Abstract
Peroxisome is a single-membrane-bounded ubiquitous organelle containing a hundred different enzymes that catalyze various metabolic pathways such as β-oxidation of very long-chain fatty acids and synthesis of plasmalogens. To investigate peroxisome biogenesis and human peroxisome biogenesis disorders (PBDs) including Zellweger syndrome, more than a dozen different complementation groups of Chinese hamster ovary (CHO) cell mutants impaired in peroxisome biogenesis are isolated as a model experimental system. By taking advantage of rapid functional complementation assay of the CHO cell mutants, successful cloning of PEX genes encoding peroxins required for peroxisome assembly invaluably contributed to the accomplishment of cloning of pathogenic genes responsible for PBDs. Peroxins are divided into three groups: 1) peroxins including Pex3p, Pex16p and Pex19p, are responsible for peroxisome membrane biogenesis via Pex19p- and Pex3p-dependent class I and Pex19p- and Pex16p-dependent class II pathways; 2) peroxins that function in matrix protein import; 3) those such as Pex11pβ are involved in peroxisome division where DLP1, Mff, and Fis1 coordinately function.
Keywords: CHO cell mutants, peroxisome biogenesis, protein import machinery, pathogenic genes, peroxins, Zellweger syndrome
1. Introduction
Peroxisomes are single-membrane-bounded ubiquitous organelles containing a hundred different enzymes that catalyze various metabolic pathways, including β-oxidation of very long-chain fatty acids, the synthesis of ether lipids such as plasmalogens, and bile-acid metabolism1) (Table 1). They were discovered in 1954, named peroxisomes in 1965, and defined that peroxisomes contain one or more enzymes that use molecular oxygen to remove hydrogen atoms and form hydrogen peroxide from organic substrates.2) Catalase, a typical marker enzyme of peroxisomal matrix, degrades hydrogen peroxides.
Table 1.
Functions of peroxisomea
| 1. | Respiration: H2O2-producing oxidases, catalase |
| 2. | Fatty acid β-oxidation: acyl-CoA oxidase, bifunctional protein (hydratase-dehydrogenase), thiolase |
| 3. | Ether glycerolipid (plasmalogen) biosynthesis: DHAP-ATPase, alkyl-DHAP synthase |
| 4. | Transamination and oxidation (gluconeogenesis): serine-pyruvate aminotransferase (alanine-glyoxylate aminotransferase) |
| 5. | Purine catabolism |
| 6. | Polyamine catabolism |
| 7. | Bile acid biosynthesis |
| 8. | Pipecolic acid catabolism |
| 9. | Phytanic acid catabolism |
aRepresentative functions in mammalian peroxisomes are listed, where peroxisomal enzymes involved in the functions (1 to 4) are also described.
Peroxisomes are thought to form by the division of pre-exiting peroxisomes after the import of newly synthesized proteins.3) Molecular mechanisms of peroxisome biogenesis, including peroxisomal import of newly synthesized matrix and membrane proteins, have been extensively investigated basically by several eukaryotic systems. Studies at the molecular level on both peroxisome assembly and peroxisome biogenesis disorders (PBDs, Table 2) rapidly progressed in the last three to four decades. The identification and isolation of over 30 essential genes termed PEXs encoding peroxisome biogenesis factors named peroxins, have been performed by means of the genetic phenotype-complementation of peroxisome assembly-deficient cell mutants, named pex mutants impaired in PEX genes. Such mutants from Chinese hamster ovary (CHO) cells (Table 3; see below),4,5) several yeast species including Saccharomyces cerevisiae,6) Pichia pastoris,7,8) Hansenula polymorpha,9) and Yarrowia lipolytica10) (also see reviews11–16)), and plant Arabidopsis thaliana17) have been successfully contributing to the investigations of peroxisome biogenesis and protein traffics in eukaryotes.18,19) I herein review and address peroxisome biogenesis and human deficiency disorders by making use of mammalian model cell systems.
Table 2.
Peroxisomal disease
| Peroxisome biogenesis disorders (PBDs) |
|---|
| Zellweger spectrum disorders |
| Zellweger syndrome (ZS) |
| Neonatal adrenoleukodystrophy (NALD) |
| Infantile Refsum disease (IRD) |
| Rhizomelic chondrodysplasia punctata (RCDP) |
| Single-enzyme deficiencies |
| Adrenoleukodystrophy (ALD) |
| Acyl-CoA oxidase deficiency |
| D-Bifunctional protein deficiency |
| 3-Ketoacyl-CoA thiolase deficiency |
| Refsum disease (phytanyl-CoA hydroxylase deficiency), α-Methylacyl-CoA racemase deficiency |
| Hyperoxaluria type I (alanine glyoxylate aminotransferase deficiency) |
| Mevalonate kinase deficiency |
| Glutaric aciduria 3 (glutaryl-CoA oxidase deficiency) |
| Acatalasemia |
Table 3.
Complementation groups (CGs) and PEX genes of peroxisome deficiencies
| Gene | CG | PBD | CHO mutants | Ps-memb. biogenesisa | Peroxin | ||
|---|---|---|---|---|---|---|---|
| US/EU | Japan | (kDa) | Characteristics | ||||
| PEX1 | 1 | E | ZS, NALD*, IRD* | Z24, ZP107 | + | 143 | AAA family |
| PEX2 | 10 | F | ZS, IRD* | Z65 | + | 35 | PMP, RING |
| PEX3 | 12 | G | ZS | ZPG208 | − | 42 | PMP, PMP-DP |
| PEX5 | 2 | ZS, NALD | ZP105*, ZP139 | + | 68 | PTS1 receptor, TPR family | |
| PEX6 | 4(6) | C | ZS, NALD* | ZP92 | + | 104 | AAA family |
| PEX7 | 11 | R | RCDP | ZPG207 | + | 36 | PTS2 receptor, WD motif |
| PEX10 | 7(5) | B | ZS, NALD | + | 37 | PMP, RING | |
| PEX11β | 16 | ZS | + | 28 | PMP | ||
| PEX12 | 3 | ZS, NALD, IRD | ZP109 | + | 40 | PMP, RING | |
| PEX13 | 13 | H | ZS, NALD* | ZP128 | + | 44 | PMP, PTS1-DP, SH3 |
| PEX14 | 15 | K | ZS | ZP110 | + | 41 | PMP, PTS1-DP, PTS2-DP |
| PEX16 | 9 | D | ZS | − | 39 | PMP, PMP-DP | |
| PEX19 | 14 | J | ZS | ZP119 | − | 33 | CAAX motif, PMP receptor |
| PEX26 | 8 | A | ZS, NALD*, IRD* | ZP124, ZP167 | + | 34 | PMP, Pex1p-Pex6p recruiter |
| ZP114 | + | ||||||
*, temperature-sensitive phenotype. aPeroxisomal membrane assembly is normal (+) or impaired (−).
PBD, peroxisomal biogenesis disorders; ZS, Zellweger syndrome; IRD, infantile Refsum disease; NALD, neonatal adrenoleukodystrophy; RCDP, rhizomelic chondrodysplasia punctata; DP, docking protein; PMP, peroxisome membrane protein; TPR, tetratricopeptide repeat.
2. Genetic approaches to studying mammalian peroxisome biogenesis
Two mutually complementary genetic approaches used for isolation of PEX genes encoding peroxins were genetic phenotype-complementation of peroxisome assembly-defective mutants of CHO cells and a combination of the human orthologue isolation by homology search on the human expressed sequence tag database using yeast PEX genes and cells from patients with PBDs of more than a dozen different genotypes, i.e., complementation groups (CGs) (Table 3; see below).4,5,20–22)
2.1. Mammalian cell mutants deficient of peroxisome.
2.1.1. Cell lines from patients with PBDs.
The PBDs include Zellweger syndrome (ZS), neonatal adrenoleukodystrophy (NALD), infantile Refsum disease (IRD), which are called Zellweger syndrome spectrum, and rhizomelic chondrodysplasia punctata (RCDP)23,24) (Table 2). Patients with ZS show severe neurological abnormalities, characteristic dysmorphism and hepatomegaly, and rarely survive with an average life of only 6 months. NALD patients have the symptoms similar to ZS patients, but they survive a little longer, early childhood. By contrast, patients with IRD do not manifest significant abnormalities in the central nervous system, and survive with the longest average life, 3–11 years.23) RCDP patients show distinct phenotypic characteristics such as severe growth failure and rhizomelia. Genetic heterogeneity consisting of 14 CGs has been identified in PBDs by cell-fusion CG analysis using fibroblast cell lines derived from PBD patients5,20,25–27) (Table 3), where the primary cause for PBDs was revealed to be the impaired biogenesis of peroxisomes.5,20)
2.1.2. Isolation of CHO cell lines.
Two methods were developed for the isolation of mammalian somatic cell mutants defective in peroxisome biogenesis: (i) colony autoradiographic screening with a phenotypic marker, dihydroxyacetonephosphate acyltransferase (DHAP-ATase) deficiency;28,29) and (ii) the photo-sensitized selection method using 9-(1′-pyrene)nonanol (P9OH) and an exposure to long wave-length ultraviolet (UV) light which kills wild-type cells incorporating P9OH as a fatty alcohol into plasmalogens and survive cell mutants deficient in such activity.30,31) We so far isolated 12 CGs of peroxisome-deficient CHO cell mutants by these methods (Table 3). All of CHO cell mutants showed a typical phenotype of deficiency in peroxisome biogenesis, such as the impaired protein import, no catalase latency, severely affected DHAP-ATase activity, as noted in fibroblasts from PBD patients.5,29)
A complete set of CG analyses by cell-fusion between 12 CGs of CHO cell mutants and 13 CGs of fibroblasts from patients with PBDs revealed that 11 CGs of CHO mutants represent the human PBD CGs5,29) (Table 3). A PBD patient of the 14th CG, CG16, was recently identified.32) Together, genetic heterogeneities comprising 15 CGs are currently reported in mammals including humans and CHO cells.
2.2. Peroxisome biogenesis genes.
2.2.1. Genetic phenotype-complementation screening.
PEXs were isolated by genetic phenotype-complementation of peroxisome biogenesis-deficient mutants of mammalian somatic cells such as CHO cells (Fig. 1A) and of several species of yeast including S. cerevisiae, P. pastoris, Y. lipolytica, and H. polymorpha.12,24,33,34) Forward genetics method using animal somatic cell mutants such as CHO cell mutants was shown to be a highly effective approach for isolating essential genes including the peroxin genes, PEXs.
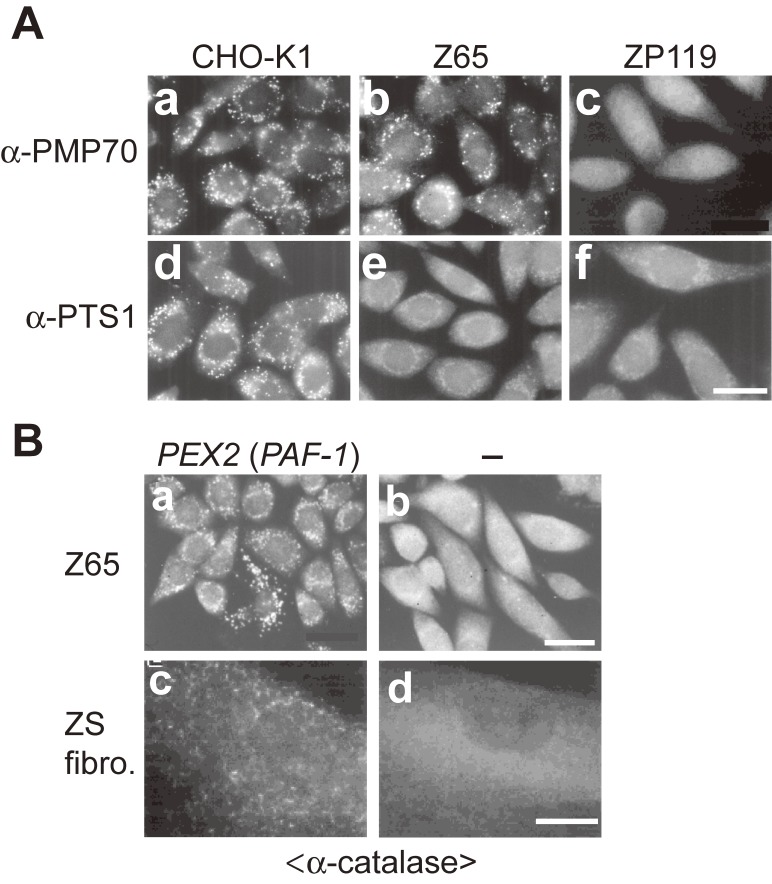
Figure 1.
Morphology of peroxisomes in CHO cell mutants defective in peroxisome biogenesis and pathogenic gene cloning of PBDs. (A) CHO cells are stained with antibodies to PMP70 (a-c) and PTS1 (d-f). Cells are as indicated at the top. Scale bar, 20 µm. pex2 Z65 contains PMP70-positive peroxisomal remnants, whereas pex19 ZP119 is absent from such peroxisome ghosts, indicative of the defect of membrane protein import. PTS1 proteins are discernible in the cytosol in pex2 Z65 and pex19 ZP119 cells, in contrast to the wild-type CHO-K1 cells where PTS1 proteins are in peroxisomes. (B) Cloning of pathogenic gene responsible for PBD. Peroxisome-restoring PEX genes were isolated by functional phenotype-complementation assay using CHO mutants. Restoration of peroxisomes was searched by transfection of rat liver cDNA library (a) in Z65 (b). Transformed cells positive in catalase import contained PEX2 (formerly PAF-1). In fibroblasts from a patient with ZS of CG10 (d), expression of PEX2 restored the impaired import of catalase (c). Scale bar, 20 µm (a and b); 30 µm (c and d).
We searched for the gene encoding a factor complementing the impaired peroxisome biogenesis of one, Z65, of the CHO cell mutants by transfecting a rat liver cDNA library.35) Transfectants were selected by the 12-(1′-pyrene) dodecanoic acid (P12)/UV method,36) showing peroxisomes as verified by staining with anti-catalase antibody (Fig. 1B). An open reading frame encoded a novel 35-kDa peroxisomal integral membrane protein with two membrane-spanning segments and a RING finger motif, C3HC4,37) termed peroxisome assembly factor-1 (PAF-1)35) (Table 3; Fig. 1B). PAF-1 was unified as PEX2 in 1996.33) Expression of PEX2 (called Zellweger gene) in fibroblasts from a ZS patient of CG10 (F) complemented the impaired peroxisome biogenesis38) (Fig. 1B). Dysfunction of PEX2 caused by a homozygous nonsense point mutation at R119ter was shown for the first time to be responsible for ZS, a prototype of the PBDs.38)
A more practical approach, i.e., a transient expression assay skipping the revertant selection by P12/UV,39) was also developed for further isolation of PEX cDNAs including nine others, PEX1, PEX3, PEX5, PEX6, PEX12, PEX13, PEX14, PEX19, and PEX2621,34,40–48) (Table 3; Fig. 2). Human PEX5,49,50) PEX14,51) and PEX19 (PXF)52) were earlier identified. These PEXs were shown to be the pathogenic genes involved in PBDs of nine CGs22,24,34,53,54) (Table 3).
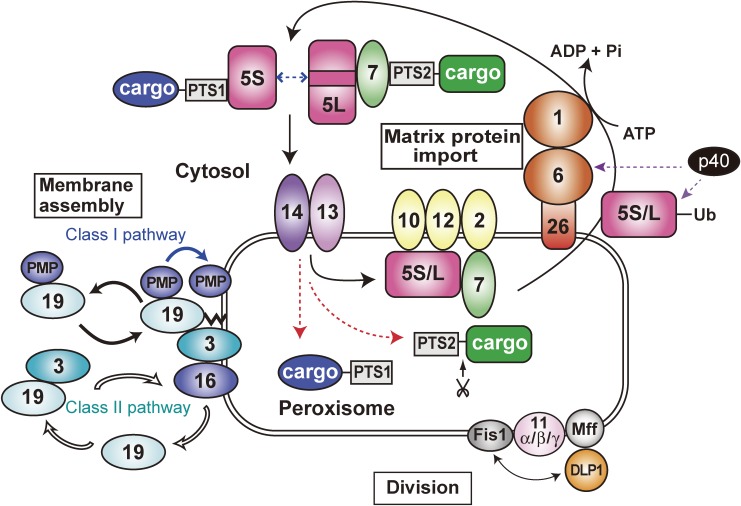
Figure 2.
A schematic view of peroxisome biogenesis in mammalian cells. The subcellular localization and molecular characteristics of peroxins are shown. Peroxins are classified into three groups: 1) peroxins including Pex3p, Pex16p and Pex19p that are responsible for peroxisome membrane assembly via classes I and II pathways required for matrix protein import; 2) those required for matrix protein import; 3) those such as three forms of Pex11p, Pex11pα, Pex11pβ, and Pex11pγ, apparently involved in peroxisome division where DLP1, Mff, and Fis1 coordinately function. PTS1 and PTS2 matrix proteins are recognized by Pex5p and Pex7p, respectively, in the cytoplasm. Two isoforms, Pex5pS and Pex5pL with an internal 37-amino-acid insertion, of Pex5p are identified in mammals. PTS1 proteins are transported by homo- and hetero-oligomers of Pex5pS and Pex5pL to peroxisomes, where Pex14p functions as a convergent, initial docking site of the ‘protein import machinery’ translocon. Pex5pL directly interacts with the PTS2 receptor, Pex7p, carrying its cargo PTS2 protein in the cytosol and translocates the Pex7p-PTS2 protein complex to Pex14p. PTS1 and PTS2 proteins are then released at the inner surface and/or inside of peroxisomes, downstream Pex14p and upstream Pex13p. Pex5p and Pex7p subsequently translocate to other translocon constituents, named translocation complex consisting of the RING peroxins, Pex2p, Pex10p, and Pex12p. Both Pex5p and Pex7p finally shuttle back to the cytosol. At the terminal step of the matrix protein import reaction, Pex1p and Pex6p of the AAA family catalyze the export of Pex5p, where Cys-monoubiquitination of Pex5p is a prerequisite to the Pex5p exit. Moreover, a cytosolic factor, AWP1/ZFAND6 (p40), is involved in the export of Ub-Pex5p in mammals.
2.2.2. Expressed sequence tag homology search.
An alternative strategy, i.e., the homology search by screening the expressed sequence tag database using yeast PEX genes, successfully made it feasible to isolate human orthologue genes responsible for PBDs:22,24,34) PEX1,55,56) PEX3,57) PEX5,58) PEX6,59) PEX7,60–62) PEX10,63,64) PEX12,65) PEX13,66) PEX14,67) and PEX16.68,69)
A PBD patient of the 14th CG, CG16, was recently identified with pathogenic gene PEX11β.32) Therefore, 14 PEXs are now shown to be responsible for PBDs of 14 distinct CGs5,22,24,27,53,70) (Table 3).
2.2.3. Genotypes of RCDP.
Several recent findings classified RCDP into five genotypes, of which responsible pathogenic genes are delineated. RCDP type 1 is caused by mutation of PEX7 encoding the peroxisome-targeting signal 2 (PTS2) receptor;60–62) types 2, 3, and 4 are impaired in three genes, DHAPAT, ADAPS, and FAR1, encoding peroxisomal enzymes, DHAP-ATase, alkyl-DHAP synthase, and fatty acyl-CoA reductase, respectively, involved in the synthesis of plasmalogens;71–73) type 5 manifesting a mutation of Pex5pL74) that transports PTS1 proteins and Pex7p-PTS2 protein complex to peroxisomes.75)
2.2.4. Genotype–Phenotype Relationships.
Patients with milder form of PBDs, NALD and IRD, tend to manifest less severe biochemical abnormalities, whose specimen including skin fibroblasts likely contain residual peroxisomes, occasionally termed mosaicism. However, clinical severity or prognosis of patients with PBDs cannot be readily predicted only on the basis of biochemical analyses. Various types of mutations such as nonsense point mutations, missense mutations, insertion and deletion of nucleotides mostly with concomitant frameshifts, splicing defects, etc. in both homozygotic and heterozygotic alleles have been identified in PBD patients. Patients with severe ZS tend to carry severe mutation such as nonsense mutations, frameshifts, and deletions, while many patients with NALD or IRD patients frequently harbor missense mutations.76,77) There is also a relationship between severe phenotype and absence of peroxisomal ghosts. Defects of PEX3, PEX16 and PEX19 encoding membrane-assembly peroxins lead to absence of ghosts and cause ZS phenotypes.22,24,34) Many cell lines from milder PBD patients, those with NALD and IRD with missense PEX mutations, showed a temperature-sensitive (ts) phenotype, restoration of peroxisome biogenesis at 30 ℃78–81) (Table 3).
Search for pathogenic genes responsible for all PBD CGs is accomplished.82) Prenatal DNA diagnosis using PEX genes is now possible for PBDs of all 14 CGs.
3. Biogenesis of peroxisomes
3.1. Membrane biogenesis.
3.1.1. Peroxins essential for membrane assembly of peroxisomes.
Three mammalian peroxins, Pex3p, Pex16p, and Pex19p, exclusively required for peroxisomal membrane assembly were isolated by the functional phenotype-complementation assay on pex3 and pex19 CHO cell mutants41,47) and the EST database search using yeast PEX genes.52,57,68,69) Malfunctions of Pex3p, Pex16p, and Pex19p, causes the most severe PBD, ZS, of three CGs, CG12 (G), CG9 (D), and CG14 (J), respectively22,24,34,83) (Table 3).
Pex3p, Pex16p, and Pex19p were identified as essential factors for assembly of peroxisomal integral membrane proteins (PMPs) in several species including humans25,47,68,69,84–89) (Fig. 2). They function as essential factors in the transport process of membrane proteins and membrane vesicle assembly in a concerted manner. Pex19p is 33-kDa farnesylated protein harboring farnesylation CAAX box motif and localized mostly in the cytosol and only partly anchored to peroxisomal membranes.47) Pex19p has a chaperone-like role in the cytosol or at the peroxisome membrane and/or functions as a cycling import receptor for newly synthesized PMPs.90,91) Pex19p forms stable Pex19p-PMP complexes except for Pex3p in the cytosol with a broad PMP-binding specificity.91–93) Pex3p, 42-kDa integral membrane protein of peroxisomes, serves as the membrane-anchoring site for Pex19p-PMP complexes, termed Class I pathway.91,94) Very recently, we demonstrated that translocation of PMPs including topologically distinct PMPs such as multi-membrane spanning PMPs and an N-terminally signal-anchored protein via the class I pathway is a common event in mammalian cells.91)
Pex16p, a protein absent in most yeasts,69,95) functions as the receptor for Pex19p complexes with newly synthesized Pex3p,94) named Class II pathway (Fig. 2). The function of Pex16p is not conserved between different species. It is noteworthy that C-tailed anchor-type peroxin Pex26p, the recruiter of Pex1p-Pex6p complex, is transported in a class I pathway,96) which is distinct from the GET3-dependent topogenesis of yeast Pex15p, a functional orthologue of Pex26p.97)
At the step of docking of a cytosolic Pex19p-PMP complex onto Pex3p, Pex19p unloads the cargo PMP and shuttles back to the cytosol for a next round of PMP transport, while the released PMP integrates into the membrane. The membrane insertion of PMPs proceeds in the absence of ATP.96,98–100) Pex19p and Pex3p apparently facilitate the insertion of transmembrane domains in a concerted manner.101,102) Investigation of molecular mechanisms underlying the membrane integration of the cargo PMPs is under way.
3.1.2. ER is involved in peroxisome biogenesis?
In regard to peroxisomal membrane assembly, the concepts of the Pex19p- and Pex16p-dependent direct import as well as the ER-dependent indirect import have recently emerged.94,103) ER was postulated to provide the initial ‘seed’ for recruiting Pex3p and Pex16p required for peroxisome assembly.104–106) Several groups suggested a different view of peroxisomal membrane biogenesis that peroxisomes are formed from ER107,108) upon induction of Pex3p;104,109,110) another study111) proposed that all peroxisomal membrane proteins are transported via ER. Several peroxisomal membrane proteins might be transported to peroxisomes via ER,112–114) implying a semi-autonomous property of peroxisomes. A recent proximity-specific ribosome profiling suggested that many PMPs are translated at the ER in both mammalian and yeast cells, implying that they are plausible substrates for the indirect route.115) Interestingly, several PMPs seem to target to peroxisomes both directly from the cytosol91,94,96,116) and indirectly via the ER.103,117,118)
However, the significance of such observations remains under debate. A study119) suggested that peroxisomes are generally formed by growth and division under normal conditions and that only under a condition where no peroxisome is present in a cell, they can be formed from the ER after the expression of the complementing PEX gene. Meanwhile, we demonstrated that Pex3p, the membrane receptor for Pex19p-complexes with PMPs including Pex16p, is directly targeted to peroxisomes in a Pex19p-Pex16p dependent class II pathway in mammalian cells.94) Moreover, we very recently provided several lines of evidence that most, if not all, mammalian PMPs are indeed authentic substrates for the Pex19p- and Pex3p-mediated class I direct pathway.91) At any event, future investigations on whether the two distinct routes exist simultaneously in cells and when cells use these routes are required for comprehensive understanding of PMP biogenesis.24,83,105,106)
3.2. Matrix protein import.
Ten peroxins including Pex1p, Pex2p, Pex5p, Pex6p, Pex7p, Pex10p, Pex12p, Pex13p, Pex14p, and Pex26p are involved in protein import into peroxisomal matrix24,34,90) (Fig. 2).
3.2.1. PTS import receptors.
PTS1 and PTS2 proteins are recognized by Pex5p and Pex7p, respectively, in the cytoplasm. Two isoforms of Pex5p, Pex5pS and Pex5pL with an internal 37-amino-acid insertion, are identified in mammals. PTS1 proteins are transported by homo- and hetero-oligomers of Pex5pS and Pex5pL to peroxisomes, where Pex14p of an 800-kDa complex functions as the initial Pex5p-docking site (Fig. 2). Pex5pL translocates the Pex7p-PTS2 protein complex to Pex14p.120,121) After releasing the cargoes, Pex5p and Pex7p translocate to a 500-kDa ‘translocation complex’ consisting of the RING peroxins, Pex2p, Pex10p, and Pex12p.121) Both Pex5p and Pex7p finally translocate back to the cytosol.121–126) At the terminal step of the protein import reaction, AAA peroxins, Pex1p and Pex6p, recruited to Pex26p (Pex15p in yeast) on peroxisomes catalyze the ATP-dependent export of Pex5p.121,124,127)
3.2.2. Peroxisome-cytoplasmic shuttling of import receptors.
Mono-ubiquitination via the thioester bond of the conserved cysteine residue at position 11 in the N-terminal region of Pex5p (Ub-Pex5p) is a prerequisite for the Pex5p recycling, i.e., in the export step from peroxisomes to the cytosol,128–131) as in yeast132,133) (Fig. 2). Moreover, a cytosolic factor, AWP1/ZFAND6 involved in the export of Ub-Pex5p is identified in mammals;131) USP9X and Ubp15 are suggested as a potential deubiquitinase in mammals134) and yeast,135) respectively. A distinct redox state may affect the recycling of Pex5p requiring Cys-ubiquitination, thereby being as a possible cause to the phenotype of deficiency in matrix protein import in PEX-defective cells.136)
4. Gene defects of proteins for peroxisomal morphogenesis
Three isoforms of Pex11p family, Pex11pα,137,138) Pex11pβ,139–142) and Pex11pγ,138,143) are identified as factors involved in morphogenesis of peroxisomes in mammals.142,144–147) In mammalian cells, dynamin-like protein 1 (DLP1),148–151) Fis1,144,152) and mitochondrial fission factor (Mff)147,153–155) are shown to be involved in the fission of peroxisomes156) (Fig. 2).
We first reported a CHO cell mutant ZP121 in mammalian cells that was impaired in DLP1 with one point dominant-negative mutation at G363D in the middle region.150) With respect to peroxisomal dysmorphogenesis in humans, only three patients have been identified with a different defect in two proteins involved in the proliferation and division of peroxisomes. The first reported patient was a severely affected female patient, who died one month after the birth and postmortally was found to have a dominant-negative heterozygous mutation at G395D in DLP1 resulted in a severe fission defect of both peroxisomes and mitochondria.151) The second patient with dysfunctional Dnm1L (DLP1) harboring G362D mutation was most recently reported.157) The first patient with a defect of peroxisomal division due to a homozygous non-sense mutation in the PEX11β gene was recently reported as the 14th CG (CG16) of PBDs32) (Table 3).
5. Turnover of peroxisomes
Several hundred peroxisomes in mammalian cells are maintained by peroxisome homeostasis, a balance between the biogenesis and turnover of peroxisomes. A form of autophagy specific for peroxisomes, named pexophagy, is the main pathway for peroxisome degradation in mammals.158) Pexophagy is well studied at a molecular level in yeast because the strong peroxisome-induction condition and the sensitive detection and gene screening systems of pexophagy are established.159–161) In contrast to the yeast system, however, molecular mechanisms of mammalian pexophagy remained largely unknown for long time.
In recent years, several reports described six different types of inducing conditions for pexophagy in mammalian cells by different stimuli, including (a) nutrient-replenishment for short period of starvation,162,163) (b) Ub-anchored peroxisomal membrane proteins,164) (c) NBR1, one of the autophagy adaptor proteins,165) (d) Pex3p,166) (e) mono-Ub-Pex5p,167) and (f) H2O2.168) Common aspects include that peroxisomal ubiquitination is recognized by autophagy adaptor proteins, p62 and/or NBR1, and that peroxisomes are then connected to autophagy machineries. Such useful and effective systems will shed light to mechanisms of mammalian pexophagy.
6. Perspective
Mammalian cell mutants of 15 CGs defective of peroxisome biogenesis have been identified, including PBD patients’ fibroblasts and CHO mutant cell lines (Table 3). Pathogenic genes are now elucidated for all 14 CGs of PBDs. Biochemical functions of peroxins involved in the import of matrix proteins are better elucidated, whilst molecular mechanisms underlying the membrane assembly are less understood. Defects in peroxisomal morphogenesis have also been reported. Investigations using the cloned peroxins and pex mutants including CHO cell mutants, cell lines from PBD patients, and PEX gene-knockout mice141,169–172) will shed light on the mechanisms involved in biogenesis, morphogenesis, and homeostasis of peroxisomes and pathogenesis of PBDs.
Acknowledgments
I thank all of my colleagues of the Fujiki laboratory who contributed to the work discussed in this review. I also thank K. Shimizu for Figure illustrations. This work was supported in part by Grants-in-Aid for Scientific Research: 24247038, 25112518, 25116717, 26116007, and 15K14511 from the Ministry of Education, Culture, Sports, Science and Technology of Japan and grants from the Takeda Science Foundation, the Naito Foundation, and the Japan Foundation for Applied Enzymology.
Abbreviations
- CG
complementation group
- CHO
Chinese hamster ovary
- DHAP-ATase
dihydroxyacetonephosphate acyltransferase
- DLP1
dynamin-like protein 1
- IRD
infantile Refsum disease
- NALD
neonatal adrenoleukodystrophy
- PBD
peroxisome biogenesis disorder
- PMP
peroxisomal integral membrane protein
- PTS
peroxisome-targeting signal
- RCDP
rhizomelic chondrodysplasia punctata
- ZS
Zellweger syndrome
Profile
Yukio Fujiki was born in Fukuoka Prefecture in 1948 and graduated from Kyushu University in 1971. He received Ph.D. degree in 1976 and worked as a post-doctoral fellow in Cornell University Medical College and then as a research associate at C. de Duve’s lab in the Rockefeller University, where he became an Assistant Professor in 1980. He came back to Japan in 1985 to work for Meiji Institute of Health Science and served as a Head and Chief Scientist at Department of Molecular Cell Biology. He became a Professor at Faculty of Science, Kyushu University in 1994 and then was appointed Distinguished Professor in 2009 and Professor Emeritus in 2013. He also served as Executive Vice President of Kyushu University from 2010 to 2014, and as Administrative Director of International Institute for Carbon-Neutral Energy Research, Kyushu University from 2013 to 2014. He continues his research at Medical Institute of Bioregulation as Professor from 2014.
He established one-step membrane isolation method called sodium carbonate or alkaline extraction method. This method has been widely used for cell membrane isolation and for assessing the integration of proteins into membrane. He has been working on intracellular organelle homeostasis by taking peroxisome as a model system. The functional consequence of human peroxisomes is highlighted by fatal genetic peroxisome biogenesis disorders (PBD), including Zellweger (cerebro-hepato-renal) syndrome, all of which are linked to a failure of peroxisome assembly. Peroxisome assembly in mammals including humans requires more than 14 PEX gene products termed peroxins. Fujiki and his colleagues isolated 11 PEX genes responsible for PBD. Fujiki’s group tackles the problems involving membrane assembly, matrix protein import, morphogenesis, and homeostasis of peroxisomes, which are tightly linked to cell functions.
References
- 1).Wanders R.J.A., Waterham H.R. (2006) Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332. [DOI] [PubMed] [Google Scholar]
- 2).de Duve C., Baudhuin P. (1966) Peroxisomes (microbodies and related particles). Physiol. Rev. 46, 323–357. [DOI] [PubMed] [Google Scholar]
- 3).Lazarow P.B., Fujiki Y. (1985) Biogenesis of peroxisomes. Annu. Rev. Cell Biol. 1, 489–530. [DOI] [PubMed] [Google Scholar]
- 4).Fujiki Y. (1997) Molecular defects in genetic diseases of peroxisomes. Biochim. Biophys. Acta 1361, 235–250. [DOI] [PubMed] [Google Scholar]
- 5).Fujiki Y. (2000) Peroxisome biogenesis and peroxisome biogenesis disorders. FEBS Lett. 476, 42–46. [DOI] [PubMed] [Google Scholar]
- 6).Erdmann R., Veenhuis M., Mertens D., Kunau W.-H. (1989) Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 86, 5419–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Gould S.J., McCollum D., Spong A.P., Heyman J.A., Subramani S. (1992) Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast 8, 613–628. [DOI] [PubMed] [Google Scholar]
- 8).Liu H., Tan X., Veenhuis M., McCullum D., Cregg J.M. (1992) An efficient screen for peroxisome-deficient mutants of Pichia pastoris. J. Bacteriol. 174, 4943–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Cregg J.M., Vankiel I.J., Sulter G.J., Veenhuis M., Harder W. (1990) Peroxisome-deficient mutants of Hansenula polymorpha. Yeast 6, 87–97. [Google Scholar]
- 10).Nuttley W.M., Brade A.M., Gaillardin C., Eitzen G.A., Glover J.R., Aitchison J.D., Rachubinski R.A. (1993) Rapid identification and characterization of peroxisomal assembly mutants in Yarrowia lipolytica. Yeast 9, 507–517. [Google Scholar]
- 11).Kunau W.-H. (1998) Peroxisome biogenesis from yeast to man. Curr. Opin. Microbiol. 1, 232–237. [DOI] [PubMed] [Google Scholar]
- 12).Subramani S., Koller A., Snyder W.B. (2000) Import of peroxisomal matrix and membrane proteins. Annu. Rev. Biochem. 69, 399–418. [DOI] [PubMed] [Google Scholar]
- 13).Tabak H.F., Braakman I., Distel B. (1999) Peroxisomes: simple in function but complex in maintenance. Trends Cell Biol. 9, 447–453. [DOI] [PubMed] [Google Scholar]
- 14).van der Klei I.J., Veenhuis M. (1996) Peroxisome biogenesis in the yeast Hansenula polymorpha: a structural and functional analysis. Ann. N. Y. Acad. Sci. 804, 47–59. [DOI] [PubMed] [Google Scholar]
- 15).Titorenko V.I., Rachubinski R.A. (2001) The life cycle of the peroxisome. Nat. Rev. Mol. Cell Biol. 2, 357–368. [DOI] [PubMed] [Google Scholar]
- 16).Lazarow P.B. (2003) Peroxisome biogenesis: advances and conundrums. Curr. Opin. Cell Biol. 15, 489–497. [DOI] [PubMed] [Google Scholar]
- 17).Hayashi M., Nishimura M. (2006) Arabidopsis thaliana — A model organism to study plant peroxisomes. Biochim. Biophys. Acta-Mol. Cell Res. 1763, 1382–1391. [DOI] [PubMed] [Google Scholar]
- 18).Schatz G., Dobberstein B. (1996) Common principles of protein translocation across membranes. Science 271, 1519–1526. [DOI] [PubMed] [Google Scholar]
- 19).Wickner W., Schekman R. (2005) Protein translocation across biological membranes. Science 310, 1452–1456. [DOI] [PubMed] [Google Scholar]
- 20).Gould S.J., Valle D. (2000) Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet. 16, 340–345. [DOI] [PubMed] [Google Scholar]
- 21).Fujiki, Y. (2003) Peroxisome biogenesis disorders. In Nature Encyclopedia of the Human Genome (ed. Cooper, D.N.). vol. 4, Nature Publishing Group, London, pp. 541–547. [Google Scholar]
- 22).Weller S., Gould S.J., Valle D. (2003) Peroxisome biogenesis disorders. Annu. Rev. Genomics Hum. Genet. 4, 165–211. [DOI] [PubMed] [Google Scholar]
- 23).Gould, S.J., Raymond, G.V. and Valle, D. (2001) The peroxisome biogenesis disorders. In The Metabolic and Molecular Bases of Inherited Disease (eds. Scriver, C.R. Beaudet, A.L., Sly, W.S. and Valle, D.). 8th ed., McGraw Hill, New York, pp. 3181–3217. [Google Scholar]
- 24).Fujiki Y., Okumoto K., Mukai S., Honsho M., Tamura S. (2014) Peroxisome biogenesis in mammalian cells. Front. Physiol. 5, article 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Ghaedi K., Honsho M., Shimozawa N., Suzuki Y., Kondo N., Fujiki Y. (2000) PEX3 is the causal gene responsible for peroxisome membrane assembly-defective Zellweger syndrome of complementation group G. Am. J. Hum. Genet. 67, 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Matsumoto N., Tamura S., Moser A., Moser H.W., Braverman N., Suzuki Y., Shimozawa N., Kondo N., Fujiki Y. (2001) The peroxin Pex6p gene is impaired in peroxisome biogenesis disorders of complementation group 6. J. Hum. Genet. 46, 273–277. [DOI] [PubMed] [Google Scholar]
- 27).Shimozawa N., Tsukamoto T., Nagase T., Takemoto Y., Koyama N., Suzuki Y., Komori M., Osumi T., Jeannette G., Wanders R.J.A., Kondo N. (2004) Identification of a new complementation group of the peroxisome biogenesis disorders and PEX14 as the mutated gene. Hum. Mutat. 23, 552–558. [DOI] [PubMed] [Google Scholar]
- 28).Zoeller R.A., Raetz C.R. (1986) Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc. Natl. Acad. Sci. U.S.A. 83, 5170–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Tsukamoto T., Yokota S., Fujiki Y. (1990) Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J. Cell Biol. 110, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Morand O.H., Allen L.-A.H., Zoeller R.A., Raetz C.R.H. (1990) A rapid selection for animal cell mutants with defective peroxisomes. Biochim. Biophys. Acta 1034, 132–141. [DOI] [PubMed] [Google Scholar]
- 31).Shimozawa N., Tsukamoto T., Suzuki Y., Orii T., Fujiki Y. (1992) Animal cell mutants represent two complementation groups of peroxisome-defective Zellweger syndrome. J. Clin. Invest. 90, 1864–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Ebberink M.S., Koster J., Visser G., van Spronsen F., Stolte-Dijkstra I., Smit G.P.A., Fock J.M., Kemp S., Wanders R.J.A., Waterham H.R. (2012) A novel defect of peroxisome division due to a homozygous non-sense mutation in the PEX11β gene. J. Med. Genet. 49, 307–313. [DOI] [PubMed] [Google Scholar]
- 33).Distel B., Erdmann R., Gould S.J., Blobel G., Crane D.I., Cregg J.M., Dodt G., Fujiki Y., Goodman J.M., Just W.W., Kiel J.A.K.W., Kunau W.-H., Lazarow P.B., Mannaerts G.P., Moser H., Osumi T., Rachubinski R.A., Roscher A., Subramani S., Tabak H.F., Tsukamoto T., Valle D., van der Klei I., van Veldhoven P.P., Veenhuis M. (1996) A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 135, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Fujiki Y., Okumoto K., Kinoshita N., Ghaedi K. (2006) Lessons from peroxisome-deficient Chinese hamster ovary (CHO) cell mutants. Biochim. Biophys. Acta-Mol. Cell Res. 1763, 1374–1381. [DOI] [PubMed] [Google Scholar]
- 35).Tsukamoto T., Miura S., Fujiki Y. (1991) Restoration by a 35K membrane protein of peroxisome assembly in a peroxisome-deficient mammalian cell mutant. Nature 350, 77–81. [DOI] [PubMed] [Google Scholar]
- 36).Zoeller R.A., Morand O.H., Raetz C.R.H. (1988) A possible role for plasmalogens in protecting animal cells against photosensitized killing. J. Biol. Chem. 263, 11590–11596. [PubMed] [Google Scholar]
- 37).Saurin A.J., Borden K.L.B., Boddy M.N., Freemont P.S. (1996) Does this have a familiar RING? Trends Biochem. Sci. 21, 208–214. [PubMed] [Google Scholar]
- 38).Shimozawa N., Tsukamoto T., Suzuki Y., Orii T., Shirayoshi Y., Mori T., Fujiki Y. (1992) A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science 255, 1132–1134. [DOI] [PubMed] [Google Scholar]
- 39).Tsukamoto T., Miura S., Nakai T., Yokota S., Shimozawa N., Suzuki Y., Orii T., Fujiki Y., Sakai F., Bogaki A., Yasumo H., Osumi T. (1995) Peroxisome assembly factor-2, a putative ATPase cloned by functional complementation on a peroxisome-deficient mammalian cell mutant. Nat. Genet. 11, 395–401. [DOI] [PubMed] [Google Scholar]
- 40).Tamura S., Okumoto K., Toyama R., Shimozawa N., Tsukamoto T., Suzuki Y., Osumi T., Kondo N., Fujiki Y. (1998) Human PEX1 cloned by functional complementation on a CHO cell mutant is responsible for peroxisome-deficient Zellweger syndrome of complementation group I. Proc. Natl. Acad. Sci. U.S.A. 95, 4350–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Ghaedi K., Tamura S., Okumoto K., Matsuzono Y., Fujiki Y. (2000) The peroxin Pex3p initiates membrane assembly in peroxisome biogenesis. Mol. Biol. Cell 11, 2085–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Otera H., Okumoto K., Tateishi K., Ikoma Y., Matsuda E., Nishimura M., Tsukamoto T., Osumi T., Ohashi K., Higuchi O., Fujiki Y. (1998) Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: Studies with PEX5-defective CHO cell mutants. Mol. Cell. Biol. 18, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Okumoto K., Fujiki Y. (1997) PEX12 encodes an integral membrane protein of peroxisomes. Nat. Genet. 17, 265–266. [DOI] [PubMed] [Google Scholar]
- 44).Okumoto K., Shimozawa N., Kawai A., Tamura S., Tsukamoto T., Osumi T., Moser H., Wanders R.J.A., Suzuki Y., Kondo N., Fujiki Y. (1998) PEX12, the pathogenic gene of group III Zellweger syndrome: cDNA cloning by functional complementation on a CHO cell mutant, patient analysis, and characterization of Pex12p. Mol. Cell. Biol. 18, 4324–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Toyama R., Mukai S., Itagaki A., Tamura S., Shimozawa N., Suzuki Y., Kondo N., Wanders R.J.A., Fujiki Y. (1999) Isolation, characterization, and mutation analysis of PEX13-defective Chinese hamster ovary cell mutants. Hum. Mol. Genet. 8, 1673–1681. [DOI] [PubMed] [Google Scholar]
- 46).Shimizu N., Itoh R., Hirono Y., Otera H., Ghaedi K., Tateishi K., Tamura S., Okumoto K., Harano T., Mukai S., Fujiki Y. (1999) The peroxin Pex14p: cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization, and functional analysis. J. Biol. Chem. 274, 12593–12604. [DOI] [PubMed] [Google Scholar]
- 47).Matsuzono Y., Kinoshita N., Tamura S., Shimozawa N., Hamasaki M., Ghaedi K., Wanders R.J.A., Suzuki Y., Kondo N., Fujiki Y. (1999) Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger syndrome, and potential role in peroxisomal membrane assembly. Proc. Natl. Acad. Sci. U.S.A. 96, 2116–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Matsumoto N., Tamura S., Fujiki Y. (2003) The pathogenic peroxin Pex26p recruits the Pex1p-Pex6p AAA ATPase complexes to peroxisomes. Nat. Cell Biol. 5, 454–460. [DOI] [PubMed] [Google Scholar]
- 49).Fransen M., Brees C., Baumgart E., Vanhooren J.C., Baes M., Mannaerts G.P., Veldhoven P.P.V. (1995) Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J. Biol. Chem. 270, 7731–7736. [DOI] [PubMed] [Google Scholar]
- 50).Wiemer E.A., Nuttley W.M., Bertolaet B.L., Li X., Francke U., Wheelock M.J., Anne U.K., Johnson K.R., Subramani S. (1995) Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J. Cell Biol. 130, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Fransen M., Terlecky S.R., Subramani S. (1998) Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc. Natl. Acad. Sci. U.S.A. 95, 8087–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Kammerer S., Arnold N., Gutensohn W., Mewes H.-W., Kunau W.-H., Heofler G., Roscher A.A., Braun A. (1997) Genomic organization and molecular characterization of a gene encoding HsPxF, a human peroxisomal farnesylated protein. Genomics 45, 200–210. [DOI] [PubMed] [Google Scholar]
- 53).Matsumoto N., Tamura S., Furuki S., Miyata N., Moser A., Shimozawa N., Moser H.W., Suzuki Y., Kondo N., Fujiki Y. (2003) Mutations in novel peroxin gene PEX26 that cause peroxisome-biogenesis disorders of complementation group 8 provide a genotype-phenotype correlation. Am. J. Hum. Genet. 73, 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Fujiki, Y. (2011) Peroxisome biogenesis disorders. In Encyclopedia of Life Sciences. John Wiley & Sons, Chichester, U.K., pp. 1–9. [Google Scholar]
- 55).Reuber B.E., Germain-Lee E., Collins C.S., Morrell J.C., Ameritunga R., Moser H.W., Valle D., Gould S.J. (1997) Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat. Genet. 17, 445–448. [DOI] [PubMed] [Google Scholar]
- 56).Portsteffen H., Beyer A., Becker E., Epplen C., Pawlak A., Kunau W.-H., Dodt G. (1997) Human PEX1 is mutated in complementation group 1 of the peroxisome biogenesis disorders. Nat. Genet. 17, 449–452. [DOI] [PubMed] [Google Scholar]
- 57).Kammerer S., Holzinger A., Welsch U., Roscher A.A. (1998) Cloning and characterization of the gene encoding the human peroxisomal assembly protein Pex3p. FEBS Lett. 429, 53–60. [DOI] [PubMed] [Google Scholar]
- 58).Dodt G., Braverman N., Wong C., Moser A., Moser H.W., Watkins P., Valle D., Gould S.J. (1995) Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat. Genet. 9, 115–125. [DOI] [PubMed] [Google Scholar]
- 59).Yahraus T., Braverman N., Dodt G., Kalish J.E., Morrell J.C., Moser H.W., Valle D., Gould S.J. (1996) The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 15, 2914–2923. [PMC free article] [PubMed] [Google Scholar]
- 60).Braverman N., Steel G., Obie C., Moser A., Moser H., Gould S.J., Valle D. (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 15, 369–376. [DOI] [PubMed] [Google Scholar]
- 61).Motley A.M., Hettema E.H., Hogenhout E.M., Brites P., ten Asbroek A.L.M.A., Wijburg F.A., Baas F., Heijmans H.S., Tabak H.F., Wanders R.J.A., Distel B. (1997) Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat. Genet. 15, 377–380. [DOI] [PubMed] [Google Scholar]
- 62).Purdue P.E., Zhang J.W., Skoneczny M., Lazarow P.B. (1997) Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat. Genet. 15, 381–384. [DOI] [PubMed] [Google Scholar]
- 63).Okumoto K., Itoh R., Shimozawa N., Suzuki Y., Tamura S., Kondo N., Fujiki Y. (1998) Mutation in PEX10 is the cause of Zellweger peroxisome deficiency syndrome of complementation group B. Hum. Mol. Genet. 7, 1399–1405. [DOI] [PubMed] [Google Scholar]
- 64).Warren D.S., Morrell J.C., Moser H.W., Valle D., Gould S.J. (1998) Identification of PEX10, the gene defective in complementation group 7 of the peroxisome-biogenesis disorders. Am. J. Hum. Genet. 63, 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Chang C.-C., Lee W.-H., Moser H., Valle D., Gould S.J. (1997) Isolation of the human PEX12 gene, mutated in group 3 of the peroxisome biogenesis disorders. Nat. Genet. 15, 385–388. [DOI] [PubMed] [Google Scholar]
- 66).Gould S.J., Kalish J.E., Morrell J.C., Bjorkman J., Urquhart A.J., Crane D.I. (1996) Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTS1 receptor. J. Cell Biol. 135, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Will G.K., Soukupova M., Hong X., Erdmann K.S., Kiel J.A.K.W., Dodt G., Kunau W.-H., Erdmann R. (1999) Identification and characterization of the human orthologue of yeast Pex14p. Mol. Cell. Biol. 19, 2265–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Honsho M., Tamura S., Shimozawa N., Suzuki Y., Kondo N., Fujiki Y. (1998) Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am. J. Hum. Genet. 63, 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).South S.T., Gould S.J. (1999) Peroxisome synthesis in the absence of preexisting peroxisomes. J. Cell Biol. 144, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Fujiki, Y. (2003) Functional complementation. In Nature Encyclopedia of the Human Genome (ed. Cooper, D.N.). 2, Nature Publishing Group, London, pp. 539–543. [Google Scholar]
- 71).Sztriha L., Al-Gazali L.I., Wanders R.J.A., Ofman R., Nork M., Lestringant G.G. (2000) Abnormal myelin formation in rhizomelic chondrodysplasia punctate type 2 (DHAPAT-deficiency). Dev. Med. Child Neurol. 42, 492–495. [DOI] [PubMed] [Google Scholar]
- 72).Bams-Mengerink A.M., Majoie C.B., Duran M., Wanders R.J., Van Hove J., Scheurer C.D., Barth P.G., Poll-The B.T. (2006) MRI of the brain and cervical spinal cord in rhizomelic chondrodysplasia punctata. Neurology 66, 798–803. [DOI] [PubMed] [Google Scholar]
- 73).Buchert R., Tawamie H., Smith C., Uebe S., Innes A.M., Al Hallak B., Ekici A.B., Sticht H., Schwarze B., Lamont R.E., Parboosingh J.S., Bernier F.P., Abou Jamra R. (2014) A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am. J. Hum. Genet. 95, 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Barøy T., Koster J., Strømme P., Ebberink M.S., Misceo D., Ferdinandusse S., Holmgren A., Hughes T., Merckoll E., Westvik J., Woldseth B., Walter J., Wood N., Tvedt B., Stadskleiv K., Wanders R.J.A., Waterham H.R., Frengen E. (2015) A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum. Mol. Genet. 24, 5845–5854. [DOI] [PubMed] [Google Scholar]
- 75).Matsumura T., Otera H., Fujiki Y. (2000) Disruption of interaction of the longer isoform of Pex5p, Pex5pL, with Pex7p abolishes the PTS2 protein import in mammals: Study with a novel PEX5-impaired Chinese hamster ovary cell mutant. J. Biol. Chem. 275, 21715–21721. [DOI] [PubMed] [Google Scholar]
- 76).Weller S., Cajigas I., Morrell J., Obie C., Steel G., Gould S.J., Valle D. (2005) Alternative splicing suggests extended function of PEX26 in peroxisome biogenesis. Am. J. Hum. Genet. 76, 987–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Steinberg S.J., Dodt G., Raymond G.V., Braverman N.E., Moser A.B., Moser H.W. (2006) Peroxisome biogenesis disorders. Biochim. Biophys. Acta-Mol. Cell Res. 1763, 1733–1748. [DOI] [PubMed] [Google Scholar]
- 78).Imamura A., Tamura S., Shimozawa N., Suzuki Y., Zhang Z., Tsukamoto T., Orii T., Kondo N., Osumi T., Fujiki Y. (1998) Temperature-sensitive mutation in PEX1 moderates the phenotypes of peroxisome deficiency disorders. Hum. Mol. Genet. 7, 2089–2094. [DOI] [PubMed] [Google Scholar]
- 79).Imamura A., Tsukamoto T., Shimozawa N., Suzuki Y., Zhang Z., Imanaka T., Fujiki Y., Orii T., Kondo N., Osumi T. (1998) Temperature-sensitive phenotypes of peroxisome assembly processes represent the milder forms of human peroxisome-biogenesis disorders. Am. J. Hum. Genet. 62, 1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Geisbrecht B.V., Collins C.S., Reuber B.E., Gould S.J. (1998) Disruption of a PEX1-PEX6 interaction is the most common cause of the neurologic disorders Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile Refsum disease. Proc. Natl. Acad. Sci. U.S.A. 95, 8630–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Walter C., Gootjes J., Mooijer P.A., Portsteffen H., Klein C., Waterham H.R., Barth P.G., Epplen J.T., Kunau W.-H., Wanders R.J.A., Dodt G. (2001) Disorders of peroxisome biogenesis due to mutations in PEX1: phenotypes and PEX1 protein levels. Am. J. Hum. Genet. 69, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Fujiki, Y., Okumoto, K., Mukai, S. and Tamura, S. (2014) Molecular basis for peroxisome biogenesis disorders. In Molecular Machines Involved in Peroxisomes Maintenance (eds. Brocard, C. and Hartig, A.). Springer-Verlag, Berlin, pp. 91–110. [Google Scholar]
- 83).Fujiki Y., Yagita Y., Matsuzaki T. (2012) Peroxisome biogenesis disorders: Molecular basis for impaired peroxisomal membrane assembly - In metabolic functions and biogenesis of peroxisomes in health and disease. Biochim. Biophys. Acta-Mol. Basis Disease 1822, 1337–1342. [DOI] [PubMed] [Google Scholar]
- 84).Sacksteder K.A., Jones J.M., South S.T., Li X., Liu Y., Gould S.J. (2000) PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J. Cell Biol. 148, 931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).South S.T., Sacksteder K.A., Li X., Liu Y., Gould S.J. (2000) Inhibitors of COPI and COPII do not block PEX3-mediated peroxisome synthesis. J. Cell Biol. 149, 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Baerends R.J.S., Rasmussen S.W., Hilbrands R.E., van der Heide M., Faber K.N., Reuvekamp P.T.W., Klei J.A.K.W., Cregg J.M., van der Klei I.J., Veenhuis M. (1996) The Hansenula polymorpha PER9 gene encodes a peroxisomal membrane protein essential for peroxisome assembly and integrity. J. Biol. Chem. 271, 8887–8894. [DOI] [PubMed] [Google Scholar]
- 87).Götte K., Girzalsky W., Linkert M., Baumgart E., Kammerer S., Kunau W.-H., Erdmann R. (1998) Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol. Cell. Biol. 18, 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Hettema E.H., Girzalsky W., van den Berg M., Erdmann R., Distel B. (2000) Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Otzen M., Perband U., Wang D., Baerends R.J., Kunau W.H., Veenhuis M., Van der Klei I.J. (2004) Hansenula polymorpha Pex19p is essential for the formation of functional peroxisomal membranes. J. Biol. Chem. 279, 19181–19190. [DOI] [PubMed] [Google Scholar]
- 90).Fujiki Y., Matsuzono Y., Matsuzaki T., Fransen M. (2006) Import of peroxisomal membrane proteins: The interplay of Pex3p- and Pex19p-mediated interactions. Biochim. Biophys. Acta-Mol. Cell Res. 1763, 1639–1646. [DOI] [PubMed] [Google Scholar]
- 91).Liu Y., Yagita Y., Fujiki Y. (2016) Assembly of peroxisomal membrane proteins via the direct Pex19p-Pex3p pathway. Traffic 17, 433–455. [DOI] [PubMed] [Google Scholar]
- 92).Fang Y., Morrell J.C., Jones J.M., Gould S.J. (2004) PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J. Cell Biol. 164, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Matsuzono Y., Matsuzaki T., Fujiki Y. (2006) Functional domain mapping of peroxin Pex19p: interaction with Pex3p is essential for function and translocation. J. Cell Sci. 119, 3539–3550. [DOI] [PubMed] [Google Scholar]
- 94).Matsuzaki T., Fujiki Y. (2008) The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J. Cell Biol. 183, 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Eitzen G.A., Szilard R.K., Rachubinski R.A. (1997) Enlarged peroxisomes are present in oleic acid-grown Yarrowia lipolytica overexpressing the PEX16 gene encoding an intraperoxisomal peripheral membrane peroxin. J. Cell Biol. 137, 1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Yagita Y., Hiromasa T., Fujiki Y. (2013) Tail-anchored PEX26 targets peroxisomes via a PEX19-dependent and TRC40-independent class I pathway. J. Cell Biol. 200, 651–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Schuldiner M., Metz J., Schmid V., Denic V., Rakwalska M., Schmitt H.D., Schwappach B., Weissman J.S. (2008) The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Diestelkotter P., Just W.W. (1993) In vitro insertion of the 22-kD peroxisomal membrane protein into isolated rat liver peroxisomes. J. Cell Biol. 123, 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Imanaka T., Shiina Y., Takano T., Hashimoto T., Osumi T. (1996) Insertion of the 70-kDa peroxisomal membrane protein into peroxisomal membranes in vivo and in vitro. J. Biol. Chem. 271, 3706–3713. [DOI] [PubMed] [Google Scholar]
- 100).Pinto M.P., Grou C.P., Alencastre I.S., Oliveira M.E., Sa-Miranda C., Fransen M., Azevedo J.E. (2006) The import competence of a peroxisomal membrane protein is determined by Pex19p before the docking step. J. Biol. Chem. 281, 34492–34502. [DOI] [PubMed] [Google Scholar]
- 101).Chen Y., Pieuchot L., Loh R.A., Yang J., Kari T.M., Wong J.Y., Jedd G. (2014) Hydrophobic handoff for direct delivery of peroxisome tail-anchored proteins. Nat. Commun. 5, article 5790. [DOI] [PubMed] [Google Scholar]
- 102).Schmidt F., Dietrich D., Eylenstein R., Groemping Y., Stehle T., Dodt G. (2012) The role of conserved PEX3 regions in PEX19-binding and peroxisome biogenesis. Traffic 13, 1244–1260. [DOI] [PubMed] [Google Scholar]
- 103).Hua R., Gidda S.K., Aranovich A., Mullen R.T., Kim P.K. (2015) Multiple domains in PEX16 mediate its trafficking and recruitment of peroxisomal proteins to the ER. Traffic 16, 832–852. [DOI] [PubMed] [Google Scholar]
- 104).Kim P.K., Mullen R.T., Schumann U., Lippincott-Schwartz J. (2006) The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J. Cell Biol. 173, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Ma C., Agrawal G., Subramani S. (2011) Peroxisome assembly: matrix and membrane protein biogenesis. J. Cell Biol. 193, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Tabak H.F., Braakman I., van der Zand A. (2013) Peroxisome formation and maintenance are dependent on the endoplasmic reticulum. Annu. Rev. Biochem. 82, 723–744. [DOI] [PubMed] [Google Scholar]
- 107).Titorenko V.I., Ogrydziak D.M., Rachubinski R.A. (1997) Four distinct secretory pathways serve protein secretion, cell surface growth, and peroxisome biogenesis in the yeast Yarrowia lipolytica. Mol. Cell. Biol. 17, 5210–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Titorenko V.I., Rachubinski R.A. (1998) The endoplasmic reticulum plays an essential role in peroxisome biogenesis. Trends Biochem. Sci. 23, 231–233. [DOI] [PubMed] [Google Scholar]
- 109).Hoepfner D., Schildknegt D., Braakman I., Philippsen P., Tabak H.F. (2005) Contribution of the endoplasmic reticulum to peroxisome formation. Cell 122, 85–95. [DOI] [PubMed] [Google Scholar]
- 110).Kragt A., Voorn-Brouwer T., van den Berg M., Distel B. (2005) Endoplasmic reticulum-directed Pex3p routes to peroxisomes and restores peroxisome formation in a Saccharomyces cerevisiae pex3Δ strain. J. Biol. Chem. 280, 34350–34357. [DOI] [PubMed] [Google Scholar]
- 111).van der Zand A., Braakman I., Tabak H.F. (2010) Peroxisomal membrane proteins insert into the endoplasmic reticulum. Mol. Biol. Cell 21, 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112).Agrawal G., Joshi S., Subramani S. (2011) Cell-free sorting of peroxisomal membrane proteins from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 108, 9113–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Lam S.K., Yoda N., Schekman R. (2010) A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 107, 21523–21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114).Yonekawa S., Furuno A., Baba T., Fujiki Y., Ogasawara Y., Yamamoto A., Tagaya M., Tani K. (2011) Sec16B is involved in the endoplasmic reticulum export of the peroxisomal membrane biogenesis factor peroxin 16 (Pex16) in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 108, 12746–12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Jan C.H., Williams C.C., Weissman J.S. (2014) Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science 346, 1257521-1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Matsuzono Y., Fujiki Y. (2006) In vitro transport of membrane proteins to peroxisomes by shuttling receptor Pex19p. J. Biol. Chem. 281, 36–42. [DOI] [PubMed] [Google Scholar]
- 117).Aranovich A., Hua R., Rutenberg A.D., Kim P.K. (2014) PEX16 contributes to peroxisome maintenance by constantly trafficking PEX3 via the ER. J. Cell Sci. 127, 3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Mayerhofer P.U., Bañó-Polo M., Mingarro I., Johnson A.E. (2016) Human peroxin PEX3 is co-translationally integrated into the ER and exits the ER in budding vesicles. Traffic 17, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119).Motley A.M., Hettema E.H. (2007) Yeast peroxisomes multiply by growth and division. J. Cell Biol. 178, 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Otera H., Setoguchi K., Hamasaki M., Kumashiro T., Shimizu N., Fujiki Y. (2002) Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: conserved Pex5p WXXXF/Y motifs are critical for matrix protein import. Mol. Cell. Biol. 22, 1639–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Miyata N., Fujiki Y. (2005) Shuttling mechanism of peroxisome targeting signal type 1 receptor Pex5: ATP-independent import and ATP-dependent export. Mol. Cell. Biol. 25, 10822–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Dammai V., Subramani S. (2001) The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell 105, 187–196. [DOI] [PubMed] [Google Scholar]
- 123).Gouveia A.M., Guimaraes C.P., Oliveira M.E., Reguenga C., Sa-Miranda C., Azevedo J.E. (2003) Characterization of the peroxisomal cycling receptor, Pex5p, using a cell-free in vitro import system. J. Biol. Chem. 278, 226–232. [DOI] [PubMed] [Google Scholar]
- 124).Platta H.W., Grunau S., Rosenkranz K., Girzalsky W., Erdmann R. (2005) Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat. Cell Biol. 7, 817–822. [DOI] [PubMed] [Google Scholar]
- 125).Miyata N., Hosoi K., Mukai S., Fujiki Y. (2009) In vitro import of peroxisome-targeting signal 2 (PTS2) receptor Pex7p into peroxisomes. Biochim. Biophys. Acta-Mol. Cell Res. 1793, 860–870. [DOI] [PubMed] [Google Scholar]
- 126).Nair D.M., Purdue P.E., Lazarow P.B. (2004) Pex7p translocates in and out of peroxisomes in Saccharomyces cerevisiae. J. Cell Biol. 167, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127).Tamura S., Matsumoto N., Takeba R., Fujiki Y. (2014) AAA peroxins and their recruiter Pex26p modulate the interactions of peroxins involved in peroxisomal protein import. J. Biol. Chem. 289, 24336–24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Carvalho A.F., Pinto M.P., Grou C.P., Alencastre I.S., Fransen M., Sá-Miranda C., Azevedo J.E. (2007) Ubiquitination of mammalian Pex5p, the peroxisomal import receptor. J. Biol. Chem. 282, 31267–31272. [DOI] [PubMed] [Google Scholar]
- 129).Grou C.P., Carvalho A.F., Pinto M.P., Huybrechts S.J., Sá-Miranda C., Fransen M., Azevedo J.E. (2009) Properties of the ubiquitin-Pex5p thiol ester conjugate. J. Biol. Chem. 284, 10504–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Okumoto K., Misono S., Miyata N., Matsumoto Y., Mukai S., Fujiki Y. (2011) Cysteine ubiquitination of PTS1 receptor Pex5p regulates Pex5p recycling. Traffic 12, 1067–1083. [DOI] [PubMed] [Google Scholar]
- 131).Miyata N., Okumoto K., Mukai S., Noguchi M., Fujiki Y. (2012) AWP1/ZFAND6 functions in Pex5 export by interacting with Cys-monoubiquitinated Pex5 and Pex6 AAA ATPase. Traffic 13, 168–183. [DOI] [PubMed] [Google Scholar]
- 132).Williams C., van den Berg M., Sprenger R.R., Distel B. (2007) A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p. J. Biol. Chem. 282, 22534–22543. [DOI] [PubMed] [Google Scholar]
- 133).Platta H.W., Magraoui F.E., Bäumer B.E., Schlee D., Girzalsky W., Erdmann R. (2009) Pex2 and Pex12 function as protein-ubiquitin ligases in peroxisomal protein import. Mol. Cell. Biol. 29, 5505–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Grou C.P., Francisco T., Rodrigues T.A., Freitas M.O., Pinto M.P., Carvalho A.F., Domingues P., Wood S.A., Rodríguez-Borges J.E., Sá-Miranda C., Fransen M., Azevedo J.E. (2012) Identification of ubiquitin-specific protease 9X (USP9X) as a deubiquitinase acting on ubiquitin-peroxin 5 (PEX5) thioester conjugate. J. Biol. Chem. 287, 12815–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).Debelyy M.O., Platta H.W., Saffian D., Hensel A., Thoms S., Meyer H.E., Warscheid B., Girzalsky W., Erdmann R. (2011) Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery. J. Biol. Chem. 286, 28223–28234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136).Yano T., Oku M., Akeyama N., Itoyama A., Yurimoto H., Kuge S., Fujiki Y., Sakai Y. (2010) A novel fluorescent sensor protein for visualization of redox states in the cytoplasm and in peroxisomes. Mol. Cell. Biol. 30, 3758–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137).Abe I., Okumoto K., Tamura S., Fujiki Y. (1998) Clofibrate-inducible, 28-kDa peroxisomal integral membrane protein is encoded by PEX11. FEBS Lett. 431, 468–472. [DOI] [PubMed] [Google Scholar]
- 138).Li X., Baumgart E., Dong G.-X., Morrell J.C., Jimenez-Sanchez G., Valle D., Smith K.D., Gould S.J. (2002) PEX11α is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor alpha-mediated peroxisome proliferation. Mol. Cell. Biol. 22, 8226–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139).Abe I., Fujiki Y. (1998) cDNA cloning and characterization of a constitutively expressed isoform of the human peroxin Pex11p. Biochem. Biophys. Res. Commun. 252, 529–533. [DOI] [PubMed] [Google Scholar]
- 140).Schrader M., Reuber B.E., Morrell J.C., Jimenez-Sanchez G., Obie C., Stroh T.A., Valle D., Schroer T.A., Gould S.J. (1998) Expression of PEX11β mediates peroxisome proliferation in the absence of extracellular stimuli. J. Biol. Chem. 273, 29607–29614. [DOI] [PubMed] [Google Scholar]
- 141).Li X., Baumgart E., Morrell J.C., Jimenez-Sanchez G., Valle D., Gould S.J. (2002) PEX11β deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol. Cell. Biol. 22, 4358–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Yoshida Y., Niwa H., Honsho M., Itoyama A., Fujiki Y. (2015) Pex11p mediates peroxisomal proliferation by promoting deformation of the lipid membrane. Biol. Open 4, 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Tanaka A., Okumoto K., Fujiki Y. (2003) cDNA cloning and characterization of the third isoform of human peroxin Pex11p. Biochem. Biophys. Res. Commun. 300, 819–823. [DOI] [PubMed] [Google Scholar]
- 144).Kobayashi S., Tanaka A., Fujiki Y. (2007) Fis1, DLP1, and Pex11p coordinately regulate peroxisome morphogenesis. Exp. Cell Res. 313, 1675–1686. [DOI] [PubMed] [Google Scholar]
- 145).Delille H.K., Agricola B., Guimaraes S.C., Borta H., Lüers G.H., Fransen M., Schrader M. (2010) Pex11pβ-mediated growth and division of mammalian peroxisomes follows a maturation pathway. J. Cell Sci. 123, 2750–2762. [DOI] [PubMed] [Google Scholar]
- 146).Koch J., Pranjic K., Huber A., Ellinger A., Hartig A., Kragler F., Brocard C. (2010) PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J. Cell Sci. 123, 3389–3400. [DOI] [PubMed] [Google Scholar]
- 147).Itoyama A., Michiyuki S., Honsho M., Yamamoto T., Moser A., Yoshida Y., Fujiki Y. (2013) Mff functions with Pex11pβ and DLP1 in peroxisomal fission. Biol. Open 2, 998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148).Koch A., Thiemann M., Grabenbauer M., Yoon Y., McNiven M.A., Schrader M. (2003) Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597–8605. [DOI] [PubMed] [Google Scholar]
- 149).Li X., Gould S.J. (2003) The dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11. J. Biol. Chem. 278, 17012–17020. [DOI] [PubMed] [Google Scholar]
- 150).Tanaka A., Kobayashi S., Fujiki Y. (2006) Peroxisome division is impaired in a CHO cell mutant with an inactivating point-mutation in dynamin-like protein 1 gene. Exp. Cell Res. 312, 1671–1684. [DOI] [PubMed] [Google Scholar]
- 151).Waterham H.R., Koster J., van Roermund C.W.T., Mooyer P.A.W., Wanders R.J.A., Leonard J.V. (2007) A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 356, 1736–1741. [DOI] [PubMed] [Google Scholar]
- 152).Koch A., Yoon Y., Bonekamp N.A., McNiven M.A., Schrader M. (2005) A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 16, 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153).Gandre-Babbe S., van der Bliek A.M. (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 19, 2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154).Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 191, 1141–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155).Koch J., Brocard C. (2012) PEX11 proteins attract Mff and human Fis1 to coordinate peroxisomal fission. J. Cell Sci. 125, 3813–3826. [DOI] [PubMed] [Google Scholar]
- 156).Fujiki, Y., Itoyama, A., Abe, Y. and Honsho, M. (2014) Molecular complex coordinating peroxisome morphogenesis in mammalian cells. In Molecular Machines Involved in Peroxisomes Maintenance (eds. Brocard, C. and Hartig, A.). Springer-Verlag, Berlin, pp. 391–401. [Google Scholar]
- 157).Vanstone J.R., Smith A.M., McBride S., Naas T., Holcik M., Antoun G., Harper M.-E., Michaud J., Sell E., Chakraborty P., Tetreault M., Care4Rare Consortium. Majewski J., Baird S., Boycott K.M., Dyment D.A., MacKenzie A., Lines M.A. (2016) DNM1L-related mitochondrial fission defect presenting as refractory epilepsy. Eur. J. Hum. Genet. 24, 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158).Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).Till A., Lakhani R., Burnett S.F., Subramani S. (2012) Pexophagy: the selective degradation of peroxisomes. Int. J. Cell Biol. 2012, 512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Farre J.-C., Manjithaya R., Mathewson R.D., Subramani S. (2008) PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev. Cell 14, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161).Motley A.M., Nuttall J.M., Hettema E.H. (2012) Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 31, 2852–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162).Hara-Kuge S., Fujiki Y. (2008) The peroxin Pex14p is involved in LC3-dependent degradation of mammalian peroxisomes. Exp. Cell Res. 314, 3531–3541. [DOI] [PubMed] [Google Scholar]
- 163).Jiang L., Hara-Kuge S., Yamashita S., Fujiki Y. (2015) Peroxin Pex14p is the key component for coordinated autophagic degradation of mammalian peroxisomes by direct binding to LC3-II. Genes Cells 20, 36–49. [DOI] [PubMed] [Google Scholar]
- 164).Kim P.K., Hailey D.W., Mullen R.T., Lippincott-Schwartz J. (2008) Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. U.S.A. 105, 20567–20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165).Deosaran E., Larsen K.B., Hua R., Sargent G., Wang Y., Kim S., Lamark T., Jauregui M., Law K., Lippincott-Schwartz J., Brech A., Johansen T., Kim P.K. (2013) NBR1 acts as an autophagy receptor for peroxisomes. J. Cell Sci. 126, 939–952. [DOI] [PubMed] [Google Scholar]
- 166).Yamashita S., Abe K., Tatemichi Y., Fujiki Y. (2014) The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy. Autophagy 10, 1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167).Nordgren M., Francisco T., Lismont C., Hennebel L., Brees C., Wang B., Van Veldhoven P.P., Azevedo J.E., Fransen M. (2015) Export-deficient monoubiquitinated PEX5 triggers peroxisome removal in SV40 large T antigen-transformed mouse embryonic fibroblasts. Autophagy 11, 1326–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168).Zhang J., Tripathi D.N., Jing J., Alexander A., Kim J., Powell R.T., Dere R., Tait-Mulder J., Lee J.-H., Paull T.T., Pandita R.K., Charaka V.K., Pandita T.K., Kastan M.B., Walker C.L. (2015) ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat. Cell Biol. 17, 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169).Faust P.L., Hatten M.E. (1997) Targeted deletion of the PEX2 peroxisome assembly gene in mice provides a model for Zellweger syndrome, a human neuronal migration disorder. J. Cell Biol. 139, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170).Baes M., Gressens P., Baumgart E., Carmeliet P., Casteels M., Fransen M., Evrard P., Fahimi D., Declercq P.E., Collen D., van Veldhoven P.P., Mannaerts G.P. (1997) A mouse model for Zellweger syndrome. Nat. Genet. 17, 49–57. [DOI] [PubMed] [Google Scholar]
- 171).Maxwell M., Bjorkman J., Nguyen T., Sharp P., Finnie J., Paterson C., Tonks I., Paton B.C., Kay G.F., Crane D.I. (2003) Pex13 inactivation in the mouse disrupts peroxisome biogenesis and leads to a Zellweger syndrome phenotype. Mol. Cell. Biol. 23, 5947–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172).Kassmann C.M., Lappe-Siefke C., Baes M., Brügger B., Mildner A., Werner H.B., Natt O., Michaelis T., Prinz M., Frahm J., Nave K.A. (2007) Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 39, 969–976. [DOI] [PubMed] [Google Scholar]