Abstract
Complex microbial communities within the human body, constituting the microbiome, have a broad impact on human health and disease. A growing body of research now examines the role of the microbiome in patients with critical illness, such as sepsis and acute respiratory failure. In this article, we provide an introduction to microbiome concepts and terminology and we systematically review the current evidence base of the critical-illness microbiome, including 51 studies in animal models and pediatric and adult critically-ill patients. We further examine how this emerging scientific discipline may transform the way we manage infectious and inflammatory diseases in intensive care units. The evolving molecular, culture-independent techniques offer the ability to study microbial communities in unprecedented depth and detail, and in the short-term, may enable us to diagnose and treat infections in critical care more precisely and effectively. Longer-term, these tools may also give us insights in the underlying pathophysiology of critical illness and reveal previously unsuspected targets for innovative, microbiome-targeted therapeutics. We finally propose a roadmap for future studies in the field for transforming critical care from its current isolated focus on the host to a more personalized paradigm addressing both human and microbial contributions to critical illness.
Keywords: sepsis, acute respiratory failure, acute respiratory distress syndrome, microbiome, microbiota, dysbiosis
Introduction
The advent of molecular, culture-independent techniques to study micro-organisms revealed that the human host harbors approximately 40 trillion microbes [1], including bacteria and their phages, viruses, fungi and archaea. These microbes, organized in complex communities and contributing an enormous amount of genomic information, are clearly important, yet their roles are largely uncharacterized [2]. An exponentially growing body of literature explores the role of the microbiome across a vast array of human pathologies, while the microbiome in critical care has not been studied as extensively. We have just begun to explore how microbiota perturbations (dysbiosis) are involved in the development, evolution and outcome of critical illness, and such microbiome research in patients in intensive care units (ICUs) holds tremendous potential. In the short-term, molecular techniques may allow us to provide more timely, accurate and personalized management of infections compared to our current practice directed by traditional microbial cultures. With deeper understanding of host-microbe interactions, microbiome research may reveal new targets for groundbreaking therapeutics for inflammatory syndromes, such as sepsis and the acute respiratory distress syndrome (ARDS), since efforts so far to modify host responses (without considering their microbial counterparts) have not delivered any efficacious therapies [3,4]. In this article, we review the current state of knowledge on dysbiosis with critical illness and we also discuss important research challenges and strategies to move the field forward. We also provide a synopsis of available microbiome evidence for two common and serious clinical syndromes requiring care in general ICUs [5] - sepsis and acute respiratory failure.
Definitions
While prior reviews have extensively summarized key concepts in microbiome research for clinicians and investigators [6–10], we provide the basic, widely-accepted definitions [11] necessary for comprehending the microbiome literature in Table 1.
Table 1.
Terminology used in human and animal microbiome research presented in order of experimental and analytical workflow.
| General Terms | Microbiome | The totality of human (or other host) body's micro-organisms (including bacteria, viruses, fungi, archaea, protozoa), their genomes and molecular products, and the surrounding environmental conditions. |
| Microbiota | The assemblage of microorganisms present in a defined environment (e.g. human lung microbiota) | |
| “Meta-omics”: | The total content of a community of microbiota in terms of: | |
| Metagenome | - genomic DNA | |
| Metatranscriptome | - transcribed RNA | |
| Metaproteome | - entire protein complement | |
| Metabolome | - metabolite pool | |
| Commensal microbiota | Microbes that provide benefits to the (human) host without being affected by it | |
| Symbiotic microbiota | Microbes in a mutually beneficial relationship with the (human) host | |
| Dysbiosis | A condition in which the normal structure and function of the microbiome has been disturbed and which is considered to be detrimental for the host | |
| Experimental Analysis Terms | Culture-independent techniques | Molecular techniques that analyze the DNA (or other biologic material) directly from a sample rather than from individually cultured microbes |
| Marker | A DNA sequence that identifies the genome that contains it | |
| Amplicon sequencing | Amplification (with PCR) and sequencing of specific markers | |
| 16s rRNA | (or 16S rDNA) 16S ribosomal subunit gene, unique to prokaryotic cells, with highly preserved sequence and hypervariable regions, which are amplified and used as markers for bacterial identification | |
| Whole Metagenome Shotgun sequencing | Sequencing of short, random DNA/RNA fragments in an undirected whole-genome fashion | |
| Bioinformatic Analysis Terms | Operational Taxonomic Units (OTUs) | A common classification used for the amplicon sequences, which are clustered based on a similarity threshold (e.g.>97%) as a proxy for species-level taxonomic assignment. |
| Abundance | Prevalence of a particular taxonomic group in a microbial community | |
| Diversity | Taxonomic distribution within a community, including both the number of distinct taxa and their relative distribution | |
| Richness | Number of taxonomic groups in a microbial community | |
| Evenness | Relative abundance of different taxonomic groups | |
| Dominance | Emergence of a single, overtly abundant taxonomic group | |
| Alpha-diversity | Within-sample taxonomic diversity (including richness and evenness) as a summary statistic of a single population | |
| Beta-diversity | Between-sample taxonomic diversity describing absolute or relative taxonomic overlap between samples | |
| Community structure | Taxonomic composition of a microbial community | |
| Functional metagenomics | Computational or experimental analysis of a microbial community with respect to the molecular activities of its composite genome | |
| Interventional Studies Terms | Germ-free animal | Host animal containing no microorganisms |
| Gnotobiotic animal | Host animal containing artificially transferred humanized microbiota | |
| Prebiotic | A nutrient promoting the growth of symbiotic or commensal microbes | |
| Probiotic | A live microbe introduced in the host with the intention to preserve or restore symbiosis | |
| Symbiotics | Combination of both prebiotics and probiotics | |
Abbreviations: PCR: polymerase chain reaction
Why should we study the microbiome in critical illness?
Contemporary “study of the microbiome” in the ICU essentially equates the use of molecular, culture-independent techniques to profile microbial communities in human samples (e.g. sputum or stool) as opposed to cultures that require ex-vivo growth of organisms. Although the ICU microbiome field is in its infancy, its importance for critical care research and practice is detailed below.
1. Epidemiologic evidence of dysbiosis in critically-ill patients
Accumulating epidemiologic evidence has provided indirect evidence for the presence of dysbiosis in critical illness, even prior to the application of culture-independent techniques [10]. For example, non-infectious acute insults increase the risk for subsequent infections, as with bacterial peritonitis in cirrhotic patients following gastrointestinal bleeding [12] or ventilator-associated pneumonia (VAP) following ARDS [13]. Acute infections such as influenza disrupt respiratory epithelia homeostasis, immune mechanisms and bacterial colonization, leading to secondary infections [14]. In the best clinically-accepted example of dysbiosis, Clostridium difficile colitis, large-scale epidemiologic data show that immediately following Clostridium difficile colitis, patients are at 70% increased risk for rehospitalization with sepsis [15], highlighting again the impact of a disturbed microbial ecosystem.
2. Critical-care interventions disrupt the microbiome
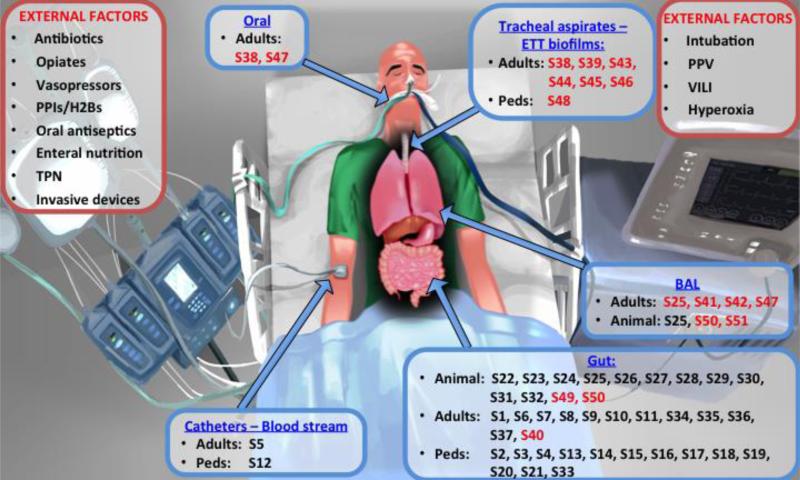
The effects of iatrogenic forces applied during ICU care (Figure 1) cannot be overemphasized, even if they are not yet completely understood [10]. The most profound effects are likely accounted for by antibiotics, which, even if “tailored” to culture-identified pathogens, can have community-wide effects. Antibiotics can indiscriminately ablate commensal microbiota (i.e. indigenous microbes that provide benefits to the human host), with resultant increased vulnerability to secondary pathogen intrusion, and enrichment for antibiotic-resistance genes [16]. Host nutrition is also likely important, because intestinal microbiota rely mainly on availability of enteral nutrients for their survival, and critical illness places them in an acute starvation state [17]. Additionally, pharmacological interventions may alter specific body-site conditions (e.g. skin decontamination, gastric acid suppression therapies) and invasive procedures may disrupt natural barrier mechanisms (e.g. endotracheal intubation, intravascular catheters) opening ports for microbial entry and proliferation. Finally, the ICU environmental ecosystem, including room surfaces, devices or even the hands of healthcare providers may form reservoirs of microbes that can colonize vulnerable patients, as shown in the case of gut colonization of very low birth weight infants by bacteria present in their room environment [18]. Overall, we have only limited knowledge of the impact of ICU care on the microbiome [17].
Figure 1.
Factors that may alter the microbiome in the ICU (shown in red boxes) and references of reviewed microbiome studies according to body site sampled and study subjects (animal, adult or pediatric patients) (shown in blue boxes). Reference numbers (prefaced by “S”) correspond to the reference list provided in the Online Data Supplement. References for studies in sepsis are presented in black font and for studies in acute respiratory failure in red font.
Abbreviations: ETT: endotracheal tube; PPIs: proton-pump inhibitors; H2B: histamine-2 receptor blockers; TPN: total parenteral nutrition; PPV: positive pressure ventilation; VILI: ventilator-induced lung injury; Peds: pediatrics
3. The microbiome as an organ-system in critical illness
If we think of the microbiome as an internalized organ with physiologically important functions, then it becomes evident that microbiome disruptions can be harmful, similar to other “organ failures” in the ICU with damage both by the “organ” function being lost and also the aberrant physiology replacing that function. In this context, the organs being lost are the commensal microbial communities that help metabolize drugs, nutrients and hormones, modulate immune responses, and maintain mucosal barrier homeostasis. By losing commensal microbes, the host also loses protection against invading pathogens, offered either by direct inhibition with antimicrobial peptides (bacteriocins) or through nutrient resource competition [16,19]. Finally, the “aberrant physiology” is represented by emerging pathogens that dominate microbial communities to cause dysregulated inflammatory responses, end-organ damage, and even systemically invade the critically-ill patient to cause sepsis [20].
4. Impact of dysbiosis on the critically-ill host
Disruption of the microbial communities within the human body can have metabolic, immune and even neurocognitive disturbances for the critically-ill host. A major metabolic role of gut microbes is the fermentation of dietary fiber into short-chain fatty acids (SCFA), among which butyrate serves as a primary energy source for the colonic epithelium and preserves gut integrity [19]. With a rapid and persistent drop in fecal SCFA concentration with sepsis [21], the mucus epithelial barrier is degraded opening up ports for pathogen translocation, and epithelial apoptosis occurs resulting in malabsorption of nutrients, diarrhea and fecal energy loss [17,22]. Intestinal microbiota are also considered major tonic activators of host immunity against infections, involving both innate (via granulopoiesis stimulation and antimicrobial peptide production) and adaptive (through regulatory and Th17 T cell differentiation) mechanisms [23]. Following sepsis onset, the disturbed (in content, quantity or function) microbial communities can potentially injure the host both by excessive inflammation with end-organ damage driven by dominant pathogens, and by immune exhaustion with super-infections due to loss of specific microbial signals in the gut (such as segmented filamentous bacteria in mouse models) necessary for the normal maintenance of T-helper cells [24]. Finally, microbial products acting on human brain receptors (gut-brain axis) are responsible for the well-known encephalopathy in cirrhotic patients [25], but have also been implicated in the development of delirium among the most vulnerable elderly patients [26].
5. Utility of culture-independent techniques for diagnosis of infections
While we currently rely heavily on cultures of biological samples to guide clinical management of infections, our gold-standard technique is not fast or accurate enough: cultures take 48-72hr to result and are negative 30-40% of the time despite a high clinical index of suspicion for infection [27]. Negative cultures result not only due to pathogen growth inhibition by antibiotics administered prior to sample acquisition, but also because several human microbes are considered to be uncultivable. Although recent research showed that most of these previously considered uncultivable gut [28] and lung [29] microbiota can in fact be cultured by using a variety of media and conditions, the conventional growth conditions used in clinical laboratories inevitably have limited sensitivity [30]. In the end, delayed or negative cultures lead to empiric broad-spectrum antibiotic regimens in the ICU, which can be disproportionately intense, unnecessary or ineffective for individual patients, and thus contribute to increased toxicity, costs and emergence of antibiotic resistance [31].
Culture-independent sequencing techniques (Table 1) can overcome some of the limitations of cultures and may enable us to deliver more personalized care of infections in the ICU. With direct (and thus timely) amplification of microbial DNA from samples, sequencing offers a comprehensive profile of the microbial communities in question, with insightful quantitative information of abundances of microbial taxa. With further research in this setting, we may be able to use such quantitative taxonomic information for etiologic inference on causative organisms (e.g. when community dominance is accompanied by absolute supra-threshold bacterial loads) or for effectively ruling out an infectious process when diverse communities are uncovered [32]. Furthermore, antibiotic resistance could also become predictable based on sequenced genes [33]. Nonetheless, several methodological issues of next-generation sequencing remain before clinical implementation, including biases with DNA extraction, primer targeting in amplicon studies, polymerase chain reaction contamination or artifacts, and sequencing depth biases. In addition, amplification of microbial DNA does not necessarily signify microbial viability, as both viable and non-viable bacteria can be detected [34,35]. While ongoing methodological research is addressing such limitations, the development of portable, point-of-care sequencing devices, as utilized on-field during the Ebola epidemic in Western Africa demonstrates the potential feasibility of bedside sequencing [36]. Thus, microbiome-based diagnostic testing in the ICU is a field ripe for investigations to transform our current crude management and help promote antibiotic stewardship [37].
6. Promise for microbiome-based therapeutics
Beyond diagnostic applications, microbiome research may also open new avenues for treating critical illness. Early efforts to manipulate the microbiome in the ICU during the “pre-microbiome era” showed considerable promise [10], despite the fact that tested interventions had limited specificity in microbial targets. For example, selective digestive decontamination with antibiotics for intestinal pathogen suppression is the most efficacious VAP preventive measure [38], yet has limited clinical adoption due to concerns for inducing antibiotic resistance. Extensive research supports that probiotics are safe, and potentially efficacious in several critical care settings [39]. However, notable safety exceptions, as in the case of acute pancreatitis [40] and lack of efficacy in recent phase II [41] or III [42] clinical trials highlight the need for refinement of probiotic design, strain and dosage selection and host-microbiome targeting.
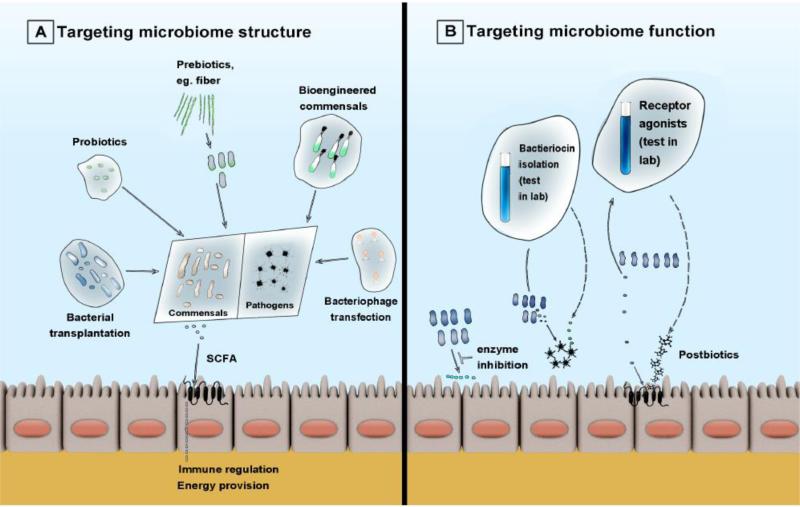
Manipulation of the microbiome for patients’ benefit, either by targeting the microbial community structure or by modifying the function of existing microbiota (Figure 2) represents an active area of research. Therapies such as microbial replacement (as in the case of fecal microbiota transplantation), genetic engineering of modified strains to outcompete pathogens, selective nutrient or prebiotic administration, or engineered bacteriophages may steer the microbiome structure towards a healthy phenotype and alter the course of critical illness [43]. Potential therapeutics targeting modification of microbial function might include targeted small molecule inhibition of specific enzymes [44], harvesting microbial bacteriocins to use as novel antibiotics [20], or administration of microbial product analogs such as receptor agonists (postbiotics) to emulate beneficial actions of microbiota. Such interventions tailored to an individual's microbiome may truly represent a new frontier in precision medicine.
Figure 2.
Overview of therapeutic strategies for targeted microbiome manipulations. A. Strategies targeting microbiome structure include commensal enrichment approaches (bacterial transplantation, probiotics or prebiotics) and pathogen suppression approaches (with bioengineered commensals designed to outperform pathogens or bacteriophage transfection). B. Strategies targeting microbiome function include direct bacterial enzyme inhibition, isolation of bacteriocins as naturally occurring antibiotics or isolation of small molecule agonists of host receptors (postbiotics) to emulate the beneficial effects of commensal microbes.
Abbreviations: SCFA: short-chain fatty acids.
The current state of the microbiome literature in critical care
To synthesize this accumulating literature, we performed a systematic review of culture-independent microbiome studies for sepsis and acute respiratory failure in humans (adult and pediatric patients) and in animal models. We defined acute respiratory failure broadly as the requirement for invasive mechanical ventilation in an ICU, to include the clinically-defined ARDS [45], mechanically-ventilated patients for any indication, serious complications such as VAP, and also corresponding experimental models of ARDS. We provide detailed methods, graphical and qualitative summaries (Evidence Map) in Figure 1 and in the Online Data Supplement. In the next sections, we present the major findings of primary studies for sepsis and acute respiratory failure.
The microbiome in sepsis
Dysbiosis in the gut is considered a central orchestrator in sepsis (gut-origin sepsis), both in triggering pathogen invasion (microbial translocation) and in mediating distal end-organ damage by inflammatory mediators (gut-lymph hypothesis) [46,47]. Current theories consider interactions at the interface between the intestinal mucosal layer and the indigenous microbiome, with barrier integrity failure on the epithelial side, and pathogen expansion on the micro-organism end. The latter occurs as microbes continuously monitor their environment and the density of surrounding bacteria (quorum sensing system) and in response to injury signals (e.g. luminal hypophosphatemia [48], depletion of carbohydrate nutrients [49]) pathogenic bacteria proliferate, increase their virulence, and alter the micro-environment to their benefit. While alternative sources of microbial translocation are plausible in critically-ill patients (e.g. from the skin, mouth or lung), our systematic search identified almost exclusively studies focused on the gut microbiome in accordance to the gut-origin sepsis theory.
Sepsis in animal models
Animal model studies have provided insights into the mechanisms of dysbiosis in sepsis, especially when systemic antibiotics disturb the microbiome. In a study of mice subjected to chemical-induced intestinal injury, systemic expansion of intestinal Escherichia coli resulted in sepsis and organ dysfunction through activation of IL-1β via the inflammasome. These effects were observed only in mice pre-treated with antibiotics [50]. In another mouse model of neonatal sepsis, perinatal antibiotics decreased intestinal microbial diversity and impaired IL-17A-mediated granulopoiesis leading to sepsis vulnerability. The detrimental antibiotic effects were partially reversed with fecal transplantation from normal donors [51]. Antibiotic-induced dysbiosis in mice has been shown to lead to translocation of both pathogenic and commensal Enterobacteriacae through transcytotic routes [52]. In a recent study, two experimental murine models of sepsis both resulted in enrichment of the lung microbiome with gut bacteria, including unculturable anaerobes, suggesting a plausible translocation mechanism [53].
Study of investigational dietary therapies in sepsis animal models showed predictable shifts in microbiota, but variable clinical effects. For example, whey-based peptide diets encouraged the growth of protective microbiota like Lactobacillus and improved intestinal atrophy and permeability [54]. In contrast, omega-3 fatty acid supplementation, despite promoting an anti-inflammatory microbial composition, led to worse outcomes with experimental sepsis [55,56]. This research emphasizes the need for future explorations to assess not only the nutritional demands of the critically-ill host, but also of the indigenous gut microbiota (feed the microbiome concept).
Sepsis in adults
Cumulatively, observational studies have analyzed approximately 400 adults in ICUs before (at risk for) or after the onset of sepsis. Clinically evident sepsis was associated with a significant loss of intestinal microbial diversity over time, with resultant abundance of particular pathogens. Among dominant pathogens, Enterococcal species dominated in general ICU [57], hematopoietic stem cell transplant [58,59] or burn injury [60] patients, and appeared to predict subsequent bacteremia and multiple organ failure [57]. As expected, antibiotics were associated with specific microbiome signatures. For example, metronidazole was associated with a 3-fold increased risk of Enterococcal dominance whereas fluoroquinolones decreased Proteobacterial dominance by as much as 10-fold [59]. More recent evidence highlights extreme patterns of dysbiosis in the gut microbiome of 115 critically-ill patients in general ICUs, with progressive depletion of “health-promoting” organisms, such as Faecalibacterium, and conversely increased abundance of “pro-inflammatory” taxa of the Enterobacteriaceae family [61]. With a broader assessment of taxonomic composition at the phylum level, a smaller study in critically-ill adults showed that the ratio of Bacteriodetes to Firmicutes phyla (B/F ratio) was associated with hospital mortality, since the development of a B/F ratio >10 was more common in patients who died [62].
Sepsis in neonates and infants
Neonatal ICUs offer a unique setting for studying the microbiome, as sampling can begin at birth (before the onset of sepsis) and be repeated prospectively as the neonatal microbiome evolves overtime. Bacterial populations in fecal samples have been analyzed from a total of 600 preterm infants in 13 individual studies that classified sepsis into early-onset (<72hr from birth), late-onset (>72hr from birth) and sepsis in the setting of necrotizing enterocolitis (NEC), as the pathophysiology of these syndromes is distinct. For early-onset sepsis, a microbial link has been established between the amniotic fluid, cord blood and neonatal blood stream, with the same uncultured species detected in all three specimens in septic neonates [63]. In contrast, research in late-onset sepsis points towards a gut origin with loss of intestinal diversity preceding sepsis onset [64,65]. Often, there is eventual dominance of Staphylococcus and Enterobacteriaceae taxa [65–68] or lack of Bifidobacteria [69]. Development of NEC-related sepsis has not been associated with a clear-cut taxonomic composition. Dominant microbial profiles across different cohorts of premature infants have been variable. Abundant organisms included Enterococcus alone [70] or in combination with Staphylococcus [64], Sphingomonas [71], Escherichia [72], and Clostridium Perfringens A [73].
Empiric antibiotics are often prescribed in the first week of life in preterm infants and have sustained effects on the intestinal microbiome. Antibiotic administration is associated with reduced diversity, increased abundance of Enterobacter and Staphylococcus species, and overall increased risk for sepsis and NEC [67], suggesting that this commonly used practice may have unintended effects on the microbiome that should be factored into treatment decisions.
In summary, available research highlights a pattern of intestinal diversity loss with abundance of pathogens in septic adults, indicates different mechanisms of dysbiosis for sepsis subtypes in neonates, and provides a concerning signal for the effects of early life antibiotics. Animal studies in sepsis have offered an experimental platform to study mechanisms of dysbiosis-related inflammation, with corroboration of microbial composition patterns observed in humans.
The microbiome in acute respiratory failure and ARDS
The role of the lung microbiome in acute respiratory failure syndromes and especially the most serious form of ARDS has a less established theoretical and experimental evidence base compared to sepsis. The prevailing theories consider how changes in the alveolar space, which is inflamed and flooded by protein-rich edema, can affect microbial growth (nutritional homeostasis and interkingdom signaling models), as extensively reviewed elsewhere [74]. Three animal studies examined sterile ARDS models (intratracheal lipopolysaccharide (LPS)), whereas in adult humans only one study examined ARDS and the remainder studies enrolled mechanically ventilated patients with acute respiratory failure (Online Data Supplement), as discussed below.
ARDS in animal models
In LPS-induced lung injury in mice [75], bacterial load increased 5-fold in bronchoalveolar lavages (BALs), accounted by a bloom of indigenous Proteobacteria capable of metabolizing the nutrients of the BAL fluid. Notably, intratracheal administration of BAL microbiota from LPS-treated mice did not cause ARDS in naïve mice, but further intensified IL-6-induced lung inflammation in mice treated with IL-6, suggesting that the altered microbiome can act as an effect modifier in ARDS following an initial insult [75].
ARDS in adults
The first evidence that alterations in the lung microbiome are related to the systemic and alveolar inflammation characteristic of ARDS comes from the single available study in 68 adult patients with ARDS [53]. Lung communities were enriched with an uncultured, anaerobic member of the Bacteroides genus. This lung enrichment with gut-specific bacteria was significantly correlated with systemic inflammation, measured by serum TNF-α concentration, whereas alveolar inflammation (by BAL TNF-α concentration) was positively correlated with abundance of the Proteobacteria phylum, even in the absence of clinically-identified pneumonia.
Acute respiratory failure in adults
Mechanically-ventilated patients with respiratory failure showed a pattern of decreased alpha diversity around the time of intubation, with further diversity decline overtime while on ventilatory support [76]. With clinical suspicion of VAP, comparisons of dominant taxa by sequencing versus organisms grown in microbial cultures of lower respiratory tract specimens had overall limited concordance [77–79], and in certain cases indicted previously unsuspected organisms as VAP culprits (e.g. Dialister pneumosintes [78] or Ureaplasma parvum [76]).
Neonatal respiratory distress syndrome
In the single available study in neonates, VAP development was associated with decreased diversity and profound time-related shifts in the abundance of pathogenic species in tracheal aspirates, including Klebsiella, Acinetobacter and Serratia. Overall, these shifts in bacterial abundance did not follow a predictable pattern [80].
In summary, the available evidence highlights generally limited concordance of VAP molecular analyses with culture-based techniques, progressive diversity reduction in the airspace, and nutrient-related bacterial proliferation and propagation of inflammation. Rigorous investigation is needed to examine how lung microbiota perturbations could be modified to prevent development of VAP and ongoing alveolar injury in ARDS.
Challenges and opportunities for microbiome research in the ICU
Microbiome studies often are faced with technical and analytical challenges. Such studies in the ICU are particularly difficult, as the acuity of illness makes the design and conduct of translational –omics research challenging. In the next paragraphs, we consider relevant research challenges and opportunities, and we propose a roadmap for future microbiome research in critical care (Box 1) [81,82].
At a conceptual level, the dynamic nature of microbial shifts, high inter-individual variability and specificity of dysbiosis patterns in particular subphenotypes of critical illness may limit our capacity to identify broad actionable patterns in diverse populations. Thus, microbiome information will have to be highly individualized in order to be helpful in clinical practice. Experimental design in the ICU is also challenging. Cross-sectional studies are of limited value, yet they account for about half of the published evidence. With the evolution of critical illness and corresponding ICU interventions tightly interweaved, it becomes difficult to infer causality and direction of effects for observed dysbiosis. Large sample sizes and advanced statistical methods are needed to account for the multiple confounders at play, whereas randomized clinical trials to dissect the causal effects of ICU therapies on microbiota are hard to undertake. Consortium efforts may improve our ability to rapidly and reliably generate rich microbiome data in critical care, and microbiome outcomes should be considered for inclusion in the design of future clinical trials.
Inherent to critical care research are challenges related to the ability to obtain timely informed consent and high quality biological samples from body sites of interest (e.g. BAL samples from ARDS patients on high ventilator support). Particularly in low biomass samples such as the lung microbiome, it is necessary to control for experimental sequencing noise from contamination by using stringent analytical control protocols. As causal inference from human studies is difficult, animal models (including gnotobiotic and germ-free mice) are indispensable for mechanistic studies. At the same time, further research is also needed to determine the applicability of animal microbiome studies to human disease. Most microbiome studies have been limited to examination of bacteria, but other organisms (i.e. viruses, archaea, and fungi) likely play a role and community interactions may be critical. Although there are methodological challenges, future studies should consider broadening the scope of microbes examined in multiple body sites including the lung, gut, oral and skin microbiome.
Perhaps the biggest challenge pertains to the analysis and integration of microbiome “Big Data”. The critical care literature consists predominantly of a first wave of descriptive studies with 16S rRNA gene sequencing that capture broad taxonomic information. Further advancements in our understanding of pathophysiology require moving beyond taxonomic comparisons by examining the microbial genome comprehensively, by defining interactions of microbial and host gene expression, and by determining effects of bacterial metabolic processing on the host. Advancing to the level of predictive modeling and functional analytics is not an easy task and will require cross-disciplinary collaborations between clinical scientists, bioinformatics experts, statisticians, and systems biologists.
Conclusions – A call for critical care microbiome research
The evolving field of microbiome research is likely to transform the current culture-based paradigm of clinical practice in the ICU. These studies promise to open new avenues for diagnosing, treating or even preventing critical illnesses. Ultimately, clinical translation will require transition from a descriptive/correlative phase to causal modeling and targeted interventions. While formidable challenges for advancing this research agenda exist, the momentum is such that the microbiome is the current big revolution in the post-genomics era. The call for national and global initiatives on microbiome research is encouraging and can catalyze its maturation [83,84].
We anticipate opportunities for selective microbiome manipulation in critical illnesses, even before the microbiome function has been fully elucidated. Such innovative microbiome-directed interventions (including symbiotics, nutritional supplements, fecal transplant, etc.) can potentially be applied in the ICU, even if their exact mechanisms remain to be clarified [85]. We can envision a time in the not-too-distant future when the microbiome will be viewed as yet another organ system of the critically-ill patient, requiring special attention and plan of care during our daily ICU rounds.
Supplementary Material
All authors meet the following authorship criteria:
Substantial contributions to the conception and design of the work
Drafting the work and revising it critically for important intellectual content
Final approval of the version submitted
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Box 1: A roadmap for future microbiome research in critical care.
A. Conceptual Design:
Standardization of human disease phenotypes for microbiome research
Leverage of microbiome profiles for determining disease subphenotypes (e.g. microbiome in infectious versus “non-infectious” ARDS)
Public education on the microbiome, stakeholder engagement, development of accelerated consent mechanisms for microbiome research
Transition to whole metagenome sequencing from amplicon studies
Functional assessments of the microbiome with metabolomics/proteomics approaches
Mechanistic studies in animal models, including gnotobiotic and germ-free models
B. Experimental Design and Conduct:
Prospective studies with serial microbiome sampling
Detailed metadata (clinical data) recording, including key external variables (i.e. antibiotics, nutrition, vasopressors etc.)
Standardization of sampling sites, techniques (e.g. oral swabs, bronchoalveolar lavage sites etc.) and analytical (negative) controls
Incorporation of microbiome sampling from ongoing clinical trials in critical care and design of new studies with microbiome readouts
C. Analytics:
Standardization of microbial composition metrics (e.g. expressions of taxonomic abundances, diversity metrics)
Methodological/analytical innovation for optimal analyses of multidimensional Big Data
D. Information synthesis, validation and dissemination:
Consortium efforts – creation of large cohorts of patients
External validation of results in independent cohorts
Evidence synthesis approaches for pooling individual patient data across cohorts and analytical platforms
Public availability of data and programming codes to ensure reproducibility
Acknowledgments
Supported by: T32 HL007563 (GDK); K24 HL123342 and U01 HL098962 (AM); PPG - P01HL114453 and R01HL097376 (BJM); K23HL130641 and UL1TR000433 (RPD)
Abbreviations
- ICU
intensive care unit
- ARDS
acute respiratory distress syndrome
- ALI
acute lung injury
- VAP
ventilator-associated pneumonia
- SCFA
short-chain fatty acid
- NEC
necrotizing enterocolitis
- LPS
lipopolysaccharide
- BAL
bronchoalveolar lavage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
No conflict of interest
Bibliography
- 1.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–40. doi: 10.1016/j.cell.2016.01.013. doi:10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Shukla SK, Murali NS, Brilliant MH. Personalized medicine going precise: from genomics to microbiomics. Trends Mol Med. 2015;21:461–2. doi: 10.1016/j.molmed.2015.06.002. doi:10.1016/j.molmed.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166. doi: 10.1186/1741-7015-11-166. doi:10.1186/1741-7015-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. doi:10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 5.Barrett ML, Smith MW, Elixhauser A, Honigman LS, Pines JM. HCUP Statistical Brief #185. Agency for Healthcare Research and Quality; Rockville, MD: 2014. Utilization of Intensive Care Services, 2011. [PubMed] [Google Scholar]
- 6.Morgan XC, Huttenhower C. Human microbiome analysis. PLoS Comput Biol. 2012;8:e1002808. doi: 10.1371/journal.pcbi.1002808. Chapter 12, doi:10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui L, Morris A, Huang L, Beck JM, Twigg HL, von Mutius E, et al. The microbiome and the lung. Annals of the American Thoracic Society. 2014;11(Suppl 4):S227–32. doi: 10.1513/AnnalsATS.201402-052PL. doi:10.1513/AnnalsATS.201402-052PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YJ, Charlson ES, Collman RG, Colombini-Hatch S, Martinez FD, Senior RM. The role of the lung microbiome in health and disease. A National Heart, Lung, and Blood Institute workshop report. Am J Respir Crit Care Med. 2013;187:1382–7. doi: 10.1164/rccm.201303-0488WS. doi:10.1164/rccm.201303-0488WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal LN, Rom WN, Weiden MD. Lung microbiome for clinicians. New discoveries about bugs in healthy and diseased lungs. Annals of the American Thoracic Society. 2014;11:108–16. doi: 10.1513/AnnalsATS.201310-339FR. doi:10.1513/AnnalsATS.201310-339FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson RP. The microbiome and critical illness. The Lancet Respiratory Medicine. 2016;4:59–72. doi: 10.1016/S2213-2600(15)00427-0. doi:10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. doi:10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2015 doi: 10.1002/hep.28330. doi:10.1002/hep.28330. [DOI] [PubMed] [Google Scholar]
- 13.Markowicz P, Wolff M, Djedaïni K, Cohen Y, Chastre J, Delclaux C, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med. 2000;161:1942–8. doi: 10.1164/ajrccm.161.6.9909122. doi:10.1164/ajrccm.161.6.9909122. [DOI] [PubMed] [Google Scholar]
- 14.Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Infect Immun. 2015;83:3764–70. doi: 10.1128/IAI.00298-15. doi:10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization Type and Subsequent Severe Sepsis. Am J Respir Crit Care Med. 2015;192:581–8. doi: 10.1164/rccm.201503-0483OC. doi:10.1164/rccm.201503-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124:4212–8. doi: 10.1172/JCI72333. doi:10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morowitz MJ, Carlisle EM, Alverdy JC. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg Clin North Am. 2011;91:771–85. viii. doi: 10.1016/j.suc.2011.05.001. doi:10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks B, Firek BA, Miller CS, Sharon I, Thomas BC, Baker R, et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome. 2014;2:1. doi: 10.1186/2049-2618-2-1. doi:10.1186/2049-2618-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenney PT, Pamer EG. From Hype to Hope: The Gut Microbiota in Enteric Infectious Disease. Cell. 2015;163:1326–32. doi: 10.1016/j.cell.2015.11.032. doi:10.1016/j.cell.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreyra JA, Ng KM, Sonnenburg JL. The Enteric Two-Step: nutritional strategies of bacterial pathogens within the gut. Cell Microbiol. 2014;16:993–1003. doi: 10.1111/cmi.12300. doi:10.1111/cmi.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada T, Shimizu K, Ogura H, Asahara T, Nomoto K, Yamakawa K, et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J Parenter Enteral Nutr. 2015;39:569–77. doi: 10.1177/0148607114529596. doi:10.1177/0148607114529596. [DOI] [PubMed] [Google Scholar]
- 22.Demehri FR, Barrett M, Ralls MW, Miyasaka EA, Feng Y, Teitelbaum DH. Intestinal epithelial cell apoptosis and loss of barrier function in the setting of altered microbiota with enteral nutrient deprivation. Front Cell Infect Microbiol. 2013;3:105. doi: 10.3389/fcimb.2013.00105. doi:10.3389/fcimb.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hand TW. The Role of the Microbiota in Shaping Infectious Immunity. Trends Immunol. 2016 doi: 10.1016/j.it.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–58. doi: 10.1038/mi.2009.132. doi:10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397–403. doi: 10.4161/gmic.28684. doi:10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mello AM, Paroni G, Daragjati J, Pilotto A. Gastrointestinal Microbiota and Their Contribution to Healthy Aging. Dig Dis. 2016;34:194–201. doi: 10.1159/000443350. doi:10.1159/000443350. [DOI] [PubMed] [Google Scholar]
- 27.Esperatti M, Ferrer M, Theessen A, Liapikou A, Valencia M, Saucedo LM, et al. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010;182:1533–9. doi: 10.1164/rccm.201001-0094OC. doi:10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
- 28.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, et al. Culturing of “unculturable” human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–6. doi: 10.1038/nature17645. doi:10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, et al. Application of a neutral community model to assess structuring of the human lung microbiome. MBio. 2015:6. doi: 10.1128/mBio.02284-14. doi:10.1128/mBio.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol. 2014;52:3605–13. doi: 10.1128/JCM.01028-14. doi:10.1128/JCM.01028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98. doi: 10.1016/S1473-3099(13)70318-9. doi:10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 32.Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7:245–57. doi: 10.1586/ers.13.24. doi:10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judge K, Harris SR, Reuter S, Parkhill J, Peacock SJ. Early insights into the potential of the Oxford Nanopore MinION for the detection of antimicrobial resistance genes. J Antimicrob Chemother. 2015;70:2775–8. doi: 10.1093/jac/dkv206. doi:10.1093/jac/dkv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiergeist A, Gläsner J, Reischl U, Gessner A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015;56:228–40. doi: 10.1093/ilar/ilv017. doi:10.1093/ilar/ilv017. [DOI] [PubMed] [Google Scholar]
- 35.Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–93. doi: 10.1111/1469-0691.12023. doi:10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 36.Quick J, Loman NJ, Duraffour S, Simpson JT, Severi E, Cowley L, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530:228–32. doi: 10.1038/nature16996. doi:10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitsios GD, Morris A, McVerry BJ. Antibiotic de-escalation: observational causal inference and culture dependence. Intensive Care Med. 2016 doi: 10.1007/s00134-016-4443-z. doi:10.1007/s00134-016-4443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price R, MacLennan G, Glen J. SuDDICU Collaboration. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. doi: 10.1136/bmj.g2197. doi:10.1136/bmj.g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2015;19:262. doi: 10.1186/s13054-016-1434-y. doi:10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. doi:10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 41. [September 8, 2016];ECOSPOR Clinical Trial. 2016 http://www.serestherapeutics.com/clinical-trials/ecospor-trial.
- 42.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. The Lancet. 2016;387:649–60. doi: 10.1016/S0140-6736(15)01027-2. doi:10.1016/S0140-6736(15)01027-2. [DOI] [PubMed] [Google Scholar]
- 43.Waldor MK, Tyson G, Borenstein E, Ochman H, Moeller A, Finlay BB, et al. Where next for microbiome research? PLoS Biol. 2015;13:e1002050. doi: 10.1371/journal.pbio.1002050. doi:10.1371/journal.pbio.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–9. doi: 10.1038/nature12503. doi:10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. doi:10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 46.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214–23. doi: 10.1016/j.molmed.2013.08.004. doi:10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10:350–6. doi: 10.1016/j.surge.2012.03.003. doi:10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morowitz MJ, Babrowski T, Carlisle EM, Olivas A, Romanowski KS, Seal JB, et al. The human microbiome and surgical disease. Ann Surg. 2011;253:1094–101. doi: 10.1097/SLA.0b013e31821175d7. doi:10.1097/SLA.0b013e31821175d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–41. doi: 10.1038/nature13823. doi:10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18:799–806. doi: 10.1038/nm.2729. doi:10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–30. doi: 10.1038/nm.3542. doi:10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu LC, Shih YA, Wu LL, Lin YD, Kuo WT, Peng WH, et al. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G824–35. doi: 10.1152/ajpgi.00070.2014. doi:10.1152/ajpgi.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickson RP, Singer BH, Newstead MH, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the Lung Microbiome with Gut Bacteria in Sepsis and the Acute Respiratory Distress Syndrome. Nature Microbiology. 2016 doi: 10.1038/nmicrobiol.2016.113. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsutsumi R, Horikawa YT, Kume K, Tanaka K, Kasai A, Kadota T, et al. Whey Peptide-Based Formulas With ω-3 Fatty Acids Are Protective in Lipopolysaccharide-Mediated Sepsis. JPEN J Parenter Enteral Nutr. 2015;39:552–61. doi: 10.1177/0148607114520993. doi:10.1177/0148607114520993. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, DeCoffe D, Brown K, Rajendiran E, Estaki M, Dai C, et al. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE. 2013;8:e55468. doi: 10.1371/journal.pone.0055468. doi:10.1371/journal.pone.0055468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myles IA, Pincus NB, Fontecilla NM, Datta SK. Effects of parental omega-3 fatty acid intake on offspring microbiome and immunity. PLoS ONE. 2014;9:e87181. doi: 10.1371/journal.pone.0087181. doi:10.1371/journal.pone.0087181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iapichino G, Callegari ML, Marzorati S, Cigada M, Corbella D, Ferrari S, et al. Impact of antibiotics on the gut microbiota of critically ill patients. J Med Microbiol. 2008;57:1007–14. doi: 10.1099/jmm.0.47387-0. doi:10.1099/jmm.0.47387-0. [DOI] [PubMed] [Google Scholar]
- 58.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–41. doi: 10.1172/JCI43918. doi:10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–14. doi: 10.1093/cid/cis580. doi:10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, et al. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS ONE. 2015;10:e0129996. doi: 10.1371/journal.pone.0129996. doi:10.1371/journal.pone.0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, et al. Extreme Dysbiosis of the Microbiome in Critical Illness. 2016:1. doi: 10.1128/mSphere.00199-16. doi:10.1128/mSphere.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, et al. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Dig Dis Sci. 2016;61:1628–34. doi: 10.1007/s10620-015-4011-3. doi:10.1007/s10620-015-4011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS ONE. 2013;8:e56131. doi: 10.1371/journal.pone.0056131. doi:10.1371/journal.pone.0056131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart CJ, Marrs EC, Magorrian S, Nelson A, Lanyon C, Perry JD, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012;101:1121–7. doi: 10.1111/j.1651-2227.2012.02801.x. doi:10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 65.Shaw AG, Sim K, Randell P, Cox MJ, McClure ZE, Li MS, et al. Late-Onset Bloodstream Infection and Perturbed Maturation of the Gastrointestinal Microbiota in Premature Infants. PLoS ONE. 2015;10:e0132923. doi: 10.1371/journal.pone.0132923. doi:10.1371/journal.pone.0132923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drell T, Lutsar I, Stšepetova J, Parm U, Metsvaht T, Ilmoja ML, et al. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes. 2014;5:304–12. doi: 10.4161/gmic.28849. doi:10.4161/gmic.28849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165:23–9. doi: 10.1016/j.jpeds.2014.01.010. doi:10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madan JC, Salari RC, Saxena D, Davidson L, O'Toole GA, Moore JH, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F456–62. doi: 10.1136/fetalneonatal-2011-301373. doi:10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE. 2013;8:e52876. doi: 10.1371/journal.pone.0052876. doi:10.1371/journal.pone.0052876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156:20–5. doi: 10.1016/j.jpeds.2009.06.063. doi:10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart CJ, Nelson A, Scribbins D, Marrs EC, Lanyon C, Perry JD, et al. Bacterial and fungal viability in the preterm gut: NEC and sepsis. Arch Dis Child Fetal Neonatal Ed. 2013;98:F298–303. doi: 10.1136/archdischild-2012-302119. doi:10.1136/archdischild-2012-302119. [DOI] [PubMed] [Google Scholar]
- 72.Stewart CJ, Marrs EC, Nelson A, Lanyon C, Perry JD, Embleton ND, et al. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS ONE. 2013;8:e73465. doi: 10.1371/journal.pone.0073465. doi:10.1371/journal.pone.0073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li MS, et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis. 2015;60:389–97. doi: 10.1093/cid/ciu822. doi:10.1093/cid/ciu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1047–55. doi: 10.1152/ajplung.00279.2015. doi:10.1152/ajplung.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poroyko V, Meng F, Meliton A, Afonyushkin T, Ulanov A, Semenyuk E, et al. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L76–83. doi: 10.1152/ajplung.00061.2014. doi:10.1152/ajplung.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly BJ, Imai I, Bittinger K, Laughlin A, Fuchs BD, Bushman FD, et al. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:7. doi: 10.1186/s40168-016-0151-8. doi:10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huebinger RM, Liu MM, Dowd SE, Rivera-Chavez FA, Boynton J, Carey C, et al. Examination with next-generation sequencing technology of the bacterial microbiota in bronchoalveolar lavage samples after traumatic injury. Surg Infect (Larchmt) 2013;14:275–82. doi: 10.1089/sur.2012.095. doi:10.1089/sur.2012.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahrani-Mougeot FK, Paster BJ, Coleman S, Barbuto S, Brennan MT, Noll J, et al. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007;45:1588–93. doi: 10.1128/JCM.01963-06. doi:10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bousbia S, Papazian L, Saux P, Forel JM, Auffray JP, Martin C, et al. Repertoire of intensive care unit pneumonia microbiota. PLoS ONE. 2012;7:e32486. doi: 10.1371/journal.pone.0032486. doi:10.1371/journal.pone.0032486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu W, Yu J, Ai Q, Liu D, Song C, Li L. Increased constituent ratios of Klebsiella sp., Acinetobacter sp., and Streptococcus sp. and a decrease in microflora diversity may be indicators of ventilator-associated pneumonia: a prospective study in the respiratory tracts of neonates. PLoS ONE. 2014;9:e87504. doi: 10.1371/journal.pone.0087504. doi:10.1371/journal.pone.0087504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ravel J, Wommack KE. All hail reproducibility in microbiome research. Microbiome. 2014;2:8. doi: 10.1186/2049-2618-2-8. doi:10.1186/2049-2618-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NCI-NHGRI Working Group on Replication in Association Studies. Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, et al. Replicating genotypephenotype associations. Nature. 2007;447:655–60. doi: 10.1038/447655a. doi:10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 83.Alivisatos AP, Blaser MJ, Brodie EL, Chun M, Dangl JL, Donohue TJ, et al. MICROBIOME. A unified initiative to harness Earth's microbiomes. Science. 2015;350:507–8. doi: 10.1126/science.aac8480. doi:10.1126/science.aac8480. [DOI] [PubMed] [Google Scholar]
- 84.Dubilier N, McFall-Ngai M, Zhao L. Microbiology: Create a global microbiome effort. Nature. 2015;526:631–4. doi: 10.1038/526631a. doi:10.1038/526631a. [DOI] [PubMed] [Google Scholar]
- 85.Knight R. Why microbiome treatments could pay off soon. Nature. 2015;518:S5. doi: 10.1038/518S5a. doi:10.1038/518S5a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.