabetes, other important variables may have contributed to the reduced VO2max, such as poor glycemic control (Vanninen et al. 1992; Demir et al. 2001; Fang et al. 2005), presence of LVDD (Poirier et al. 2000; Poanta et al. 2011), reduced heart rate variability (Erdogan et al. 2011; Poanta et al. 2011), impaired lung diffusion capacity or early signs of cardiac impairment altering pumping capacity (Saltin 1985; Cerretelli and Di Prampero 1987) and the presence of comorbidities associated with type 2 diabetes (Estacio et al. 1998). The presence of type 2 diabetes did not add additional burden to the majority of variables related to resting cardiopulmonary function in the present study. Since the cardiopulmonary function has an important influence on exercise performance in healthy individuals (Saltin 1985), the absence of a deleterious impact of well‐controlled, uncomplicated type 2 diabetes on VO2max in this study is not surprising. It is noteworthy that our participants with type 2 diabetes were treated with diet (n = 8) or metformin alone (n = 2) reinforcing the fact that their diabetes was not longstanding which may have required a more aggressive pharmacological intervention. Since metformin has been associated with a small reduction in maximal aerobic capacity (Braun et al. 2008), it is worth mentioning that exclusion of the two diabetics using metformin from our analyses (data not shown) did not influence our findings.
In addition, type 2 diabetes is usually associated with altered LV diastolic function (Shishehbor et al. 2003; Boyer et al. 2004; Baldi et al. 2006; Erdogan et al. 2011). The negative impact of LVDD on submaximal exercise responses (Regensteiner et al. 2009) and maximal exercise capacity (Poirier et al. 2000) has been reported. Although higher LV filling pressures were observed in diabetics versus controls, five participants in each group had LVDD, which could partly contribute to the comparable maximal exercise capacity between the two groups. What is still ambiguous pertaining to LVDD in diabetics is whether its appearance coincides with the development of diabetes or occurs earlier, since several variables such as blood pressure, age, sedentary lifestyle, obesity, hyperinsulinemia and LV systolic failure may modulate the development of LVDD (Borlaug and Kass 2006; Poirier et al. 2006; Prasad et al. 2007). Accordingly, the results of this study do not exclude that LVDD has a negative influence on exercise capacity in patients with type 2 diabetes (Baldi et al. 2016). Further research is necessary to isolate the influence of LVDD on VO2max in these patients.
The evaluation of VO2 and HR kinetics provides supplemental mechanistic information on how a clinical population, in this case well‐characterized men with well‐controlled type 2 diabetes with short known disease duration, adapt to a transition from low to moderate intensity exercise. Compared to VO2max, VO2 and HR kinetics provide a more accurate evaluation of how participants adapt to everyday life activity, considering that they rarely perform activities at maximal exercise intensity. As VO2 kinetics represents the efficiency of the cardiovascular and metabolic systems to respond to changes in demand, a slower VO2 or HR adjustment at the onset of exercise is associated with a lower functional capacity and harder perceived effort when performing regular activities (Jones and Poole 2005). A slower response may be the result of impaired O2 delivery, inappropriate distribution of O2 to the working muscles, other muscular factors (metabolic inertia), or more likely, a combination of these factors (Jones and Poole 2005).
In our study, no difference in VO2 kinetics was observed between groups. This contrasts with previous studies conducted in patients with type 2 diabetes, in which slower VO2 kinetics has been reported (Regensteiner et al. 1998; Nadeau et al. 2009; MacAnaney et al. 2011; O'Connor et al. 2015). Factors that could have contributed to the slower VO2 kinetics reported in previous studies include non‐optimal short and long‐term glycemic control, comparison with a lean control group, reduced heart rate variability and/or other signs of cardiac impairment, such as the presence of LVDD in diabetics. Of note, LVDD was not associated with slower VO2 kinetics in this study, but this could be related to our small sample size. However, our results support those of Wilkerson et al. (2011) who reported similar VO2 kinetics between older men with diabetes of longer disease duration versus control subjects. This similar VO2 adjustment was attributed to altered blood flow compensated by adaptive mechanisms with long‐disease duration, such as O2 extraction (Bailey et al. 2011). Interestingly, the slowed VO2 kinetics reported in uncomplicated type 2 diabetics could attain a plateau early following the onset of the disease, without a further detrimental impact of aging (O'Connor et al. 2015).
The evaluation of HR kinetics provides a measure of central blood flow adjustment, and O2 delivery, at the onset of moderate exercise (Jones and Poole 2005). Previous studies having investigated HR kinetics in type 2 diabetics provided equivocal observations. The adjustment of HR seems slower in pre‐ and post‐menopausal women with type 2 diabetes (Regensteiner et al. 1998; O'Connor et al. 2012), as well as in older men with type 2 diabetes with longer disease duration (Wilkerson et al. 2011) or with suboptimal glycemic control (O'Connor et al. 2012). The difference between those results and ours may be attributable to the shorter disease duration of our cohort (<5 years), the mean age of our participants and the optimal short and long‐term glycemic control. In addition, diabetics involved in this study were treated with diet or metformin alone, compared to more advanced treatment (O'Connor et al. 2012), which often reflects a more advanced form of diabetes or the presence of complications or comorbidities. To our knowledge, the present study is the first to show that well‐characterized optimally controlled men with type 2 diabetes and of a relatively short known disease duration (<5 years) do not have slower HR kinetics compared to control subjects. In addition, it appears that the slowed HR kinetics may be influenced to a greater extent by other variables, such as age, fitness, or the presence of comorbidities rather than the disease processes, at least in the early stage of diabetes.
This study has limitations that need to be discussed. We acknowledge that the number of participants in the present study was relatively low, although comparable to several other studies (Regensteiner et al. 1995, 1998; Brandenburg et al. 1999), only composed of men and the study did not include a lean control group. These factors limit the generalization of our results. Although 8 participants per group would have been necessary to report a statistically significant difference of 15 ± 10 sec in τVO2 between our groups based on previous reported data (Regensteiner et al. 1998; Brandenburg et al. 1999), we did not reach such a sample size for the evaluation of VO2 and HR kinetics (diabetic group: n = 6 and control group: n = 7). However, it is highly unlikely that τVO2 would have been different with the addition of two participants in the diabetic group and one participant in the control group taking into consideration that the difference in τVO2 between groups was far from the ~15 sec reported in the literature. By study design, participants in both groups had similar age and body mass index. They also had similar long‐term glycemic control, lipid profile, resting blood pressure, pulmonary function as well as cardiac autonomic system modulation. The majority of participants with diabetes were recently diagnosed, and all of them were well‐controlled, while control participants may not be considered as completely “healthy” participants, considering their age, the presence of obesity in a majority of participants, borderline prediabetes in three participants, and the fact that they were physically inactive. For example, normal aging (Hees et al. 2004), as well as overweight/obesity [normotensive individuals (Grandi et al. 2000), patients with normal LV ejection fraction (Aljaroudi et al. 2012) and community‐based elderly cohort (Russo et al. 2011)] are known to have an influence on the development of LVDD. We consider that the similarities observed between our groups is a strength of this study which permit to assess the impact of type 2 diabetes per se, and not the related complications, on submaximal and maximal exercise performance.
The number of square‐wave exercise protocols used to model VO2 and HR kinetics is also a strength of this study. Indeed, at least three transitions seem necessary to get an adequate signal‐to‐noise ratio (Spencer et al. 2011) and previous studies that investigated VO2 or HR kinetics in patients with type 2 diabetes did not all reach that prerequisite, which considerably reduces the precision in the modeling of the underlying physiologic responses during an exercise transition. Another important issue is that we allowed 48 h between each exercise trial for the evaluation of VO2 and HR kinetics, to ensure that insulin sensitivity induced by exercise returned to baseline value (Mikines et al. 1988), and that fatigue would not interfere with the results, considering the low level of physical fitness of our population. Finally, these results warrant further studies investigating the influence of well‐controlled, uncomplicated type 2 diabetes on exercise performance. Still, these results have potential clinical implications. If the presence of type 2 diabetes eventually leads to a lower capacity to perform exercise (Fang et al. 2005), the present study suggests that well‐controlled type 2 diabetes is not always associated with a reduction in submaximal (VO2 and HR kinetics) or maximal exercise performance (VO2max). A more likely interpretation of these results may be that exercise performance is already impaired prior to the diagnosis of type 2 diabetes, as observed in participants with metabolic syndrome and healthy first‐degree relatives of patients with type 2 diabetes, in whom subclinical metabolic and/or cardiovascular abnormalities are present (Berntorp and Lindgarde 1985; Nyholm et al. 1996; LaMonte et al. 2005). In the eventuality that these findings are supported in further studies, it will reinforce the importance of strong early therapeutic actions in individuals prone to develop type 2 diabetes in order to delay the appearance of this metabolic disorder and preserve the individual's exercise capacity and quality of life.
In conclusion, the findings from this study suggest that well‐controlled type 2 diabetes does not necessarily result in a reduction of VO2max and a slowing of VO2/HR kinetics over and above what can be expected in obese and sedentary individuals.
Conflict of Interest
None declared.
Acknowledgments
Paul Poirier is a senior clinician‐scientist of the Fonds de recherche du Québec ‐ Santé (FRQS). Annie Ferland was supported by the Canadian Institutes of Health Research (CIHR). Patrice Brassard is a Junior 1 Research Scholar of the Fonds de recherche du Québec ‐ Santé (FRQS).
Caron J., duManoir G. R., Labrecque L., Chouinard A., Ferland A., Poirier P., Legault S., Brassard P.. Impact of type 2 diabetes on cardiorespiratory function and exercise performance. Physiol Rep, 5 (4), 2017, e13145, doi: 10.14814/phy2.13145
Funding Information
This study has been supported in part by the Canadian Diabetes Association.
References
- Aljaroudi, W. , Halley C., Houghtaling P., Agarwal S., Menon V., Rodriguez L., et al. 2012. Impact of body mass index on diastolic function in patients with normal left ventricular ejection fraction. Nutr Diabetes 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association . 2007. Diagnosis and classification of diabetes mellitus. Diabetes Care 30(Suppl. 1):S42–S47. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society . 1995. Standardization of spirometry, 1994 update. Am. J. Respir. Crit. Care Med. 152:1107–1136. [DOI] [PubMed] [Google Scholar]
- Bailey, S. J. , Vanhatalo A., DiMenna F. J., Wilkerson D. P., and Jones A. M.. 2011. Fast‐start strategy improves VO2 kinetics and high‐intensity exercise performance. Med. Sci. Sports Exerc. 43:457–467. [DOI] [PubMed] [Google Scholar]
- Baldi, J. C. , Aoina J. L., Oxenham H. C., Bagg W., and Doughty R. N.. 2003. Reduced exercise arteriovenous O2 difference in Type 2 diabetes. J. Appl. Physiol. 94:1033–1038. [DOI] [PubMed] [Google Scholar]
- Baldi, J. C. , Aoina J. L., Whalley G. A., Carrick‐Ranson G., Walsh H. A., O'Shaughnessy H., et al. 2006. The effect of type 2 diabetes on diastolic function. Med. Sci. Sports Exerc. 38:1384–1388. [DOI] [PubMed] [Google Scholar]
- Baldi, J. C. , Wilson G. A., Wilson L. C., Wilkins G. T., and Lamberts R. R.. 2016. The type 2 diabetic heart: its role in exercise intolerance and the challenge to find effective exercise interventions. Sports Med. 46:1605–1617. [DOI] [PubMed] [Google Scholar]
- Beaver, W. L. , Wasserman K., and Whipp B. J.. 1986. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 60:2020–2027. [DOI] [PubMed] [Google Scholar]
- Berntorp, K. , and Lindgarde F.. 1985. Impaired physical fitness and insulin secretion in normoglycaemic subjects with familial aggregation of type 2 diabetes mellitus. Diabetes Res 2:151–156. [PubMed] [Google Scholar]
- Bogaty, P. , Dagenais G. R., Poirier P., Boyer L., Auclair L., Pepin G., et al. 2003. Effect of atorvastatin on exercise‐induced myocardial ischemia in patients with stable angina pectoris. Am. J. Cardiol. 92:1192–1195. [DOI] [PubMed] [Google Scholar]
- Booth, F. W. , Chakravarthy M. V., and Spangenburg E. E.. 2002. Exercise and gene expression: physiological regulation of the human genome through physical activity. J. Physiol. 543:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, B. A. , and Kass D. A.. 2006. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc. Med. 16:273–279. [DOI] [PubMed] [Google Scholar]
- Boyer, J. K. , Thanigaraj S., Schechtman K. B., and Perez J. E.. 2004. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 93:870–875. [DOI] [PubMed] [Google Scholar]
- Brandenburg, S. L. , Reusch J. E., Bauer T. A., Jeffers B. W., Hiatt W. R., and Regensteiner J. G.. 1999. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care 22:1640–1646. [DOI] [PubMed] [Google Scholar]
- Brassard, P. , Ferland A., Bogaty P., Desmeules M., Jobin J., and Poirier P.. 2006. Influence of glycemic control on pulmonary function and heart rate in response to exercise in subjects with type 2 diabetes mellitus. Metabolism 55:1532–1537. [DOI] [PubMed] [Google Scholar]
- Braun, B. , Eze P., Stephens B. R., Hagobian T. A., Sharoff C. G., Chipkin S. R., et al. 2008. Impact of metformin on peak aerobic capacity. Appl. Physiol. Nutr. Metab. 33:61–67. [DOI] [PubMed] [Google Scholar]
- Canadian Cardiovascular Society and the Canadian Hypertension Society . 1995. Guidelines on the echocardiographic determination of left ventricular mass. Task Force of the Echocardiography Section. Can. J. Cardiol. 11:391–395. [PubMed] [Google Scholar]
- Cerretelli, P. , and Di Prampero P. E.. 1987. Hanbook of Physiology Pp. 297–339 in Fishman A. P., Farhi L. E., Tenney S. M. and Geiger S. R., eds. Gas exchange in exercise. American Physiological Society, Bethesda, MD. [Google Scholar]
- Demir, I. , Ermis C., Altunbas H., and Balci M. K.. 2001. Serum HbA1c levels and exercise capacity in diabetic patients. Jpn. Heart J. 42:607–616. [DOI] [PubMed] [Google Scholar]
- Dumesnil, J. G. , Gaudreault G., Honos G. N., and Kingma J. G. Jr. 1991. Use of Valsalva maneuver to unmask left ventricular diastolic function abnormalities by Doppler echocardiography in patients with coronary artery disease or systemic hypertension. Am. J. Cardiol. 68:515–519. [DOI] [PubMed] [Google Scholar]
- Dumesnil, J. G. , Paulin C., Pibarot P., Coulombe D., and Arsenault M.. 2002. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J. Am. Soc. Echocardiogr. 15:1226–1231. [DOI] [PubMed] [Google Scholar]
- Erdogan, D. , Akcay S., Ersoy I. H., Icli A., Yucel H., Kutlucan A., et al. 2011. Cardiac determinants of impaired exercise performance in patients with type 2 diabetes mellitus. Int. J. Cardiol. 152:143–146. [DOI] [PubMed] [Google Scholar]
- Estacio, R. O. , Regensteiner J. G., Wolfel E. E., Jeffers B., Dickenson M., and Schrier R. W.. 1998. The association between diabetic complications and exercise capacity in NIDDM patients. Diabetes Care 21:291–295. [DOI] [PubMed] [Google Scholar]
- European Society of Cardiology and the North American Society of Pacing and Electrophysiology . 1996. Heart rate variability: standards of measurements, physiological interpretation and clinical use. Circulation 93:1043–1065. [PubMed] [Google Scholar]
- Fang, Z. Y. , Sharman J., Prins J. B., and Marwick T. H.. 2005. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care 28:1643–1648. [DOI] [PubMed] [Google Scholar]
- Friedewald, W. T. , Levy R. I., and Fredrickson D. S.. 1972. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18:499–502. [PubMed] [Google Scholar]
- Grandi, A. M. , Zanzi P., Piantanida E., Gaudio G., Bertolini A., Guasti L., et al. 2000. Obesity and left ventricular diastolic function: noninvasive study in normotensives and newly diagnosed never‐treated hypertensives. Int. J. Obes. Relat. Metab. Disord. 24:954–958. [DOI] [PubMed] [Google Scholar]
- Gusso, S. , Hofman P., Lalande S., Cutfield W., Robinson E., and Baldi J. C.. 2008. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia 51:1317–1320. [DOI] [PubMed] [Google Scholar]
- Hees, P. S. , Fleg J. L., Dong S. J., and Shapiro E. P.. 2004. MRI and echocardiographic assessment of the diastolic dysfunction of normal aging: altered LV pressure decline or load? Am. J. Physiol. Heart Circ. Physiol. 286:H782–H788. [DOI] [PubMed] [Google Scholar]
- Jones, A. M. , and Poole D. C., eds. 2005. Oxygen uptake kinetics in sport, exercise and medicine. New York: Routledge. p. 405. [Google Scholar]
- Kitahara, Y. , Hattori N., Yokoyama A., Yamane K., Sekikawa K., Inamizu T., et al. 2010. The influence of lung function on exercise capacity in patients with type 2 diabetes. Hiroshima J. Med. Sci. 59:7–13. [PubMed] [Google Scholar]
- Kjaer, M. , Hollenbeck C. B., Frey‐Hewitt B., Galbo H., Haskell W., and Reaven G. M.. 1990. Glucoregulation and hormonal responses to maximal exercise in non‐insulin‐dependent diabetes. J. Appl. Physiol. 68:2067–2074. [DOI] [PubMed] [Google Scholar]
- Lalande, S. , Gusso S., Hofman P. L., and Baldi J. C.. 2008. Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med. Sci. Sports Exerc. 40:612–617. [DOI] [PubMed] [Google Scholar]
- LaMonte, M. J. , Barlow C. E., Jurca R., Kampert J. B., Church T. S., and Blair S. N.. 2005. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 112:505–512. [DOI] [PubMed] [Google Scholar]
- MacAnaney, O. , Reilly H., O'Shea D., Egana M., and Green S.. 2011. Effect of type 2 diabetes on the dynamic response characteristics of leg vascular conductance during exercise. Diab Vasc Dis Res 8:12–21. [DOI] [PubMed] [Google Scholar]
- Maser, R. E. , and Lenhard M. J.. 2005. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J. Clin. Endocrinol. Metab. 90:5896–5903. [DOI] [PubMed] [Google Scholar]
- Mikines, K. J. , Sonne B., Farrell P. A., Tronier B., and Galbo H.. 1988. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am. J. Physiol. 254:E248–E259. [DOI] [PubMed] [Google Scholar]
- Murias, J. M. , Spencer M. D., Kowalchuk J. M., and Paterson D. H.. 2011. Influence of phase I duration on phase II VO2 kinetics parameter estimates in older and young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301:R218–R224. [DOI] [PubMed] [Google Scholar]
- Nadeau, K. J. , Zeitler P. S., Bauer T. A., Brown M. S., Dorosz J. L., Draznin B., et al. 2009. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J. Clin. Endocrinol. Metab. 94:3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm, B. , Mengel A., Nielsen S., Skjaerbaek C., Moller N., Alberti K. G., et al. 1996. Insulin resistance in relatives of NIDDM patients: the role of physical fitness and muscle metabolism. Diabetologia 39:813–822. [DOI] [PubMed] [Google Scholar]
- O'Connor, E. , Kiely C., O'Shea D., Green S., and Egana M.. 2012. Similar level of impairment in exercise performance and oxygen uptake kinetics in middle‐aged men and women with type 2 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303:R70–R76. [DOI] [PubMed] [Google Scholar]
- O'Connor, E. , Green S., Kiely C., O'Shea D., and Egana M.. 2015. Differential effects of age and type 2 diabetes on dynamic vs. peak response of pulmonary oxygen uptake during exercise. J Appl Physiol (1985) 118:1031–1039. [DOI] [PubMed] [Google Scholar]
- Ommen, S. R. , Nishimura R. A., Appleton C. P., Miller F. A., Oh J. K., Redfield M. M., et al. 2000. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation 102:1788–1794. [DOI] [PubMed] [Google Scholar]
- Poanta, L. , Porojan M., and Dumitrascu D. L.. 2011. Heart rate variability and diastolic dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. 48:191–196. [DOI] [PubMed] [Google Scholar]
- Poirier, P. , Garneau C., Bogaty P., Nadeau A., Marois L., Brochu C., et al. 2000. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well‐controlled type 2 diabetes mellitus. Am. J. Cardiol. 85:473–477. [DOI] [PubMed] [Google Scholar]
- Poirier, P. , Bogaty P., Garneau C., Marois L., and Dumesnil J. G.. 2001. Diastolic dysfunction in normotensive men with well‐controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24:5–10. [DOI] [PubMed] [Google Scholar]
- Poirier, P. , Bogaty P., Philippon F., Garneau C., Fortin C., and Dumesnil J. G.. 2003. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well‐controlled type 2 diabetes. Metabolism 52:1056–1061. [DOI] [PubMed] [Google Scholar]
- Poirier, P. , Giles T. D., Bray G. A., Hong Y., Stern J. S., Pi‐Sunyer F. X., et al. 2006. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113:898–918. [DOI] [PubMed] [Google Scholar]
- Prasad, A. , Popovic Z. B., Arbab‐Zadeh A., Fu Q., Palmer D., Dijk E., et al. 2007. The effects of aging and physical activity on Doppler measures of diastolic function. Am. J. Cardiol. 99:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski, H. , Appleton C., Chan K. L., Dumesnil J. G., Honos G., Jue J., et al. 1996. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J. Am. Soc. Echocardiogr. 9:736–760. [DOI] [PubMed] [Google Scholar]
- Regensteiner, J. G. , Sippel J., McFarling E. T., Wolfel E. E., and Hiatt W. R.. 1995. Effects of non‐insulin‐dependent diabetes on oxygen consumption during treadmill exercise. Med. Sci. Sports Exerc. 27:661–667. [PubMed] [Google Scholar]
- Regensteiner, J. G. , Bauer T. A., Reusch J. E., Brandenburg S. L., Sippel J. M., Vogelsong A. M., et al. 1998. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J. Appl. Physiol. 85:310–317. [DOI] [PubMed] [Google Scholar]
- Regensteiner, J. G. , Bauer T. A., Reusch J. E., Quaife R. A., Chen M. Y., Smith S. C., et al. 2009. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med. Sci. Sports Exerc. 41:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, T. M. , Peterson H. R., Snider H. L., Cyrus J., Broadstone V. L., Fell R. D., et al. 1989. Autonomic influence on cardiovascular performance in diabetic subjects. Am. J. Med. 87:382–388. [DOI] [PubMed] [Google Scholar]
- Russo, C. , Jin Z., Homma S., Rundek T., Elkind M. S., Sacco R. L., et al. 2011. Effect of obesity and overweight on left ventricular diastolic function: a community‐based study in an elderly cohort. J. Am. Coll. Cardiol. 57:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin, B. 1985. Hemodynamic adaptations to exercise. Am. J. Cardiol. 55:42D–47D. [DOI] [PubMed] [Google Scholar]
- Schneider, S. H. , Amorosa L. F., Khachadurian A. K., and Ruderman N. B.. 1984. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non‐insulin‐dependent) diabetes. Diabetologia 26:355–360. [DOI] [PubMed] [Google Scholar]
- Shishehbor, M. H. , Hoogwerf B. J., Schoenhagen P., Marso S. P., Sun J. P., Li J., et al. 2003. Relation of hemoglobin A1c to left ventricular relaxation in patients with type 1 diabetes mellitus and without overt heart disease. Am. J. Cardiol. 91:1514–1517. A1519. [DOI] [PubMed] [Google Scholar]
- Spencer, M. D. , Murias J. M., Lamb H. P., Kowalchuk J. M., and Paterson D. H.. 2011. Are the parameters of VO2, heart rate and muscle deoxygenation kinetics affected by serial moderate‐intensity exercise transitions in a single day? Eur. J. Appl. Physiol. 111:591–600. [DOI] [PubMed] [Google Scholar]
- Vanninen, E. , Uusitupa M., Remes J., Siitonen O., Laitinen J., Lansimies E., et al. 1992. Relationship between hyperglycaemia and aerobic power in men with newly diagnosed type 2 (non insulin‐dependent) diabetes. Clin. Physiol. 12:667–677. [DOI] [PubMed] [Google Scholar]
- Wilkerson, D. P. , Poole D. C., Jones A. M., Fulford J., Mawson D. M., Ball C. I., et al. 2011. Older type 2 diabetic males do not exhibit abnormal pulmonary oxygen uptake and muscle oxygen utilization dynamics during submaximal cycling exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300:R685–R692. [DOI] [PubMed] [Google Scholar]


