Abstract
Due to intrinsically low levels of antioxidant enzyme expression and activity, insulin producing pancreatic β-cells are particularly susceptible to free radical attack. In diabetes mellitus, which is accompanied by high levels of oxidative stress, this feature of β-cells significantly contributes to their damage and dysfunction. In light of the documented pro-survival effect of chemokine C-X-C Ligand 12 (CXCL12) on pancreatic β-cells, we examined its potential role in antioxidant protection. We report that CXCL12 overexpression enhanced the resistance of rat insulinoma (Rin-5F) and primary pancreatic islet cells to hydrogen peroxide (H2O2). CXCL12 lowered the levels of DNA damage and lipid peroxidation and preserved insulin expression. This effect was mediated through an increase in catalase (CAT) activity. By activating downstream p38, Akt and ERK kinases, CXCL12 facilitated Nrf2 nuclear translocation and enhanced its binding to the CAT gene promoter, inducing constitutive CAT expression and activity that was essential for protecting β-cells from H2O2.
Keywords: CXCL12, catalase, pancreatic β-cells, Nrf2, H2O2 treatment
Introduction
Oxidative stress is a major contributor to insulin producing pancreatic β-cell damage and dysfunction in diabetes mellitus. Elevated concentrations of reactive oxygen species (ROS) (which include the superoxide anion (O2−), hydroxyl radical (•OH), hydrogen peroxide (H2O2)), and reactive nitrogen species (RNS) (which include nitric oxide radical (NO•) and peroxynitrite (ONOO−)) are observed in systemic oxidative stress that accompanies both diabetes types 1 and 2.1) Pancreatic β-cells are at greater risk of oxidative damage than other tissues due to the intrinsically low levels of activities of antioxidant enzymes in these cells.2) While the expression level of O2− eliminating superoxide dismutase (SOD) isoenzymes (MnSOD and CuZnSOD) in β-cells is about 50% lower than in the liver, the expression levels of the H2O2-inactivating enzymes, catalase (CAT) and glutathione peroxidase (GPx) contribute to less than 2% of their levels of expression in the liver,3) rendering β-cells particularly vulnerable to increased concentrations of H2O2.
The low antioxidant capacity provides pancreatic β-cells with an enhanced responsiveness to ROS-mediated signaling.4) As a small, uncharged, freely diffusible molecule, H2O2 is an efficient intracellular messenger that can be synthesized and degraded rapidly in response to external stimuli.5) The H2O2 which is produced during glucose metabolism in β-cells serves as a metabolic signal for glucose-stimulated insulin secretion (GSIS).4) While low levels of ROS stimulate insulin release from β-cells, increased ROS levels reduce insulin expression and secretion, leading to β-cell damage. Therefore, maintenance of redox balance is critical for proper β-cell functioning. Slight stimulation of antioxidative enzyme expression exerts positive effects on β-cells by protecting them from oxidative stress, without hindering their ability to secrete insulin.6,7) In this regard, the stimulation of the endogenous antioxidant defenses in β-cells can be included in potential therapeutic approaches aimed at alleviating the harmful effects of oxidative stress on β-cells in diabetes. Any such consideration requires an understanding of the molecular events that underlie the regulation of antioxidant enzyme expression and activity.
Recent studies have stressed the important role of chemokine CXCL12 (C-X-C motif Ligand 12) in enhanced survival and regeneration of pancreatic β-cells.8) CXCL12 binds to the CXC receptor 4 (CXCR4) and 7 (CXCR7), initiating signal transduction that elicits a variety of biological responses.9) The main signaling pathways that are upregulated downstream of CXCL12 are phosphatidylinositol 3 kinase/Akt kinase (PI3K/Akt) and mitogen activated protein kinases (MAPK), such as extracellular signal regulated protein kinase (ERK) and p38 kinase.10,11) Activated PI3K/Akt kinases have a prosurvival role, primarily by inhibiting apoptotic pathways.12) Activated ERK kinase also promotes cell survival,13) while p38, depending on the type of activating stress, is involved in the inhibition of cell growth and induction of apoptosis,14) but also promotes cell survival.15)
Positive effects of CXCL12 on β-cells were initially hinted by Yano et al.16) who showed that β-cells overexpressing CXCL12 in RIP-SDF-1 transgenic mice are resistant to streptozotocin (STZ)-induced β-cell apoptosis and diabetes. Furthermore, when islet β-cells are injured by different stimuli (STZ, cytokines, thapsigargin and glucotoxicity), they induce expression and secretion of CXCL12 that changes the biological function of adjacent α-cells. The affected α-cells cease producing glucagon and start to produce glucagon-like peptide-1 (GLP-1) which, in combination with CXCL12, promotes the growth, survival and viability of β-cells.17) In our previous publications, we showed that the CXCL12-overexpressing insulinoma β-cell line (Rin-5F) is more resistant to treatments with either STZ18) or H2O219) in comparison to wild-type (wt) Rin-5F cells. In addition, we showed that pretreatment of wt and primary rat islet cells with recombinant CXCL12 improved their viability and insulin gene expression after H2O2 treatment. Even though these results showed that CXCL12 overexpression redirects H2O2-induced cell death from the necrotic to the apoptotic pathway mediated by Akt kinase,19) they strongly suggest that CXCL12 overexpressing β-cells, when compared with wt cells, were more resistant to oxidative stress mediated by H2O2, with their cellular functions remaining preserved.
To extend our previous studies, the aim of this work was to examine the mechanism of the proposed CXCL12-mediated increased resistance to H2O2 in β-cells. We analyzed the expression and activity of CAT, MnSOD and CuZnSOD enzymes in the CXCL12 overexpressing Rin-5F cell line and in rat islets exogenously supplemented with CXCL12, in the control and H2O2-induced state of oxidative stress. To assess the underlying mechanisms of antioxidative enzyme regulation by CXCL12, we analyzed the activities of the CXCL12-induced downstream kinases (Akt, ERK and p38) and their target transcription factors (Sp1, C/EBPβ, STAT3, NFκB, Nrf2). We report for the first time that CXCL12, by activating Akt, ERK and p38 and inducing nuclear translocation and binding of Nrf2 to the CAT gene promoter, promotes CAT expression and activity, thus contributing to the functional resistance of β-cells to H2O2.
Materials and methods
Cell culture and treatment.
We used the rat pancreatic islet tumor cell line Rin-5F (ATCC-CRL-2058) wild-type (wt), the same cell line with a stable transfected human gene for CXCL12 (#1) in which CXCL12 expression is 170-fold higher than endogenous CXCL12 expression in the rat18,20) and empty vector-transfected Rin-5F (mock) cells. Cells were cultured in RPMI medium supplemented with 10% FBS, 2 mM L-glutamine, penicillin (100 U/mL) and streptomycin (100 µg/mL) in a humidified (95%) atmosphere and 5% CO2 at 37 ℃. All cell culture reagents were obtained from PAA Laboratories GmbH, Austria. The cells were treated with an IC50 dose of H2O2 for 1 h (unless otherwise indicated). Recovering cells were transferred in complete medium and incubated for an additional 2 h or 12 h after the H2O2 treatment. The IC50 concentrations of H2O2 were 75 µM for wt and 150 µM for #1 cells, as described.19)
Preparation of rat pancreatic islet cells.
All animal procedures were in compliance with Directive 2010/63/EU on the protection of animals used for experimental and other scientific purposes, and were approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research “Siniša Stanković”, University of Belgrade. Adult (2.5-month-old) male albino Wistar rats weighing 220–250 g were kept under controlled environmental conditions (12 h light/dark cycle, 22 ± 2 ℃, 50% relative humidity). Standard food pellets and tap water were provided ad libitum. The islets were isolated in Hank’s balanced salt solution (HBSS): 115 mM NaCl, 10 mM NaHCO3, 5 mM KCl, 1.2 mM NaH2PO4, 25 mM HEPES, 1.1 mM MgCl2, supplemented with 1% bovine serum albumin (BSA, fraction V, Sigma) and 5 mM glucose, according to the slightly modified procedure previously described.19,21) Islet cells were pretreated with 80 ng/ml of recombinant murine CXCL12alpha (Sigma) for 30 min and/or treated for 1 h with 45 µM H2O2 (IC50).19)
Alkaline comet assay.
To estimate and compare the levels of DNA damage in different experimental groups we used the Comet assay as already described.18) DNA damage was quantified by measuring the displacement of the genetic material between the nucleus (‘comet head’) and the resulting ‘comet tail’. Images were analyzed with TriTekCometScoree Freeware version 1.5 (http://www.AutoComet.com).
Lipid peroxidation assay.
The level of lipid peroxidation was estimated by measuring the concentration of the reactive by-product malondialdehyde (MDA) in the thiobarbituric acid-reactive substance assay (TBARS) as described.22) The concentration of MDA was expressed as nM MDA/mg proteins. Protein concentrations were determined according to Lowry et al.23)
Superoxide dismutase and catalase activities.
The cells were resuspended in sucrose buffer (0.25 M sucrose, 1 mM EDTA and 0.05 M Tris-HCl, pH 7.4), sonicated for 30 s at 20 Hz on ice, and the obtained homogenates were centrifuged for 1 min at 14,000 × g at 4 ℃. Aliquots of the obtained supernatants were used for protein concentration determination and measurement of CAT and SOD activities. Total SOD activity was measured by the epinephrine method.24) MnSOD activity was assessed after preincubation with 8 mM KCN. CuZnSOD activity was the difference between total SOD and MnSOD activities. CAT activity was measured by the rate of H2O2 decomposition.25) SOD and CAT activities were expressed as U/mg proteins.
Immunoblot analysis.
Cell cytosol, nuclear fractions and lysates were prepared using the ProteoJET Cytoplasmic and Nuclear Protein Extraction Kit and ProteoJET Mammalian Cell Lysis Reagent (Fermentas), respectively, according to the manufacturer’s instructions. Proteins (20 µg) separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) were transferred onto polyvinylidene difluoride membranes, blocked for 1 h at room temperature with 5% non-fat dry milk in blotto base buffer (0.1% Tween 20, 20 mM Tris–HCl pH 7.6, 137 mM NaCl) and examined by immunoblot analysis using the following antibodies: anti-MnSOD, anti-CuZnSOD, anti-Akt 1/2/3, anti-pAkt 1/2/3, anti-ERK 1/2, anti-pERK, anti-p38, anti-p-p38, anti-Nrf2, anti-NFκB-p65, anti-STAT3, anti-Sp1, anti-C/EBPβ, anti-lamin B (Santa Cruz Biotechnology), anti-catalase and anti-β-actin (Abcam). Staining was performed by the chemiluminescent technique according to the manufacturer’s instructions (Amersham Pharmacia Biotech). Quantification of immunoreactive bands was performed using TotalLab (Phoretix) electrophoresis software (ver. 1·10).
RNA isolation and real-time quantitative PCR analysis (RT-qPCR).
Total RNA was prepared from Rin-5F (wt and #1) and islet cells using the GeneJET RNA Purification Kit (Thermo Fisher Scientific). Cells were treated with H2O2 (IC50) for 1 h and allowed to recover in the medium either for 2 or 12 h. For complementary DNA synthesis, total RNA (1 µg) was treated with DNAse I and reverse transcribed with RevertAid First Strand cDNA Synthesis Kit (Fermentas) using oligo(dT) primers. mRNA levels were quantified by RT-qPCR using an ABI Prism 7000 Sequence Detection System (Applied Biosystems) and a Maxima SYBR Green/ROX qPCR Master Mix (Fermentas). The fragments were amplified using the following primers (Invitrogen): forward 5′-CAGATCATGCAGCTGCACCA-3′ and reverse 5′-TCAGTCCAGGCTGAAGAGCA-3′ for the rat MnSOD gene; forward 5′-GCAGAAGGCAAGCGGTGAAC-3′ and reverse 5′-CGGCCAATGATGGAATGCTC-3′ for the rat CuZnSOD gene; forward 5′-GCGAATGGAGAGGCAGTGTAC-3′ and reverse 5′-GAGTGACGTTGTCTTCATTAGCACTG-3′ for the rat CAT gene; forward 5′-ATGGCCCTGTGGATGCGCTT-3′ and reverse 5′-ACAATGCCACGCTTCTGCCG-3′ for the rat insulin 1 gene (Ins1); forward 5′-AGATTACTGCCCTGGCTCCT-3′ and reverse 5′-ACATCTGCTGGAAGGTGGAC-3′ for the rat β-actin gene. The programme for RT-qPCR was as follows: initial denaturation step at 95 ℃ for 10 min; two-step PCR program at 95 ℃ for 15 s and at 60 ℃ for 60 s, 40 cycles. Negative controls without the template were used in all RT-qPCR reactions. The expression levels of the target genes were related to the averaged expression level of rat β-actin as the housekeeping gene. RT-qPCR reactions were carried out in triplicate.
Chromatin immunoprecipitation (ChIP).
Chromatin was prepared from Rin-5F cells subjected to a 2 h recovery period after treatment with an IC50 dose of H2O2. The ChIP assay was performed using the ChIP-IT Express kit (Active Motif) according to the manufacturer’s. The cells were fixed with 1% formaldehyde for 10 min at room temperature prior to lysis and isolation of nuclei. Chromatin was sheared by sonication on ice with 10 pulses (each consisted of a 20 s sonication at 50 Hz followed by a 30 s rest on ice). The sheared chromatin yielded a smear between 200–1100 bp; this was used in an immunoprecipitation reaction performed with 3 µg of anti-Nrf2 antibody (Santa Cruz) and 20 µg of DNA for all of the analyzed chromatin samples, so that the initial amounts of DNA were equal for all ChIP reactions. After pelleting the immune complexes by Protein G magnetic beads, the bound DNA was eluted, and the cross-links were reversed by heating the samples in Reverse Cross-linking Buffer at 95 ℃ for 15 min, followed by incubation with 1 µg of proteinase K for 1 h at 37 ℃. To validate ChIP methodology, the same immunoprecipitation procedure was performed using antibodies for RNA Pol II (Active Motif) and IgG (Santa Cruz), which served as positive and negative controls, respectively. The DNA samples from the ChIP reaction and the “input” DNA corresponding to the DNA aliquots that were not submitted to immunoprecipitation were amplified in a Mastercycler pro (Eppendorf) using the optimized number of cycles during the linear phase of amplification for agarose gel electrophoresis. After an initial melt step at 95 ℃ for 3 min, 33 cycles of: 95 ℃ for 20 s, 59 ℃ for 30 s and 72 ℃ for 30 s, and a hold cycle at 10 ℃ were performed. Quantification of ChIP analysis was performed using RT-qPCR in the ABI Prism 7000 Sequence Detection System (Applied Biosystems) according to the ChIP-IT Express kit (Active Motif) method. Average Ct values for Nrf2 samples and IgG controls were expressed as the percentage of the input (% input). The primers were as follows: forward 5′-TACCACATCTTTGTCAAATC-3′ and reverse 5′-AATATTTGGTGACTTTGTCG-3′ for the ARE1 region (201 bp amplicons); forward 5′-GTCCACCTCCCGGAGCCCAC-3′ and reverse 5′-CAGGCCCGCTGGAGTGGTGA-3′ for the ARE2 region (149 bp amplicons).
Bioinformatic procedure.
Nrf2 binding sites in the rat CAT gene promoter element (−1418/+22 bp) were predicted by ALGGEN-PROMO software (www.alggen.Isi.upc.es) and Genomatix MatInspector software (www.genomatix.de). The analyzed rat CAT gene promoter element, characterized by Nakashima et al.26) is available on: NCBI/GenBank:AH004967.1/Rat Catalase gene/exon1; Rattus norvegicus, Location: Chromosome 3 (3q32-q34).
Statistical analysis.
The results are presented as means ± SEM of three independent experiments (run in duplicate). Statistical significance was evaluated by Student’s t test or one-way ANOVA for multiple comparisons; the statistical significance level was at p < 0.05.
Results
CXCL12 overexpression reduces H2O2-mediated oxidative stress in the β-cell line.
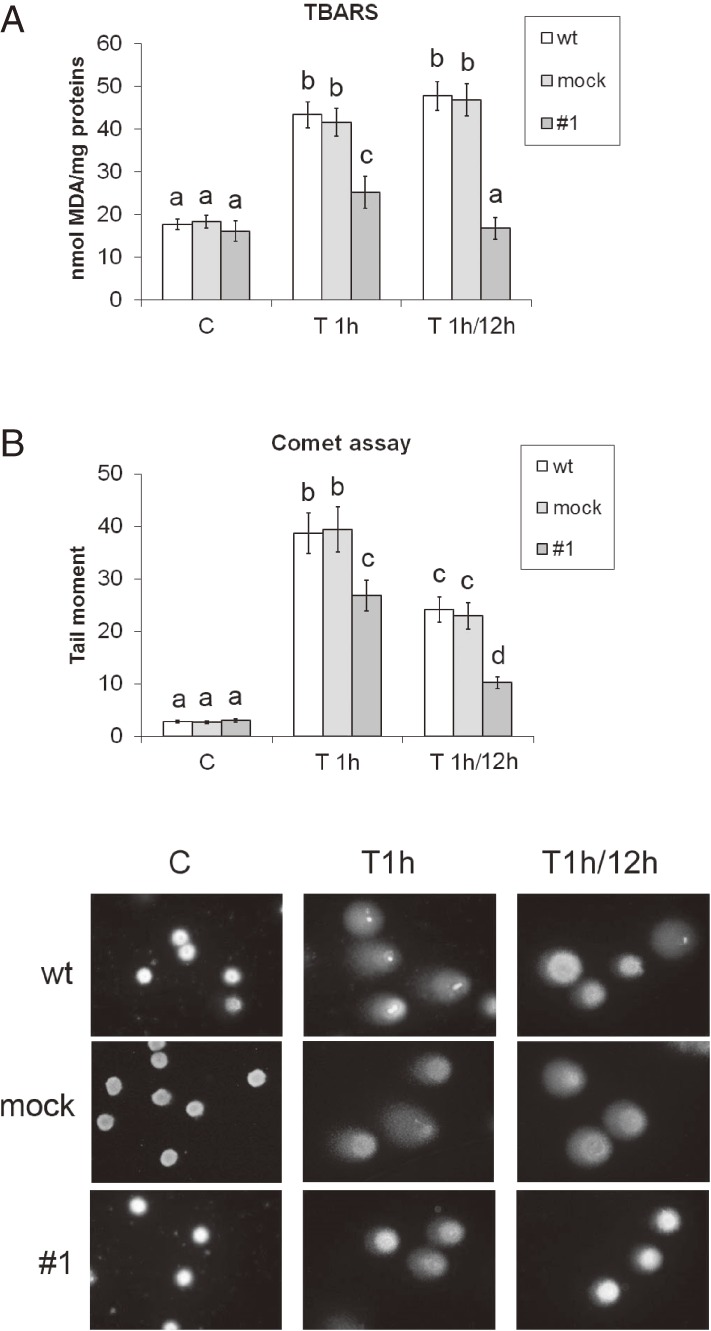
To assess the degree of oxidative stress induced by H2O2 in Rin-5F wt, mock and CXCL12 overexpressing cells (#1), we analyzed the levels of lipid peroxidation and DNA damage (Fig. 1). Examination of MDA concentrations revealed that the H2O2 treatment led to an increase in lipid peroxidation in wt and mock (2.4-fold and 2.3-fold respectively), and in #1 (1.6-fold) cells compared to matching controls (Fig. 1A). To assess cell recovery from the oxidative stress, the cells were incubated for 12 h in complete medium. This step was performed to obtain an estimate of their ability to survive and repair the damage caused by the exposure to H2O2. The recovery period was accompanied by further induction of lipid peroxidation in wt and mock cells, which was 2.7-fold and 2.6-fold, respectively, higher than in matching controls. In contrast, the concentration of MDA in #1 was at the control level after recovery. The results of the Comet assay (Fig. 1B) show that the comet tail moment, a measure of DNA damage, was extremely low and similar in all cell lines under control conditions. After a 1 h H2O2 treatment, the tail moment was 13.5-, 14.4- and 8.7 fold higher in wt, mock and #1 cells, respectively, as compared to matching controls. After the 12 h recovery period, the tail moment remained increased and in wt and mock cells it was 8-fold higher, whereas in #1 cells it was 3-fold higher, as compared to matching controls. The significantly lower level of DNA-damage and lipid peroxidation in #1 cells compared to wt and mock cells suggests that CXCL12 overexpressing #1 cells possess a higher level of endogenous antioxidant protection than wt cells. This could explain the increased survival of #1 cells after the H2O2 treatment reported previously.19)
Figure 1.
Overexpression of CXCL12 attenuates oxidative stress in Rin-5F cells after H2O2 treatment. (A) The level of lipid peroxidation, determined by the TBARS assay. (B) DNA integrity was determined by the Comet assay according to which the tail moment serves as the measure of DNA damage. Representative images of cell comets are presented. wt – wild-type Rin-5F; empty vector-transfected Rin-5F – mock; #1 – CXCL12 overexpressing Rin-5F; C – control; T1h – H2O2 treatment for (IC50; 1 h); T1h/12h – treatment with H2O2 (IC50) for 1 h followed by a 12 h recovery in standard medium. IC50 concentrations of H2O2 are explained in the Materials and Methods. Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
Effects of CXCL12 on β-cell line and islet cell antioxidant enzyme activities and protein levels.
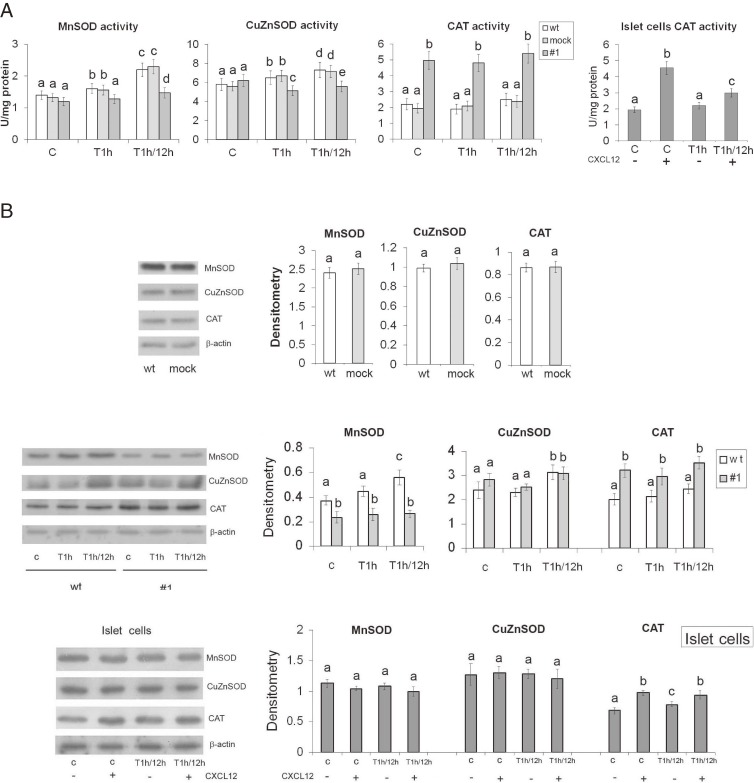
To assess the antioxidant protection status of CXCL12 overexpressing #1 cells as compared to wt and mock cells, we measured the activities and protein levels of MnSOD, CuZnSOD and CAT (Fig. 2). Analysis of the activity and expression of antioxidant enzymes at 1 h after H2O2 treatment allowed us to compare the abilities of the cells to immediately respond to the H2O2 attack, whereas the 12 h recovery period served to monitor and compare the different H2O2-induced molecular signals and their effects on the activities and expression of antioxidant enzymes in the remaining viable cells. Given that no differences between wt and mock cells were recorded (Fig. 2A,B), the activities and protein levels of antioxidant enzymes in CXCL12 overexpressing #1 cells were expressed in relation to wt cells. Under control conditions, the activity of MnSOD was at the same level in wt and #1 cells. The 1 h H2O2 treatment induced MnSOD activity only in wt cells by 15% (Fig. 2A). In cells that were allowed to recover for 12 h after the H2O2 treatment, MnSOD activity was induced in both cell lines, and was 33% higher in wt cells than in #1 cells. Likewise, the activity of CuZnSOD was at a similar level in both cell lines under control conditions. The H2O2 treatment significantly affected CuZnSOD activity in both cell lines, increasing it by 12% in wt cells and reducing it for 17% in #1 cells relative to matching controls. As observed for MnSOD, cell recovery was accompanied by an increase in CuZnSOD activity in both cell lines so that CuZnSOD activity was 23% higher in wt than in #1 cells. Under control conditions, CAT activity was increased by 125% in #1 cells as compared to wt cells. H2O2-treatment caused a slight decrease in CAT activity in both cell lines, with CAT activity being higher in #1 cells by 153% as compared to wt cells. After recovery from the H2O2 treatment, CAT activity remained slightly elevated in both cell lines as compared to matching control cells; it was higher by 116% in #1 cells than in wt cells. These results indicate that the differences in CAT activity between wt and #1 cell lines were more pronounced than the differences between the activities of SOD entities. CAT activity was more than 2-fold higher in #1 cells than in wt cells under the analyzed conditions, suggesting that CAT provided increased protection in CXCL12 overexpressing #1 cells.
Figure 2.
CXCL12 overexpression modifies the endogenous antioxidant enzymes’ activities and protein levels in Rin-5F and islet cells in basal and oxidative stress conditions. (A) CuZnSOD, MnSOD and CAT enzyme activities in pancreatic cells. (B) Relative protein levels of CuZnSOD, MnSOD and CAT in pancreatic cells estimated by immunoblot analysis of homogenates. Representative blots from three independent experiments are shown. The results of quantification of the immunoreactive bands relative to β-actin loading controls are presented in the graphs. wt – wild-type Rin-5F; empty vector-transfected Rin-5F – mock; #1 – CXCL12 overexpressing Rin-5F; (+) and (−) – islet cells with or without pretreatment with recombinant CXCL12 (80 ng/ml; 30 min). C – control; T1h – H2O2 (IC50) treated for 1 h. T1h/12h – H2O2 (IC50) treatment for 1 h followed by 12 h recovery. Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
We next assessed CAT activity in primary cells isolated from rat pancreatic islets. Prior to exposure to H2O2 (45 µM/1 h), the cells were incubated with recombinant CXCL12 protein (80 ng/ml) for 30 min. This was followed by cell recovery in complete medium for 12 h (Fig. 2A). The pretreatment with CXCL12 increased CAT activity by 134% in control cells and by 30% in H2O2-treated cells. Under control conditions, the CXCL12-mediated elevation of CAT activity in islet cells was similar to that recorded in CXCL12 overexpressing #1 cells (about 2-fold). While CXCL12-stimulated CAT activity by about 30% in H2O2-treated islet cells was not increased to the same extent as in Rin-5F cells, in which it was increased by 116%, this increase was significant. Together, these results suggest that stimulation of CAT activity is a CXCL12-mediated mechanism that enhances β-cell viability and functionality in H2O2 induced oxidative stress.
Western blot analysis of cell homogenates showed that the level of MnSOD protein was higher in wt than in #1 cells under all analyzed conditions, and even more so after the 12 h recovery (Fig. 2B). The MnSOD protein level was higher by 37% in the control, whereas after 12 h of cell recovery it was increased by 53% in wt cells compared to #1 cells. The relative amounts of CuZnSOD proteins were similar between wt and #1 cells under analyzed conditions. After the 12 h recovery period, the level of CuZnSOD protein increased in both cell lines when compared to matching controls. The level of CAT protein was consistently significantly higher in #1 cells than in wt cells in all of the above experimental groups, despite the slight decrease in CAT protein in #1 cells after the H2O2 treatment. In control conditions, the level of CAT protein was 60% higher in #1 than in wt cells, whereas after recovery from the H2O2 treatment it was 44% higher. When primary islet cells were pretreated with CXCL12 (80 ng/ml; 30 min) the level of CAT protein was significantly increased by 42%. After the CXCL12-pretreatment of islet cells before the H2O2 treatment (45 µM/1 h), followed by incubation in complete medium for 12 h, the level of CAT protein was increased for 19% when compared to CXCL12 non-pretreated cells. MnSOD was slightly higher in non-pretreated islet cells but unlike in Rin-5F cells, this increase was not significant. The relative amount of CuZnSOD protein was similar under all analyzed conditions (Fig. 2B).
The effect of CXCL12 on antioxidant enzyme gene expression in β-cells.
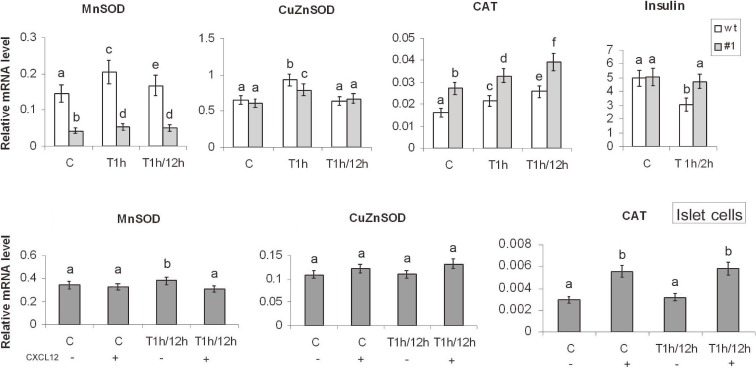
RT-qPCR was used to examine the effect of CXCL12 overexpression on the levels of antioxidative enzyme gene expression (Fig. 3). Since transfection of Rin-5f cells with empty-vector (mock cells) had no effect on the cell’s resistance to H2O2 (as judged by the TBARS and Comet assays), and neither on CAT and Mn/Cu/ZnSOD activities or protein levels, further analyses were performed using wt and #1 cells. mRNA was isolated from control, H2O2-treated (1 h) and cells whose potential for recovery after the H2O2 treatment was monitored after 12 h. The level of MnSOD mRNA was 3-fold higher in wt cells than in #1 cells under control conditions. This difference was more pronounced after the H2O2 treatment, which induced MnSOD gene expression by 41% in wt cells and by 26% in #1 cells relative to matching controls. Although the level of MnSOD mRNA decreased in wt cells after the 12 h recovery period, it remained 3-fold higher than in #1 cells. The levels of CuZnSOD mRNA in wt and #1 cells were very similar under control conditions. After the H2O2 treatment, CuZnSOD mRNA increased in wt cells by 43% and in #1 by 30% in comparison with matching controls. After the 12 h recovery period, CuZnSOD mRNA returned to the control level in both cell lines. In contrast, under control conditions, the CAT mRNA level was higher by 69% in #1 cells as compared to wt cells. After the H2O2 treatment, CAT mRNA was significantly induced in both cell lines: in wt cells by 32% and in #1 cells by 20%. After the post-treatment recovery period, further increases in CAT mRNA (by 59% in wt cells and 42% in #1 cells) were observed. While CAT mRNA was significantly induced in both cell lines (both after the treatment and the post-treatment recovery period), the level of CAT mRNA was about 50% higher in #1 than in wt cells.
Figure 3.
CXCL12-mediated changes in antioxidant enzyme gene expression in Rin-5F and islet cells in basal and oxidative stress conditions. Insulin, CuZnSOD, MnSOD and CAT mRNA levels in pancreatic cells were determined by RT-qPCR; graphs show changes in mRNA levels relative to β-actin. wt – wild-type Rin-5F; #1 – CXCL12 overexpressing Rin-5F; (+) and (−) – islet cells with or without pretreatment with recombinant CXCL12 (80 ng/ml; 30 min). C – control; T1h – H2O2 (IC50) treated for 1 h; T1h/2h – H2O2 (IC50) treated for 1 h, followed by 2 h recovery in standard medium. T1h/12h – H2O2 (IC50) treatment for 1 h followed by 12 h recovery. Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
To estimate the effect of CXCL12 on SOD and CAT mRNA levels in islet cells, mRNA was prepared from control and islet cells that were pretreated with CXCL12 (80 ng/ml; 30 min), exposed to H2O2 (45 µM/1 h) and allowed to recover for 12 h (Fig. 3). Exogenous CXCL12 induced a nearly 2-fold increase in CAT mRNA in both control and H2O2-treated islet cells and suppressed MnSOD gene induction by H2O2. Application of CXCL12 caused a slight increase in CuZnSOD mRNA that was not statistically significant.
Insulin gene expression served as a measure of the functional viability of islet cells. Rat pancreas expresses two insulin genes (Ins1 and Ins2) which are regulated coordinately in normal rat pancreas, but are differentially regulated in response to different stimuli that cause changes in the level of total rat insulin mRNA.27) Considering that selective expression of the Ins1 gene is a general feature of all Rin cell lines despite the presence of both normal insulin genes (Ins1 and Ins2),28) next we analyzed Ins1 expression. Examination of the effect of CXCL12 on Ins1 gene expression revealed stable levels of Ins1 mRNA in wt and #1 cells under control conditions (Fig. 3). After the 2 h recovery period, a 39% decrease in Ins1 mRNA was observed in wt cells, whereas in #1 cells it remained at the control level, indicating that CXCL12 overexpression did not disrupt but supported Ins1 gene expression in Rin-5F cells after the H2O2 treatment. Together, these results suggest that CXCL12 protected β-cells from H2O2-induced stress through CAT induction. This is in agreement with the fundamental role CAT plays in H2O2 removal.
The effect of CXCL12 on factors potentially involved in the transcriptional regulation of CAT in β-cells.
Considering that the differences in CAT gene expression that were observed in β-cell lines and primary islet cells after the pretreatment step with CXCL12 as compared to untreated cells could be linked to CXCL12 overexpression, we analyzed the main signaling pathways downstream from the chemokine receptor.
Effect of CXCL12 on kinase phosphorylation.
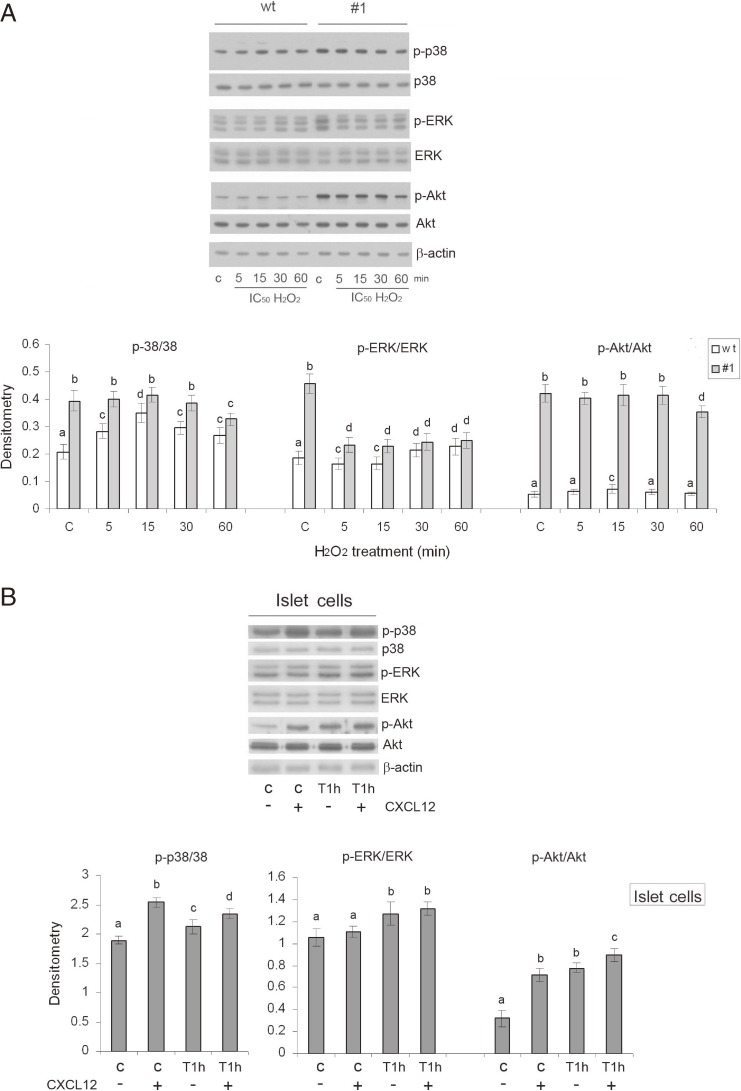
CXCL12 achieves its effects by activating the downstream kinases ERK, Akt and p38. In cells in which oxidative stress was induced by exposure to H2O2 we examined the levels of the phosphorylated forms of these kinases which reveal their activation. Maximal kinase phosphorylation/activation was reported to be attained within 60 min after the H2O2 treatment.29) In subsequent experiments, cells were exposed to H2O2 for 5, 15, 30 and 60 min, and whole cells lysates were analyzed by Western blotting (Fig. 4A). The relative level of phosphorylated p38 (p-p38) was higher in #1 than in wt cells under control conditions, as well as after the H2O2 treatment at all-time points. In wt cells, maximal p-p38 level was observed 15 min after initiation of the H2O2 treatment, while in #1 cells p-p38 was slightly decreased 60 min after the H2O2 treatment. The highest level of p-ERK1/2 was detected in the #1 cell line under control conditions, and was significantly higher than in control wt cells. The H2O2 treatment was accompanied by an increase in p-ERK1/2 in wt cells after 30 and 60 min, while in #1 cells the level of p-ERK1/2 was reduced at all analyzed time points, while remaining higher in #1 as compared to wt cells. Phosphorylation of Akt was consistently higher in #1 cells than in wt cells at all time points. In wt cells, the maximal level of p-Akt was observed 15 min after initiation of the H2O2 treatment. To assess the effect of CXCL12 on kinase activation in islet cells, the relative levels of the phosphorylated forms of the examined kinases were monitored in the control and H2O2-induced state of oxidative stress in islet cells pretreated with CXCL12 (80 ng/ml for 30 min) (Fig. 4B). A 30 min pretreatment with CXCL12 was applied because maximal kinase activation by phosphorylation is induced by CXCL12 after exposure that lasts 1–120 min.10) Under control conditions, the pretreatment with CXCL12 was accompanied by significantly increased p-p38 and p-Akt levels and a slight increase in p-ERK. After the H2O2 treatment (45 µM/1 h), the levels of p-p38 and p-Akt were higher in cells pretreated with CXCL12. The level of p-ERK was uniformly increased by the H2O2 treatment in CXCL12 non-pretreated and pretreated cells.
Figure 4.
Enhanced expression of CXCL12 activates downstream p38, ERK and Akt kinases in Rin-5F and islet cells in the basal state and after H2O2 treatment. Relative protein levels of p38, ERK and Akt and their phosphorylated forms (designated as p-p38, p-ERK and p-Akt, respectively) were determined by immunoblot analysis of cell lysates isolated from (A) Rin-5F and (B) islet cells. wt – wild-type Rin-5F; #1 – CXCL12 overexpressing Rin-5F; (+) and (−) – islet cells with or without pretreatment with recombinant CXCL12 (80 ng/ml; 30 min); C – control; 5, 15, 30, 60 – H2O2 (IC50)-treated Rin-5F cells for 5, 15, 30 and 60 min; T1h – H2O2 (IC50) treatment for 1 h. Representative blots from three independent experiments are shown. The results of quantification of the immunoreactive bands are presented in the graph depicting changes in p-kinase protein levels relative to matching kinase and β-actin loading controls. Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
These results suggest that CXCL12 induces phosphorylation of p38, ERK and Akt kinases in the basal state. Activated kinases could mediate the activation of downstream transcription factors that are involved in the induction of CAT gene expression.
Effect of CXCL12 on the activities of transcription factors.
To further elucidate the principal molecular mechanisms that are responsible for induced CAT gene expression, we characterized the molecular factors that operate at the transcriptional level and are known to be involved in the regulation of CAT, MnSOD and CuZnSOD gene transcription. We analyzed the presence and cellular localization of STAT3, NFκB-p65, Sp1, C/EBPβ and Nrf2 transcription factors (Fig. 5). To obtain insight into transcription factor activation and subsequent translocation from the cytoplasm to the nucleus after the activation of different molecular signals by H2O2, cell fractions were isolated from wt and #1 cells that were maintained under the following conditions: control (basal), from cells 1 h after initiation of the H2O2 treatment, and from cells that were allowed to recover from the H2O2 treatment for 2 h in complete medium. Based on the nuclear translocation, we concluded that STAT3 and NFκB-p65 were more active in wt than in #1 cells under both control and oxidative stress conditions (Fig. 5A). NFκB-p65 was significantly induced in wt cells immediately after the 1 h treatment with H2O2 and remained increased after the 2 h recovery period. In contrast, in #1 cells only a faint induction of NFκB-p65 was observed after recovery. In wt cells, the slight increase in nuclear translocation of STAT3 after the H2O2 treatment was maximal after the recovery period. In #1 cells, the level of STAT3 in the nuclear fraction was reduced after the 2 h recovery period.
Figure 5.
Enhanced expression of CXCL12 differentially modulates activities of transcription factors in pancreatic cells in the basal state and after H2O2 treatment. (A) Cellular localization of STAT3, NFκB-p65, Sp1, C/EBPβ and Nrf2; the transcription factors were analyzed in the cytosolic and nuclear protein fractions of Rin-5F cells. (B) CXCL12-stimulated Nrf2 nuclear translocation in islet cells; Lamin B and β-actin served as loading controls for the nuclear and cytosolic fractions, respectively. wt – wild-type Rin-5F; #1 – CXCL12 overexpressing Rin-5F; (+) and (−) – islet cells with or without pretreatment with recombinant CXCL12 (80 ng/ml; 30 min); C – control; T1h – H2O2 (IC50) treatment for 1 h; T1h/2h – H2O2 (IC50) treatment for 1 h, followed by 2 h recovery in medium. Representative blots from three independent experiments are shown. The results of quantification of immunoreactive bands are presented in the graph showing changes in the nuclear to cytoplasmic ratios of the protein levels relative to the appropriate nuclear (lamin B) and cytosol protein (β-actin) loading controls. Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
Sp1 displayed a higher increase in nuclear translocation in #1 than in wt cells under control conditions; this relative increase was more pronounced at 1 h after the H2O2 treatment. Nuclear translocation of Sp1 was uniform in wt cells under all examined conditions. C/EBPβ was detected only in the nuclear fraction. In the control state, the nuclear level of C/EBPβ was higher in #1 cells than in wt cells. The H2O2 treatment induced an increase in the level of C/EBPβ in nuclei of both cell types, although it was higher in #1 cells than in wt cells. The recovery period was characterized by a decrease in C/EBPβ in wt cells, and its maximal induction in #1 cells. Nuclear translocation of Nrf2 was significantly and persistently higher in #1 cells than in wt cells in the control state and oxidative stress conditions (about 2-fold). In wt cells, Nrf2 nuclear translocation did not change under different conditions. In #1 cells, maximal Nrf2 activation was detected after the 2 h recovery period (after the H2O2 treatment).
The obtained data strongly suggest roles for Sp1, C/EBPβ and Nrf2 in the upregulation of CAT gene transcription in CXCL12 overexpressing cells. Given that C/EBPβ and Sp1 are established regulators of CAT gene expression, whereas the activity of Nrf2 on the CAT gene promoter is insufficiently explored, the role of Nrf2 in CAT gene regulation was examined further. Nuclear translocation of Nrf2 was induced in islets cells pretreated with CXCL12 (80 ng/ml for 30 min) that were maintained under control conditions (for 96%), or after the H2O2 treatment (45 µM/1 h) and the 2 h recovery period (for 64%) (Fig. 5B). This result further supports the hypothesis that the CXCL12-stimulated nuclear translocation of Nrf2 was responsible for the induction of CAT expression which was examined next.
CXCL12 overexpression enhances Nrf2 binding affinity towards the CAT gene promoter in pancreatic β-cell lines.
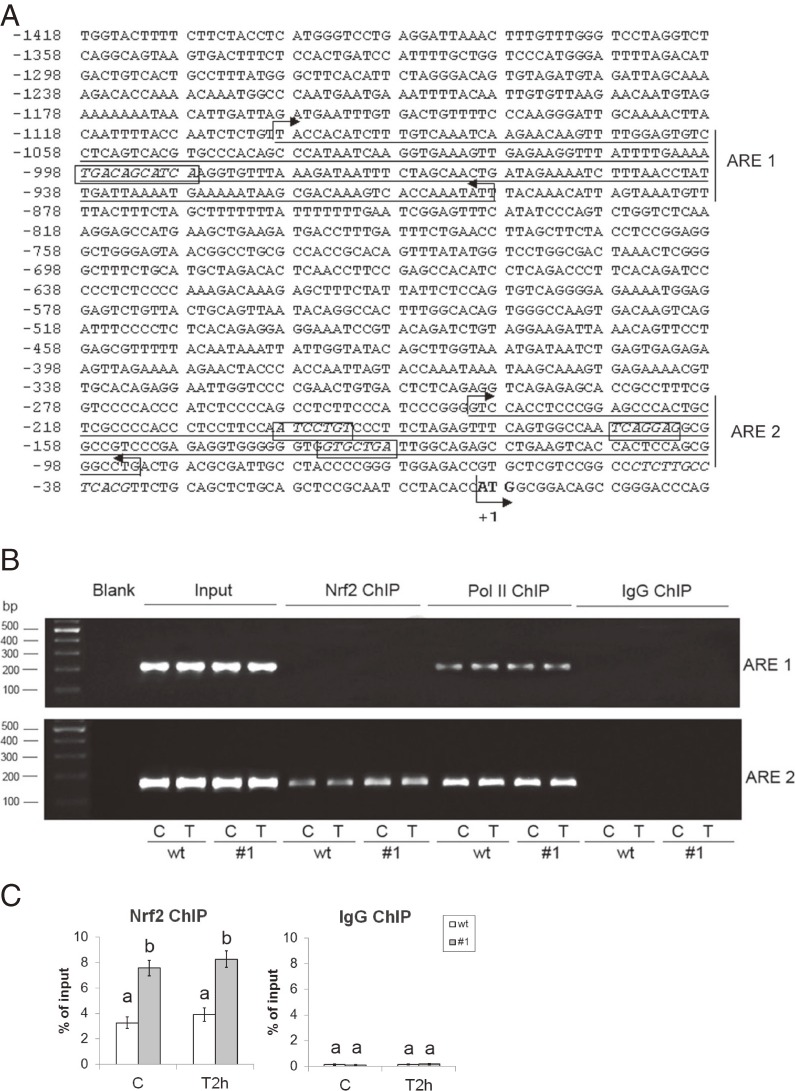
Analysis of the CAT gene promoter revealed the presence of several potential Nrf2 binding sites (Fig. 6A). One site was identified as a putative Nrf2 binding site (−998/−988 bp) that contains a potential Nrf2/MafK binding site (−996/−990 bp). Three additional sites (at positions: −199/−193 bp; −168/−162 bp; −135/−129 bp) were identified as putative Nrf2/MafK binding sites. The binding of Nrf2 to the CAT gene promoter was examined by ChIP analysis with anti-Nrf2 antibody (Fig. 6B). Chromatin was prepared from control and cells that were allowed to recover for 2 h after the H2O2 treatment. The anti-Nrf2 antibody precipitated the part of the CAT promoter that contained three putative Nrf2/MafK binding sequences (annotated as the ARE2 region); it did not precipitate the putative Nrf2 binding sequence (annotated as the ARE1 region) within the CAT promoter (Fig. 6B). Quantification of the ChIP assay by RT-qPCR analysis performed with primers for the ARE2 region that contains all three predicted Nrf2/MafK binding sites revealed that the binding affinity of Nrf2 for ARE2 was significantly higher than control IgG binding (Fig. 6C). The level of control IgG binding affinity for the ARE2 region did not change in wt and #1 cells, in control conditions and after the H2O2 treatment. However, Nrf2 binding was higher in #1 cells than in wt cells; under control conditions it was increased 2.3-fold, and after the H2O2 treatment it was increased 2.1-fold (Fig. 6C). The binding affinity of Nrf2 for the ARE2 region did not statistically change at 2 h after initiation of the H2O2 treatment in either wt or #1 cells. This result points to a role for Nrf2 in basal transcriptional regulation of CAT. However, we cannot exclude the possibility of increased Nrf2 binding to the CAT promoter at other time points after the H2O2 treatment.
Figure 6.
CXCL12 overexpression enhances Nrf2 binding to the rat CAT gene promoter in the basal state and after H2O2 treatment in Rin-5F cells. (A) Putative Nrf2 binding sites in the rat CAT gene promoter element (−1418/+22 bp) were predicted by computer search as described in the Materials and Methods. The Nrf2 and Nrf2/MafK binding sites are in rectangles, the ATG translation start site is in bold (with an arrow, marked as +1 bp). Parts of the CAT promoter containing the putative Nrf2 and three Nrf2/MafK binding sequences were annotated as ARE1 and ARE2 regions, respectively, due to their similarity to ARE. The positions of primers used in ChIP analysis are indicated as forward and reverse arrows as follows: the underlined ARE1 region (201 bp) is enclosed with arrows from −1099 (forward arrow) to −899 bp (reverse arrow) corresponding to forward and reverse primers; the underlined ARE2 region (149 bp) is enclosed with arrows from −241 (forward arrow) to −93 bp (reverse arrow), which corresponds to the forward and reverse primers, respectively. (B) ChIP assay for Nrf2 binding to the rat CAT promoter analyzed by electrophoresis. Pol II and IgG antibodies were used for ChIP validation. Nrf2 ChIP – DNA from the ChIP assay, probed with Nrf2 antibody; Pol II ChIP – DNA from the ChIP assay, probed with RNA pol II antibody (positive control); IgG ChIP – DNA from the ChIP assay, probed with IgG antibody (negative control); Input (genomic DNA) and Blank (water-only) served as positive and negative controls, respectively, in the PCR reaction; ARE1 – amplicons (201 bp) obtained with primer pairs enclosing the Nrf2-binding site; ARE2 – amplicons (149 bp) obtained with primer pairs enclosing the Nrf2/MafK-binding sites; wt – wild-type Rin-5F; #1 – CXCL12 overexpressing Rin-5F; C – control; T and T2h – cells subjected to 2 h recovery after 1 h treatment with an IC50 dose of H2O2. (C) ChIP assay for Nrf2 binding to the ARE 2 region of the CAT promoter analyzed by RT-qPCR. Average Ct values for Nrf2 samples and IgG controls are expressed as the percentage of the input (% input). Results are expressed as means ± SEM. Means not sharing a common letter are significantly different between groups (p < 0.05).
Discussion
A growing body of evidence indicates that CXCL12 plays an important role in pancreatic β-cell survival. In line with its ability to improve the survival and functioning of β-cells, we reported that CXCL12 promotes a switch of the H2O2-activated cell death in β-cells from the necrotic to the apoptotic pathway.19) In the present work, we provide evidence that CXCL12 protects pancreatic β-cells from the toxic effects of H2O2 by enhancing CAT activity and expression. We propose that the observed induction of CAT gene expression by CXCL12 was mediated by the activation of Akt, ERK and p38 kinases which are responsible for the phosphorylation/activation of Nrf2 and its nuclear translocation. Consequently, the binding of activated Nrf2 to the CAT gene promoter stimulated CAT expression and activity, contributing to the functional resistance of β-cells to H2O2.
H2O2 exerts its toxic effects by inducing single- and double-strand DNA breaks30) and through generation of ROS species, such as the hydroxyl radical and singlet oxygen, which cause lipid peroxidation.31) The significantly lower levels of DNA damage and lipid peroxidation in #1 cells compared to wt cells after H2O2 treatment reveal that CXCL12 overexpressing #1 cells possess a higher level of H2O2 endogenous defense than wt cells. The results presented here indicate that CXCL12 overexpression enhances the resistance of β-cells to H2O2 through the induction of CAT which is primarily responsible for H2O2 elimination.31,32) The extremely low endogenous CAT activity renders pancreatic β-cells vulnerable not only to virtually all ROS-producing toxins, but also to H2O2 produced by SOD catalyzed reactions.33) Herein we have shown that following H2O2 treatment, wt cells displayed higher levels of MnSOD and CuZnSOD activities than CXCL12 overexpressing #1 cells. This could be explained by the lower capacity of wt cells to neutralize H2O2-provoked ROS generation. Enhanced CAT activity in #1 cells in the basal state, which was accompanied by lower MnSOD and CuZnSOD activities most likely protected them from the toxic effects of H2O2.
The constitutively elevated nuclear translocation of STAT3 and NFκB-p65 in wt as compared to #1 cells could be implicated in the induction of MnSOD gene expression in wt cells. This assumption is in agreement with the STAT3 regulation of constitutive MnSOD gene expression in mouse neuronal cells,34) and its participation in the induction of MnSOD transcription in rat cardiomyocytes.35) NFκB responsive elements have been identified in the promoter regions of SOD genes,36,37) and NFκB is a major transcriptional regulator of MnSOD induction.38,39) While a role for NFκB in CAT gene regulation has not been determined, the results presented here support the assumption that NFκB activation is negatively correlated with CAT expression.40) STAT3 and NFκB are activated in response to increased ROS levels,41,42) and H2O2-induced activation of NFκB has been described.43) In contrast to #1 cells, the H2O2 treatment promoted an additional increase in STAT3 and NFκB-p65 nuclear translocation in wt cells. This could be explained by the lower capacity of wt cells to eliminate H2O2. Such an activation of STAT3 and NFκB-p65 in wt cells could have contributed to the increase in MnSOD and CuZnSOD gene expression. In addition, activation of the redox-sensitive transcription factor NFκB is a reflection of the higher level of H2O2-induced oxidative stress in wt cells in comparison to #1 cells. On the other hand, considering that CXCL12 signaling through CXCR4 activates the JAK2/STAT3/SOCS3 pathway, the observed decrease in nuclear STAT3 in response to CXCL12 signaling in #1 cells in comparison to wt cells could result from CXCL12-stimulated SOCS3 upregulation which is a negative feedback regulator of STAT3 activation through direct inhibition of JAK.44,45)
The constitutively elevated nuclear levels of Sp1 and C/EBPβ, and in particular the increased nuclear translocation of Nrf2 in #1 cells as compared to wt cells could very likely play an essential role in the high level of constitutive CAT expression and activity in CXCL12 overexpressing cells. This is in agreement with the role Sp1 plays in the induction of CAT transcription,46) while basal CAT gene expression is regulated by C/EBPβ.47) We speculate that the H2O2-promoted increase in nuclear C/EBPβ in wt cells contributed to CAT gene induction. Whereas Sp1 and C/EBPβ contributed to enhanced CAT gene expression in #1 cells in the basal state, the activation of Sp1 and C/EBPβ in #1 cells in response to the H2O2 treatment points to their important role in the induction of CAT gene expression in response to stress. Considering the role of Sp1 in constitutive and inducible expression of MnSOD and CuZnSOD,48) it is possible that Sp1 activation in #1 cells after the H2O2 treatment caused the increase in MnSOD and CuZnSOD gene expression. Regarding the involvement of C/EBPβ in the transcriptional regulation of CuZnSOD,49) the H2O2-promoted induction of CuZnSOD gene expression in wt and #1 cells could be correlated with C/EBPβ activation.
Nrf2 is negatively regulated by actin-binding protein Keap1 (Kelch-like ECH-associated protein 1), which binds to and sequesters Nrf2 in the cytoplasm, leading to its ubiquitination and proteasome-mediated degradation.50) Obstruction of the Nrf2-Keap1 interaction allows Nrf2 release, nuclear translocation and activation. In the nucleus, Nrf2 dimerizes with proteins such as Jun (c-Jun, Jun-B, Jun-D), c-Fos, Fra1, Nrf1 and small Maf proteins (Musculo aponeurotic fibrosarcoma; MafG, MafK, MafF), forming a transactivation complexes that binds antioxidant response elements (ARE) and participates in the transcriptional activation of antioxidant defense genes.51) Although a Nrf2 binding element has not been identified in the CAT gene promoter, it was shown that Nrf2 positively affects CAT gene expression.43,52–54) Our results point to an essential role of Nrf2 signaling in increased CAT gene expression in CXCL12 stimulated cells, rendering them more prepared for H2O2 attack in comparison to control cells. This is in correlation with the findings that the basal level of CAT was significantly lower in Nrf2 knockout cardiac fibroblasts (Nrf2−/−) as compared to Nrf2 expressing wt cells (Nrf2+/+).52) In contrast, the basal levels of SOD and GPx did not differ between Nrf2+/+ and Nrf2−/− cells, suggesting that Nrf2 signaling was not involved in the regulation of the basal expression of these two antioxidative enzymes. Aside from CAT and SOD, Nrf2 targets other genes that could also provide protection to β-cells from oxidative stress. We therefore examined the mRNA levels of the H2O2 eliminating enzymes, GPx1 and peroxiredoxin 3 (Prdx3), and of two antioxidant proteins from the phase 2 detoxifying enzyme family, glutathione S-transferase alpha 1 (GSTA1) and heme oxygenase-1 (HMOX-1). These genes were selected in view of their expression in the pancreas and their potential role in β-cell protection from H2O2 toxicity. While GPx1 expression was higher in wt cells than in #1 cells in control conditions and after the H2O2 treatment, the expression patterns of Prdx3, GSTA1 and HMOX-1 were similar in both cell lines (results not shown). While further analysis of CXCL12-mediated signaling effects on these proteins is warranted, our preliminary data support the importance of CAT induction in CXCL12-mediated protection of β-cells from H2O2-induced stress.
The interaction between Nrf2 and the CAT gene promoter described herein provides evidence for the involvement of Nrf2 in the regulation of CAT gene transcription in rat pancreatic β-cells. Although the role of small Maf proteins as transcriptional activators is controversial, results obtained using small-Maf-factor-knockout mice support the assertion that small Maf proteins serve as functional heterodimeric partner molecules of Nrf2 in vivo.55) The constitutively higher Nrf2 binding affinity for the CAT gene promoter in #1 cells than in wt cells that persisted after the H2O2 treatment, suggests that CXCL12 stimulated Nrf2 activation and Nrf2-mediated induction of CAT.
Activation of CXCL12 downstream kinases could facilitate the transcriptional activation of Nrf2. Namely, Nrf2 nuclear translocation is supported by the activities of several kinases, including ERK, p38, and PI3K/Akt, that have been shown to participate in signal transduction from antioxidants and xenobiotics to the ARE.56–58) The constitutively higher levels of p-p38, p-ERK and p-Akt in #1 than in wt cells could contribute to constitutive Nrf2 activity. Although the H2O2 treatment induced a transient induction of kinases only in wt cells and a slight reduction of kinase phosphorylation in #1 cells, kinase activity was consistently higher in #1 cells than in wt cells at all of the analyzed points. The observed higher level of p38 and Akt phosphorylation in CXCL12-pretreated cells when compared to non-pretreated cells, points to the activation of equivalent mechanisms in pancreatic islet cells. These findings point to a potential role of the CXCL12 downstream signaling pathways in Nrf2 activation and increased oxidative stress protection of β-cells through CAT activation. The improved protection against oxidative stress in β-cells through CXCL12-mediated Nrf2 activation is in agreement with recent data describing the therapeutic potential of Nrf2 activation as a promising approach to pancreatic β-cell protection against oxidative damage in diabetes.59–62)
The presented results, together with findings presented previously, strongly suggest that slight stimulation of CAT expression has a positive effect on β-cells. It provides protection from oxidative stress without impeding insulin secretion. CAT overexpression does not interfere with the glucose responsiveness of insulin-secreting INS-1E cells and rat islets.7) Increased CAT activity in β-cells of transgenic mice was observed to provide marked protection of their insulin secretion against H2O2, and significantly reduced the diabetogenic effect of streptozocin (STZ) in vivo.33) On the other hand, strong induction of endogenous antioxidant enzymes in response to oxidative stress may blunt ROS signaling, leading to reduced GSIS.4) Accordingly, it has been shown that exogenously applied CAT to permeable cells inhibits glucose-stimulated H2O2 accumulation and GSIS.63) These findings suggest that the realization of a balance between H2O2 production and elimination in β-cells is critical for their survival, regeneration and proper functioning in diabetes. Therefore, CXCL12-stimulated doubling of CAT expression and activity may have relevant biological consequence in terms of β-cell protection against H2O2-induced toxicity and preservation of insulin gene expression in both diabetes types 1 and 2.
In conclusion, we present a model that describes the increased protection of β-cells from H2O2-induced oxidative stress through CXCL12-stimulated CAT expression (Fig. 7). Central to this model is the transduction pathway, which includes CXCL12 stimulation of downstream p38, Akt and ERK kinases, Nrf2 activation and resulting ARE-mediated upregulation of CAT transcription. In addition to the previously described positive effects of CXCL12 on pancreatic β-cell survival, the results of our study confirm the therapeutic potential of CXCL12 in diabetes treatment. Thus, stimulated expression of CXCL12 could increase CAT expression and activity in β-cells of diabetic patients and prevent their oxidative damage and ultimate demise.
Figure 7.
Proposed mechanism for CXCL12-stimulated Nrf2-ARE-mediated CAT transcription. The two upper panels describe the differences between the regulatory mechanisms that support constitutive CAT expression in wt and #1 cells (left and right panels, respectively). The lower panels describe the potential mechanisms in oxidative stress, after exposure of wt and #1 cells (left and right panels, respectively) to H2O2. Under basal conditions (upper panels), Keap1 targets Nrf2 for ubiquitin-dependent degradation and in this way represses Nrf2-dependent gene expression. In #1 cells (upper right panel), CXCL12 binding to its receptor CXCR4 activates protein kinases (p38, Akt and ERK) and Nrf2 phosphorylation, inducing nuclear translocation of Nrf2 to a greater extent than in wt cells where the kinase activities are considerably lower. Consequently, in CXCL12-overexpressing #1 cells, more activated nuclear Nrf2 binds to the CAT gene promoter and maintains higher levels of CAT expression and enzymatic activity than in wt cells. After exposure to H2O2 (lower left panel), transient induction of p38, Akt and ERK kinases in wt cells was not accompanied by Nrf2 activation. The absence of Nrf2 activation could be attributed to the inhibitory effect of NFκB-p65 which was more abundant in the cytosol of wt than #1 cells after H2O2 treatment. By interacting with Keap1, NFκB-p65 represses Nrf2 dissociation from Keap1 and suppresses the Nrf2-ARE pathway.64) In #1 cells (lower right panel), the CXCL12 driven increase in nuclear translocation of Nrf2 remained after the H2O2 treatment probably because of high kinase activity. Nuclear translocation of Nrf2 was not disrupted by the redox-sensitive factor NFκB-p65. This is probably because of efficient H2O2 elimination in #1 cells as a result of the much higher level of basal CAT activity, which results in a lower level of oxidative stress.
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant No. 173020. We are very grateful to Professor Dr. Ludwig Wagner (Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria) for providing a Rin-5F cells and for all scientific help, suggestions and unlimited support. The authors declare no potential conflict of interest.
Abbreviations
- Sp1
Specificity protein 1
- C/EBPβ
CCAAT enhancer-binding protein beta
- STAT3
Signal Transducers and Activators of Transcription 3
- NFκB
Nuclear factor κB
- Nrf2
Nuclear factor erythroid 2-related factor
- ARE
Antioxidant Response Element
References
- 1).Lenzen S. (2008) Oxidative stress: the vulnerable beta-cell. Biochem. Soc. Trans. 36, 343–347. [DOI] [PubMed] [Google Scholar]
- 2).Robertson R.P., Zhou H., Zhang T., Harmon J.S. (2007) Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 48, 139–146. [DOI] [PubMed] [Google Scholar]
- 3).Lenzen S., Drinkgern J., Tiedge M. (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20, 463–466. [DOI] [PubMed] [Google Scholar]
- 4).Pi J., Zhang Q., Fu J., Woods C.G., Hou Y., Corkey B.E., Collins S., Andersen M.E. (2010) ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 244, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Rhee S.G. (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883. [DOI] [PubMed] [Google Scholar]
- 6).Chen H., Li X., Epstein P.N. (2005) MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes 54, 1437–1446. [DOI] [PubMed] [Google Scholar]
- 7).Lortz S., Gurgul-Convey E., Naujok O., Lenzen S. (2013) Overexpression of the antioxidant enzyme catalase does not interfere with the glucose responsiveness of insulin-secreting INS-1E cells and rat islets. Diabetologia 56, 774–782. [DOI] [PubMed] [Google Scholar]
- 8).Nti B.K., Markman J.L., Bertera S., Styche A.J., Lakomy R.J., Subbotin V.M., Trucco M., Zorina T.D. (2012) Treg cells in pancreatic lymph nodes: the possible role in diabetogenesis and beta cell regeneration in a T1D model. Cell. Mol. Immunol. 9, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Teicher B.A., Fricker S.P. (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927–2931. [DOI] [PubMed] [Google Scholar]
- 10).Tilton B., Ho L., Oberlin E., Loetscher P., Baleux F., Clark-Lewis I., Thelen M. (2000) Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J. Exp. Med. 192, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Badr G., Al-Sadoon M.K., El-Toni A.M., Daghestani M. (2012) Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFkappaB and ERK signaling. Lipids Health Dis. 11, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ward S.G. (2006) T lymphocytes on the move: chemokines, PI 3-kinase and beyond. Trends Immunol. 27, 80–87. [DOI] [PubMed] [Google Scholar]
- 13).Xia Z., Dickens M., Raingeaud J., Davis R.J., Greenberg M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- 14).Hui L., Bakiri L., Stepniak E., Wagner E.F. (2007) p38alpha: a suppressor of cell proliferation and tumorigenesis. Cell Cycle 6, 2429–2433. [DOI] [PubMed] [Google Scholar]
- 15).Thornton T.M., Rincon M. (2009) Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int. J. Biol. Sci. 5, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Yano T., Liu Z., Donovan J., Thomas M.K., Habener J.F. (2007) Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes 56, 2946–2957. [DOI] [PubMed] [Google Scholar]
- 17).Liu Z., Stanojević V., Avadhani S., Yano T., Habener J.F. (2011) Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia 54, 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Marković J., Grdović N., Dinić S., Karan-Djurašević T., Uskoković A., Arambašić J., Mihailović M., Pavlović S., Poznanović G., Vidaković M. (2013) PARP-1 and YY1 are important novel regulators of CXCL12 gene transcription in rat pancreatic beta cells. PLoS One 8, e59679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Grdović N., Dinić S., Mihailović M., Uskoković A., Arambašić-Jovanović J., Poznanović G., Wagner L., Vidaković M. (2014) CXC chemokine ligand 12 protects pancreatic beta-cells from necrosis through Akt kinase-mediated modulation of poly(ADP-ribose) polymerase-1 activity. PLoS One 9, e101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ilhan A., Nabokikh A., Maj M., Vidakovć M., Nielsen J.H., Prikoszovich T., Niederle B., Base W., Luger A., Wagner L. (2009) CXCL12/SDF-1 over-expression in human insulinomas and its biological relevance. Mol. Cell. Endocrinol. 298, 1–10. [DOI] [PubMed] [Google Scholar]
- 21).Nadal A., Rovira J.M., Laribi O., Leon-Guinto T., Andreu E., Ripoll C., Soria B. (1998) Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB J. 12, 1341–1348. [DOI] [PubMed] [Google Scholar]
- 22).Ohkawa H., Ohishi N., Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. [DOI] [PubMed] [Google Scholar]
- 23).Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- 24).Misra H.P., Fridovich I. (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170–3175. [PubMed] [Google Scholar]
- 25).Claiborne, A. (1984) Catalase activity. In Handbook of Methods for Oxygen Radical Research (ed. Greenwald, R.A.). CRC Press Inc., Boca Raton, FL, pp. 283–284. [Google Scholar]
- 26).Nakashima H., Yamamoto M., Goto K., Osumi T., Hashimoto T., Endo H. (1989) Isolation and characterization of the rat catalase-encoding gene. Gene 79, 279–288. [DOI] [PubMed] [Google Scholar]
- 27).Giddings S.J., Carnaghi L.R., Fischer L.J., Miller C.P. (1991) Differential regulation of rat insulin I and II messenger RNA synthesis: effects of fasting and cyproheptadine. Mol. Endocrinol. 5, 549–554. [DOI] [PubMed] [Google Scholar]
- 28).Fiedorek F.T., Jr., Carnaghi L.R., Giddings S.J. (1990) Selective expression of the insulin I gene in rat insulinoma-derived cell lines. Mol. Endocrinol. 4, 990–999. [DOI] [PubMed] [Google Scholar]
- 29).Zhou Y., Wang Q., Evers B.M., Chung D.H. (2005) Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr. Res. 58, 1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Driessens N., Versteyhe S., Ghaddhab C., Burniat A., De Deken X., Van Sande J., Dumont J.E., Miot F., Corvilain B. (2009) Hydrogen peroxide induces DNA single- and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocr. Relat. Cancer 16, 845–856. [DOI] [PubMed] [Google Scholar]
- 31).Chance B., Sies H., Boveris A. (1979) Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605. [DOI] [PubMed] [Google Scholar]
- 32).Halliwell, B.G. and Gutteridge, J.M.C. (1999) Free Radicals in Biology and Medicine, third ed. Oxford University Press, New York. [Google Scholar]
- 33).Xu B., Moritz J.T., Epstein P.N. (1999) Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radic. Biol. Med. 27, 830–837. [DOI] [PubMed] [Google Scholar]
- 34).Jung J.E., Kim G.S., Narasimhan P., Song Y.S., Chan P.H. (2009) Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J. Neurosci. 29, 7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Negoro S., Kunisada K., Fujio Y., Funamoto M., Darville M.I., Eizirik D.L., Osugi T., Izumi M., Oshima Y., Nakaoka Y., Hirota H., Kishimoto T., Yamauchi-Takihara K. (2001) Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104, 979–981. [DOI] [PubMed] [Google Scholar]
- 36).Hoffmann A., Natoli G., Ghosh G. (2006) Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25, 6706–6716. [DOI] [PubMed] [Google Scholar]
- 37).Xu Y., Kiningham K.K., Devalaraja M.N., Yeh C.C., Majima H., Kasarskis E.J., St Clair D.K. (1999) An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 18, 709–722. [DOI] [PubMed] [Google Scholar]
- 38).Eastgate J., Moreb J., Nick H.S., Suzuki K., Taniguchi N., Zucali J.R. (1993) A role for manganese superoxide dismutase in radioprotection of hematopoietic stem cells by interleukin-1. Blood 81, 639–646. [PubMed] [Google Scholar]
- 39).St Clair D.K., Porntadavity S., Xu Y., Kiningham K. (2002) Transcription regulation of human manganese superoxide dismutase gene. Methods Enzymol. 349, 306–312. [DOI] [PubMed] [Google Scholar]
- 40).Schreiber J., Jenner R.G., Murray H.L., Gerber G.K., Gifford D.K., Young R.A. (2006) Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 103, 5899–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Simon A.R., Rai U., Fanburg B.L., Cochran B.H. (1998) Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 275, C1640–C1652. [DOI] [PubMed] [Google Scholar]
- 42).Janssen-Heininger Y.M., Poynter M.E., Baeuerle P.A. (2000) Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 28, 1317–1327. [DOI] [PubMed] [Google Scholar]
- 43).Vaziri N.D. (2012) Protective effect of Nrf2 and catalase in maternal diabetes-induced perinatal hypertension and kidney disease. Diabetes 61, 2400–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Yasukawa H., Hoshijima M., Gu Y., Nakamura T., Pradervand S., Hanada T., Hanakawa Y., Yoshimura A., Ross J., Jr., Chien K.R. (2001) Suppressor of cytokine signaling-3 is a biomechanical stress–inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J. Clin. Invest. 108, 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Ozawa Y., Nakao K., Shimazaki T., Shimmura S., Kurihara T., Ishida S., Yoshimura A., Tsubota K., Okano H. (2007) SOCS3 is required to temporally fine-tune photoreceptor cell differentiation. Dev. Biol. 303, 591–600. [DOI] [PubMed] [Google Scholar]
- 46).Nenoi M., Ichimura S., Mita K., Yukawa O., Cartwright I.L. (2001) Regulation of the catalase gene promoter by Sp1, CCAAT-recognizing factors, and a WT1/Egr-related factor in hydrogen peroxide-resistant HP100 cells. Cancer Res. 61, 5885–5894. [PubMed] [Google Scholar]
- 47).Taniguchi M., Hashimoto M., Hori N., Sato K. (2005) CCAAT/enhancer binding protein-beta (C/EBP-beta), a pivotal regulator of the TATA-less promoter in the rat catalase gene. FEBS Lett. 579, 5785–5790. [DOI] [PubMed] [Google Scholar]
- 48).Miao L., St Clair D.K. (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med. 47, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Seo S.J., Kang S.S., Cho G., Rho H.M., Jung G. (1997) C/EBP alpha and C/EBPbeta play similar roles in the transcription of the human Cu/Zn SOD gene. Gene 203, 11–15. [DOI] [PubMed] [Google Scholar]
- 50).Itoh K., Wakabayashi N., Katoh Y., Ishii T., O’Connor T., Yamamoto M. (2003) Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8, 379–391. [DOI] [PubMed] [Google Scholar]
- 51).Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322. [DOI] [PubMed] [Google Scholar]
- 52).Zhu H., Itoh K., Yamamoto M., Zweier J.L., Li Y. (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 579, 3029–3036. [DOI] [PubMed] [Google Scholar]
- 53).Dreger H., Westphal K., Weller A., Baumann G., Stangl V., Meiners S., Stangl K. (2009) Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 83, 354–361. [DOI] [PubMed] [Google Scholar]
- 54).Aminzadeh M.A., Nicholas S.B., Norris K.C., Vaziri N.D. (2013) Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 28, 2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Motohashi H., Katsuoka F., Engel J.D., Yamamoto M. (2004) Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc. Natl. Acad. Sci. U.S.A. 101, 6379–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Zipper L.M., Mulcahy R.T. (2000) Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem. Biophys. Res. Commun. 278, 484–492. [DOI] [PubMed] [Google Scholar]
- 57).Lee J.M., Hanson J.M., Chu W.A., Johnson J.A. (2001) Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J. Biol. Chem. 276, 20011–20016. [DOI] [PubMed] [Google Scholar]
- 58).Rojo A.I., Sagarra M.R., Cuadrado A. (2008) GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 105, 192–202. [DOI] [PubMed] [Google Scholar]
- 59).Song M.Y., Kim E.K., Moon W.S., Park J.W., Kim H.J., So H.S., Park R., Kwon K.B., Park B.H. (2009) Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol. Appl. Pharmacol. 235, 57–67. [DOI] [PubMed] [Google Scholar]
- 60).Bhakkiyalakshmi E., Shalini D., Sekar T.V., Rajaguru P., Paulmurugan R., Ramkumar K.M. (2014) Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 171, 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Uruno A., Furusawa Y., Yagishita Y., Fukutomi T., Muramatsu H., Negishi T., Sugawara A., Kensler T.W., Yamamoto M. (2013) The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 33, 2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Yagishita Y., Fukutomi T., Sugawara A., Kawamura H., Takahashi T., Pi J., Uruno A., Yamamoto M. (2014) Nrf2 protects pancreatic beta-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63, 605–618. [DOI] [PubMed] [Google Scholar]
- 63).Pi J., Bai Y., Zhang Q., Wong V., Floering L.M., Daniel K., Reece J.M., Deeney J.T., Andersen M.E., Corkey B.E., Collins S. (2007) Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56, 1783–1791. [DOI] [PubMed] [Google Scholar]
- 64).Yu M., Li H., Liu Q., Liu F., Tang L., Li C., Yuan Y., Zhan Y., Xu W., Li W., Chen H., Ge C., Wang J., Yang X. (2011) Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 23, 883–892. [DOI] [PubMed] [Google Scholar]