Abstract
Inflammatory bowel diseases (IBD) are represented by ulcerative colitis (UC) and Crohn’s disease (CD), both of which involve chronic intestinal inflammation. Recent evidence has indicated that gut immunological homeostasis is maintained by the interaction between host immunity and intestinal microbiota. A variety of innate immune cells promote or suppress T cell differentiation and activation in response to intestinal bacteria or their metabolites. Some commensal bacteria species or bacterial metabolites enhance or repress host immunity by inducing T helper (Th) 17 cells or regulatory T cells. Intestinal epithelial cells between host immune cells and intestinal microbiota contribute to the separation of these populations and modulate host immune responses to intestinal microbiota. Therefore, the imbalance between host immunity and intestinal microbiota caused by host genetic predisposition or abnormal environmental factors promote susceptibility to intestinal inflammation.
Keywords: innate immunity, intestinal microbiota, mucosal barrier, inflammatory bowel disease
Introduction
The gut is a unique organ, in which many commensal microbes and foreign materials exist. Gut homeostasis is ingeniously maintained by intestinal environmental factors and host immunity. Inflammatory bowel diseases (IBD) include Crohn’s disease (CD) and ulcerative colitis (UC), which involve chronic inflammation of all or part of the digestive tract. In Japan, the number of patients with IBD has increased tremendously in the last 20 years, in association with a shift towards a more Westernized diet. However, the pathogenesis of IBD remains unclear, and therefore there is no definitive treatment for the diseases.
Recent evidence has indicated that both abnormal environmental factors and host immune dysregulation underlying genetic predisposition contribute to IBD development.1) Intestinal environmental factors include gut microbiota, food antigens and metabolites from various organisms in the intestine. Several recent studies have revealed that certain gut microbiota and metabolites largely influence the host innate and adaptive immunity in the intestine by inducing immune cell activity and differentiation or the expression of several molecules involved in barrier function.2) Therefore, alteration of microbial composition caused by pathogenic bacterial infection, Westernized foods or antimicrobial drugs contributes to aberrant immune responses and inflammatory cytokine production in the intestine.3)
In the intestinal lamina propria, various kinds of myeloid and lymphoid cells are present. These cells orchestrate gut immune system by communicating with one another through cytokine production or cell-cell contact.4) There are numerous CD4+ T cells in the lamina propria, most of which are effector or memory T cells. The number and activity of effector T cells including T helper (Th) 1 and Th 17 cells are exquisitely regulated by several mechanisms. Foxp3+ regulatory T (Treg) cells, abundant in the lamina propria, play a central role in the suppression of inflammatory response. IL-10, derived from Treg cells, decreases the production of the Th1 cytokines, interferon (IFN)-γ and IL-12, and regulates intestinal myeloid cell activity. Accordingly, IL-10-deficient mice show spontaneous colitis accompanied by enhanced effector T cell activity.5)
Many reports have indicated that several innate immune cell subsets modulate intestinal homeostasis in human and murine intestine by enhancing or suppressing T cell immune responses. The function of innate immune cells such as dendritic cells (DCs) and macrophages is tightly maintained by several mechanisms, and the excessive activation of innate immune cells results in IBD development.6,7)
Between intestinal environmental factors and host immunity, intestinal epithelial cells exist. Intestinal epithelial cells include absorptive epithelial cells, goblet cells and Paneth cells, each of which has characteristic features (Fig. 1). The mucosal barriers including antimicrobial peptides (AMPs) and the mucus layer constructed by intestinal epithelial cells, separate the intestinal microbiota and epithelial layers, contributing to the prevention of intestinal inflammation.8) Intestinal epithelial cells can also directly modulate immune cell responses by producing inflammatory mediators including cytokines and chemokines, or presenting antigens to DCs or T cells.8,9)
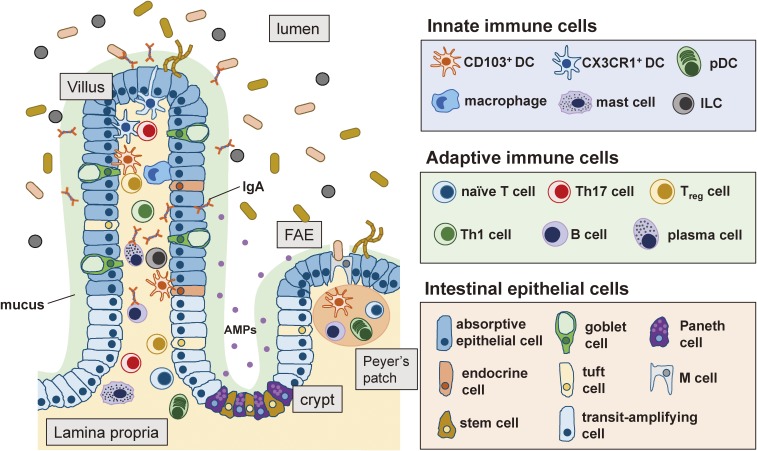
Figure 1.
Players in the immune system of the small intestine. Various types of cells, such as innate immune cells, adaptive immune cells and intestinal epithelial cells, are involved in the gut immune system. Innate immune cells include DCs, macrophages and innate lymphoid cells (ILCs), and adaptive immune cells include T cells, B cells and plasma cells. Intestinal epithelial cells are divided into seven types: absorptive epithelial cells, goblet cells, Paneth cells, endocrine cells, tuft cells, M cells and stem cells. Transit-amplifying cells are progenitor cells that differentiate into mature epithelial cell types. The cross talk among these players is critical for the maintenance of the gut homeostasis. FAE: follicle-associated epithelium, AMPs: antimicrobial peptides, pDC: plasmacytoid DC.
In the intestine, the crosstalk among microbiota, epithelial cells and immune cells is essential to maintain homeostasis and regulate intestinal inflammation. In this review, we focus on the roles of innate immunity, intestinal microbiota and intestinal epithelial cells and the interaction of these players in gut homeostasis and inflammation.
1. Innate immunity and gut homeostasis
Several studies have identified a variety of innate immune cells that maintain gut homeostasis by promoting or suppressing T cell differentiation and activation.10,11) These include myeloid cells such as CD103+ DCs, CX3CR1+ DCs, macrophages and CX3CR1high regulatory myeloid (Mreg) cells (Fig. 2). These cells have been well characterized in mice. In addition, recent reports have identified some human counterparts to murine innate immune cells in the intestine.
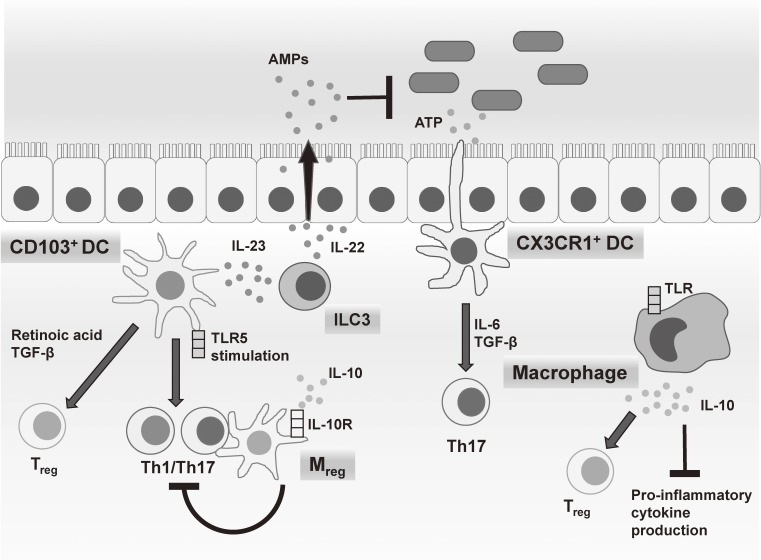
Figure 2.
Innate immune subsets in the gut mucosa. Several myeloid cell subsets, including CD103+ DCs, CX3CR1+ DCs, macrophages and Mreg cells, regulate T cell differentiation and proliferation in the intestinal lamina propria by the cytokine production in response to commensal bacteria or their metabolites. ILC3 regulates epithelial cell function through IL-22 production.
1). DCs.
Recent studies, including those from our group, have identified several DC subsets that induce T cell subset differentiation and proliferation. Murine intestinal DCs are divided into three major groups: CD103+ DCs, CX3CR1+ DCs and plasmacytoid DCs (pDCs).
i) CD103+ DCs.
CD103+ DCs possess various functions, which include inducing CD4+ and CD8+ T cell proliferation and facilitating Foxp3+ Treg cell differentiation through the production of retinoic acid and TGF-β to induce gut immune tolerance.12,13) In addition, retinoic acid produced by DCs in gut-associated lymphoid tissues (GALT) including CD103+ DCs induces IgA class switching in naïve B cells, and upregulates the expression of gut homing receptors including α4β7 and CC-chemokine receptor 9 (CCR9) in IgA+ B cells.14) Our group demonstrated that Bifidobacterium breve, one of probiotic bacteria, activates CD103+ DCs to produce IL-10 and IL-27 via the TLR2/Myd88 pathway thereby inducing IL-10 producing type 1 regulatory T (Tr1) cells.15) On the other hand, CD103+ DCs activated by TLR5 signaling induce Th1/Th17 cell development,16) and produce IL-23 to induce IL-22 production from type 3 ILC (ILC3).17,18) IL-22 promotes the production of AMPs from intestinal epithelial cells to regulate intestinal microbiota.19,20) Some recent studies have revealed that CD103+ DCs can be divided into two subsets, a Batf3- and IRF8-dependent subset of CD103+ CD11b− DCs and an IRF4- and Notch2-dependent subset of CD103+ CD11b+ DCs.21) CD103+ CD11b− DCs have a high capacity to cross-present antigen and function as a platform for CD4+ T cell-dependent CD8+ T cell responses.22,23) In contrast, CD103+ CD11b+ DCs are dominant CD103+ DC population in the small intestinal lamina propria and have an integral role in facilitating mucosal Th2 and Th17 cell responses.24–26)
ii) CX3CR1+ DCs.
In the murine intestine, CX3C chemokine receptor 1 (CX3CR1)-expressing cells are subdivided into CD11c− CX3CR1+ cells, CD11c+ CX3CR1+ CD68+ F4/80+ cells and CD11c+ CX3CR1+ CD68− F4/80− cells, based on surface marker expression.27) Our group revealed that CX3CR1intermediate CD70+ CD11b+ DCs promote Th17 development by producing IL-6, IL-23 and TGF-β in response to ATP derived from commensal bacteria.28)
In the human intestine, HLA-DRhigh CD14+ CD163low cells, the counterparts to murine CX3CR1intermediate CD70+ CD11b+ DCs, induce Th17 cell differentiation by producing IL-6, IL-23 and tumor necrosis factor (TNF)-α through TLR2, TLR4 and TLR5 signaling.29)
iii) Plasmacytoid DCs (pDCs).
pDCs are a unique population of bone-marrow-derived immune cells with the ability to produce large amounts of type I interferon. pDCs can differentiate into antigen-presenting DCs through the stimulation of TLR7 or TLR9 by pathogen-derived nucleic acid. pDCs localize in the lamina propria and GALTs of the small intestine and bridge the innate and adaptive immune systems resulting in a concerted immune response against pathogens.30) pDCs in GALTs strongly induce IgA class switch recombination in B cells in a T cell independent manner by producing a proliferation-inducing ligand (APRIL) and B cell-activating factor of the tumor necrosis factor family (BAFF).31)
2). Macrophages.
Intestinal macrophages (CX3CR1+ CD11b+ F4/80+ cells) have various functions for the prevention of intestinal inflammation. Our groups reported that intestinal CD11b+ CD11c− macrophages in the large intestine produce large amounts of IL-10 in response to commensal bacteria via the TLR signaling pathway.32) IL-10 suppresses the production of pro-inflammatory cytokines from activated myeloid cells in an IL-10/Stat3 signal dependent manner.6) Accordingly, LysM-Cre; Stat3flox/flox mice spontaneously develop intestinal inflammation. In addition, IL-10 derived from intestinal macrophages acts on Treg cells to maintain Foxp3 expression and suppressive function and promote Treg cell proliferation, thereby contributing to the prevention of intestinal inflammation.33,34) Moreover, CX3CR1+ macrophages induce GM-CSF production from ILC3 via production of IL-1β in response to commensal microbes, which in turn control DCs and macrophages to maintain colonic Treg cell homeostasis.35)
3). Mreg cells.
CD11b+ CD11c+ cells in the large intestine can be divided into three subsets based on CX3CR1 expression level. Our group has reported that CX3CR1high CD11b+ CD11c+ cells, termed Mreg cells, suppress T cell proliferation in a cell-cell contact dependent manner.36) Mreg cells express several macrophage-related molecules including CD14, CD68, and F4/80, as well as DC-related molecules including CD11c and DEC205, indicating Mreg cells are a different population from CX3CR1+ CD11b+ CD11c+ DCs or CX3CR1+ CD11b+ CD11c− macrophages in the large intestine. Mreg cells, in which CD80/86 expression is severely suppressed via IL-10/Stat3 signaling, preferentially contact T cells through highly expressed adhesion molecules, such as ICAM-1 and VCAM-1, and maintain the anergic state of effector T cells. We demonstrated that transfer of Mreg cells prevented T cell-dependent colitis, and ameliorated colitis development in LysM-Cre; Stat3flox/flox mice. These results indicate that Mreg cell dysfunction is involved in the pathogenesis of intestinal inflammation.
A variety of innate myeloid cells in the intestine have been identified by several studies including those from our group. These studies demonstrate that each of innate myeloid cells directly or indirectly stimulated with commensal microorganisms exerts characteristic functions to maintain the gut immune system by enhancing or suppressing T cell activity or differentiation.
2. Intestinal microbiota and gut homeostasis
A huge number of microbiota inhabit the mammalian gut. Recent findings have demonstrated that commensal bacteria contribute to the maintenance of gut homeostasis by modulating not only nutrient metabolism, but also the gut immune system (Fig. 3).37) Indeed, in germ-free mice, which have no intestinal bacteria, the size of gut-associated lymphoid tissue (GALT) such as Peyer’s patches and isolated lymphoid follicles, and mesenteric lymph nodes (MLNs) is dramatically reduced.38) In addition, the number of IgA-producing plasma cells and Th17 cells in the intestinal lamina propria is severely decreased in germ-free mice.39,40) Therefore, these mice are susceptible to enteric bacterial infection.41) The gnotobiotic approach, in which germ-free animals are colonized with defined microorganisms, is used to analyze the interaction between the host immune system and microorganisms. Recent studies using this approach have identified several microbial populations that modulate host immunity.
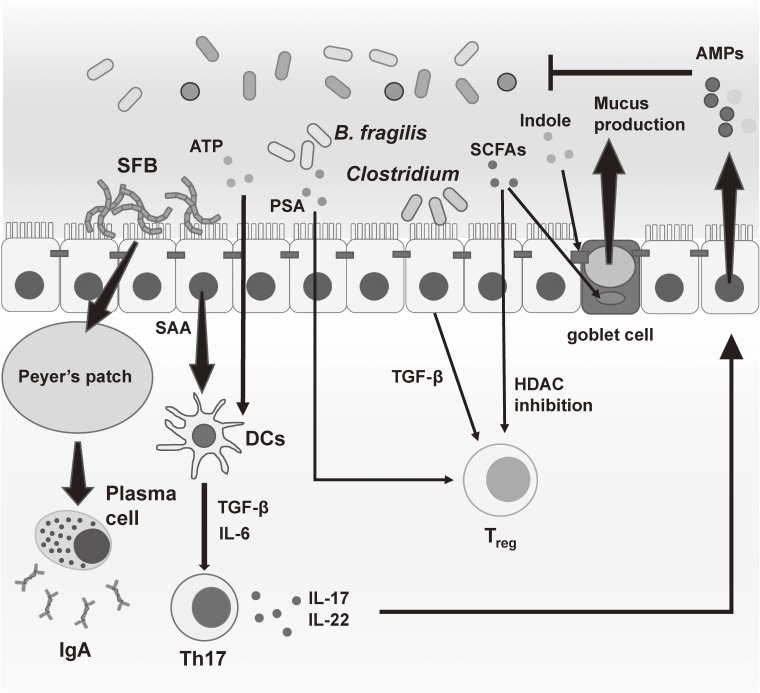
Figure 3.
Intestinal microbiota shapes the gut immune response. Tremendous numbers of commensal bacteria exist in the intestine. Several kinds of commensal microbiota or metabolites from intestinal microorganisms are known to induce T cell immune responses or enhance the mucosal barrier function in the gut. These immune responses lead to host defense or occasionally intestinal inflammation. SFB: segmented filamentous bacteria, ATP: adenosine triphosphate, PSA: Polysaccharide A, SCFAs: short-chain fatty acids, SAA: serum amyloid A.
Segmented filamentous bacteria (SFB), intestinal bacteria found in mice and rats, specifically induce Th17 cells in the intestinal lamina propria by promoting the production of serum amyloid A (SAA) and reactive oxygen species (ROS) from intestinal epithelial cells.40,42) In addition, SFB colonization promote the development of Peyer’s patch and IgA-producing cells, resulting in a much higher IgA level in the gut lumen.43) Accordingly, SFB colonization enhances resistance to pathogenic bacteria, such as Citrobacter rodentium.40) In contrast, Th17 cells induced by SFB colonization trigger autoimmune arthritis and enhance experimental autoimmune encephalomyelitis development, indicating that SFB colonization is involved in the development of autoimmune diseases.44,45)
In contrast, some commensal bacteria have repressive activities on host immunity by facilitating the development of Foxp3+ Treg cells in the intestinal lamina propria. Clostridium species belonging to cluster XIVa and IV promote the development of Foxp3+ Treg in the large intestine by inducing TGF-β production from intestinal epithelial cells.46) Oral inoculation of Clostridium during the early life of conventionally reared mice enhances resistance to intestinal inflammation. Bacteroides fragilis also protect mice against experimental colitis by initiating Foxp3+ Treg development. Polysaccharide A (PSA) of B. fragilis induces Foxp3+ Treg cells through TLR2 signaling in CD4+ T cells to promote immunologic tolerance.47)
Metabolites derived from commensal bacteria, such as short chain fatty acids (SCFAs), secondary bile acids and vitamins, can also modulates the host gut immune system and contribute to shaping gut microbiome consortium. Our group reported that ATP derived from commensal bacteria drives Th17 differentiation in the intestine.32) In addition, we previously reported that indole, which is produced by commensal bacteria possessing tryptophanase, enhances epithelial barrier function in the colon by increasing the expression of both tight junction- and adherens junction-associated molecules.48)
SCFAs including butyrate, propionate and acetate are produced by commensal bacteria during dietary fiber fermentation in the colon. SCFAs are not only energy sources for the host, but also immunomodulators for the gut immune systems. For example, butyrate and propionate induce Foxp3+ Treg cell differentiation and their suppressive ability by inhibiting histone deacetylase (HDAC) activity. Consequently, this then promotes histone acetylation in the promoter and conserved non-coding sequence regions of the Foxp3 locus.49,50) Butyrate also suppresses the production of pro-inflammatory cytokines from macrophages via HDAC inhibition.51) On the other hand, acetate promotes intestinal epithelial cell proliferation and mucus production, resulting in the enhancement of mucosal barrier function.
Many studies have demonstrated “dysbiosis”, which means significant changes in the gut microbiota, occurs in patients with IBD.52) It is a subject of controversy whether dysbiosis is a cause or consequence of IBD. However, mouse studies have revealed that alteration of microbial composition, resulting from co-housing with mice showing dysbiosis of their gut microbiome or high-fat diet intake causes high susceptibility to intestinal inflammation.53,54) Additionally, dysbiosis-related conditions promote CX3CR1+ macrophages ability to capture luminal bacteria and activate effector T cells, contributing to excessive immune responses to commensal bacteria.55) These findings clearly indicate that intestinal microbiota is critically involved in the maintenance of gut homeostasis.
3. Intestinal epithelial cells and gut homeostasis
The intestinal mucosa is protected from commensal microbes and pathogenic microorganisms by various types of barriers.8) These barriers, constructed by several kinds of intestinal epithelial cells such as absorptive epithelial cells, goblet cells and Paneth cells, consist of physical barriers and chemical barriers (Fig. 4). Physical barriers include mucus layer, glycocalyx and cell junction. Chemical barriers consist of a variety of AMPs produced by intestinal epithelial cells, in particular Paneth cells. Recent genome-wide association studies (GWAS) revealed that mucosal barrier-related genes including FUT2, MUC19 and NOD2 are identified as IBD susceptibility genes.56) Indeed, decreased mucosal barrier function, such as reduced production of AMPs or mucus, is observed in the intestines of patients with IBD.57) Additionally, genetically-modified mice showing mucosal barrier dysfunction are highly susceptible to intestinal inflammation.58–60) These results indicate that the mucosal barriers established by intestinal epithelial cells is essential for the maintenance of gut homeostasis.
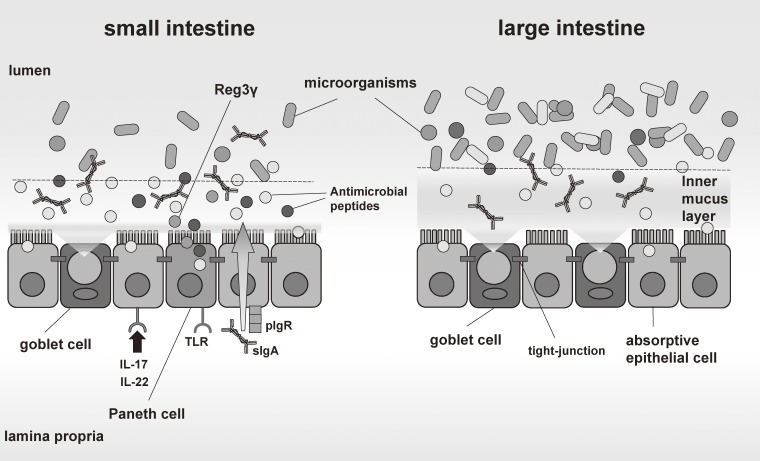
Figure 4.
Mucosal barriers in the gut. Mucosal barriers in the gut consist of physical barriers and chemical barriers. In the small intestine, chemical barriers including AMPs produced by Paneth cells mainly segregate intestinal bacteria and intestinal epithelial cells. In the large intestine, mucus layers are thick and composed of two layers: the outer mucus layer and the inner mucus layer. Whereas Paneth cell-like cells which can produce a large amount of AMPs do not exist in the large intestine, there is no bacterium in the inner mucus layer.
The mucus layer, one of physical barriers, is produced by intestinal secretory cells. Mucus is a viscous fluid enriched in mucin glycoproteins forming larger net-like polymers. The mucus layers play an indispensable role in the prevention of enteric pathogen infection and intestinal inflammation. Intestinal epithelial cells in the colon, where tremendous numbers of microorganisms inhabit, are covered by thick mucus composed of two layers: the inner firm mucus layer and the outer loose mucus layer.61) A number of commensal microorganisms are present in the outer mucus layer, whereas the inner mucus layer is free from them. The separation of commensal bacteria and colonic epithelia by the inner mucus layer is critically involved in the prevention of intestinal inflammation. For example, Muc2-deficient mice, in which the mucus layer is defective, develop spontaneous colitis.58) The deficiency of cooperation of core 1 synthase (C1galt), which synthesizes the major constituent of the O-glycan core structure, allows bacteria to penetrate into the inner mucus layer, resulting in spontaneous colitis.62) In addition, mice lacking Nlrp6, an inflammasome-associated molecule, show the disappearance of bacterial free zone in the inner mucus layer and thus highly sensitive to DSS-induced intestinal inflammation and pathogenic bacterial infection.53,63) However, the mechanism by which the inner mucus layer prevents bacterial access to the epithelial surface of the large intestine remained unclear.
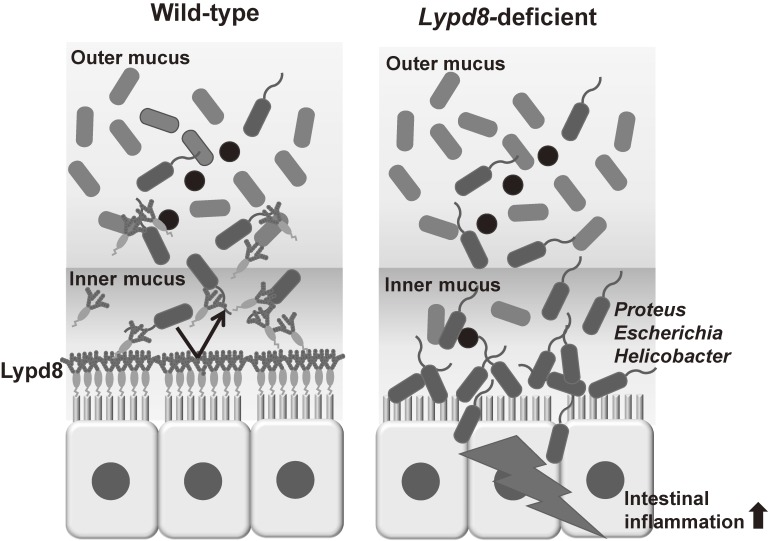
Our group identified a novel molecule termed Ly6/Plaur domain containing 8 (Lypd8), which is a GPI-anchored protein, selectively expressed on the uppermost epithelial layer of the colon.64) Immunohistochemical analysis revealed that highly glycosylated Lypd8 is constitutively shed into the intestinal lumen. Lypd8-deficient mice showed the disappearance of bacterial free space just above the epithelial layer of the colon and high susceptibility to DSS-induced intestinal inflammation, indicating that Lypd8 is critical for the segregation of intestinal bacteria and colonic epithelia. In the absence of Lypd8, a lot of flagellated bacteria, such as Escherichia, Proteus and Helicobacter, invaded the colonic epithelia. An in-vitro analysis demonstrated that Lypd8 preferentially binds to flagellated bacteria by binding to flagella and thereby inhibits their motility. These results indicate that Lypd8 maintain gut homeostasis by suppressing bacterial invasion of the intestinal mucosa by flagellated bacteria (Fig. 5).
Figure 5.
Lypd8 segregates intestinal bacteria and colonic epithelia. Lypd8 is a highly glycosylated GPI-anchored protein, and highly and selectively expressed on the uppermost layer of the colonic epithelial cells and shed into the intestinal lumen. Lypd8 inhibits invasion of flagellated bacteria to the colonic mucosa by suppressing their motility, and thereby regulates the intestinal inflammation.
In the small intestine, where the number of gobet cells is fewer than in the large intestine, a variety of cationic AMPs including α, β-defensin and regenerating islet-derived 3 (Reg3) family proteins, produced by intestinal epithelial cells including Paneth cells, mainly contribute to the segregation of intestinal bacteria and epithelial cells.65,66) Most AMPs are small basic amino acid-rich cationic proteins, which can bind to the microbial cell membrane and form pore-like membrane defects. The production of Reg3γ, a member of the Reg3 family, from Paneth cells is enhanced by TLR/Myd88 signaling.67) Reg3γ is active against gram-positive bacteria and contributes to the spatial separation of gram-positive bacteria and the host in the small intestine.66,68,69)
4. Interaction of intestinal epithelial cells with immune cells
Intestinal epithelial cells indirectly or directly interact with innate and adaptive immune cells by presenting antigens to DCs or T cells, or by expressing cytokines, chemokines, hormones and enzymes.8,9,70) We previously reported that ecto-nucleoside triphosphate diophosphohydrolase (E-NTPD) 7, highly expressed in intestinal epithelial cells of the small intestine, controls ATP concentrations in the intestinal lumen and make a contribution to the regulation of Th17 cell responses in the lamina propria.71) Enterocytes produce SAA or ROS in response to SFB or pathogenic bacterial adhesion, which leads to Th17 cell differentiation.42) Muc2, produced by goblet cells, not only organize the mucus layer but also constrains the immunogenicity of gut antigens by delivering tolerogenic signals to DCs taking in Muc2 glycans.72) M cells, which are found in follicle-associated epithelia (FAE), is a specialist to deliver antigens in the lumen to antigen-presenting cells including DCs and T cells and play an important role in antigen-specific IgA production.73) Recent studies demonstrated that tuft cells, which is a member of intestinal epithelial cells, contribute largely to elimination of helminths by producing IL-25 following infection. IL-25 further activates ILC2 to secrete IL-13 which acts on epithelial progenitors to promote differentiation of tuft and goblet cells for protection against parasite infection.74–76) Cholecystokinin, secreted by intestinal endocrine cells, a minor population of intestinal epithelial cells, can control macrophage activations by inhibiting inducible nitric oxide synthase (iNOS) production.77)
Immune cells also act on intestinal epithelial cells by secreting cytokines. As previously mentioned, IL-22 produced by ILC3 or Th17 cells upregulate AMPs secretion from epithelial cells. IL-17 also enhances the expression of AMPs cooperative with IL-22.20) IL-6 derived from intraepithelial lymphocytes promotes intestinal epithelial proliferation contributing to healing from mucosal injury.78) In contrast, pro-inflammatory cytokines such as TNF-α and IFN-γ inhibits epithelial cell proliferation through suppression of β-catenin/T cell factor (TCF) signaling.79) In the case of mucosal injury, inflammatory monocytes are recruited into the mucosal wound site after neutrophil infiltration to help recovery of the mucosal barrier. Activated macrophages differentiated from recruited monocytes stimulates the colonic epithelial progenitor niche to promote epithelial regeneration.80) Th2 cytokines such as IL-4 and IL-13 facilitate colonic wound healing by inducing alternative activation of macrophages, which contribute to epithelial proliferation.81)
Conclusion
Recent mouse studies have revealed that multiple players, including host immune cells, intestinal epithelial cells and intestinal microorganisms, play critical roles in the maintenance of gut homeostasis by communicating with one another. Both of abnormal environmental factors and gut immune system dysfunction cause inflammation in the intestine. In addition, recent genome-wide association studies have identified IBD susceptibility loci and have contributed to the understanding of IBD pathogenesis. However, there are a number of differences in the immune system and the gut microbial composition between mice and humans.82,83) Therefore the pathogenesis of human IBD is still not fully elucidated. Further investigation of the human gut immune system and intestinal environmental factors may promote advances in the understanding of IBD pathogenesis and new therapeutic approaches for IBD.
Profile
Kiyoshi Takeda was born in 1966 and graduated from Osaka University School of Medicine in 1992. He conducted his Ph.D. work at the Graduate School of Medicine, Osaka University under the supervision of Prof. Shizuo Akira. He was an assistant professor in Hyogo College of Medicine, and Research Institute for Microbial Diseases, Osaka University, where he worked on the mechanisms for Toll-like receptor-dependent pathogen recognition. In 2003, he became a professor at Medical Institute of Bioregulation, Kyushu University, and then moved to Graduate School of Medicine, Osaka University in 2007. He is also a professor at WPI Immunology Frontier Research Center, Osaka University. His present research activity is focused on understanding the pathogenesis of inflammatory bowel diseases, particularly the analysis on how intestinal homeostasis is maintained by mucosal innate immune cells and epithelial cells.
References
- 1).Goto Y., Kurashima Y., Kiyono H. (2015) The gut microbiota and inflammatory bowel disease. Curr. Opin. Rheumatol. 27, 388–396. [DOI] [PubMed] [Google Scholar]
- 2).Samuelson D.R., Welsh D.A., Shellito J.E. (2015) Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 6, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Bellaguarda E., Chang E.B. (2015) IBD and the gut microbiota--from bench to personalized medicine. Curr. Gastroenterol. Rep. 17, 15. [DOI] [PubMed] [Google Scholar]
- 4).Kayama H., Takeda K. (2016) Functions of innate immune cells and commensal bacteria in gut homeostasis. J. Biochem. 159, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Kuhn R., Lohler J., Rennick D., Rajewsky K., Muller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274. [DOI] [PubMed] [Google Scholar]
- 6).Takeda K., Clausen B.E., Kaisho T., Tsujimura T., Terada N., Förster I., Akira S. (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49. [DOI] [PubMed] [Google Scholar]
- 7).Kobayashi M., Kweon M.N., Kuwata H., Schreiber R.D., Kiyono H., Takeda K., Akira S. (2003) Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Peterson L.W., Artis D. (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153. [DOI] [PubMed] [Google Scholar]
- 9).Vitale S., Picascia S., Gianfrani C. (2016) The cross-talk between enterocytes and intraepithelial lymphocytes. Mol Cell Pediatr. 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Strober W. (2009) The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity 31, 377–388. [DOI] [PubMed] [Google Scholar]
- 11).Laffont S., Powrie F. (2009) Immunology: Dendritic-cell genealogy. Nature 462, 732–733. [DOI] [PubMed] [Google Scholar]
- 12).Johansson-Lindbom B., Svensson M., Pabst O., Palmqvist C., Marquez G., Förster R., Agace W.W. (2005) Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., Rajewsky K., Adams D.H., von Andrian U.H. (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160. [DOI] [PubMed] [Google Scholar]
- 15).Jeon S.G., Kayama H., Ueda Y., Takahashi T., Asahara T., Tsuji H., Tsuji N.M., Kiyono H., Ma J.S., Kusu T., Okumura R., Hara H., Yoshida H., Yamamoto M., Nomoto K., Takeda K. (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 8, e1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Uematsu S., Fujimoto K., Jang M.H., Yang B.G., Jung Y.J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K.J., Akira S. (2008) Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776. [DOI] [PubMed] [Google Scholar]
- 17).Kinnebrew M.A., Buffie C.G., Diehl G.E., Zenewicz L.A., Leiner I., Hohl T.M., Flavell R.A., Littman D.R., Pamer E.G. (2012) Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Sonnenberg G.F., Monticelli L.A., Elloso M.M., Fouser L.A., Artis D. (2011) CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Sonnenberg G.F., Fouser L.A., Artis D. (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390. [DOI] [PubMed] [Google Scholar]
- 20).Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Edelson B.T., Wumesh K.C., Juang R., Kohyama M., Benoit L.A., Klekotka P.A., Moon C., Albring J.C., Ise W., Michael D.G., Bhattacharya D., Stappenbeck T.S., Holtzman M.J., Sung S.S., Murphy T.L., Hildner K., Murphy K.M. (2010) Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 207, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Eickhoff S., Brewitz A., Gerner M.Y., Klauschen F., Komander K., Hemmi H., Garbi N., Kaisho T., Germain R.N., Kastenmüller W. (2015) Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Immunity 39, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Hor J.L., Whitney P.G., Zaid A., Brooks A.G., Heath W.R., Mueller S.N. (2015) Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43, 554–565. [DOI] [PubMed] [Google Scholar]
- 24).Gao Y., Nish S.A., Jiang R., Hou L., Licona-Limón P., Weinstein J.S., Zhao H., Medzhitov R. (2013) Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 39, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Persson E.K., Uronen-Hansson H., Semmrich M., Rivollier A., Hägerbrand K., Marsal J., Gudjonsson S., Håkansson U., Reizis B., Kotarsky K., Agace W.W. (2013) IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969. [DOI] [PubMed] [Google Scholar]
- 26).Schlitzer A., McGovern N., Teo P., Zelante T., Atarashi K., Low D., Ho A.W., See P., Shin A., Wasan P.S., Hoeffel G., Malleret B., Heiseke A., Chew S., Jardine L., Purvis H.A., Hilkens C.M., Tam J., Poidinger M., Stanley E.R., Krug A.B., Renia L., Sivasankar B., Ng L.G., Collin M., Ricciardi-Castagnoli P., Honda K., Haniffa M., Ginhoux F. (2013) IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Niess J.H., Adler G. (2010) Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 184, 2026–2037. [DOI] [PubMed] [Google Scholar]
- 28).Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812. [DOI] [PubMed] [Google Scholar]
- 29).Ogino T., Nishimura J., Barman S., Kayama H., Uematsu S., Okuzaki D., Osawa H., Haraguchi N., Uemura M., Hata T., Takemasa I., Mizushima T., Yamamoto H., Takeda K., Doki Y., Mori M. (2013) Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 145, 1380–1391. [DOI] [PubMed] [Google Scholar]
- 30).Lombardi V.C., Khaiboullina S.F. (2014) Plasmacytoid dendritic cells of the gut: relevance to immunity and pathology. Clin. Immunol. 153, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Tezuka H., Abe Y., Asano J., Sato T., Liu J., Iwata M., Ohteki T. (2011) Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34, 247–257. [DOI] [PubMed] [Google Scholar]
- 32).Ueda Y., Kayama H., Jeon S.G., Kusu T., Isaka Y., Rakugi H., Yamamoto M., Takeda K. (2010) Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int. Immunol. 22, 953–962. [DOI] [PubMed] [Google Scholar]
- 33).Murai M., Turovskaya O., Kim G., Madan R., Karp C.L., Cheroutre H., Kronenberg M. (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Müller W., Sparwasser T., Förster R., Pabst O. (2011) Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246. [DOI] [PubMed] [Google Scholar]
- 35).Mortha A., Chudnovskiy A., Hashimoto D., Bogunovic M., Spencer S.P., Belkaid Y., Merad M. (2014) Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Kayama H., Ueda Y., Sawa Y., Jeon S.G., Ma J.S., Okumura R., Kubo A., Ishii M., Okazaki T., Murakami M., Yamamoto M., Yagita H., Takeda K. (2012) Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc. Natl. Acad. Sci. U.S.A. 109, 5010–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Palm N.W., de Zoete M.R., Flavell R.A. (2015) Immune-microbiota interactions in health and disease. Clin. Immunol. 159, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Pollard M., Sharon N. (1970) Responses of the Peyer’s Patches in Germ-Free Mice to Antigenic Stimulation. Infect. Immun. 2, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Macpherson A.J., Gatto D., Sainsbury E., Harriman G.R., Hengartner H., Zinkernagel R.M. (2000) A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226. [DOI] [PubMed] [Google Scholar]
- 40).Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Mtller C.P., Bohnhoff M. (1963) Changes in the mouse’s enteric microflora associated with enhanced susceptibility to Salmonella infection following streptomycin treatment. J. Infect. Dis. 113, 59–66. [DOI] [PubMed] [Google Scholar]
- 42).Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., Suda W., Imaoka A., Setoyama H., Nagamori T., Ishikawa E., Shima T., Hara T., Kado S., Jinnohara T., Ohno H., Kondo T., Toyooka K., Watanabe E., Yokoyama S., Tokoro S., Mori H., Noguchi Y., Morita H., Ivanov I.I., Sugiyama T., Nuñez G., Camp J.G., Hattori M., Umesaki Y., Honda K. (2015) Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Umesaki Y., Setoyama H., Matsumoto S., Imaoka A., Itoh K. (1999) Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect. Immun. 67, 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Wu H.J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. (2010) Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Berer K., Mues M., Koutrolos M., Rasbi Z.A., Boziki M., Johner C., Wekerle H., Krishnamoorthy G. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. [DOI] [PubMed] [Google Scholar]
- 46).Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Shimada Y., Kinoshita M., Harada K., Mizutani M., Masahata K., Kayama H., Takeda K. (2013) Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 8, e80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N.N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J.M., Topping D.L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. [DOI] [PubMed] [Google Scholar]
- 50).Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Chang P.V., Hao L., Offermanns S., Medzhitov R. (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U.S.A. 111, 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Michail S., Durbin M., Turner D., Griffiths A.M., Mack D.R., Hyams J., Leleiko N., Kenche H., Stolfi A., Wine E. (2012) Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 18, 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Elinav E., Strowig T., Kau A.L., Henao-Mejia J., Thaiss C.A., Booth C.J., Peaper D.R., Bertin J., Eisenbarth S.C., Gordon J.I., Flavell R.A. (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Diehl G.E., Longman R.S., Zhang J.X., Breart B., Galan C., Cuesta A., Schwab S.R., Littman D.R. (2013) Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., Essers J., Mitrovic M., Ning K., Cleynen I., Theatre E., Spain S.L., Raychaudhuri S., Goyette P., Wei Z., Abraham C., Achkar J.P., Ahmad T., Amininejad L., Ananthakrishnan A.N., Andersen V., Andrews J.M., Baidoo L., Balschun T., Bampton P.A., Bitton A., Boucher G., Brand S., Büning C., Cohain A., Cichon S., D’Amato M., De Jong D., Devaney K.L., Dubinsky M., Edwards C., Ellinghaus D., Ferguson L.R., Franchimont D., Fransen K., Gearry R., Georges M., Gieger C., Glas J., Haritunians T., Hart A., Hawkey C., Hedl M., Hu X., Karlsen T.H., Kupcinskas L., Kugathasan S., Latiano A., Laukens D., Lawrance I.C., Lees C.W., Louis E., Mahy G., Mansfield J., Morgan A.R., Mowat C., Newman W., Palmieri O., Ponsioen C.Y., Potocnik U., Prescott N.J., Regueiro M., Rotter J.I., Russell R.K., Sanderson J.D., Sans M., Satsangi J., Schreiber S., Simms L.A., Sventoraityte J., Targan S.R., Taylor K.D., Tremelling M., Verspaget H.W., De Vos M., Wijmenga C., Wilson D.C., Winkelmann J., Xavier R.J., Zeissig S., Zhang B., Zhang C.K., Zhao H., International IBD Genetics Consortium (IIBDGC) Silverberg M.S., Annese V., Hakonarson H., Brant S.R., Radford-Smith G., Mathew C.G., Rioux J.D., Schadt E.E., Daly M.J., Franke A., Parkes M., Vermeire S., Barrett J.C., Cho J.H. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Jager S., Stange E.F., Wehkamp J. (2013) Inflammatory bowel disease: an impaired barrier disease. Langenbecks Arch. Surg. 398, 1–12. [DOI] [PubMed] [Google Scholar]
- 58).Van der Sluis M., De Koning B.A., De Bruijn A.C., Velcich A., Meijerink J.P., Van Goudoever J.B., Büller H.A., Dekker J., Van Seuningen I., Renes I.B., Einerhand A.W. (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129. [DOI] [PubMed] [Google Scholar]
- 59).Tanaka H., Takechi M., Kiyonari H., Shioi G., Tamura A., Tsukita S. (2015) Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut. 64, 1529–1538. [DOI] [PubMed] [Google Scholar]
- 60).Biswas A., Liu Y.J., Hao L., Mizoguchi A., Salzman N.H., Bevins C.L., Kobayashi K.S. (2010) Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc. Natl. Acad. Sci. U.S.A. 107, 14739–14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U.S.A. 105, 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Fu J., Wei B., Wen T., Johansson M.E., Liu X., Bradford E., Thomsson K.A., McGee S., Mansour L., Tong M., McDaniel J.M., Sferra T.J., Turner J.R., Chen H., Hansson G.C., Braun J., Xia L. (2011) Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Wlodarska M., Thaiss C.A., Nowarski R., Henao-Mejia J., Zhang J.P., Brown E.M., Frankel G., Levy M., Katz M.N., Philbrick W.M., Elinav E., Finlay B.B., Flavell R.A. (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Okumura R., Kurakawa T., Nakano T., Kayama H., Kinoshita M., Motooka D., Gotoh K., Kimura T., Kamiyama N., Kusu T., Ueda Y., Wu H., Iijima H., Barman S., Osawa H., Matsuno H., Nishimura J., Ohba Y., Nakamura S., Iida T., Yamamoto M., Umemoto E., Sano K., Takeda K. (2016) Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature 532, 117–121. [DOI] [PubMed] [Google Scholar]
- 65).Ayabe T., Satchell D.P., Wilson C.L., Parks W.C., Selsted M.E., Ouellette A.J. (2000) Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118. [DOI] [PubMed] [Google Scholar]
- 66).Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. (2011) The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Brandl K., Plitas G., Schnabl B., DeMatteo R.P., Pamer E.G. (2007) MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204, 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Mukherjee S., Zheng H., Derebe M.G., Callenberg K.M., Partch C.L., Rollins D., Propheter D.C., Rizo J., Grabe M., Jiang Q.X., Hooper L.V. (2014) Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 505, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Worthington J.J. (2015) The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 43, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Kusu T., Kayama H., Kinoshita M., Jeon S.G., Ueda Y., Goto Y., Okumura R., Saiga H., Kurakawa T., Ikeda K., Maeda Y., Nishimura J., Arima Y., Atarashi K., Honda K., Murakami M., Kunisawa J., Kiyono H., Okumura M., Yamamoto M., Takeda K. (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J. Immunol. 190, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Shan M., Gentile M., Yeiser J.R., Walland A.C., Bornstein V.U., Chen K., He B., Cassis L., Bigas A., Cols M., Comerma L., Huang B., Blander J.M., Xiong H., Mayer L., Berin C., Augenlicht L.H., Velcich A., Cerutti A. (2013) Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. (2013) Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6, 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Gerbe F., Sidot E., Smyth D.J., Ohmoto M., Matsumoto I., Dardalhon V., Cesses P., Garnier L., Pouzolles M., Brulin B., Bruschi M., Harcus Y., Zimmermann V.S., Taylor N., Maizels R.M., Jay P. (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).von Moltke J., Ji M., Liang H.E., Locksley R.M. (2016) Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Howitt M.R., Lavoie S., Michaud M., Blum A.M., Tran S.V., Weinstock J.V., Gallini C.A., Redding K., Margolskee R.F., Osborne L.C., Artis D., Garrett W.S. (2016) Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Saia R.S., Mestriner F.L., Bertozi G., Cunha F.Q., Carnio E.C. (2014) Cholecystokinin inhibits inducible nitric oxide synthase expression by lipopolysaccharide-stimulated peritoneal macrophages. Mediators Inflamm. 2014, 896029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Mann E.R., Landy J.D., Bernardo D., Peake S.T., Hart A.L., Al-Hassi H.O., Knight S.C. (2013) Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol. Lett. 150, 30–40. [DOI] [PubMed] [Google Scholar]
- 79).Nguyen T.L., Vieira-Silva S., Liston A., Raes J. (2015) How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Kuhn K.A., Manieri N.A., Liu T.C., Stappenbeck T.S. (2014) IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One 9, e114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Capaldo C.T., Beeman N., Hilgarth R.S., Nava P., Louis N.A., Naschberger E., Stürzl M., Parkos C.A., Nusrat A. (2012) IFN-γ and TNF-α-induced GBP-1 inhibits epithelial cell proliferation through suppression of β-catenin/TCF signaling. Mucosal Immunol. 5, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. (2005) Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. U.S.A. 102, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Seno H., Miyoshi H., Brown S.L., Geske M.J., Colonna M., Stappenbeck T.S. (2009) Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]