Abstract
Alport syndrome is the result of mutations in any of three type IV collagen genes, COL4A3, COL4A4, or COL4A5. Because the three collagen chains form heterotrimers, there is an absence of all three proteins in the basement membranes where they are expressed. In the glomerulus, the mature glomerular basement membrane type IV collagen network, normally comprised of two separate networks, α3(IV)/α4(IV)/α5(IV) and α1(IV)/α2(IV), is comprised entirely of collagen α1(IV)/α2. This review addresses the current state of our knowledge regarding the consequence of this change in basement membrane composition, including both the direct, via collagen receptor binding, and indirect, regarding influences on glomerular biomechanics. The state of our current understanding regarding mechanisms of glomerular disease initiation and progression will be examined, as will the current state of the art regarding emergent therapeutic approaches to slow or arrest glomerular disease in Alport patients.
Introduction
Alport syndrome is a basement membrane disorder that presents with delayed onset and progressive glomerular disease associated with progressive sensorineural hearing loss and sometimes with retinal flecks, anterior lenticonis, and rarely with aortic aneurism [1]. It is caused by mutations in type IV collagen genes with approximately 80% of cases associated with mutations in the COL4A5 gene (X-linked) and the remaining cases associated with mutations in either the COL4A3 or COL4A4 genes. Due to the fact that all three of the encoded type IV collagen α-chains are required to form the heterotrimeric protomers, debilitating mutations in any of these type IV collagen genes results in the complete absence of the α3(IV)/α4(IV)/α5(IV) network in the GBM. The resulting mature GBM in Alport syndrome is thinner and has only α1(IV)/α2(IV) networks.
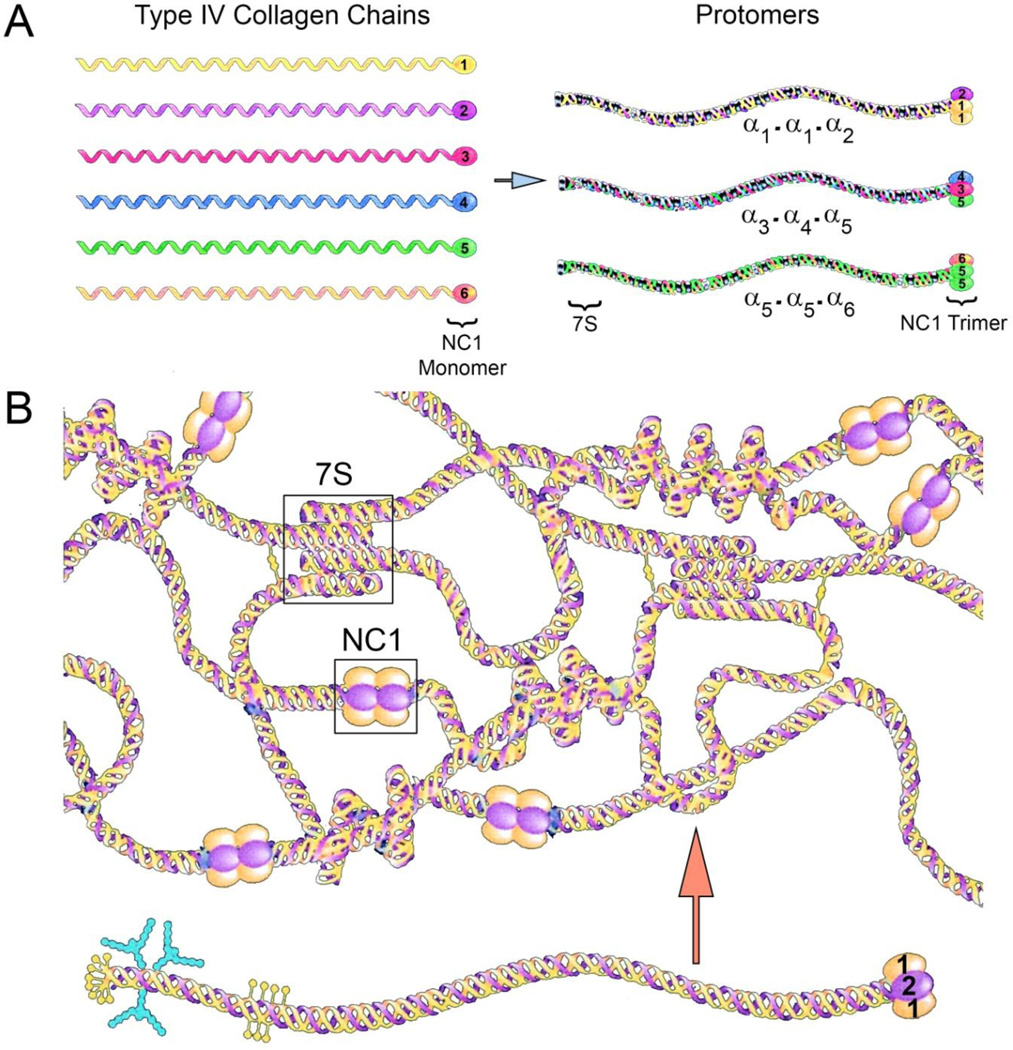
Type IV collagen networks are the structural foundation for all basement membranes. There are six different genes encoding type IV collagen chains designated COL4A1-COL4A6 [2]. The encoded proteins, α1(IV)-α6(IV), form heterotrimers (protomers) with compositions pre-determined by precise interactions of the carboxy-terminal globular NC1 domains [3,4]. Protomer assembly is initiated via NC1 domain interactions [5]. Assembly of the type IV collagen network occurs via protomer interactions at the NC1 domains and at the amino-terminal 7S domains. While these associations are almost always between protomers with the same type IV collagen chain composition, smooth muscle basement membrane is an exception, where α1(IV)/α2(IV) protomer NC1 domains interact with α5(IV)/α6(IV) protomer NC1 domains [6]. Protomers comprised of two α1(IV) chains and one α2(IV) chain are found in all basement membranes, while protomers comprised of one each α3(IV) α4(IV) and α5(IV) or two α5(IV) chains and one α6(IV) chain have a more limited tissue distribution. The density of disulfide interchain crosslinks is significantly greater in networks comprised of α3(IV)/α4(IV)/α5(IV) protomers compared to networks comprised of α1(IV)/α2(IV) protomers, suggesting these networks impart distinct biomechanical properties to the basement membranes where they are found [7] A summary of the chain associations to form protomers and network assembly are provided in Figure 1. An up to date summary of the mutations that cause Alport syndrome can be found in the Leiden Open Variant Database which is accessible at http://www.lovd.nl/3.0/home. While there are no mutational “hot spots” it is notable that there is indeed an association between the severity of the mutation and the severity of the disease [8,9]. Missense mutations that result in glycine substitutions are generally associated with a less severe form of the disease, while out of frame deletions, stop codons, and other mutations that result in chain termination are generally associated with the more severe phenotypes. It was recently shown that a glycine substitution mutation resulted in the activation of unfolded protein response and ER stress, suggesting that this class of mutations might be amenable to therapeutic approaches using chemical chaperones [10].
Figure 1.
Assembly of type IV collagen networks in basement membranes. Panel A. There are 6 different collagen α-chains that assemble via specific NC1 domain interactions into three distinct heterotrimeric protomers. The protomers further assemble into the basement membrane superstructure via NC1 and 7S domain interactions. Interchain disulfide crosslinks provide further stability to the network. Modified from Hudson et al. [4] with permission.
The glomerular basement membrane in mature mammals actually contains two separate networks of type IV collagen. The sub-endothelial GBM is comprised of α1(IV)/α2(IV) networks, while the thicker sub-epithelial layer is comprised of α3(IV)/α4(IV)/α5(IV) networks [7,11]. Collagen α1(IV)/α2(IV) is synthesized by endothelial cells, mesangial cells, and podocytes of immature glomeruli, while collagen α3α4α5(IV) originates solely from podocytes [12]. The GBM together with the fenestrated endothelium and the slit diaphragms contribute to the filtration barrier of the glomerulus to albumin. These components are essential for the permselectivity of the glomerular capillary wall [13].
How the change in GBM type IV collagen composition may influence Alport glomerular pathology
Direct effects
This change in GBM type IV collagen composition is what causes the delayed onset and progressive glomerular disease in Alport syndrome, however mechanistically how the change elicits the initiation and drives the progression of the disease is less clear. A recent study employed super resolution microscopy to imply that the α3(IV)/α4(IV)/α5(IV) network in normal GBM is too distant from the podocyte pedicle interface to interact with the network, while in Alport mice the α1(IV)/α2(IV) network is proximal enough to interact with podocyte receptors [14]. Thus the change in type IV collagen composition might be expected, under this scenario, to directly influence cell signaling in podocytes via engagement of type IV collagen receptors (summarized in Figure 1), or due to the loss of cell signaling due to the absence of type IV collagen α3/α4/α5 mediated effects. Two such collagen receptors are expressed on podocytes; the discoidin domain receptor 1 (DDR1) and integrin α2β1 [15,16]. While α2 integrin-deficient mice show no renal phenotype, DDR1-deficient mice show areas of the GBM with isodense thickening and foot process effacement associated with proteinuria [17,18]. The phenotype in these mice takes 9 months to develop, however, suggesting its influence on glomerular homeostasis is mild in wild type mice. Alport mice that are also deficient in DDR1 showed improved renal function and a 50% increase in lifespan, while Alport mice haploinsufficient for DDR1 survived 29% longer suggesting that this receptor does indeed influence the progression of Alport glomerular disease, apparently in a dose specific manner [19]. It is however unclear whether this effect is due to deletion of DDR1 in podocytes or in other glomerular cell types. Integrin α2-deficient Alport mice resulted in milder renoprotection with only marginally improved GBM ultrastructure and a 20% increase in lifespan [20]. Integrin α2-deficient mice also show attenuated progression for other renal disease models, including injury with adriamycin and partial ablation [21]. In these models, the type IV collagen composition of the GBM is normal, suggesting that the effects of α2β1 integrin deletion in Alport mice might not involve aberrant collagen signaling.
Indirect effects
While it is logical to assume that podocytes exposed to a different type IV collagen network might have altered collagen receptor signaling, and that this change might contribute to podocyte dysfunction associated with Alport glomerular disease progression, it is naïve to presume that Alport syndrome is a podocyte-centric disease. The GBM in Alport mice/humans before the onset of proteinuria is much thinner than normal GBM. As mentioned above, the α1(IV)/α2(IV) networks are much less crosslinked than α3(IV)/α4(IV)/α5(IV) networks [7]. The structure of the capillary loop is stabilized by the attachment of the GBM to the mesangium/mesangial matrix at the mesangial angles combined with the elastile nature of the GBM and the structural reinforcement of the podocyte pedicles, attached to the GBM via integrin mediated adhesion, and slit diaphragms [22]. These structural components create counterforces to the expansile forces of high capillary pressure. When any one of these structural components is compromised, it would be expected that all three glomerular cells would be subjected to elevated biomechanical strain. Such a scenario for Alport glomeruli is predicted by modeling studies [23], and implied by direct measurements of glomerular deformability using glomeruli from preproteinuric Alport mice [24]. Such appears to be the case in Alport mice, where elevated blood pressure following treatment with L-NAME salts resulted in accelerated progression of the disease with earlier onset and faster progression of proteinuria associated with more severe ultrastructural defects in the GBM [25]. Conversely, treatment of Alport mice with the angiotensin converting enzyme inhibitor ramipril slowed the progression of the disease and doubled the lifespan of Alport mice [26]. It should be noted that in the latter study the connection with reduced blood pressure was not established, although our lab has since demonstrated that ramipril treatment does in fact significantly lower blood pressure in these mice [27]. An interesting correlate to the influences of biomechanical strain on the glomerular capillary tuft is the CD151 knockout mouse. CD151 is a co-receptor for laminin binding integrins, including integrin α3β1, and functions to enhance integrin adhesion strength [28]. Podocyte integrin α3β1 adhesion to laminin 521 in the GBM is essential to glomerular health, and disruption of this interaction results in total foot process effacement and post-natal renal failure in mice [29,30]. CD151-null mice develop renal disease with GBM dysmorphology and a molecular pathology that is strikingly similar to Alport mice [31,32]. Like Alport mice, CD151 knockout mice respond to elevated blood pressure with accelerated glomerular disease, and to lower blood pressure with slowed glomerular disease [33]. Thus, the CD151 phenotype provides a second example where compromising the structural integrity of the glomerular capillary tuft results in delayed onset progressive glomerular disease. The fact that this model is strikingly similar in so many ways to Alport glomerular disease suggests a role for biomechanical stress as a potential important underlying driver of glomerular pathogenesis in Alport syndrome.
What we know about the mechanism of Alport glomerular disease onset
Alport syndrome is a delayed onset and progressive disease. Before the onset of proteinuria, the GBM in both Alport mice and humans is thinner than normal GBM, but has very few areas where the characteristic irregular thickening and splitting is observed [34]. Thus, despite the fact that the GBM has abnormal type IV collagen composition at birth, the renal glomerulus in Alport syndrome functions “normally” for a while, several years in humans. This fact has important implications with regard to the mechanism of disease initiation. If we can identify what triggers disease onset, we can devise therapeutic approaches to delay or prevent the triggering mechanism. One clue as to what this might be is the fact that ramipril treatment significantly delayed the onset of proteinuria in Alport mice and prolonged the lifespans of both mice and humans with Alport syndrome [26,35]. Ramipril inhibits the renin-angiotensin-aldosterone system (RAAS), which regulates blood pressure via the vasoconstrictive properties of angiotensin II. Thus, blockade or RAAS would be expected to reduce glomerular blood pressure, which is why RAAS blockade reduces proteinuria and slows renal disease progression for a large number of renal diseases [36]. The RAAS system is complex, and angiotensin II can have a number of direct effects in addition to vasoconstriction, including induction of chemokines and reactive oxygen species and well as extracellular matrix homeostasis [37]. Beyond its effects on blood pressure, these influences need to be considered when evaluating the renoprotective effects of RAAS blockade on Alport renal disease. Along these lines, angiotensin II has been found to be up-regulated in Alport mice, while expression of angiotensin 1–7 was reduced. In addition, both angiotensinogen and renin expression levels were increased in Alport mice [37]. This modulation of the renin/angiotensin components must also be considered when evaluating the molecular effects of RAAS blockade on Alport renal pathology.
Conversely, as mentioned, hypertension is associated with early onset proteinuria, faster progression of GBM dysmorphology, and maladaptive gene expression in Alport glomeruli [25]. This work, combined with the RAAS blockade work, suggests that biomechanical strain may play an important role in the earliest events of Alport glomerular disease. The question is how, given that all three glomerular cell types are subjected to biomechanical forces. Which cell type(s) might be responsible for strain-mediated damage was unclear. A clue was published 16 years ago where it was described that laminin 211, a laminin normally restricted to the mesangial matrix, was accumulating in a punctate and progressive manner in the GBM of Alport mice, dogs, and humans [38,39]. Deletion of a mesangial cell specific integrin, integrin α1β1, attenuated the accumulation of laminin 211 in the GBM [38]. These observations suggested that the source of GBM laminin 211 in Alport glomeruli might be mesangial cell processes that are invading the subendothelial spaces. This was shown to be likely the case using a mesangial cell-specific marker, integrin α8 [40]. The process of mesangial filopodial invasion of the glomerular capillaries was blocked by preventing the activation of CDC42, suggesting that these processes are filopodia. In this same manuscript it was also demonstrated that CD151 knockout mice show mesangial invasion of the glomerular capillaries, suggesting that CDC42 activation is mediated by biomechanical strain. Whether this was a direct effect of mesangial cell stretching or some indirect mechanism was unclear.
It was previously shown that CDC42 can be activated in cultured mesangial cells by treating the cells with endothelin-1 [41]. CDC42 activation induces actin cytoskeletal rearrangement and the formation of filopodia [42]. When cultured primary mesangial cells are treated with endothelin-1 they form drebrin-positive actin microspikes [27]. Drebrin is functionally involved in the clustering of actin during filopodia formation [43]. Blocking CDC42 activation in vivo prevented the accumulation of integrin α8 immunopositivity in the glomerular capillaries, implying that this pathway is essential for mesangial filopodial invasion [40].
The cellular source of endothelin-1 in Alport glomeruli appears to be the glomerular endothelial cells. Endothelial cell expression of endothelin-1 is significantly higher in Alport glomeruli (and urine) compared to wild type glomeruli. In Alport glomeruli, but not wild type glomeruli, endothelin-1 expression is further up-regulated by hypertension in pre-proteinuric mice from both the 129sv autosomal Alport mouse model and the C57Bl/6 X-linked Alport mouse model [28]. The formation of mesangial filopodia can be blocked both in vivo and in vitro using small molecule inhibitors for the endothelin A receptor [27].
One consequence of mesangial filopodial invasion of the GBM is the progressive deposition of mesangial proteins, including laminin α2, in the GBM. A functional consequence of laminin α2 in the GBM is the activation of focal adhesion kinase (FAK) in glomerular podocytes, which results in activation and nuclear translocation of the NFkappaB transcription factor resulting in a pro-inflammatory response [44]. NF-kappaB activation was linked to a broad spectrum of maladaptive gene dysregulation in podocytes, including cytokines, chemokines, growth factors, and metalloproteinases [44]. Blocking FAK activation normalizes maladaptive gene regulation, reduces proteinuria, and prevents GBM destruction in the mouse model. Consistent with this, it was recently shown that deletion of p53 resulted in the acceleration of Alport glomerular disease progression [45]. Given that p53 is a repressor for NF-kappaB activation [46], its deletion might influence the activation state of NF-kappaB, exacerbating expression of NF-kappaB-responsive gene expression. It is worth noting that the podocyte receptor activated by laminin α2 remains unknown.
What we know about Alport renal disease progression
The glomerulus
Early on it was recognized that Alport glomerular disease progression was associated with elevated expression of TGF-β1 and ECM proteins in podocytes [47]. Inhibiting TGF-β1 using a soluble receptor decoy prevented irregular thickening of the GBM, but not podocyte foot process effacement, proteinuria, or lifespan [38]. Elevated expression of matrix metalloproteinases in Alport glomeruli was also reported, and inhibition of MMPs by way of either genetic ablation or broad spectrum small molecule inhibitors attenuated progression of the glomerular disease progression and improved lifespan [48,49]. These effects were blunted if the drugs were administered after glomerular disease had already progressed, suggesting that early intervention is necessary. Genetic ablation of MMP-9 alone, which is significantly elevated in Alport glomeruli, had no effect on Alport glomerular disease progression [50]. The GBM in Alport syndrome is more susceptible to proteolysis than wild type GBM, suggesting that the elevated MMP expression may indeed result in GBM damage [51]. Irregularly thickened regions of the Alport GBM are also abnormally permeable, which might be expected if the GBM were proteolytically damaged [52]. Inhibition of bone morphogenetic proteins (BMPs) by way of uterine sensitization associated gene 1 (USAG-1) reduced MMP expression and attenuated glomerular disease progression in Alport mice, providing additional evidence that MMPs influence Alport glomerular disease [53].
There is emerging evidence that ER stress in podocytes might contribute to glomerular disease progression for missense mutations that result in aberrant protein folding [10]. This evidence is based primarily on the observed induction of unfolded protein related markers in podocytes overexpressing a COL4A3 cDNA containing a missense mutation known to be associated with Alport syndrome.
The interstitium
All glomerular diseases, including Alport syndrome, result in interstitial fibrosis, however the functional link between glomerular disease and tubulointerstitial injury is not clear. It has long been proposed that proteinuria directly and progressively damages tubulointerstitial cells and activates pro-inflammatory pathways [54]. Genetic deletion of albumin in Alport mice does indeed ameliorate renal disease and extend lifespan; however the primary site of albumin-induced injury in this study appeared to be glomerular podocytes [55].
Some studies do suggest that targeting mechanisms of tubulointerstitial fibrosis might improve overall renal function in Alport mice. Function blocking and genetic ablation studies for αvβ6 integrin, which is induced in cortical tubular epithelial cells in Alport mice, ameliorated renal disease progression, improved glomerular architecture, and increased lifespan [56]. Anti-microRNA-21 oligonucleotides markedly improved glomerular function and interstitial disease and increased lifespan in Alport mice. This effect was attributed to reducing miR-21-mediated reactive oxygen species generation and reducing inflammatory signaling in the tubulointerstitium [57]. Increased miR-21 expression in both glomerular and tubular portions of Alport kidneys makes it difficult to parse out just where the renoprotective effects of anti-miR-21 therapy are dominant. Lastly, the BMP-7 negative regulator, USAG-1, is expressed primarily in the macula densa of the distal tubules, which are contact with mesangial cells. Ablation of USAG-1 in Alport mice ameliorated the disease, suggesting crosstalk may exist between kidney tubules and the glomerulus [53].
Interstitial fibrosis is associated with the progressive accumulation of both leukocytes and myofibroblasts. In Alport mice, monocytes were found to promote myofibroblast accumulation and to secrete TGF-β1. Blocking TGF-β1 activity prevented myofibroblast accumulation and fibrosis, but not tubular atrophy, suggesting the latter may be dominantly influenced by the presence of interstitial leukocytes [58]. Mice treated with TGF-β1 blockade did not show improved proteinuria or lifespans, suggesting a disconnect between interstitial fibrosis and glomerular pathology in the mouse model. Removal of monocytes by treatment of Alport mice with clodronate did not prevent myofibroblast accumulation, suggesting monocytes aren’t the only source of TGF-β1 in the fibrosing Alport kidney [59]. These clodronate-treated Alport mice showed no improvement in disease progression of lifespan, suggesting that treatments aiming to prevent monocyte accumulation in Alport kidneys would not be clinically beneficial, as was suggested by earlier work using neutralizing antibodies to collagen XIII [60]. T-cells and B- cells also accumulate in the interstitium of Alport mice. By producing Rag-1 deficient Alport mice, lymphocytes were depleted. These mice showed significantly reduced fibrosis, and like those treated by TGF-β1 blockade, no improvement in glomerular disease or lifespans [61]. This latter study combined with the clodronate depletion studies suggests that the lymphocytes, rather than the monocytes, might be a dominant driver of TGF-β1 mediated fibrosis in the mouse model.
Therapeutic approaches for Alport renal disease
Because the primary deficiency in Alport syndrome is the absence of type IV collagen α3/α4/α5 networks, the obvious primary approach to therapy is to replace the collagen network by introducing a functioning gene to the glomerular podocytes or by replacing the genetically defective podocytes with genotypically normal ones through a stem cell mediated approach. Indeed, gene therapy for the X-linked form of the disease was considered not long after the gene was identified. Adenovirus mediated gene transfer was achieved in 85% of glomeruli in pigs using a GFP reporter construct [62]. When this approach was attempted using the full length COL4A5 cDNA, however, the transduction efficiency was too low to be considered clinically viable [63]. In this study they did achieve expression of the collagen α5(IV) protein in the GBM, providing some level of proof of concept. No further attempts at gene therapy to treat Alport syndrome have been reported since.
As proof that gene replacement is indeed a viable approach, a yeast artificial chromosome containing the human COL4A3/4A4 locus was introduced to the COL4A3 knockout mice. These mice expressed a human/mouse chimeric type IV collagen network in the GBM that functionally rescued the phenotype [64]. A more elegant version of this approach was later used, employing a tetracycline inducible promoter to drive the murine full length COL4A3 cDNA under control of the nephrin promoter. These investigators showed that re-expression of the collagen α3/α4/α5 network, even after glomerular disease was allowed to progress somewhat, provided significant rescue with reduced proteinuria and increased lifespan. The GBM did continue to display some ultrastructural damage in these same mice [65]. This latter study is of particular importance, since it suggests that a gene therapy or stem cell based approach to re-expression of the network would likely work in patients after the initiation of the disease. This implies broader therapeutic utility in the general patient population than approaches that significantly protect renal function only if applied before the onset of proteinuria.
An alternate approach to gene therapy aiming to restore the normal type IV collagen network to the GBM in Alport syndrome is by way of stem cell therapy. This approach was first applied with limited success using bone marrow derived stem cells [66] where investigators were able to show biochemically that stem cells contributed to expression of basement membrane collagens in isolated glomeruli from treated Alport mice. Mesenchymal stem cell therapy reduced interstitial fibrosis but did not delay disease progression in this same model [67]. Interestingly, stem cells from amniotic fluid delayed the progression of the disease, but these cells did not contribute to the formation of GBM basement membrane collagens. This suggests that the cells can exert a therapeutic benefit without physically integrating into the glomerular structures, presumably by secretion of beneficial cytokines and/or growth factors [68]. A cell based therapy using either bone marrow, peripheral blood, or embryonic stem cells was able to restore de novo expression of the α3(IV) chain in the GBM, improve the phenotype and increase lifespan in COL4A3-deficient mice, although these phenotypic improvements were marginal [69]. Although stem cell therapy has shown some promise, the therapeutic benefits demonstrated thus far have been somewhat disappointing. Additional refinements of these methodologies are needed if this approach is to emerge as therapeutically viable.
Final thoughts
The very best way to treat Alport renal disease is to introduce a functional collagen to replace the one damaged by genetic mutation. The studies from Jeff Miners group [65] show that this can be highly beneficial even when the collagen chain is expressed after the onset of renal disease in the mouse model. This might be accomplished by gene therapy or a stem cell based approach, however the technology development in these two areas suggests that such a therapy is a long way off. In the meantime, chemical chaperones might provide such a therapy for patients with specific missense mutations. About 40–50% of Alport patients have missense mutations and 90% of disease causing missense mutations are Glycine substitution [70]. It is clearly important that we understand the molecular mechanisms underlying glomerular disease onset and progression, which are currently coming to light. Already such pursuits have produced therapies in various stages of pre-clinical work and even clinical trials (as is the case for anti-miR-21 therapy). All emergent targeted therapies should be tested both on their own as well as in combination with ACE inhibitors, as ACE inhibition is the current standard of care for patients with Alport syndrome.
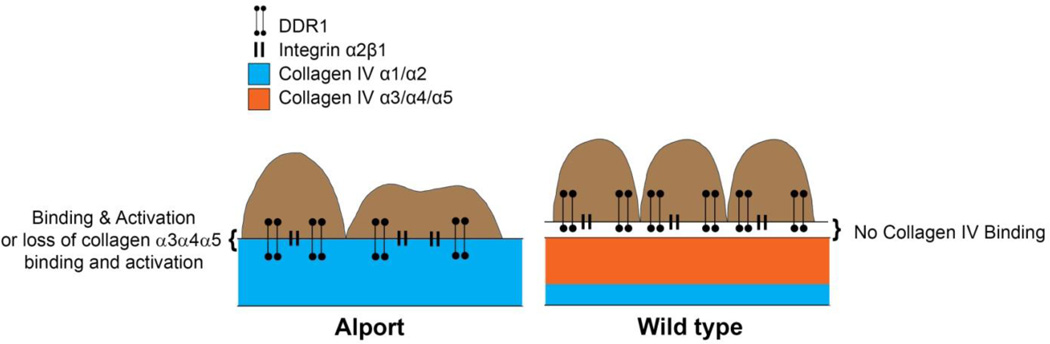
Figure 2.
Ultra-high resolution fluorescence microscopy predicts that the type IV collagen network in the Alport GBM is proximate enough to engage collagen receptors on the podocyte pedicles, while the type IV collagen network in wild type mice is not. Two known collagen receptors expressed in podocytes are DDR1 and integrin α2β1, both of which have been shown to influence Alport disease progression in mouse models.
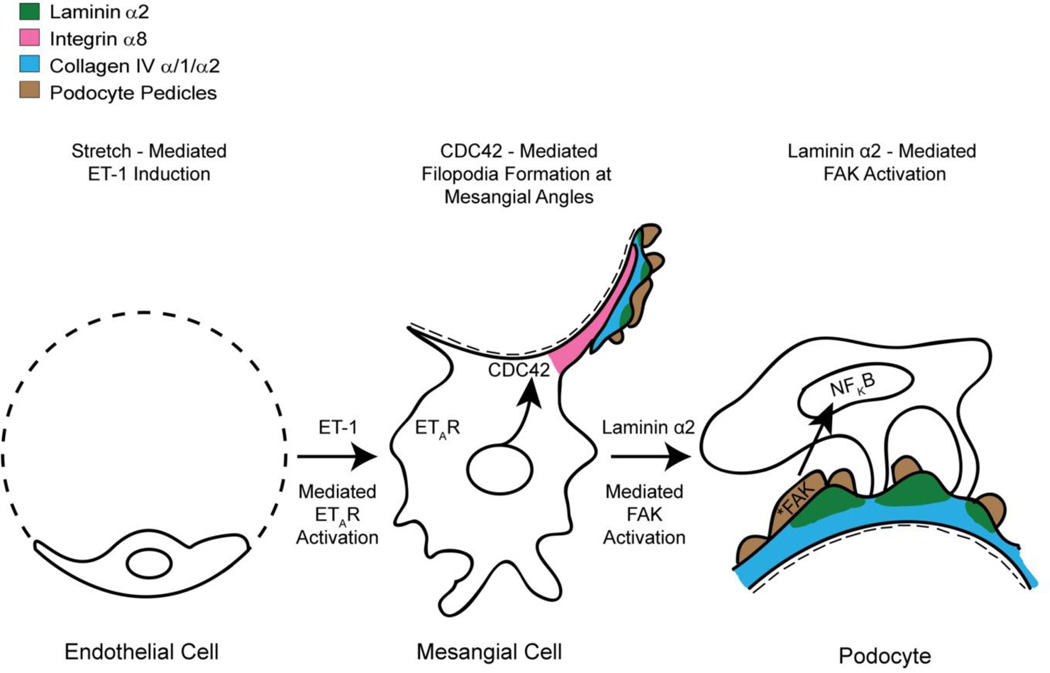
Figure 3.
Model for Alport glomerular disease initiation. The changes in GBM composition result in elevated biomechanical strain on all glomerular cells. In the endothelial cells, this results in elevated expression of endothelin-1, which activates the endothelin A receptors on mesangial cells. ETAR signaling in mesangial cells results in the activation of the small GTPase CDC42 which induces the formation of filopodia (shown in red) at the mesangial angles. These filopodia invade the sub-endothelial aspect of the GBM and deposit mesangial proteins in the GBM, including laminin α2 (shown in green). Laminin α2 activates focal adhesion kinase (*FAK) in the podocyte pedicles, which in turn activates NFkappaB, resulting in nuclear translocation and activation of pro-inflammatory genes.
Highlights.
This review addresses glomerular basement membrane pathology in the context of Alport syndrome. It aims to provide an up to date analysis of the current state of our knowledge regarding the pathobiology of Alport glomerular disease with special emphasis on the role of the GBM collagens.
Acknowledgments
We thank John Kennedy for assistance with art work. This work was funded by R01 DK055000 and R01 DC015385 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Kühn K. Basement membrane (type IV) collagen. Matrix Biol. 1995;14:439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 2.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 3.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J. Biol. Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 4.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J. Am. Soc. Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 5.Kang JK, Colon S, Hellmark T, Sado Y, Hudson BG. Identification of noncollageneous sites encoding specific interactions and quaternary assembly of α3α4α5(IV) collagen. J. Biol. Chem. 2008;283:35070–35077. doi: 10.1074/jbc.M806396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borza DB, Bondar O, Ninomiya Y, Sado Y, Naito I, Todd P, Hudson BG. The NC1 domain of collagen IV encodes a novel network composed of the alpha 1, alpha 2, alpha 5, and alpha 6 chains in smooth muscle basement membranes. J. Biol. Chem. 2001;276:28532–28540. doi: 10.1074/jbc.M103690200. [DOI] [PubMed] [Google Scholar]
- 7.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 1998;273:8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 8.Knebelmann B, Breillat C, Forestier L, Arrondel C, Jacassier D, Giatras I, Drouot L, Deschenes G, Grunfeld J-P, Broyer M, Gubler M-C, Antignac C. Spectrum of mutations in the COL4A5 collagen gene in X-linked Alport syndrome. Am. J. Human. Genet. 1996;59:1221–1232. [PMC free article] [PubMed] [Google Scholar]
- 9.Crockett DK, Pont-Kingdon G, Gedge F, Sumner K, Seamons R, Lyon E. The Alprot syndrome COL4A5 variant database. Hum. Mutation. 2010;31:E1652–E1657. doi: 10.1002/humu.21312. [DOI] [PubMed] [Google Scholar]
- 10.Pieri M, Stefenou C, Zaravinos A, Erguler K, Stylianou K, Lapathitis G, Karaiskos C, Savva I, Paraskeva R, Dweep H, Sticht C, Anastasiadou N, Zouvani I, Goumenos D, Felekkis K, Saleem M, Voskarides K, Gretz N, Deltas C, et al. Evidence for the activation of unfolded protein response in collagen IV nephropathies. J. Am. Soc. Nephrol. 2014;26:260–275. doi: 10.1681/ASN.2012121217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleppel MM, Fan W, Cheong HI, Michael AF. Evidence for separate networks of classical and novel basement membrane collagen. Characterization of alpha 3(IV)-Alport antigen heterodimer. J. Biol. Chem. 1992;267:4137–4142. [PubMed] [Google Scholar]
- 12.Abrahamson D, Hudson B, Strogonova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 2013;9:470–477. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suleiman H, Zang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staatz WD, Walsh JJ, Pexton T, Santoro SA. The alpha 2 beta 1 integrin cell surface collagen receptor binds to the alpha 1 (I)-CB3 peptide of collagen. J. Biol. Chem. 1990;265:4778–4781. [PubMed] [Google Scholar]
- 16.Mohan RR, Mohan RR, Wilson SE. Discoidin domain receptor (DDR) 1 and 2: collagen-activated tyrosine kinase receptors in the cornea. Exp. Eye. Res. 2001;72:87–92. doi: 10.1006/exer.2000.0932. [DOI] [PubMed] [Google Scholar]
- 17.Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2003;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin a2-deficient mice provide insights into the specific functions of collagen receptors in the kideney. Fibrogenesis Tissue Repair. 2010;22 doi: 10.1186/1755-1536-3-19. 1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Müller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, Eckes B, Müller GA, Gross O. Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice. Matrix. Biol. 2014;34:13–21. doi: 10.1016/j.matbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A. Inhibition of integrin α2β1 ameliorates glomerular injury. J. Am. Soc. Nephrol. 2012;23:1027–1038. doi: 10.1681/ASN.2011040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barocas VH, Dorfman KD, Segal Y. A model of strain-dependent glomerular basement membrane maintenance and its potential ramifications in health and disease. J. Biomech. Eng. 2012;134:081006. doi: 10.1115/1.4007098. [DOI] [PubMed] [Google Scholar]
- 23.Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J. Am. Soc. Nephrol. 1995;5:1731–1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- 24.Wyss HM, Henderson JM, Byfield FJ, Bruggeman LA, Ding Y, Huang C, Suhn JH, Franke T, Mele E, Pollak MR, Miner JH, Janmey PA, Weitz DA, Miller RT. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011;300:C397–C405. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meehan DT, Delimont D, Cheung L, Zallocchi M, Sansom SC, Holzclaw JD, Rao V, Cosgrove D. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int. 2009;76:968–976. doi: 10.1038/ki.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addicks K, Smyth N, Schulze-lohoff E, Weber M. Preemptive ramipril therapy delays renal failure and reduces fibrosis in COL4A3-knockout mice with Alport syndrome. 2003;63:438–446. doi: 10.1046/j.1523-1755.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- 27.Dufek B, Meehan DT, Delimont D, Linda Cheung L, Gratton MA, Phillips G, Song W-P, Liu S, Cosgrove D. Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int. 2016;90:300–310. doi: 10.1016/j.kint.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates alpha6beta1 integrin adhesion strengthening. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7616–7621. doi: 10.1073/pnas.1337546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachs N, Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013;9:200–210. doi: 10.1038/nrneph.2012.291. [DOI] [PubMed] [Google Scholar]
- 30.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 31.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am. J. Pathol. 2008;173:927–937. doi: 10.2353/ajpath.2008.071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J. Clin. Invest. 2012;122:348–358. doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidet L, Gubler MC. The renal lesions of Alport syndrome. J. Am. Soc. Nephrol. 2009;20:1210–1215. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 35.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie. Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney. Int. 2012;81:494–501. doi: 10.1038/ki.2011.407. [DOI] [PubMed] [Google Scholar]
- 36.Rüster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J. Am. Soc. Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 37.Bae EH, Konvalinka A, Fang F, Zhou X, Williams V, Maksimowski N, Song X, Zhang SL, John R, Oudit GY, Pei Y, Scholey JW. Characterization of the intrarenal renin-angiotensin system in experimental Alport syndrome. Am. J. Pathol. 2015;185:1423–1435. doi: 10.1016/j.ajpath.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am. J. Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashtan CE, Kim Y, Lees GE, Thorner PS, Virtanen I, Miner JH. Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: aberrant laminin alpha2 deposition is species independent. J. Am. Soc. Nephrol. 2001;12:252–260. doi: 10.1681/ASN.V122252. [DOI] [PubMed] [Google Scholar]
- 40.Zallocchi M, Johnson BM, Meehan DT, Delimont D, Cosgrove D. α1β1 Integrin/Rac1-dependent mesangial invasion of glomerular capillaries in Alport syndrome. Am. J. Pathol. 2013;183:1269–1280. doi: 10.1016/j.ajpath.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- 42.Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:mena complex. Curr. Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J. Neurosci. 2003;23:6586–6595. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, Cosgrove D. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS One. 2014;9:e99083. doi: 10.1371/journal.pone.0099083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda R, Suico MA, Kai Y, Omachi K, Motomura K, Koga T, Komohara Y, Koyama K, Yokota T, Taura M, Shuto T, Kai H. Podocyte p53 limits the severity of experimental Alport syndrome. J. Am. Soc. Nephrol. 2016;27:144–157. doi: 10.1681/ASN.2014111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy SH, Suzuki K, Downes M, Welch GL, De Jesus P, Miraglia LJ, Orth AP, Chanda SK, Evans RM, Verma IM. Tumor suppressor protein (p)53, is a regulator of NF-κB repression by the glucocorticoid receptor. PNAS. 2011;108:17117–17122. doi: 10.1073/pnas.1114420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayers R, Kalluri R, Rodgers KD, Shield CF, III, Meehan DT, Cosgrove D. Role for transforming growth factor-β1 in Alport renal disease progression. Kidney Int. 1999;56:1662–1673. doi: 10.1046/j.1523-1755.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 48.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D. Role for macrophage metalloelastase in glomerular basement membrane damage associated with Alport syndrome. Am. J. Pathol. 2006;169:32–46. doi: 10.2353/ajpath.2006.050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH. Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. Am. J. Pathol. 2000;157:303–311. doi: 10.1016/S0002-9440(10)64541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J. Clin. Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, St. John PL. Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J. Am. Soc. Nephrol. 2007;18:2465–2472. doi: 10.1681/ASN.2007030328. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka M, Asada M, Higashi AY, Nakamura J, Oguchi A, Tomita M, Yamada S, Asada N, Takase M, Okuda T, Kawachi H, Economides AN, Robertson E, Takahashi S, Sakurai T, Goldschmeding R, Muso E, Fukatsu A, Kita T, Yanagita M. Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. J. Clin. Invest. 2010;120:768–777. doi: 10.1172/JCI39569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 55.Jarad G, Knutsen RH, Mecham RP, Miner JH. Albumin contributes to kidney disease in Alport Syndrome. Am. J. Physiol. Renal Physiol. 2016 doi: 10.1152/ajprenal.00456.2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez lG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Invest. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodgers KD, Rao V, Meehan DT, Fager N, Gotwals P, Ryan ST, Koteliansky V, Nemori R, Cosgrove D. Monocytes may promote myofibroblast accumulation and apoptosis in Alport renal fibrosis. Kidney Int. 2003;63:1338–1355. doi: 10.1046/j.1523-1755.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Piaia A, Shenoy N, Kagan D, Gapp B, Kueng B, Weber D, Dietrich W, Ksiazek I. Progression of Alport kidney disease in Col4a3 knock out mice is independent of sex or macrophage depletion by clodronate treatment. PLoS One. 2015;10:e0141231. doi: 10.1371/journal.pone.0141231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dennis J, Meehan DT, Delimont D, Zallocchi M, Perry GA, O’Brien S, Tu H, Pihlajaniemi T, Cosgrove D. Collagen XIII induced in vascular endothelium mediates alpha1beta1 integrin-dependent transmigration of monocytes in renal fibrosis. Am. J. Pathol. 2010;177:2527–2540. doi: 10.2353/ajpath.2010.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lebleu VS, Sugimoto H, Miller CA, Gattone VH, 2nd, Kalluri R. Lymphocytes are dispensable for glomerulonephritis but required for renal interstitial fibrosis in matrix defect-induced Alport renal disease. Lab Invest. 2008;88:284–292. doi: 10.1038/labinvest.3700715. [DOI] [PubMed] [Google Scholar]
- 62.Heikkila P, Parpala T, Lukkarinen O, Weber M, Tryggvason K. Adenovirus-mediated gene transfer into kidney glomeruli using an ex vivo and in vivo kidney perfusion system – first steps towards gene therapy of Alport syndrome. Gene Ther. 1996;3:21–27. [PubMed] [Google Scholar]
- 63.Heikkilä P, Tibell A, Morita T, Chen Y, Wu G, Sado Y, Ninomiya Y, Pettersson E, Tryggvason K. Adenovirus-mediated transfer of type IV collagen alpha5 chain cDNA into swine kidney in vivo: deposition of the protein into the glomerular basement membrane. Gene Ther. 2001;8:882–890. doi: 10.1038/sj.gt.3301342. [DOI] [PubMed] [Google Scholar]
- 64.Heidet L, Borza DB, Jouin M, Sich M, Mattei MG, Sado Y, Hudson BG, Hastie N, Antignac C, Gubler MC. A human-mouse chimera of the alpha3alpha4alpha5(IV) collagen protomer rescues the renal phenotype in Col4a3−/− Alport mice. Am. J. Pathol. 2003;163:1633–1644. doi: 10.1016/s0002-9440(10)63520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin X, Suh JH, Go G, Miner JH. Feasibility of repairing glomerular basement membrane defects in Alport syndrome. J. Am. Soc. Nephral. 2014;25:687–692. doi: 10.1681/ASN.2013070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R. Bone marrow derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl. Acad. Sci. USA. 2006;103:7321–7326. doi: 10.1073/pnas.0601436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlöndorff D, Anders HJ. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 68.Sedrakyan S, Da Sacco S, Milanesi A, Shiri L, Petrosyan A, Varimezova R, Warburton D, Lemley KV, De Filippo RE, Perin L. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J. Am. Soc. Nephrol. 2012;23:661–673. doi: 10.1681/ASN.2011030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeBleu V, Sugimoto H, Mundel TM, Gerami-Naini B, Finan E, Miller CA, Gattone VH, II, Lu L, Shield CF, III, Folkman J, Kalluri R. Stem cell therapies benefit Alport syndrome. J. Am. Soc. Nephrol. 2009;20:2359–2370. doi: 10.1681/ASN.2009010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jais JP, Knebelmann B, Giatras I, DeMarchi M, Rizzoni G, Reneiri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Verellen C, Wieslan der J, Persson U, Tryggvason K, Martin P, Hettz JM, Schroder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport syndrome: natural history of 195 families and genotype-phenotype correlations in males. J. Am. Soc. Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]