Abstract
Chloroplasts move toward weak light to increase photosynthetic efficiency, and migrate away from strong light to protect chloroplasts from photodamage and eventual cell death. These chloroplast behaviors were first observed more than 100 years ago, but the underlying mechanism has only recently been identified. Ideal plant materials, such as fern gametophytes for photobiological and cell biological approaches, and Arabidopsis thaliana for genetic analyses, have been used along with sophisticated methods, such as partial cell irradiation and time-lapse video recording under infrared light to study chloroplast movement. These studies have revealed precise chloroplast behavior, and identified photoreceptors, other relevant protein components, and novel actin filament structures required for chloroplast movement. In this review, our findings regarding chloroplast and nuclear movements are described.
Keywords: actin filament, blue light, chloroplast movement, neochrome, nuclear movement, phototropin
1. Introduction
When aquatic plants evolved to survive on land, they developed strategies to tolerate new environmental conditions such as water stress, dehydration, high and/or low temperatures, high light intensity, and exposure to UV light.1) However, sessile plants not only tolerated new environments, they also used environmental factors to predict seasonal and diurnal changes and to regulate their development and physiological responses. Among environmental factors, light is the most important stimulus because in addition to its use during photosynthesis, various light characteristics, such as presence/absence, wavelength, intensity, length and direction of irradiation, and day length, can provide plants with useful information.2)
The germination of plant seeds (or spores) is mostly induced by red light. Germinated seedlings elongate in darkness; however, even short red light pulses cause seedlings to de-etiolate, induce hook opening, leaf expansion, and greening, and accelerate phototropism toward blue light. Other physiological responses affecting plant reproductive stages, including the circadian rhythm, flower opening, and the underlying gene expression, are also regulated by light.3–5) Light-mediated plant development is referred to as photomorphogenesis. To regulate photomorphogenesis, plants use various photoreceptors, such as phytochromes,6,7) which mainly absorb red and far-red light, three different blue light receptors [i.e., phototropins,8,9) cryptochromes,10,11) and the ZEITLUPE/LOV KELCH PROTEIN/FLAVIN-BINDING, KELCH REPEAT, F-BOX (ZTL/LKP/FKF)],12–14) and the recently discovered ultraviolet (UV) light receptor, UVR8.15)
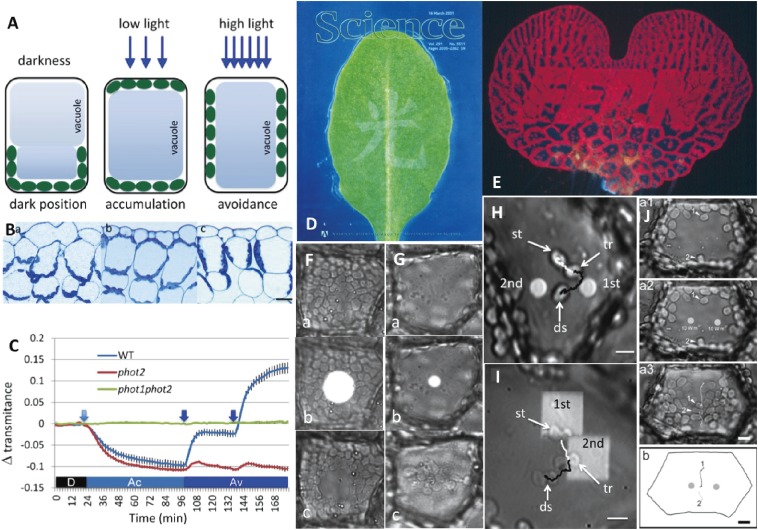
Chloroplast photorelocation movement is an important phenomenon that protects plants from high light-intensity stress, and enhances the use of light signals to promote photosynthesis.16–20) Phototropin is the main photoreceptor associated with chloroplast movement.21–23) Chloroplasts accumulate in areas irradiated with weak light to increase photosynthetic efficiency (i.e., accumulation response or weak-light response; Fig. 1A, Bb),24) and move away from areas with strong light to prevent chloroplast photodamage (i.e., avoidance response or strong-light response; Fig. 1A, Bc, D, E).25) In darkness, chloroplasts migrate to the lower periclinal or anticlinal cell walls depending on the tissue structure (i.e., dark position; Fig. 1A, Ba),26,27) although the physiological function of this movement has not been elucidated.26) Chloroplast movement is important for plants living under low light conditions (e.g., under canopies).28) However, the ecological importance of chloroplast movement in plants exposed to sunlight throughout the day has not been determined. It was recently reported that in such conditions, most chloroplasts remain on the anticlinal cell walls of long and thin palisade cells even under low light conditions.29) The ecological significance of this phenomenon will be discussed in the last part of this review.
Figure 1.
Induction, detection, and analyses of chloroplast photorelocation movement. A. Schematic drawings of chloroplast positions in darkness, and during the accumulation response under weak (low) light and avoidance response under strong (high) light in seed plant palisade cells. In darkness, chloroplasts are present at the bottom of the cells. Under weak light, they occur below the cell surface (periclinal wall) and at the bottom of the cells. Under strong light, the chloroplasts migrate to the sidewall (anticlinal wall). B. Photographs of the adaxial side of Arabidopsis thaliana leaf sections. (a) Dark position. (b) Accumulation response. (c) Avoidance response. Bar: 20 µm. C. Detection of chloroplast movement by red light transmittance through a wild-type (WT) A. thaliana leaf, and phot2 mutant and phot1phot2 double mutant leaves. The WT plant exhibited reduced transmittance under weak light because of the accumulation response (Ac), increased transmittance under strong light because of the avoidance response (Av), and an even greater increase in transmittance with stronger light provided at the time point indicated by an arrow. A phot2 mutant leaf exhibited normal chloroplast accumulation, but almost no avoidance response under strong light. There were no transmittance changes in a double mutant leaf. D. Avoidance response induced in an A. thaliana leaf by the partial irradiation with a strong light beam spelling “light” in Chinese [cover of Science, 16 March 2001 issue (courtesy of Science)]. The discovery of phot2 as the photoreceptor involved in the avoidance response was reported in this journal issue. E. Avoidance response in an Adiantum capillus-veneris gametophyte (prothallus) induced by strong light irradiation outside of the letters (FERN). F. Avoidance response in an A. capillus-veneris prothallial cell continuously irradiated with a blue microbeam (27 µm diameter) before (a), during (b), and after (c) irradiation. G. Accumulation response in a dark-adapted cell irradiated with a red microbeam (8 µm diameter) for 1 min. Other details are the same as in F. H. Accumulation response induced by sequential irradiation of red microbeams (1st and 2nd) for 1 min each in a dark-adapted A. capillus-veneris cell. A chloroplast (st) moved toward the first beam. When the chloroplast reached (tr), a second beam was provided. The chloroplast changed direction and migrated toward the second beam. The movements toward the first beam and to the destination (ds) after the second beam are indicated by white and black lines, respectively. Bar: 10 µm. I. Avoidance response induced by partial chloroplast irradiation with a continuous blue microbeam. Half of a chloroplast (st) was irradiated with a square beam (1st), and the second beam was provided when the chloroplast reached (tr). Other details are the same as in H. Bar: 5 µm. J. Two red microbeams (4 µm diameter) were simultaneously provided for 1 min. Chloroplasts at the same distance from the beams at the cell periphery moved toward the middle of the area between the two beams, but not to either beam. (b) Chloroplast movement path. Bar: 10 µm. Figures A, B, C, H, and I are reproduced from Wada 2013,16) D is from Science (16 March 2001 issue), F, G are from Wada 2013,51) J is from Tsuboi and Wada 2013,74) with copyright permission from Elsevier, Science/AAAS, British Pteridological Society, and Botanical Society of Japan, respectively.
Photorelocation movements of other organelles are so far known only in nuclei30) and peroxisomes.31) These organelles, however, show light-induced directional movement only when they attach moving chloroplasts, meaning that they do not have their own moving mechanism but chloroplasts carry them.
2. Background
Chloroplast photorelocation movement was first described in 1856 by J. A. Böhm.26) During the early 20th century, Gustav Senn studied the chloroplast distribution pattern in many species, including algae and angiosperms, mostly using thin sections of fixed materials (Fig. 1B). He determined that the distribution pattern was influenced by the light that penetrated plant tissues.26) Senn’s 1908 monograph was recently translated into Japanese by Hironao Kataoka, with an overview provided in English.32) This phenomenon was not extensively analyzed again until Jan Zurzycki of Poland and Wolfgang Haupt of Germany initiated their studies in the 1950s. Zurzycki used the moss Funaria hygrometrica and the aquatic seed plant Lemna trisulca (Monocots, Araceae),33) in which cells contain many small chloroplasts, whose movement is affected by blue light.34) On the contrary, Haupt mainly used the green alga Mougeotia scalaris (Charophyta, Zygnemataceae; Fig. 2B)35) whose cells contain one large ribbon-shaped chloroplast, and the movement of this chloroplast is primarily induced by red light,26) and reversed by far-red light, suggesting that phytochrome is the photoreceptor.36)
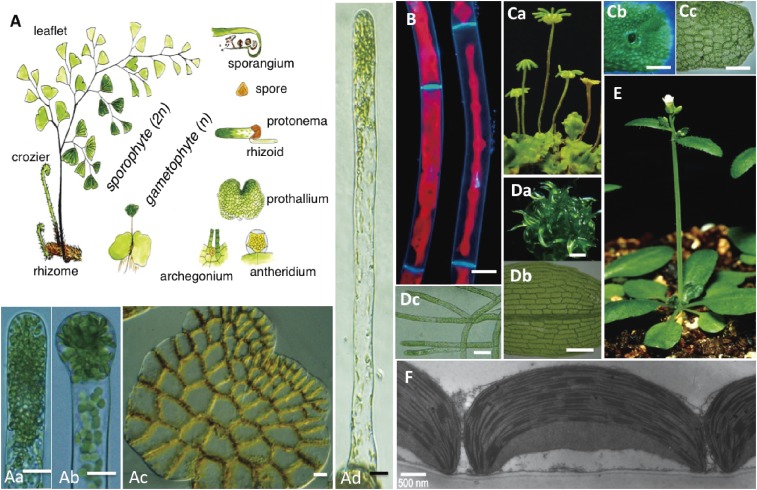
Figure 2.
Plant materials used in chloroplast movement experiments. A. Life cycle of the fern Adiantum capillus-veneris. (a) Apical part of a protonemal cell growing under red light. (b) Cell division induced by continuous white light irradiation. (c) Dark-adapted prothallus. All chloroplasts moved to the anticlinal walls. Bars of Aa, Ab, and Ac: 10 µm. (d) Long single-celled protonema grown for 6 days under continuous red light. Bar: 20 µm. B. Fluorescence micrographs of Mougeotia scalaris. Nuclei were stained with DAPI. Face (left) and profile (right) positions of chloroplasts indicated with red chlorophyll fluorescence. Bar: 20 µm. C. Marchantia polymorpha. (a) Female (left four) and male (rightmost) reproductive gametophores. (b) Gemma cup on a young thallus. Bar: 2 mm. (c) Part of a gemma. Bar: 100 µm. D. Physcomitrella patens. (a) Colony of leafy gametophores. Bar: 1 mm. (b) Part of a leaf. Bar: 100 µm. (c) Protonemata. Bar: 50 µm. E. Arabidopsis thaliana. F. Electron micrograph of cross-sectioned chloroplasts in a dark-adapted A. thaliana palisade cell. The peripheral chloroplast region is firmly attached to the plasma membrane. Bar: 500 nm. Figures A is reproduced from Wada 2007,52) Aa, Ab, and Ad are from Wada 2013,51) B is modified from Cover of PNAS (September 20, 2005 issue), E is from Suetsugu and Wada 2007,162) and F is from Wada and Kong 2011,28) with permission from Botanical Society of Japan, British Pteridological Society, Photochem Photobiol., Methods in Mol. Biol. and PNAS, respectively.
From the 1960s to the 1990s, chloroplast movement was mainly investigated in Mougeotia, especially in Germany by Haupt,37) Ekkehard Schönbohm,38) Gottfried Wagner,39) and their colleagues. Mougeotia is a filamentous alga consisting of cylindrical cells in which a large ribbon-shaped (flat) chloroplast is sandwiched between two large vacuoles (Fig. 2B). The chloroplast is set parallel to the cell polarity (i.e., along the long axes), and the chloroplast edge is attached to the plasma membrane. When a cylindrical cell is irradiated with a weak red or blue light from the side, the chloroplast rotates along the central axis of the cell so that its flat side is positioned facing the light for efficient light absorption. In contrast, when cells are exposed to strong blue light from any direction under weak red light, the chloroplast turns to assume a profile position, parallel to the incident red light, to minimize the absorption of the blue light.26,37) The interaction between red and blue light looks very complicated,38) but it is understandable because chloroplasts have to avoid strong white light (i.e., blue light) but the direction of the white light is monitored by red light. Because of these unique features of chloroplast movements in Mougeotia, German groups investigated the membrane localization of photoreceptors (i.e., phytochrome and blue light-absorbing pigment) using partial cell irradiation with polarized light. The arrangements of transition moments were predicted based on the effects of polarized light on chloroplast movement, and the mechanism underlying chloroplast rotation was analyzed in terms of the cytoskeleton and involvement of calcium.37–40) Actin filaments were seen on the front edge of rotating chloroplast, suggesting the motive force generating structure.40) However, despite the considerable efforts of researchers over a long period, the molecular mechanism mediating chloroplast movement in Mougeotia cells remains largely uncharacterized.
Instead of plant species with cells containing one large chloroplast, researchers investigating chloroplast movement currently use plants containing cells with many small chloroplasts, especially the model plant Arabidopsis thaliana. Groups in Japan,16,41) the United States of America,42,43) and Poland44) are working to characterize the mechanism regulating chloroplast movements. Japanese researchers have used unique approaches to study this phenomenon, including the induction of chloroplast movement using partial cell or partial chloroplast irradiation, precise analyses of each chloroplast movement under infrared light, identification of protein factors involved in signal transduction pathways using mutant lines, and other molecular techniques.16–18,28,45–47) For a general overview of chloroplast movement, please refer to recent comprehensive reviews written by other groups.39,48–50)
3. Plant materials
For analytical studies of the mechanisms mediating chloroplast photorelocation movement, gametophytes of the fern Adiantum capillus-veneris [linear (Fig. 2Aa, Ad) and two-dimensional (Figs. 1E, 2Ac) stages called the protonema and prothallus, respectively] are one of the best materials because they are not surrounded by any other tissues. Additionally, they are highly sensitive to light, which controls their early developmental processes.51,52) A long single-celled protonema (Fig. 2Ad) can be obtained under red light, and cell division at the apical part of the cell can be induced by short irradiation with blue or white light (Fig. 2Ab).53,54) The photosensitivity of the chloroplast movement in the resulting long basal cell of a two-celled protonema is greater than that of the cell before cell division, although the reason for this is unknown.55) The intracellular structure of the protonemal cell and the structural changes occurring during development have been well characterized in long-term studies.51,52,56–61) Two-dimensional, single-cell-layered prothallia (Figs. 1F–J, 2Ac) are also useful for the study of chloroplast movement.62–66) Following an incubation in darkness for 1 or 2 days, most chloroplasts move from the periclinal (surface) cell walls to the anticlinal (side) walls (Fig. 2Ac). Therefore, the behavior of chloroplasts remaining on the periclinal wall can be easily observed (Fig. 1G–J). This experimental system using dark-adapted cells with a few chloroplasts on the periclinal wall is hereafter referred to as a “dark-adapted cell” system.
Mutant lines defective in chloroplast movement are easily selected in mutanized fern gametophytes because of their haploid generation (Fig. 2A).51) We identified the mutated genes in some A. capillus-veneris mutant lines, such as phot2, neo1, and kac, which will be discussed in detail later in this review.65–68) However, genetic approaches including the identification of mutated genes in fern gametophytes is difficult without some information regarding candidate genes that have already been identified in A. thaliana. Effective transient transformation are available,65,66) but stable transformation of cells and gene knockouts by homologous recombination are almost impossible to achieve, probably because bombarded genes or gene fragments cannot be integrated into the genome, although the reasons for this are unknown. Therefore, we focused on photobiological, physiological, and cell biological approaches to analyze the physiological responses of each chloroplast in fern gametophytes.69–76) For genetic approaches, we used A. thaliana, which is the most widely used model plant, because its genome has been fully sequenced.77) However, for cellular- or subcellular-based biological studies, A. thaliana is not the ideal material, especially for experiments involving living cells, because its complex tissue and organ structures are surrounded by other tissues. However, advanced fluorescent microscopy techniques now enable the detection of intracellular fluorescence-labeled protein factors. Recently, the gemma (Fig. 2Cc) of the liverwort Marchantia polymorpha (Fig. 2Ca) has been used for cell biological experiments, because of its little redundancy of genes,78) the availability of various gene manipulation techniques,78,79) and the fact there is no surrounding tissue.
4. Methods
Chloroplast photorelocation movement should be observed in a dark room with a safe light (ideally infrared) that is not absorbed by plant cells. Chloroplast behavior should be analyzed under infrared light, which is not absorbed by chloroplasts. Chloroplasts move very slowly, which necessitates the use of time-lapse video recordings to monitor their migration. The most commonly used techniques in my laboratory to analyze chloroplast responses to light involve partial cell or chloroplast irradiation with a small round microbeam ranging from a few to several tens of micrometers in diameter or a small square light beam by custom-made microbeam irradiators (Fig. 1F, G).28,80) Different cell parts can be sequentially (Fig. 1H, I)16) or simultaneously (Fig. 1J)74) illuminated using two microbeams. Combining instruments and techniques has enabled the detailed observation of chloroplast behavior.
Custom-made special cuvettes to protect materials from drying during microbeam irradiation and/or for repeated protonema centrifugation in different directions have also been prepared.28,52) Adiantum capillus-veneris gametophytes were sandwiched between two round glass pieces (1.8 mm diameter) with a silicon ring spacer in between to create a culture space. The samples were then placed in a cuvette made of two stainless steel rings, tightly screwed, irradiated with a microbeam, and observed.
Cell manipulation of long single-celled protonemata is also effective for research into chloroplast behavior (Fig. 2Ad). Protonema ligation with a thin string to remove the nucleus-containing part of the cell can produce enucleated cells (Fig. 3A).81) Primary and secondary antibodies for staining cytoskeletons can be introduced into protonemal cells cut with a razor blade fragment. Because the protonemal cell wall is surrounded by a cuticle,60) immunostaining of an uncut cell is impossible.57,58)
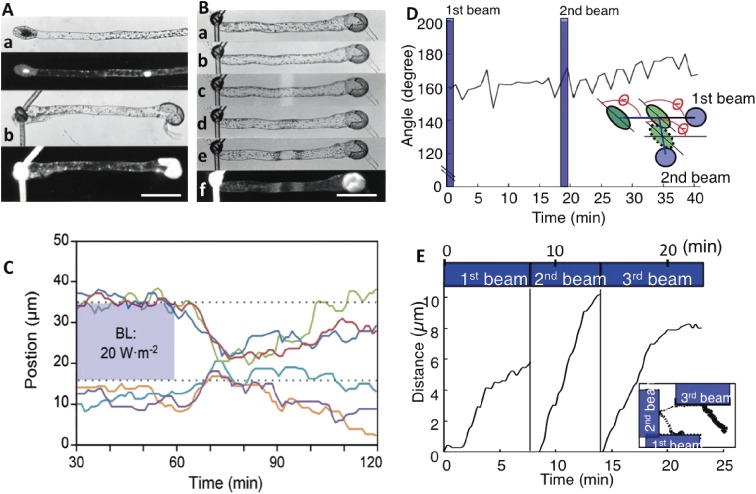
Figure 3.
Chloroplast behavior during movement. A. Two-celled protonema of Adiantum capillus-veneris. (a) Protonema with a short tip cell and a long basal cell (top), with a DAPI-stained image (below). (b) Protonema enucleated by ligation and separation of the nuclear region (top), with a DAPI-stained image (below). Bar: 100 µm. B. Chloroplast movement in an enucleated cell. (a) Enucleated cell. (b) Chloroplast movement induced by polarized blue light. Chloroplasts are on both sides of the cell. (c) Partial cell irradiation with a weak blue microbeam. (d) Induced accumulation response. (e) Avoidance response induced with a strong microbeam. (f) A lack of a nucleus in the cell was confirmed by DAPI staining. Bar: 100 µm. C. Signal lifetimes for avoidance and accumulation responses were calculated in a prothallial cell. Chloroplasts outside of the strong blue light beam migrated into the former beam-irradiated area following a lag period (i.e., duration of the avoidance response) after the light was switched off. They then started to disperse (i.e., dark positioning) after a lag period (i.e., duration of the accumulation response). Each chloroplast movement is indicated by different colored line. D. Chloroplasts change directions without rotating during the accumulation response. The angle between the long axis of chloroplasts and the direction toward the first beam was continuously measured. Even though the destination of chloroplasts changed to the second beam, the angle did not change. The inset presents a diagram of the experimental procedure. The data were obtained from Movie 1. E. Avoidance responses induced when half of a chloroplast was irradiated three times with strong and continuous blue light. The data were obtained from Movie 3. The avoidance response occurred after a short lag period. The inset presents the chloroplast movement path. Figures A and B are reproduced from Wada 1988,81) C is from Higa and Wada 2015,76) D and E are from Tsuboi et al. 200975) and Tsuboi and Wada 201171) with copyright permission from Japanese Society of Plant Physiologists, Wiley, Botanical Society of Japan, respectively.
Polarized light irradiation has been frequently used to predict the location of the intracellular photoreception site. If there are considerable differences in responses to polarized light vibrating parallel and perpendicular to the cell wall (i.e., dichroic effect), photoreceptors might be on or close to the plasma membrane and arranged parallel or perpendicular to the membrane.37,55,82–85)
A conventional method to detect overall chloroplast movement involves spectrophotometrically measuring changes to red light transmittance through the leaf blade during changes in light conditions.86–88) The data revealed the chloroplasts migrated from the periclinal wall to the anticlinal wall or vice versa throughout the whole leaf. We recently developed a more convenient system for measuring leaf transmittance using a commercially available microplate reader (Fig. 1C).28)
5. Chloroplast behavior
In liverworts, mosses, ferns, and angiosperms, many small disc-shaped (or lens-shaped) chloroplasts (approximately 5–10 µm diameter) exist in individual cells.71,89,90) Small chloroplasts, with their concave side attached to the plasma membrane (Fig. 2F), move faster than the large chloroplast in Mougeotia cells (Fig. 2A).91) The following description of chloroplast behavior is based mainly on observations of small chloroplasts in A. capillus-veneris gametophytes during chloroplast movement induced by red or blue light microbeam irradiation under infrared light.63)
5.1. Accumulation response.
The accumulation of chloroplasts in fern gametophytes can be induced with a short pulse of red or blue light (e.g., 1 Wm−2, 1 min). When a small area near a chloroplast (but not on a chloroplast) of a dark-adapted cell is irradiated with a small microbeam spot (5 or 10 µm diameter) for 1 min, the chloroplast moves toward the irradiated area in darkness (Fig. 1G, H).16,51) During the accumulation response, if a second microbeam is provided at another part of the cell, the chloroplast changes direction and migrates toward the second beam area (Fig. 1H; Movie 1).16) Chloroplast behavior during migrations under infrared light was monitored every 1 min and the speed, path, and lag time needed for the directional change, as well as the direction of the long axis of the chloroplast were analyzed. Chloroplasts did not rotate, and started to move by sliding, but not rolling, after a short lag period. These results suggest that regarding movement, chloroplasts do not have a head or tail (Fig. 3D).75) Exposure to blue and red light resulted in lag times of 2–4 min and 6–12 min, respectively.71,75) The reasons for this difference will need to be determined in future studies. The speed at which chloroplasts move is not dependent on microbeam light intensity or wavelength, but is constant around 0.3–1 µm min−1.63,71)
5.2. Avoidance response.
Avoidance response is induced when chloroplasts are illuminated with strong blue or white light, but not when a region of the cell devoid of chloroplasts is illuminated (Figs. 1F, I, 3E, Movie 2). This indicates that photoreceptors localize on the chloroplast or on the plasma membrane where chloroplasts are attached.64,71) Biochemical and fluorescent microscopy studies of A. thaliana leaves revealed that photoreceptors localize on the chloroplast membrane92,93) (refer to Section 8).
When half of a chloroplast is irradiated with strong light, the chloroplast moves to the non-irradiated side after a very short lag period (i.e., almost instantaneously in some cases) (Fig. 2C; Movie 3).71) In contrast, when a whole chloroplast is exposed to very strong light, it cannot escape from the irradiated area, especially if the chloroplast is in the center of the microbeam.94,95) This is likely because the avoidance response signal is over-saturated, making it unclear in which direction the chloroplast should move. During partial or whole chloroplast illuminations, chloroplasts stop moving as soon as they move away from the beam-irradiated area. In other words, chloroplasts exhibit the avoidance response only when they are illuminated. The speed of the avoidance response depends on the light intensity, with higher light intensities resulting in faster chloroplast migration away from the light beam63,71,95,96) (refer to Section 10.2 for the underlying mechanism). In all cases, the average speed of chloroplast movement is at most 1–1.5 µm min−1.63,73,94) Other chloroplast movement characteristics are the same as those observed during the accumulation response.
5.3. Dark positioning.
When a plant is transferred from light to complete darkness, chloroplasts assume specific positions (i.e., dark positioning). In A. thaliana palisade cells, chloroplasts in periclinal walls move to the lower cell surface (Fig. 1A).29) In A. capillus-veneris prothallia and M. polymorpha gemmae, chloroplasts move to the anticlinal walls, but not to the lower cell surface in tissues composed of a single cell layer, which is similar to the positions assumed during the avoidance response (Fig. 1A).97,98) The difference between the avoidance response and dark positioning is that chloroplasts localize in the outermost cell edge in the avoidance response, but not during dark positioning. Chloroplast behaviors, such as movement during dark positioning, were analyzed in A. capillus-veneris prothallia.72) Results indicated that chloroplasts move slowly, but directly to the nearest anticlinal wall during the first 5 hours in darkness. They subsequently exhibit staggered movement, but gradually accumulate in the anticlinal walls.
6. Signaling
6.1. Signal viability and distance transmitted.
The characteristics of signals released from photoreceptors are different for accumulation and avoidance responses. When part of a prothallial cell maintained under weak light is irradiated with a strong microbeam (either blue or white light), chloroplasts within the irradiated zone move away (Fig. 1F), but chloroplasts outside this area migrate toward the beam (Movie 4).64) This indicates that the signal for avoidance is transmitted over a short distance, but the signal for accumulation can be transmitted over longer distances.99) The accumulation and avoidance responses also differ in terms of how long signals remain active. When a strong microbeam is switched off, chloroplasts quickly move into the irradiated region (i.e., an accumulation response) (Fig. 3C).64,76) This suggests the avoidance signal activity quickly decreases following the removal of a microbeam, but the accumulation response signal remains active and is at sufficient levels to induce movement. Thus, the accumulation response overwhelms the avoidance response. Thereafter, chloroplasts gradually disperse and undergo dark positioning (Fig. 3C). In A. capillus-veneris gametophyte cells, the avoidance signals are viable for 3 or 4 min, to a maximum of about 6 min, while the accumulation signals are active for 19–28 min, to a maximum of 30–40 min.62,64,76)
6.2. Involvement of gene expression.
The importance of gene expression controlled by phytochromes and cryptochromes during photomorphogenesis has been well established.7,11) Consequently, the effects of gene expression changes on chloroplast movement have been studied using A. capillus-veneris two-celled protonemata. A non-nuclear region of the long basal cell was ligated with a thin string, and the nucleus-containing upper part of the cell was removed to produce an enucleated cell fragment (Fig. 3A).81) In these cell fragments, chloroplast accumulation and avoidance responses were induced by red or blue light using partial cell irradiation with a microbeam or polarized light irradiation of the whole cell fragment. The resulting data indicated that the expression of specific nuclear genes is unnecessary as a signal transduction pathway associated with chloroplast movement (Fig. 3B).81) The signals are transmitted along the plasma membrane or through the thin ectoplasm. These findings are consistent with the observation that phototropins do not mediate blue light-induced gene expression.100–102)
6.3. Chloroplast sensitivity to signals.
For chloroplasts to move in the appropriate direction during an accumulation response, they must detect from which direction the strongest signal is coming. Consequently, chloroplasts may have sensors around their whole peripheral region, although there is currently no evidence to support this possibility. There are several other uncertainties, including how chloroplasts sense the differences in the intensity of signals from different directions and the identity of the factor responsible for detecting signal levels (e.g., whether it is the difference in light intensity or amount, or the ratio of fluence rates). To clarify these points, two adjacent parts (50 µm width) of two-celled protonemata were irradiated with various combinations of red microbeams with a high-to-low (H/L) ratio of 1.2, 1.5, 2, and 10, and at intensities of 1, 0.1, and 0.01 Wm−2.55,61,80) Chloroplasts migrated to the side irradiated with higher intensity light if the H/L ratio was greater than 2, even at very low intensities (e.g., 0.007 Wm−2). The migration direction was unaffected by differences in fluence rates. This indicated chloroplasts can detect very small differences in signal levels between their front and rear, or their front and side.
Another important consideration for chloroplast movement is whether the direction of signal flow (e.g., streaming) or the concentration of the signal is more important. To address this, two red microbeams with the same fluence rate were provided simultaneously at the center of a dark-adapted cell.74) Chloroplasts on the cell periphery at an equal distance to the two beams moved toward the middle of the area between the beams (Fig. 1J; Movie 4), indicating that they detected the location with the highest signal level based on the sum of the signals from the two beams. Therefore, the signal flow direction may not be important.
6.4. Signal transmission speed.
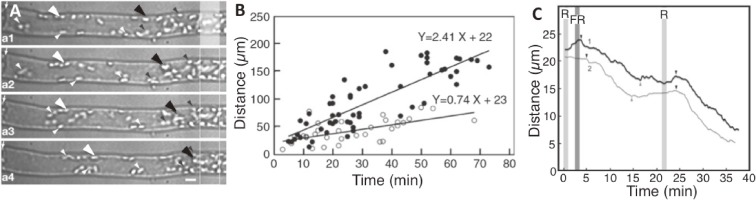
The speed of intracellular signal transmissions during the accumulation response was determined using two-celled protonemata and dark-adapted prothallia.69,70) A 20-µm region of the long basal cells was irradiated with 1 Wm−2 red light for 1 min. The time required for each chloroplast at a specific distance from the microbeam to initiate movement after the microbeam was removed was recorded (Fig. 4A).69) The resulting data indicated the signal transmission speed depended on cell polarity, with speeds of 2.3 µm min−1 from the base to the tip, and 0.6 µm min−1 in the opposite direction (Fig. 4B, Table 1). In cells lacking a clearly defined polarity, such as prothallial cells, the average signal speed was about 1 µm min−1.69,70) These speeds were relatively consistent between red and blue light, and between short pulses and continuous irradiation.70) The signal transmission speed was slow relative to the rate of diffusion of small molecules, such as ions and proteins, or the speed of organelle movements along the cytoskeleton, including microtubules and actomyosin (actin-myosin) systems. This suggests the signal transmission system associated with the accumulation response is unique.70)
Figure 4.
Characteristics of the accumulation response signal. A. Method to calculate the signal transmission speed. Part of a dark-adapted two-celled protonema was irradiated with a red microbeam (1 Wm−2; 20 µm width) for 1 min. The time required for each chloroplast (white and black arrowheads) to start migrating toward the beam with (i.e., white arrowheads) or without (i.e., black arrowheads) a lag period was measured. Photographs were taken at 20-min intervals. Bar: 10 µm. B. Relationship between the distance separating the chloroplasts from the microbeam when the light was switched off and the start times of chloroplast movement toward the irradiated area. Black and white circles indicate the direction of signal transmissions (i.e., toward the protonemal apex and base, respectively). The slopes correspond to the signal transmission speed (µm min−1). The intensity of the red microbeam was 0.1 Wm−2. Other details are the same as in A. C. Distance changes over time for two chloroplasts moving under different light conditions. Part of a dark-adapted prothallial cell was irradiated with a red (R) microbeam (10 Wm−2; 8 µm diameter; 1 min) and the whole cell was irradiated with far-red (FR) light (20 Wm−2; 1 min) 3 min later to convert the far-red light-absorbing form (Pfr) of neochrome back to red light-absorbing form (Pr). The R microbeam was provided again after 21 min. The two chloroplasts stopped moving some time after the FR light irradiation, and started again after the second R microbeam was provided. This indicates that a signal is continuously released from the photoreceptors, and that chloroplasts are continuously monitoring the signal during migrations. Figures A and B are reproduced from Tsuboi and Wada 2010,69) C is Tsuboi and Wada 2013,74) with copyright permission from Botanical Society of Japan.
Table 1.
Speed of signal transmission in chloroplast accumulation response under different light conditions
| Plant material | R 0.1 Wm−2 | R 1 Wm−2 | R 10 Wm−2 | B 1 Wm−2 | B 10 Wm−2 | |
|---|---|---|---|---|---|---|
| Adiantum capillus-veneris | contin. | 1 min | contin. | contin. | contin. | contin. |
| Protonemal cell | ||||||
| (towards apical region) | 2.41 | 2.32 | 2.37 | 2.39 | 2.28 | - |
| Protonemal cell | ||||||
| (towards basal region) | 0.74 | 0.58 | 0.73 | 0.86 | 0.80 | - |
| Prothallial cell | 1.08 | 1.13 | 0.92 | 0.95 | 1.10 | - |
| Arabidopsis thaliana | ||||||
| Uppermost palisade cell | - | - | - | - | - | 0.70 |
Dark-adapted cells were irradiated with red (R) or blue (B) light for 1 min or continuously at 25 ℃ and the speed of signal transmission was measured and is shown as (µm min−1).
-: not detected.
contin.: continuous irradiation.
This table is reproduced from Tsuboi and Wada (2010)69) with permission of Plant, Sign. Behav.
6.5. Signal monitoring.
We tested whether chloroplasts continuously monitor photoreceptor signals during movement or move by inertia once the direction is set. Red light stimulation can be canceled by far-red light. Therefore, the associated photoreceptor was believed to be phytochrome,103) although it was not the conventional phytochrome, but neochrome, which is a chimeric photoreceptor exhibiting features of phytochromes and phototropins67) (refer to Section 7.2). When far-red light was provided after a red microbeam pulse to inactivate the neochrome, the chloroplast stopped moving after a lag period, then started moving again if another red microbeam pulse was administered (Fig. 4C; Movie 5).74) This indicated the photoreceptors continuously release accumulation response signals, and that chloroplasts accumulate only when they continuously receive signals. This concept was confirmed based on how long chloroplasts are attracted to and remain within a red microbeam-irradiated area.104) Immediately after far-red light was provided following the accumulation response, chloroplasts moved away from the irradiated region. Otherwise, they remained in the area for a long time.
7. Photoreceptors
The most effective wavelengths for chloroplast movement are generally in the blue light region.26,34,103) An action spectrum for the accumulation response in A. capillus-veneris indicated the blue and red light regions are suitable for inducing chloroplast movement.103) Many non-phototropic mutants defective in red light-induced phototropism and chloroplast movement or in blue light-induced avoidance response have been generated in A. capillus-veneris gametophytes.65,68,105) However, it is almost impossible to identify the causal gene in ferns, especially if the responsible gene is new. Thus, we decided to screen chloroplast movement-defective A. thaliana mutants using high throughput methods. The chloroplast avoidance movement was induced in these mutants by irradiation with strong light through a slit. The avoidance response was complete after about 1 hour, and could be detected by the naked eye (Fig. 1D).21) The accumulation response was also induced by irradiation with low light through a slit after whole leaves were irradiated with strong light.27) Leaves lacking the avoidance or accumulation responses were considered to be from mutant plants.
7.1. Phototropin.
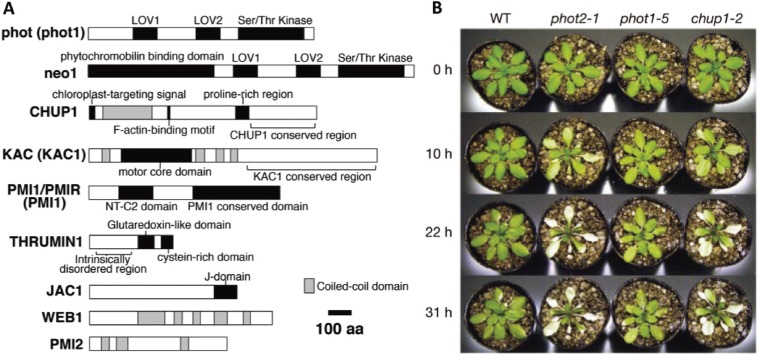
Mutants of phototropin2 (phot2) were detected among the many generated mutant plants defective in the avoidance response (Fig. 5B). Arabidopsis thaliana has two phototropins, phot1 and phot2. The N-terminal half of phototropins contains two LOV (light, oxygen, voltage) domains, which consist of about 100 amino acids that covalently bind to a chromophore, flavin mononucleotide. The C-terminal half comprises a serine/threonine kinase (Fig. 5A).106) Phot1 is responsible for the accumulation response,23,107) while phot2 regulates the avoidance and accumulation responses.21,22)
Figure 5.
A. Photoreceptors and other protein factors affecting chloroplast movement. Protein structures and the functional domains affecting chloroplast movement are presented. Phototropins (i.e., phot1 and phot2) are the main blue light receptors involved in chloroplast movement in all organisms tested so far. Neochrome1 (neo1) is a red and blue light receptor in the fern Adiantum capillus-veneris. The CHUP1 and KAC proteins polymerize or maintain cp-actin filaments. Details regarding each factor are provided in Section 10.4. B. Arabidopsis thaliana mutant phenotypes. Necrosis was observed in avoidance response-deficient phot2 and chup1 mutant plants under continuous irradiation with white light (1400 µmol m−2 s−1). Figure A is modified from Suetsugu and Wada 2016,46) B is reproduced from Kasahara et al. 200225) with copyright permission from Frontiers in Plant Sciences and Nature, respectively.
In addition to their effects on chloroplast movement, phototropins mediate phototropism,8,23) stomatal opening,108) leaf flattening,23,109) leaf palisade cell development,110) and rapid hypocotyl growth inhibition.111) These phenomena play important roles that influence photosynthetic efficiency.112) The A. capillus-veneris mutants deficient in the avoidance response were determined to carry mutations in phot2,65) confirming that phot2 is the photoreceptor related to the avoidance response in fern gametophytes.
7.2. Neochrome.
In ferns and many non-vascular plants, red light mediates chloroplast movement. During our screening of fern phytochrome genes from a genomic library, we identified a novel chimeric gene composed of part of a phytochrome gene, including the sequence corresponding to the N-terminus and chromophore-binding region, and the full phototropin gene sequence (Fig. 5A).113) We named this chimeric photoreceptor neochrome (formerly phytochrome 3),114) which can absorb blue and red light.106,113,115)
We identified mutations in the neochrome gene in most of the sequenced non-phototropic mutant lines.66,67) When a full-length neochrome gene with a cauliflower mosaic virus (CaMV) 35S promoter was inserted into mutant cells by particle bombardment, red light-induced chloroplast movement was fully complemented,67) confirming that neochrome functions as the photoreceptor during red light-induced chloroplast movement. Moreover, insertion of a neochrome DNA fragment into an A. capillus-veneris gametophyte cell inhibited red light-induced phototropism and chloroplast movement, although blue light-mediated movement occurred as normal.116) This DNA insertion technique (i.e., DNA interference) by particle bombardment represents a new gene-silencing option for fern gametophytes,116,117) and is similar to RNA interference, which is widely used for gene silencing.118)
Unexpectedly, a neochrome with an apparently different origin, but a structure that is similar to that of A. capillus-veneris neochrome, was identified in M. scalaris (Fig. 2C) and hornworts.114,119) Surprisingly, the M. scalaris neochrome was functional in A. capillus-veneris gametophytes,114) suggesting the red light receptor associated with M. scalaris chloroplast movement120) that Wolfgang Haupt predicted as a phytochrome, might be this neochrome. Recently, a phylogenetic study of phytochromes, including neochrome, was completed using a broad range of plant groups. It was hypothesized that the A. capillus-veneris neochrome originated in hornworts and was acquired by ferns through horizontal gene transfer.119,121)
7.3. Phytochrome.
In the moss Physcomitrella patens, red and blue light mediate the accumulation and avoidance responses in protonemata.122–124) However, only the red light effects on chloroplast movements are inhibited by simultaneous irradiation with far-red light. This indicates red light is perceived by phytochromes, but blue light is not. Interestingly, red light-induced chloroplast movement is significantly reduced in photA2/photB1/photB2 triple phototropin mutants,124) suggesting that phototropins are the downstream components of the P. patens phytochrome signaling pathway. It was recently reported that the far-red light-absorbing form of the P. patens phytochrome binds to phototropins. This enables the red light signal absorbed by the phytochrome to be transmitted to phototropin for the eventual mediation of protonema phototropism.125) Similar photo-signal transmissions from phytochromes to phototropins may occur during chloroplast movements in P. patens protonemata.
8. Localization of photoreceptors
The photoreceptors related to the accumulation response in A. capillus-veneris (i.e., neochrome and probably phot1 and phot2) were believed to be localized on the plasma membrane according to physiological experiments involving the dichroic effects of polarized light37,103) and the effects of microbeam irradiation on a dark-adapted gametophyte cell in the absence of chloroplasts (Fig. 1G).62,63) In contrast, chloroplasts exhibited the avoidance response when they were directly illuminated with strong light from a microbeam (Fig. 1F),64) or more effectively when one side of a chloroplast was irradiated (Fig. 1I),71) suggesting that the photoreceptor (phot2) is localized on the chloroplasts. Results of immunofluorescence microscopy investigations of A. thaliana indicated that phot1-green fluorescent protein (GFP)109) and phot2-GFP125) were located on the plasma membrane. The phot2 photoreceptor was detected biochemically in the isolated chloroplast membrane of A. thaliana leaves, while the phot2-GFP signal was observed in the chloroplast envelope.92) Furthermore, a fusion protein consisting of M. polymorpha phototropin and citrine was also localized to the chloroplast.93) Additionally, the deletion of the phot2 C-terminal region, which is important for the avoidance response,65) affected the localization of the receptor on chloroplasts.126) These observations implied that, during the avoidance response, phot2 is present in the chloroplast outer membrane, while in the accumulation response, phot1, phot2, and neochrome are localized on the plasma membrane.
9. Downstream events
It is important to identify the signals released from the photoreceptors for accumulation and avoidance responses. In A. thaliana, phot1 and phot2 influence phototropism, stomatal opening, leaf flattening, and mesophyll cell development. It is believed that different signal transduction pathways regulate these phenomena. However, they have not been characterized, except the pathway affecting the blue light-induced stomatal opening in which the phototropin substrate BLUE LIGHT SIGNALING 1 (BLUS1)127) and downstream steps have been well studied.128) Phototropins are photo-activated kinases, and auto-phosphorylation has been observed, but the substrates related to chloroplast movement have not been identified. Calcium has long been considered, but its involvement has not been confirmed.49)
10. Mechanism of movement
10.1. Chloroplast actin filaments.
An actomyosin system was thought to influence chloroplast photorelocation movement because of the existence of connections between actin filaments and chloroplasts, the presence of myosin on plastids, and the effects of myosin inhibitors.129–133) However, chloroplasts move directly toward any intra-cellular point irradiated with a microbeam light,62,73) or migrate away from the partially irradiated chloroplast side,71) after a brief (i.e., 1–2 min) lag period. These rapid movements in any direction could only be possible if fine networks of actin filaments were distributed throughout the cell cortex. Otherwise, an actin filament structure connecting chloroplasts and a microbeam-irradiated region would need to be constructed immediately after a light beam is provided. This would require a radial actin structure that extended from the irradiated spot to all chloroplasts in the cell.
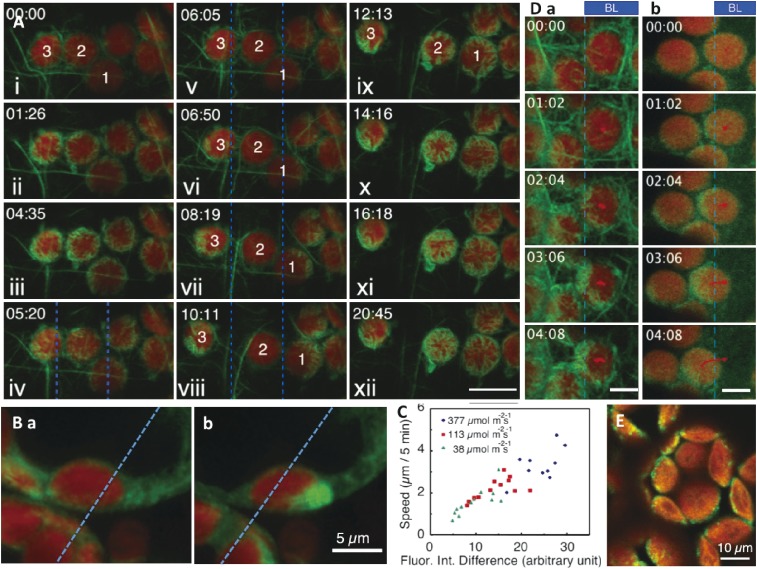
To analyze actin structures, a transgenic A. thaliana plant that produces actin filament-binding mouse talin-GFP was generated.94) A novel actin structure was observed between a chloroplast and the plasma membrane. It was located on the chloroplast periphery, but there was neither a radial actin structure nor a fine meshwork underlying the plasma membrane (Fig. 6A, B; Movies 6, 7). These structures were named chloroplast actin filaments (cp-actin filaments).94) They are similar to an actin ring structure observed surrounding the chloroplast periphery in A. capillus-veneris protonemal cells when chloroplast movement was induced by partial irradiation with a microbeam and the chloroplasts settled at the proper positions.134) During the avoidance response, the ring structure appeared on the chloroplasts accumulating on both sides of the irradiated area, while in the accumulation response, the structure was present on the chloroplasts illuminated by the light beam.134) These actin rings disappeared immediately before chloroplasts moved away from their settled position, suggesting their roles in anchoring chloroplasts to the plasma membrane.
Figure 6.
Chloroplast actin filaments and CHUP1. A. Cp-actin filaments occur around the chloroplast periphery at the interface between the chloroplast and the plasma membrane. Cp-actin filaments are scarce in dark-adapted Arabidopsis thaliana leaves (i; chloroplasts 1–3), but are more abundant following exposure to a laser scan (488 nm) (ii–iii). When part of a cell (i.e., between the two blue lines) was irradiated with strong blue light (458 nm) (vi), the cp-actin depolymerized, especially for chloroplast 2, which was in the middle of the beam. Chloroplasts 1 and 3 started to migrate away from the light, with cp-actin filaments on their front side (vi–viii). When the beam was switched off (ix), the cp-actin of chloroplast 2 started to polymerize (x–xii). Bar: 10 µm. B. Cp-actin filaments observed from the side (refer to Movie 7). (a) Before blue light irradiation. (b) During the avoidance response after blue light irradiation. Left side of blue dotted line was irradiated with blue laser beam continuously. C. The speed of chloroplast movement depended on the ratio of the abundance of cp-actin filaments in the front and rear. D. The distribution pattern of THRUM1-green fluorescent protein (GFP) (b) was similar to that of cp-actin filaments (i.e., indicated by GFP-talin) (a) during chloroplast movement. Bar: 5 µm. E. CHUP1-GFP localization on the chloroplast outer envelope. Figure A and D are reproduced from Kong et al. 2013,135) C is from Kadota et al. 2009,94) with copyright permission from PNAS.
10.2. Speed of movement.
Under steady state conditions, in which chloroplasts do not move, cp-actin filaments are scarce. In contrast, when chloroplast avoidance movement is induced, thick cp-actin filaments appear on the front side of chloroplasts, with almost none on the rear. This difference is correlated with the speed of the chloroplast avoidance movement. The bigger the difference, the faster the chloroplast migrates (Fig. 6C).94,135) Analyses of cp-actin filaments using total internal reflection fluorescence microscopy revealed that when cp-actin filaments were irradiated with strong blue light, they were degraded into small pieces (about 0.3 µm long) and then became undetectable.135) Therefore, rapid escape from strong light depends on phot2-mediated cp-actin filament degradation.135)
During the accumulation response, the cp-actin filaments occur on the front and rear of chloroplasts, with greater amounts on the front side.94,135) The presence of the cp-actin filaments during the accumulation response has been confirmed in A. capillus-veneris prothallial cells.73)
10.3. Chloroplast unusual positioning 1 (CHUP1).
Several mutant lines of chloroplast unusual positioning 1 (chup1, Fig. 5A) were obtained during a high-throughput screening of chloroplast movement.21,136) Chloroplasts of chup1 mutant plants accumulate on the lower side of palisade cells under all light conditions, while those of wild-type plants are firmly attached to the plasma membrane. The CHUP1 gene has multiple conserved regions encoding an N-terminal hydrophobic region, a coiled-coil region, an actin-binding site, a proline-rich region, and a highly conserved C-terminal region.136) The hydrophobic domain consists of 25 amino acids, and enables the targeting of the chloroplast to the outer membrane.136) If this hydrophobic domain is replaced with the N-terminal hydrophobic domain from OUTER ENVELOPE PROTEIN 7 (OEP7), chup1 transgenic plants can be complemented, although the N-terminal sequences of CHUP1 and OEP7 are different.136)
To analyze the function of the CHUP1 coiled-coil region, various transgenic lines (i.e., chup1 mutant background) either expressing the N-terminus including the coiled-coil region with various truncated C-terminal sequences or full-length CHUP1 lacking the coiled-coil region were generated.136) Chloroplasts were attached to the anticlinal wall, although chloroplast movement was not induced, in all lines expressing the truncated CHUP1 proteins with the coiled-coil region. In contrast, plants lacking the coiled-coil region exhibited the chup1 mutant phenotype, implying the coiled-coil region is important for chloroplast attachment to the plasma membrane. The coiled-coil region was also reported to be a homo-dimerization site.137) Nevertheless, CHUP1 localizes between the chloroplast and plasma membrane (Fig. 6E), and affects the anchoring of chloroplasts to the plasma membrane.
The C-terminal conserved region, which contains a proline-rich region, was predicted to be an actin polymerization site.136,138) It was reported that the proline-rich region binds to profilin, while the actin-binding site binds to filamentous actin136) and possibly soluble actin.138)
10.4. Other factors controlling chloroplast movement.
Other chloroplast movement-controlling factors (Fig. 5A) were identified, including the KINESIN-LIKE PROTEIN FOR ACTIN-BASED CHLOROPLAST MOVEMENT (KAC);139) a C2 domain protein, PLASTID MOVEMENT IMPAIRED 1 (PMI1);42) a glutaredoxin-like protein, THRUMIN 1 (THRUM1);140) an auxilin-like protein, J-DOMAIN PROTEIN REQUIRED FOR CHLOROPLAST ACCUMULATION RESPONSE 1 (JAC1);27) and two related coiled-coil proteins, PLASTID MOVEMENT IMPAIRED 2 (PMI2) and WEAK CHLOROPLAST MOVEMENT UNDER BLUE LIGHT 1 (WEB1).141,142) The physiological functions of these proteins have not been fully characterized.
There are two A. thaliana KAC proteins (i.e., KAC1 and KAC2). In kac1kac2 double mutants, chloroplast movement is almost non-existent, and cp-actin filaments are absent.139) Interestingly, the KAC motor domain is similar to those of the kinesin-14 subfamily members. However, it lacks microtubule-binding activity, and interacts with actin instead.139) The KAC proteins may be important for polymerizing or maintaining cp-actin filaments for chloroplast movement and for binding chloroplasts to the plasma membrane. In kac1kac2 plants, cells lack cp-actin filaments and the chloroplasts are highly motile, suggesting they are not attached to the plasma membrane. Similarly, the chloroplasts in A. capillus-veneris and P. patens kac mutants aggregate around the nucleus, confirming that KAC affects the association between chloroplasts and the plasma membrane.17)
The THRUM1 actin-bundling factor is another important protein influencing chloroplast movement. It binds to the plasma membrane through the S-palmitoylation and N-myristoylation motifs at its N terminus (Fig. 6Db).140) THRUM1-GFP was detected where cp-actin localized (Fig. 6D),135) suggesting that cp-actin filaments are bundled by THRUM1 and fixed on the plasma membrane. THRUM1 orthologs are present in angiosperms, but not in ferns and mosses143) in which chloroplast movement has been well studied. This implies THRUM1 is not essential for chloroplast movement, but may increase the efficiency of movement. The fact that thrum1 null mutants still exhibit accumulation and avoidance responses, albeit at lower rates,135,140) confirms that THRUM1 is non-essential for chloroplast movement.
10.5. Force generation mechanism.
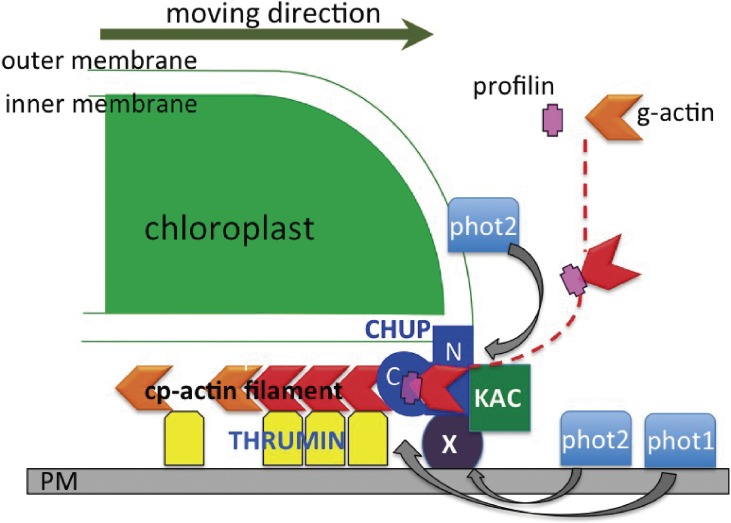
Based on the results described above, a model of the force generation mechanism for chloroplast movement was proposed (Fig. 7), in which CHUP1 is the main factor polymerizing cp-actin filaments using profilactins (i.e., profilin bound to globular actin), because CHUP1 has a Pro-rich motif that has been identified as a profilin binding motif.134) The N-terminal hydrophobic domain of CHUP1 binds to the chloroplast outer membrane, while its coiled-coil region binds to a plasma membrane protein X, thereby connecting the chloroplast to the plasma membrane. According to blue light illumination phot2 on the chloroplast (for the avoidance response) and phot1 and phot2 on the plasma membrane (for the accumulation response) may be auto-phosphorylated while simultaneously phosphorylating CHUP1, KAC, and other cp-actin filament polymerization factors. The cp-actin filaments are then polymerized by CHUP1 at the chloroplast periphery toward the center of the chloroplast along the outer envelope. The cp-actin filaments are firmly bound to the plasma membrane by THRUM1 (i.e., an actin filament-bundling factor) so that the CHUP1 complex on the edge of chloroplast outer membrane is pushed towards the front side of chloroplast by newly polymerized cp-actin filaments, resulting the chloroplast migration (indicated by an arrow on the right side of Fig. 7). In this model, actin polymerization itself is the force involved in chloroplast movement, which is similar to the Arp2/3 complex-dependent mechanism reported for bacterial movements in animal cells.144) Myosin is not involved in the force generation mechanism, as indicated by the fact that myosin XI quadruple knockout lines, which are defective in organellar transportation, exhibited normal chloroplast movement.145)
Figure 7.
Schematic model of the mechanism regulating chloroplast movement. The N-terminus of CHUP1 binds to the chloroplast outer membrane, while the CHUP1 coiled-coil domain binds to a membrane protein X to anchor the chloroplast to the plasma membrane. Upon blue light irradiation, phot1 and phot2 may phosphorylate CHUP1, KAC, and THRUM1. Using profilactins, CHUP1 polymerizes cp-actin filaments that are bundled and bound to the plasma membrane by THRUM1. Because the cp-actin filaments are bound to the plasma membrane, the CHUP1 complex is forced to move to the opposite side of the cp-actin filament as indicated with an arrow, generating the force to pull the chloroplast. Modified from Wada 2013.16)
In view of plant phylogeny, basic factors for chloroplast movement, i.e., phot, CHUP1, KAC, PMI1 are conserved from Carophytes up to Angiosperms,143) suggesting that the basic mechanisms for motive force generation are common among these organisms.
11. Nuclear photorelocation movement
11.1. Nuclear behavior.
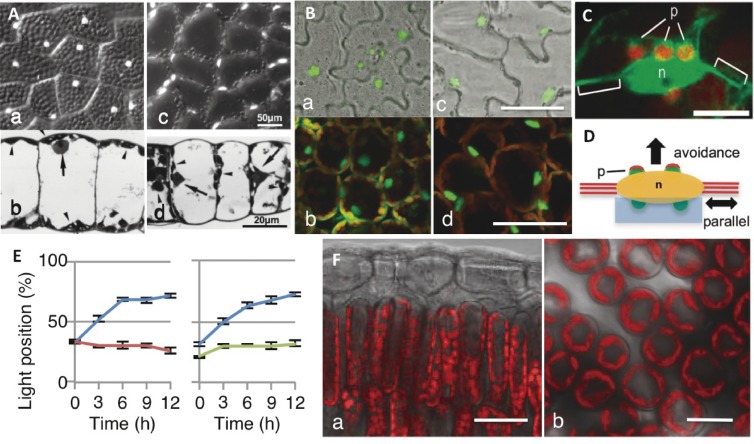
Like chloroplasts, nuclei also undergo photorelocation movement.26,30,146–150) In fern prothallial cells, nuclei localize to the center of upper periclinal walls under all light conditions (e.g., white, red, or blue light) (Fig. 8Aa) if light is not too strong. However, because of the overlying cortical chloroplasts (Fig. 8Ab), the nuclei must be fixed and stained (e.g., with DAPI) to be observed. Strong white or blue light induce nuclei to move to anticlinal walls together with the chloroplasts (Fig. 8Ac, Ad). Additionally, the nuclei along the anticlinal walls behave similarly in darkness, but the nuclei do not migrate to the outermost cell edge. Therefore, the distribution of nuclei is similar to that of chloroplasts.
Figure 8.
Nuclear photorelocation movement. A. Nuclear positioning of Adiantum capillus-veneris prothallial cells under weak (a, b) and strong (c, d) light. White dots in (a) and (c) correspond to nuclei stained with DAPI. (b, d) Photomicrographs of a prothallial section. Arrows indicate nuclei. B. Arabidopsis thaliana pavement cells in darkness (a) and under strong blue light (c), and palisade cells in darkness (b) and under strong blue light (d). Green dots indicate nuclei in the GFP:N7 line. Bar: 50 µm. C. The A. thaliana pavement cell nucleus (n) is attached to small plastids (p) that have cp-actin filaments (green). The nucleus is hung with a thick cytoplasmic strand. Bar: 10 µm. D. Schematic drawing of the following two types of nuclear movement: parallel movement involving cytoplasmic strands indicated with red lines, and a light-dependent avoidance response mediated by cp-actin filaments (red caps) on the plastids (p: green) attached to the nucleus (n: yellow). E. Time course analyses of nuclear photorelocation movement toward the side wall (i.e., light position) in wild type (blue), phot2 mutant (red), and chup1 mutant (green) lines. The presented data correspond to the light position percentage (± standard deviation). F. Photographs of fluorescence in palisade cells as observed from the side (a) and the top through the epidermis (b) of a Cocculus trilobus leaf. Note that the area of periclinal wall of thin and long palisade cells are small, so that most of chloroplasts stay on the anticlinal wall in any light condition. Bars: (a) 20 µm and (b) 10 µm. Figure A is reproduced from Wada 2008,80) B is from Higa et al. 2014,30) C, D and E are from Higa et al. 2014,151) F is from Higa and Wada 2016,29) with copyright permission from Cambridge Univ. Press, Oxford Univ. Press, PNAS, respectively.
In A. thaliana palisade cells exposed to darkness, nuclei localize at the center of the lower part of cells surrounded by many chloroplasts (Fig. 8Bb). However, they move to anticlinal positions under strong blue light (Fig. 8Bd), and exhibit the avoidance response along with chloroplasts.149) The positioning of nuclei in pavement cells is similar to that in palisade cells under light (Fig. 8Bc) or in darkness (Fig. 8Ba).149) They are also surrounded by plastids.151) Epidermal plastids contain chlorophyll, but are small and have poorly developed thylakoids.
11.2. Mechanism of nuclear movement.
Nuclear behavior cannot be easily observed in thin and long palisade cells. Moreover, nuclei may not move freely because of the surrounding chloroplasts. Therefore, epidermal large pavement cells, in which a spindle-shaped nucleus exists along with thick actin bundles and small plastids (Fig. 8C), were used for analytical studies.151) As mentioned above, the nuclei remain at the center of pavement cells in darkness, but move to the sidewalls to avoid strong blue light. Nuclear avoidance movements in pavement cells occur parallel to the thick actin bundle (mostly photosynthesis-dependent) or perpendicular to the actin bundle (mediated by phot2) (Fig. 8D).151) Interestingly, nuclear movement is absent in chloroplast movement-defective A. thaliana mutant plants (Fig. 8E),151,152) suggesting the underlying regulatory mechanisms are the same. In A. capillus-veneris, the photoreceptors associated with the accumulation of nuclei have been confirmed as neo1 and phot2, although phot1 may also be involved. The phot2 receptor is responsible for the avoidance and dark positioning responses. These are the same photoreceptors influencing chloroplast movement.148)
In A. thaliana chup1 pavement cells, nuclei exposed to strong light move faster over a greater distance (in a random direction) than wild-type nuclei, indicating the nuclei are detached from the plasma membrane. When half of a spindle-shaped nucleus in a chup1 pavement cell is irradiated with strong blue light, the nucleus rapidly migrates away along the thick cytoplasmic actin. This suggests the nucleus and plastids are not attached to the plasma membrane.151) Confocal microscopy experiments revealed the plastids exhibit a typical avoidance response with cp-actin filaments appearing on the front part of moving plastids. Additionally, even the plastids attached to the nucleus contain leading cp-actin filaments, while cp-actin-like structures are undetectable on the nucleus itself. Nuclear avoidance movement is absent in some pavement cells lacking plastids. These cells occur in the plastid division-deficient parc6 (paralog of ARC6)153) mutant plants and in the plastid division 1 (pdv1)/pdv2 double mutant.154) These findings indicate nuclei cannot move independently of plastids, and are carried by plastids.151)
12. Ecological significance of chloroplast and nuclear movements
The A. thaliana phot2 and chup1 mutant plants defective in chloroplast avoidance movement are more susceptible to photoinhibition than wild-type plants depending on the damaging effects of excess light. However, the capacity to scavenge reactive oxygen species is the same in wild-type and mutant leaves.25) The phot2-dependent avoidance response appears to offer greater protection from photodamage than non-photochemical quenching systems.155) These results regarding the effectiveness of chloroplast avoidance responses were obtained using plants cultivated under controlled conditions. However, the significance and effectiveness of chloroplast movement in terrestrial plants under natural conditions were difficult to determine. This was because analyses of real-time chloroplast movement in leaves were not possible until recently when red light transmittance through multiple mesophyll cell layers became easily detectable (Fig. 1C).
In 1973, Yorinao Inoue and Kazuo Shibata completed their pioneering work regarding chloroplast movement in 20 species of terrestrial vascular plants (both dicots and monocots) based on red light absorption by leaves. They reported that the absorption of all tested species, except Oryza sativa, was affected by weak and strong light because of intracellular chloroplast distributions, although the effects differed among species.156) Other groups subsequently studied chloroplast movement by analyzing the transmittance of red light by terrestrial plant leaves. Based on experiments using sporophytes of four fern species (i.e., A. capillus-veneris, Adiantum caudatum, Adiantum diaphanum, and Pteris cretica) grown under different light conditions, it was hypothesized that plants exposed to stable light conditions exhibit limited chloroplast movement, whereas plants grown under rapidly changing light conditions exhibit active chloroplast movement.87) The shade plant Tradescantia albiflora contains a few layers of palisade cells with actively migrating chloroplasts. This plant is more tolerant to strong light than the sun plant Pisum sativum, which has multiple layers of well-developed palisade cells.157) A correlation between chloroplast movement and cell diameter was identified among 24 tested species. The chloroplasts of shade leaves with broader and more spherical cells moved more actively than those of sun leaves with narrower and more columnar cells (i.e., less space for chloroplasts to move).158) In contrast, chloroplast movement parameters measured by red light transmittance through leaves were affected differently among species, and there was no correlation between strong light stress tolerance and chloroplast movement.159)
We recently investigated the chloroplast movement in climbing plant leaves exposed to direct sunlight during the day. The studied species included ferns, monocots, and dicots, such as Lygodium japonica (fern, deciduous), Cocculus orbiculatus (dicot, deciduous), Pueraria lobata (dicot, deciduous), Cayratia japonica (dicot, deciduous), Trachelospermum asiaticum (dicot, evergreen), Jasminum polyanthum (dicot, evergreen), Paederia scandens (dicot, deciduous), Dioscorea japonica (monocot, deciduous), and Dioscorea septemlora (monocot, deciduous). All species exhibited little if any accumulation or avoidance responses.29) Their palisade cells were cylindrical, thin, and long, and the anticlinal walls were full of chloroplasts (Fig. 8Fa). Therefore, the periclinal wall area available for chloroplasts was limited (Fig. 8Fb). However, this space was not completely filled with chloroplasts under low light conditions, implying that some regulatory factor was inhibiting the accumulation response.
Sun leaf palisade cells are generally cylindrical.158) The A. thaliana palisade cells become thin and long under strong light conditions because of phot2 regulatory activities.110) In mature palisade cells, chloroplasts are firmly attached to the plasma membrane for efficient CO2 absorption from the intercellular space.160) Based on our calculations, narrower cells have a larger plasma membrane area than wider cells, which enables more chloroplasts to attach per unit leaf area. Additionally, narrow cells have a larger plasma membrane area facing the intercellular space for more efficient CO2 exchange.29) Thus, it is better for sun leaves under strong light conditions to have thin cylindrical cells, instead of spherical cells. These cells will increase the space available for chloroplasts, leading to higher photosynthetic performance and more efficient CO2 exchange per unit leaf area, even in the absence of a chloroplast accumulation response under weak light conditions.
Regarding the importance of nuclear photorelocation movement, it was recently reported that the migration of nuclei toward anticlinal walls protects DNA from photodamage under controlled conditions.161) The efficiency of this protection in natural habitats will need to be assessed in future studies.
13. Concluding remarks
Light is arguably the most important environmental factor for plants. It serves as a source of energy for photosynthesis, but it also provides information regarding forthcoming environmental changes that will influence plant development and proliferation. A longstanding objective of researchers interested in photobiology was identifying the ultraviolet light receptor. This was recently achieved with the discovery of UV RESISTANCE LOCUS 8 (UVR8).15) This has resulted in the apparent unveiling of all types of photoreceptors in terrestrial plants. However, the precise mechanisms regulating phytochrome functions have not been fully characterized even in seed plants, despite the efforts of many researchers in the half century since phytochromes were discovered.6)
As mentioned in this review, the chloroplast photorelocation response is a good model system to study the signal transduction pathways of phototropin and neochrome which were discovered two decades ago,8,113) although they are still mostly unknown. Identifying the signal released by phototropins and determining how it is slowly transmitted to chloroplasts along the plasma membrane are challenges that will be addressed in future studies. Researchers will need to clarify whether the signal is a diffusible substance (e.g., protein or chemical) or simply information (e.g., wave on water surface or sound wave) where no substance is transmitted. I look forward to the reports of important discoveries being made by younger colleagues that will further characterize these challenging mechanisms.
Legends of movies
Acknowledgments
I would like to express my great thanks to Prof. Masamitsu Futai for recommending me to have an opportunity for writing this review. I thank MEXT and JSPS for supporting my research throughout my scientific career, and also Yamada Science Foundation, Nissan Science Foundation, and The Mitsubishi Foundation. I thank Prof. Tobias Baskin and Dr. Noriyuki Suetsugu for their careful reading and editing of this article. I also thank all of my colleagues and collaborators who worked with me over many years.
Profile
Masamitsu Wada was born in Tokyo in 1941. He graduated from the Department of Botany, Faculty of Science, The University of Tokyo in 1965. He received the degree of Master of Science in 1970 and Doctor of Science in 1972 from The University of Tokyo and worked there in the Department of Botany as an Assistant Professor from 1971 until 1981. In 1981, he moved to Tokyo Metropolitan University as an Associate Professor and was promoted to full Professor in 1989. He was appointed as an Adjunct Professor of National Institute for Basic Biology from 1998 till 2005. In 2005, he retired from Tokyo Metropolitan University and was appointed as Professor Emeritus, and moved to National Institute for Basic Biology. In 2008, he moved to Kyushu University, and in 2015, he came back to Tokyo Metropolitan University as a visiting researcher to continue his work.
He started his scientific career as a Master’s student of the Graduate School at The University of Tokyo, being a member of “Expedición Botánica a Los Andes, Universidad de Tokio” (1965–1966) organized by Professor Fumio Maekawa to find plants in Peruvian Andes related to East Asian plants. Then, he organized “Expedición Biológica a Latinoamérica, Universidad de Tokio” (1968–1969), by himself with his friends, and travelled from México down to Argentina collecting insects. The 25 thousands insect specimens collected during the trip were pinned and then donated to the National Museum of Nature and Science, Tokyo. When starting his doctoral work, he switched from fieldwork to laboratory work, studying plant morphogenesis in tissue culture, under the supervision of Dr. Masayuki Takeuchi. Next, he studied photomorphogenesis in fern gametophytes, under the guidance of Professor Masaki Furuya. After establishment of his own laboratory at Tokyo Metropolitan University in 1981, he started his main subject, the mechanism of light-induced chloroplast movement, to which he has devoted his whole life.
He was awarded the Academic Prize (2004) and the Fundamental Prize (2012) by the Botanical Society of Japan, the Corresponding Membership Award of the American Society of Plant Biologists (2005), the JSPP Award from the Japanese Society of Plant Physiologists (2006), the MIDORI Academic Prize from the Prime minister of Japan (2009), Humboldt Research Award from the Humboldt Foundation (2009), and the Finsen medal from the International Union of Photobiology (2014). In 2007, he was elected as a Fellow of the American Society of Plant Biologists.
He served as President of the Photobiology Association of Japan (1997–1998), as Vice President (2000–2004) and President (2004–2009) of the International Union of Photobiology, and President of the Botanical Society of Japan (2005–2009). He also served as Editor-in-Chief of the Journal of Plant Research published by the Botanical Society of Japan (1993–1998), and as Co-editor of The Plant Cell published by the American Society of Plant Biologists (2003–2008).
Movies of chloroplast movement are available on-line version of this article.
References
- 1).Li F.-W., Mathews S. (2016) Evolutionary aspects of plant photoreceptors. J. Plant Res. 129, 115–122. doi:10.1007/s10265-016-0785-4. [DOI] [PubMed] [Google Scholar]
- 2).Björn, L.O. and Vogelmann, T.C. (1994) Quantification of light. In Photomorphogenesis in Plants, 2nd edition (eds. Kendrick, R.E. and Kronenberg, G.H.M.). Kluwer Adademic Publisher, Netherland, pp. 17–25. [Google Scholar]
- 3).Kendrick, R.E. and Kronenberg, G.H.M. (eds.) (1986) Photomorphogenesis in Plants. Martinus Nijhoff Publishers, Kluwer Academic Publishers Group, Dordrecht, Boston, Lancaster. [Google Scholar]
- 4).Schäfer, E. and Nagy, F. (eds.) (2006) Photomorphogenesis in Plants and Bacteria 3rd Edition. Function and Signal Transduction Mechanisms. Springer, Dordrecht. [Google Scholar]
- 5).Wada, M., Shimazaki, K. and Iino, M. (eds.) (2005) Light Sensing in Plants. Springer-Verlag, Tokyo, Berlin, Heidelberg, New York. [Google Scholar]
- 6).Borthwick H.A., Hendricks S.B., Parker M.W., Toole E.H., Toole V.K. (1952) A reversible photoreaction controlling seed germination. Proc. Natl. Acad. Sci. U.S.A. 38, 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Chen M., Chory J. (2011) Phytochrome signaling mechanisms and the control of plant development. Trend. Cell Biol. 21, 664–671. doi:10.1016/j.tcb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Huala E., Oeller P.W., Liscum E., Han I.S., Larsen E., Briggs W.R. (1997) Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. doi:10.1126/science.278.5346.212. [DOI] [PubMed] [Google Scholar]
- 9).Christie J.M. (2007) Phototropin blue-light receptor. Annu. Rev. Plant Biol. 58, 21–45. doi:10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 10).Ahmad M., Cashmore A.R. (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. doi:10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 11).Liu B., Yang Z., Gomez A., Liu B., Lin C., Oka Y. (2016) Signaling mechanisms of plant cryptochromes in Arabidopsis thaliana. J. Plant Res. 129, 137–148. doi:10.1007/s10265-015-0782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Somers D.E., Schultz T.F., Milnamow M., Kay S.A. (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101, 319–329. doi:10.1016/S0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- 13).Nelson D.C., Lasswell J., Rogg L.E., Cohen M.A., Bartel B. (2000) FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101, 331–340. doi:10.1016/S0092-8674(00)80842-9. [DOI] [PubMed] [Google Scholar]
- 14).Ito M., Song Y.H., Imaizumi T. (2012) LOV domain-containing F-box proteins: Light-dependent protein degradation modules in Arabidopsis. Mol. Plant 5, 573–582. doi:10.1093/mp/sss013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Rizzini L., Favory J.-J., Cloix C., Faggionato D., O’Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G.I., Ulm R. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106. doi:10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- 16).Wada M. (2013) Chloroplast movement. Plant Sci. 210, 177–182. doi:10.1016/j.plantsci.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17).Suetsugu, N. and Wada, M. (2012) Chloroplast photorelocation movement: a sophisticated strategy for chloroplasts to perform efficient photosynthesis. In Advances in Photosynthesis — Fundamental Aspects (ed. Najafpour, M. M.). InTech, Croatia, pp. 215–234. ISBN 978-953-307-928-8. [Google Scholar]
- 18).Wada, M. and Suetsugu, N. (2013) Chloroplast motility. In The Plant Sciences — Cell Biology (eds. Assmann, S. and Liu, B.). Springer-Verlag, Berlin, Heidelberg. doi:10.1007/978-1-4614-7881-2_10-3. [Google Scholar]
- 19).Kong S.-G., Wada M. (2014) Recent advances in understanding the molecular mechanism of chloroplast photorelocation movement. Biochim. Biophys. Acta 1837, 522–530. doi:10.1016/j.bbabio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 20).Wada M., Kagawa T., Sato Y. (2003) Chloroplast movement. Annu. Rev. Plant Biol. 54, 455–468. doi:10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]
- 21).Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001) Arabidopsis NPL1: A phototropin homologue controlling the chloroplast high-light avoidance response. Science 291, 2138–2141. doi:10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 22).Jarillo J.A., Gabryś H., Capel J., Alonso J.M., Ecker J.R., Cashmore A.R. (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410, 952–954. doi:10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 23).Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. U.S.A. 98, 6969–6974. doi:10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Zurzycki J. (1955) Chloroplast arrangements as a factor of photosynthesis. Acta Soc. Bot. Pol. 24, 27–63. [Google Scholar]
- 25).Kasahara M., Kagawa T., Oikawa K., Suetsugu N., Miyao M., Wada M. (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420, 829–832. doi:10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- 26).Senn, G. (1908) Die Gestalts- und Lageveränderung der Pflanzen-Chromatophoren. Wilhelm-Engelmann, Leipzig. [Google Scholar]
- 27).Suetsugu N., Kagawa T., Wada M. (2005) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis thaliana. Plant Physiol. 139, 151–162. doi:10.1104/pp.105.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Wada, M. and Kong, S.-G. (2011) Analysis of chloroplast movement and relocation in Arabidopsis. In Methods in Molecular Biology 774, “Chloroplast Research in Arabidopsis”, Methods and Protocols. Volume 1 (ed. Jarvis, R.P.). Humana Press, Totowa, NJ, USA, pp. 87–102. doi:10.1007/978-1-61779-234-2_6. [DOI] [PubMed] [Google Scholar]
- 29).Higa T., Wada M. (2016) Chloroplast avoidance movement is not functional under strong sunlight. Plant Cell Environ. 39, 871–882. doi:10.1111/pce.12681. [DOI] [PubMed] [Google Scholar]
- 30).Higa T., Suetsugu N., Wada M. (2014) Plant nuclear photorelocation movement. J. Exp. Bot. 65, 2873–2882. doi:10.1093/jxb/ert414. [DOI] [PubMed] [Google Scholar]
- 31).Oikawa K., Kasahara M., Kiyosue T., Kagawa T., Suetsugu N., Takahashi F., Kanegae T., Niwa Y., Kadota A., Wada M. (2003) CHOLOROPLAST UNUSUAL POSITIONING1 is essential for proper chloroplast positioning. Plant Cell 15, 2805–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Kataoka H. (2015) Gustav Senn (1875–1945): The pioneer of chloroplast movement research. J. Intergr. Plant Biol. 57, 4–13. doi:10.1111/jipb.12311. [DOI] [PubMed] [Google Scholar]
- 33).Zurzycki J., Walczak T., Gabryś H., Kajfosz J. (1983) Chloroplast translocations in Lemna trisulca L. induced by continuous irradiation and by light pulses. Kinetic analysis. Planta 157, 502–510. [DOI] [PubMed] [Google Scholar]
- 34).Zurzycki J. (1962) The action spectrum for the light dependent movements of chloroplasts in Lemna trisulca L. Acta Soc. Bot. Pol. 31, 489–538. doi:10.5586/asbp.1962.036. [Google Scholar]
- 35).Haupt W. (1999) Chloroplast movement: from phenomenology to molecular biology. Prog. Bot. 60, 3–36. [Google Scholar]
- 36).Haupt W., Mörtel G., Winkelnkemper I. (1969) Demonstration of different dichroic orientation of phytochrome Pr and Pfr. Planta 88, 183–186. [DOI] [PubMed] [Google Scholar]
- 37).Haupt W., Scheuerlin R. (1990) Chloroplast movement. Plant Cell Environ. 13, 595–614. doi:10.1111/j.1365-3040.1990.tb01078.x. [Google Scholar]
- 38).Schönbohm, E. (1980) Phytochrome and non-phytochrome dependent blue light effects on intracellular movements in fresh-water algae. In The Blue Light Syndrome (ed. Senger, H.). Sprinter-Verlag, Berlin, Heidelberg, New York, pp. 69–96. [Google Scholar]
- 39).Wagner, G. (2001) Phytochrome as an algal photoreceptor. In Photomovement, Comprehensive Series in Photosciences Volume 1 (eds. Häder, D.-P. and Lebert, M.). Elsevier, Amsterdam, London, New York, Oxford, Paris, Shannon, Tokyo, pp. 421–448. doi:10.1016/S1568-461X(01)80019-3. [Google Scholar]
- 40).Mineyuki Y., Kataoka H., Masuda Y., Nagai R. (1995) Dynamic changes in the actin cytoskeleton during the high-fluence rate response of the Mougeotia chloroplast. Protoplasma 185, 222–229. [Google Scholar]
- 41).Takagi S., Takamatsu H., Sakurai-Ozato N. (2009) Chloroplast anchoring: its implications for the regulation of intracellular chloroplast distribution. J. Exp. Bot. 60, 3301–3310. doi:10.1093/jxb/erp193. [DOI] [PubMed] [Google Scholar]
- 42).DeBlasio S.L., Luesse D.L., Hangarter R.P. (2005) A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol. 139, 101–114. doi:10.1104/pp.105.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Berg R., Königer M., Schjeide B., Dikmak G., Kohler S., Harris G.C. (2006) A simple low-cost microcontroller-based photometric instrument for monitoring chloroplast movement. Photosynth. Res. 87, 303–311. doi:10.1007/s11120-005-9012-1. [DOI] [PubMed] [Google Scholar]
- 44).Trojan A., Gabrys H. (1996) Chloroplast distribution in Arabidopsis thalians (L.) depends on light conditions during growth. Plant Physiol. 111, 419–425. doi:10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Suetsugu, N. and Wada, M. (2011) Innovation of novel actin-based mechanism for chloroplasts to move. In Special Bulletin of ICPSPGE-2011, Plant Science in Post Genomic Era (eds. Biswal, B. and Panigrahi, J.). Orissa, India, pp. 7–12. [Google Scholar]
- 46).Suetsugu N., Wada M. (2016) Evolution of the cp-actin-based motility system of chloroplasts in green plants. Fronti. Plant Sci. 7, 561 doi:10.3389/fpls.2016.0056148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Kong S.-G., Wada M. (2011) New insights into dynamic actin-based chloroplast photorelocation movement. Molecular Plant 4, 771–781. Special Issue on “Cellular Dynamics”. doi:10.1093/mp/ssr061. [DOI] [PubMed] [Google Scholar]
- 48).Gabryś, H. (2012) Blue-light-activated chloroplast movements: progress in the last decade. In Progress in Botany 73 (eds. Lüttge, U., Beyschlag, W., Budel, B. and Francis, D.). Springer-Verlag, Berlin, Heidelberg, pp. 189–205. doi:10.1007/978-3-642-22746-2_7. [Google Scholar]
- 49).Banaś A.K., Aggarwal C., Łabuz J., Gabryś H. (2012) Blue light signalling in chloroplast movements. J. Exp. Bot. 63, 1559–1574. doi:10.1093/jxb/err429. [DOI] [PubMed] [Google Scholar]
- 50).Königer, M. (2014) Chloroplast movement in higher plants, ferns and bryophytes: a comparative point of view. In Photosynthesis in Bryophytes and Early Land Plants, Advances in Photosynthesis and Respiration (eds. Hanson, D.T. and Rice, S.K.). Springer Science+Business Media, Dordrecht, pp. 131–150. doi:10.1007/978-94-007-6988-5_7. [Google Scholar]
- 51).Wada M. (2013) Recent advances in the understanding of fern responses to light. Fern Gaz. 19, 97–115. [Google Scholar]
- 52).Wada M. (2007) Fern as a model system to study photomorphogenesis. J. Plant Res. 120, 3–16. doi:10.1007/s10265-006-0064-x. [DOI] [PubMed] [Google Scholar]
- 53).Wada M., Furuya M. (1972) Phytochrome action on the timing of cell division in Adiantum gametophytes. Plant Physiol. 49, 110–113. doi:10.1104/pp.49.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Wada M., Furuya M. (1978) Effects of narrow-beam irradiations with blue and far-red light on the timing of cell division in Adiantum gametophytes. Planta 138, 85–90. [DOI] [PubMed] [Google Scholar]
- 55).Yatsuhashi H., Wada M., Hashimoto T. (1987) Dichroic orientation of phytochrome and blue-light photoreceptor in Adiantum protonemata as determined by chloroplast movement. Acta Physiol. Plant. 9, 163–173. [Google Scholar]
- 56).Murata T., Wada M. (1989) Organization of cortical microtubules and microfibril deposition in response to blue-light induced apical swelling in a tip-growing Adiantum protonemal cell. Planta 178, 334–341. [DOI] [PubMed] [Google Scholar]
- 57).Murata T., Wada M. (1991) Effects of centrifugation on preprophase band formation in Adiantum protonemata. Planta 183, 391–398. [DOI] [PubMed] [Google Scholar]
- 58).Murata T., Wada M. (1992) Cell cycle specific disruption of the preprophase band of microtubules in fern protonemata: Effects of displacement of the endoplasm by centrifugation. J. Cell Sci. 101, 93–98. [Google Scholar]
- 59).Wada M., O’Brien T.P. (1975) Observations on the structure of the protonema of Adiantum capillus-veneris L. undergoing cell division following white-light irradiation. Planta 126, 213–227. [DOI] [PubMed] [Google Scholar]
- 60).Wada M., Staehelin L.A. (1981) Freez-fracture observations on the plasma membrane, the cell wall and the cuticle of growing protonemata of Adiantum capillus-veneris L. Planta 151, 462–468. [DOI] [PubMed] [Google Scholar]
- 61).Wada, M. and Sugai, M. (1994) Photobiology of Ferns. In Photomorphogenesis in Plants, 2nd edition (eds. Kendrick, R.E. and Kronenberg, G.H.M.). Kluwer Academic Publishers, Dordrecht, pp. 783–802. [Google Scholar]
- 62).Kagawa T., Wada M. (1994) Brief irradiation with red or blue light induces orientational movement of chloroplasts in dark-adapted prothallial cells of the fern Adiantum. J. Plant Res. 107, 389–398. [Google Scholar]
- 63).Kagawa T., Wada M. (1996) Phytochrome- and blue light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta 198, 488–493. [Google Scholar]
- 64).Kagawa T., Wada M. (1999) Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol. 119, 917–923. doi:10.1104/pp.119.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Kagawa T., Kasahara M., Abe T., Yoshida S., Wada M. (2004) Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol. 45, 416–426. doi:10.1093/pcp/pch045. [DOI] [PubMed] [Google Scholar]
- 66).Kanegae, T. and Wada, M. (2006) Photomorphogenesis of ferns. In Photomorphogenesis in Plants and Bacteria 3rd edition (eds. Schäfer, E. and Nagy, F.). Springer, Dordrecht, pp. 515–536. [Google Scholar]
- 67).Kawai H., Kanegae T., Christensen S., Kiyosue T., Sato Y., Imaizumi T., Kadota A., Wada M. (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421, 287–290. doi:10.1038/nature01310. [DOI] [PubMed] [Google Scholar]
- 68).Suetsugu N., Sato Y., Tsuboi H., Kasahara M., Imaizumi T., Kagawa T., Hiwatashi Y., Hasebe M., Wada M. (2012) The KAC family of kinesin-like proteins is essential for the attachment of chloroplasts to the plasma membrane in land plants. Plant Cell Physiol. 53, 1854–1865. doi:10.1093/pcp/pcs133. [DOI] [PubMed] [Google Scholar]
- 69).Tsuboi H., Wada M. (2010) Speed of signal transfer in the chloroplast accumulation response. J. Plant Res. 123, 381–390. doi:10.1007/s10265-009-0284-y. [DOI] [PubMed] [Google Scholar]
- 70).Tsuboi H., Wada M. (2010) The speed of intracellular signal transfer for chloroplast movement. Plant Signal. Behav. 5, 433–435. doi:10.4161/psb.5.4.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Tsuboi H., Wada M. (2011) Chloroplasts can move in any direction to avoid strong light. J. Plant Res. 124, 201–210. doi:10.1007/s10265-010-0364-z. [DOI] [PubMed] [Google Scholar]
- 72).Tsuboi H., Wada M. (2012) Chloroplasts move towards the nearest anticlinal walls under dark condition. J. Plant Research 125, 301–310. doi:10.1007/s10265-011-0433-y. [DOI] [PubMed] [Google Scholar]
- 73).Tsuboi H., Wada M. (2012) Distribution pattern changes of actin filaments during chloroplasts movement in Adiantum capillus-veneris. J. Plant Res. 125, 417–428. doi:10.1007/s10265-011-0444-8. [DOI] [PubMed] [Google Scholar]
- 74).Tsuboi H., Wada M. (2013) Chloroplasts continuously monitor photoreceptor signals during accumulation movement. J. Plant Res. 126, 557–566. doi:10.1007/s10265-012-0542-2. [DOI] [PubMed] [Google Scholar]
- 75).Tsuboi H., Yamashita H., Wada M. (2009) Chloroplasts do not have a polarity for light-induced accumulation movement. J. Plant Res. 122, 131–140. doi:10.1007/s10265-008-0199-z. [DOI] [PubMed] [Google Scholar]
- 76).Higa T., Wada M. (2015) Clues to the signals for chloroplast photo-relocation from the lifetimes of accumulation and avoidance responses. J. Integr. Plant Biol. 57, 120–126. doi:10.1111/jipb.12310. [DOI] [PubMed] [Google Scholar]
- 77).The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- 78).Ishizaki K., Nishihama R., Yamato K.T., Kohchi T. (2016) Molecular genetic tools and techniques for Marchantia polymorpha research. Plant Cell Physiol. 57, 262–270. doi:10.1093/pcp/pcv097. [DOI] [PubMed] [Google Scholar]
- 79).Ishizaki K., Chiyoda S., Yamato K.T., Kohchi T. (2008) Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 49, 1084–1091. doi:10.1093/pcp/pcn085. [DOI] [PubMed] [Google Scholar]
- 80).Wada, M. (2008) Photoresponses in fern gametophytes. In The Biology and Evolution of Ferns and Lycophytes (eds. Ranker, T.A. and Haufler, C.H.). Cambridge Univ. Press, Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, Sãn Paulo, Delhi, pp. 3–48. [Google Scholar]
- 81).Wada M. (1988) Chloroplast photoorientation in enucleated fern protonemata. Plant Cell Physiol. 29, 1227–1232. [Google Scholar]
- 82).Etzold H. (1965) Der Polarotropismus und Phototropismus der Chloronemen von Dryopteris filix-mas (L.) Schott. Planta 64, 254–280. [Google Scholar]
- 83).Yatsuhashi H., Hashimoto T., Wada M. (1987a) Dichroic orientation of photoreceptors for chloroplast movement in Adiantum protonemata. Non-herical orientation. Plant Sci. 51, 165–170. doi:10.1016/0168-9452(87)90189-0. [Google Scholar]
- 84).Kadota A., Wada M., Furuya M. (1985) Phytochrome-mediated polarotropism of Adiantum capillus-veneris L. protonemata as analyzed by microbeam irradiation with polarized light. Planta 165, 30–36. [DOI] [PubMed] [Google Scholar]
- 85).Kadota A., Wada M. (1986) Heart-shaped prothallia of the fern Adiantum capillus-veneris L. develop in the polarization plane of white light. Plant Cell Physiol. 27, 903–910. [Google Scholar]
- 86).Zurzycki J. (1961) The influence of chloroplast displacement on the optical properties of leaves. Acta Soc. Bot. Pol. 30, 503–527. [Google Scholar]
- 87).Augustynowicz J., Gabryś H. (1999) Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ. 22, 1239–1248. doi:10.1046/j.1365-3040.1999.00487.x. [Google Scholar]
- 88).DeBlasio S.L., Mullen J.L., Luesse D.R., Hangarter R.P. (2003) Phytochrome modulation of blue-light-induced chloroplast movement in Arabidopsis. Plant Physiol. 133, 1471–1479. doi:10.1104/pp.103.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Kiessling J., Kruse S., Rensing S.A., Harter K., Decker E.L., Reski R. (2000) Visualization of a cytoskeleton-like FtsZ network in chloroplasts. J. Cell Biol. 151, 945–950. doi:10.1083/jcb.151.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Ellis J.R., Leech R.M. (1985) Cell size and chloroplast size in relation to chloroplast replication in light-grown wheat leaves. Planta 165, 120–125. [DOI] [PubMed] [Google Scholar]
- 91).Jeong W.J., Park Y.-I., Suh K., Raven J.A., Yoo O.J., Liu J.R. (2002) A large population of small chloroplasts in tabacco leaf cells allows more effective chloroplast movement than a few elongated chloroplasts. Plant Physiol. 129, 112–121. doi:10.1104/pp.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Kong S.-G., Suetsugu N., Kikuchi S., Nakai M., Nagatani A., Wada M. (2013) Both phototropin 1 and 2 localize on the chloroplast outer membrane with distinct localization activity. Plant Cell Physiol. 54, 80–92. doi:10.1093/pcp/pcs151. [DOI] [PubMed] [Google Scholar]
- 93).Kodama Y. (2016) Time gating chloroplast autofluorescence allow clearer fluorescence imaging in planta. PLoS ONE 11, e0152484 doi:10.1371/journal.pone.0152484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Kadota A., Yamada N., Suetsugu N., Hirose M., Saito C., Shoji K., Ichikawa S., Kagawa T., Nakano A., Wada M. (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 13106–13111. doi:10.1073/pnas.0906250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Kagawa T., Wada M. (2004) Velocity of chloroplast avoidance movement is fluence rate dependent. Photochem. Photobiol. Sci. 3, 592–595. doi:10.1039/B316285K. [DOI] [PubMed] [Google Scholar]
- 96).Kimura M., Kagawa T. (2009) Blue light-induced chloroplast avoidance and phototropic responses exhibit distinct dose dependency of PHOTOTROPIN2 in Arabidopsis thaliana. Photochem. Photobiol. 85, 1260–1264. doi:10.1111/j.1751-1097.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 97).Kagawa T., Wada M. (2002) Blue light-induced chloroplast relocation. Plant Cell Physiol. 43, 367–371. doi:10.1093/pcp/pcf049. [DOI] [PubMed] [Google Scholar]
- 98).Komatsu A., Terai M., Ishizaki K., Suetsugu N., Tsuboi H., Nishihama R., Yamato K.T., Wada M., Kohchi T. (2014) Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol. 166, 411–427, doi:10.1104/pp.114.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Wada, M. (2005) Chloroplast photorelocation movement. In Light Sensing in Plants (eds. Wada, M., Shimazaki, K. and Iino, M.). Springer-Verlag, Tokyo, pp. 193–199. [Google Scholar]
- 100).Jiao Y., Yang H., Ma L., Sun N., Yu H., Liu T., Gap Y., Gu H., Chen Z., Wada M., Gerstein M., Zhao H., Qu L.-J., Deng X.W. (2004) A genome-wide analysis of blue-light regulation of Arabidopsis transcription factor gene expression during seedling development. Plant Physiol. 133, 1480–1493. doi:10.1104/pp.103.029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Ohgishi M., Saji K., Okada K., Sakai T. (2004) Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 101, 2223–2228. doi:10.1073/pnas.0305984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Lehmann P., Nöthen J., Schmidt von Braun S., Bohnsack M.T., Mirus O., Schleiff E. (2011) Transitions of gene expression induced by short-term blue light. Plant Biol. 133, 349–361. doi:10.1111/j.1438-8677.2010.00377.x. [DOI] [PubMed] [Google Scholar]
- 103).Yatsuhashi H., Kadota A., Wada M. (1985) Blue- and red-light action in photoorientation of chloroplasts in Adiantum protonemata. Planta 165, 43–50. [DOI] [PubMed] [Google Scholar]
- 104).Kagawa T., Kadota A., Wada M. (1994) Phytochrome-mediated photoorientation of chloroplasts in protonemal cells of the fern Adiantum can be induced by a brief irradiation with red light. Plant Cell Physiol. 35, 371–377. [Google Scholar]
- 105).Kadota A., Wada M. (1999) Red light-aphototropic (rap) mutants lack red light-induced chloroplast relocation movement in the fern Adiantum capillus-veneris. Plant Cell Physiol. 40, 238–247. [Google Scholar]
- 106).Christie J.M., Salomon M., Nozue K., Wada M., Briggs W.R. (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. U.S.A. 96, 8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Kagawa T., Wada M. (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol. 41, 84–93. [DOI] [PubMed] [Google Scholar]
- 108).Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660. [DOI] [PubMed] [Google Scholar]
- 109).Sakamoto K., Briggs W.R. (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14, 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Kozuka T., Kong S.-G., Doi M., Shimazaki K., Nagatani A. (2011) Tissue-autonomous promotion of palisade cell development by phototropin 2 in Arabidopsis. Plant Cell 23, 3684–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Folta K.M., Spalding E.P. (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26, 471–478. [DOI] [PubMed] [Google Scholar]
- 112).Takemiya A., Inoue S., Doi M., Kinoshita T., Shimazaki K. (2005) Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Nozue K., Kanegae T., Imaizumi T., Fukuda S., Okamoto H., Yeh K.-C., Lagarias J.C., Wada M. (1998) A phytochorme from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. U.S.A. 95, 15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114).Suetsugu N., Mittmann F., Wagner G., Hughes J., Wada M. (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc. Natl. Acad. Sci. U.S.A. 102, 13705–13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Kanegae T., Hayashida E., Kuramoto C., Wada M. (2006) A single chromoprotein with triple chromophores acts as both a phytochrome and a phototropin. Proc. Natl. Acad. Sci. U.S.A. 103, 1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Kawai-Toyooka H., Kuramoto C., Orui K., Motoyama K., Kikuchi K., Kanegae T., Wada M. (2004) DNA interference: a simple and efficient gene-silencing system for high-throughput functional analysis in the fern Adiantum. Plant Cell Physiol. 45, 1648–1657. [DOI] [PubMed] [Google Scholar]
- 117).Rutherford G., Tanurdzic M., Hasebe M., Banks J.A. (2004) A systemic gene silencing method suitable for high throughput, reverse genetic analyses of gene function in fern gametophytes. BMC Plant Biol. 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Baulcombe D. (2004) RNA silencing in plants. Nature 431, 356–363. [DOI] [PubMed] [Google Scholar]
- 119).Li F.-W., Melkonian M., Rothfels C.J., Villarreal J.C., Stevenson D.W., Graham S.W., Wong G.K.-S., Pryer K.M., Mathews S. (2015) Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat. Commun. 6, 7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Bock G., Haupt W. (1961) Die Chloroplastendrehung bei Mougeotia III. Die Frage der Lokalisierung des Hellrot-Dunkelrot-Pigmentsystems in der Zelle. Planta 57, 518–530. [Google Scholar]
- 121).Li F.-W., et al. (2014) Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc. Natl. Acad. Sci. U.S.A. 111, 6672–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Kadota A., Sato Y., Wada M. (2000) Intracellular chloroplast photorelocation in the moss Physcomitrella patens is mediated by phytochrome as well as by a blue-light receptor. Planta 210, 932–937. [DOI] [PubMed] [Google Scholar]
- 123).Sato Y., Wada M., Kadota A. (2001) Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J. Cell Sci. 114, 269–279. [DOI] [PubMed] [Google Scholar]
- 124).Kasahara M., Kagawa T., Sato Y., Kiyosue T., Wada M. (2004) Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 135, 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Jaedicke K., Lichtenthäler A.L., Meyberg R., Zeidler M., Hughes J. (2012) A phytochrome–phototropin light signaling complex at the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 109, 12231–12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Kong S.-G., Kagawa T., Wada M., Nagatani A. (2013) A carboxy-terminal membrane association domain of phototropin 2 is necessary for chloroplast movements. Plant Cell Physiol. 54, 57–68. doi:10.1093/pcp/pcs132. [DOI] [PubMed] [Google Scholar]
- 127).Takemiya A., Sugiyama N., Fujimoto H., Tsutsumi T., Yamauchi S., Hiyama A., Tada Y., Christie J.M., Shimazaki K. (2013) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat. Commun. 4, 2094 doi:10.1038/ncomms3094. [DOI] [PubMed] [Google Scholar]
- 128).Shimazaki K., Doi M., Assmann S.M., Kinoshita T. (2007) Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58, 219–247. [DOI] [PubMed] [Google Scholar]
- 129).Takamatsu H., Takagi S. (2011) Actin-dependent chloroplast anchoring is regulated by Ca2+-calmodulin in spinach mesophyll cells. Plant Cell Physiol. 52, 1973–1982. [DOI] [PubMed] [Google Scholar]
- 130).Kumatani T., Sakurai-Ozato N., Miyawaki N., Yokota E., Shimmen T., Terashima I., Takagi S. (2006) Possible association of actin filaments with chloroplasts of spinach mesophyll cells in vivo and in vitro. Protoplasma 229, 45–52. [DOI] [PubMed] [Google Scholar]
- 131).Kandasamy M., Meagher M. (1999) Actin-organelle interaction: Association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil. Cytoskeleton 44, 110–118. doi:10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 132).Anielska-Mazur A., Bernaś T., Gabryś H. (2009) In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses. BMC Plant Biol. 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Sattarzadeh A., Krahmer J., Germain A.D., Hanson M.R. (2009) A Myosin XI tail domain homologous to the yeast myosin vacuole-binding domain interacts with plastids and stromules in Nicotiana benthamiana. Mol. Plant 2, 1351–1358. [DOI] [PubMed] [Google Scholar]
- 134).Kadota A., Wada M. (1992) Photoinduction of formation of circular structures by microfilaments on chloroplasts during intracellular orientation in protonemal cells of the fern Adiantum capillus-veneris. Protoplasma 167, 97–107. [Google Scholar]
- 135).Kong S.-G., Arai Y., Suetsugu N., Yanagida T., Wada M. (2013) Rapid severing and motility of cp-actin filaments are required for the chloroplast avoidance response. Plant Cell 25, 572–590. doi:10.1105/tpc.113.109694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136).Oikawa K., Yamasato A., Kong S.-G., Kasahara M., Nakai M., Takahashi F., Ogura Y., Kagawa T., Wada M. (2008) Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 148, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137).Lehmann P., Bohnsack M.T., Schleiff E. (2011) The functional domains of the chloroplast unusual positioning protein 1. Plant Sci. 180, 650–654. [DOI] [PubMed] [Google Scholar]
- 138).Schmidt von Braun S., Schleiff E. (2008) The chloroplast outer membrane protein CHUP1 interacts with actin and profilin. Planta 227, 1151–1159. [DOI] [PubMed] [Google Scholar]
- 139).Suetsugu N., Yamada N., Kagawa T., Yonekura H., Uyeda T.Q.P., Kadota A., Wada M. (2010) Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 107, 8860–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140).Whippo C.W., Khurana P., Davis P.A., DeBlasio S.L., DeSloover D., Staiger C.J., Hangarter R.P. (2011) THRUMIN1 is a light-regulated actin-bundling protein involved in chloroplast motility. Curr. Biol. 21, 59–64. [DOI] [PubMed] [Google Scholar]
- 141).Luesse D.R., DeBlasio S.L., Hangarter R.P. (2006) Plastid Movement Impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in Arabidopsis. Plant Physiol. 141, 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Kodama Y., Suetsugu N., Kong S.-G., Wada M. (2010) Two interacting coiled-coil proteins, WEB1 and PMI2, maintain the chloroplast photorelocation movement velocity in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 107, 19591–19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Suetsugu N., Wada M. (2016) Evolution of the cp-actin-based motility system of chloroplasts in green plants. Fronti. Plant Sci. 7, 561 doi:10.3389/fpls.2016.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144).Goley E.D., Welch M.D. (2006) The ARP2/3 complex: An actin nucleator comes of age. Nat. Rev. Mol. Cell_Biol. 7, 713–726. [DOI] [PubMed] [Google Scholar]
- 145).Suetsugu N., Dolja V.V., Wada M. (2010) Why have chloroplasts developed a unique motility system? Plant Signal. Behav. 5, 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Kagawa T., Wada M. (1993) Light-dependent nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma 177, 82–85. [Google Scholar]
- 147).Kagawa T., Wada M. (1995) Polarized light induces nuclear migration in prothallial cells of Adiantum capillus-veneris L. Planta 196, 775–780. [Google Scholar]
- 148).Tsuboi H., Suetsugu N., Kawai-Toyooka H., Wada M. (2007) Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol. 48, 892–896. [DOI] [PubMed] [Google Scholar]
- 149).Iwabuchi K., Sakai T., Takagi S. (2007) Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol. 48, 1291–1298. [DOI] [PubMed] [Google Scholar]
- 150).Iwabuchi K., Takagi S. (2008) How and why do plant nuclei move in response to light? Plant Signal. Behav. 3, 266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151).Higa T., Suetsugu N., Kong S.-G., Wada M. (2014) Actin-dependent plastid movement is required for motive force generation in directional nuclear movement in plants. Proc. Natl. Acad. Sci. U.S.A. 111, 4327–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Suetsugu N., Higa T., Kong S.-G., Wada M. (2015) PLASTID MOVEMENT IMPAIRED1 and PLASTID MOVEMENT IMPAIRED-LIKE 1 mediate photorelocation movement of both chloroplasts and nuclei. Plant Physiol. 169, 1155–1167. doi:10.1104/pp.15.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153).Glynn J.M., Yang Y., Vitha S., Schmitz A.J., Hemmers M., Miyagishima S., Osteryoung K.W. (2009) PARC6, a novel chloroplast division factor, influences FtsZ assembly and is required for recruitment of PDV1 during chloroplast division in Arabidopsis. Plant J. 59, 700–711. [DOI] [PubMed] [Google Scholar]
- 154).Miyagishima S., Froehlich J.E., Osteryoung K.W. (2006) PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 18, 2517–2530.16998069 [Google Scholar]
- 155).Cazzaniga S., Dall’Osto L., Kong S.-G., Wada M., Bassi R. (2013) Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photoxidative stress in Arabidopsis. Plant J. 76, 568–579. doi:10.1111/tpj.12314. [DOI] [PubMed] [Google Scholar]
- 156).Inoue Y., Shibata K. (1973) Light-induced chloroplast rearrangements and their action spectra as measured by absorption spectrophotometry. Planta 114, 341–358. [DOI] [PubMed] [Google Scholar]
- 157).Park Y.I., Chow W.S., Anderson J.M. (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 111, 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158).Davis P.A., Caylor S., Whippo C.W., Hangarter R.P. (2011) Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ. 34, 2047–2059. [DOI] [PubMed] [Google Scholar]
- 159).Königer M., Bollinger N. (2012) Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 236, 411–426. [DOI] [PubMed] [Google Scholar]
- 160).Terashima I., Hanba Y.T., Tazoe Y., Vyas P., Yano S. (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57, 343–354. [DOI] [PubMed] [Google Scholar]
- 161).Iwabuchi K., Hidema J., Tamura K., Takagi S., Hara-Nishimura I. (2016) Plant nuclei move to escape ultraviolet-induced DNA damage and cell death. Plant Physiol. 170, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162).Suetsugu N., Wada M. (2007) Phytochrome-dependent photomovement responses mediated by phototropin family proteins in Cryptogam plants. Photochem. Photobiol. 83, 87–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.