Abstract
The generation of functional, vascularized tissues is a key challenge for the field of tissue engineering. Before clinical implantations of such tissue engineered bone constructs can succeed, tactics to promote neovascularization need to be strengthened. We have previously demonstrated that the tubular perfusion system (TPS) bioreactor is an effective culturing method to augment osteogenic differentiation and maintain viability of human mesenchymal stem cells (hMSC). Here, we devised a strategy to address the need for a functional microvasculature by designing an in vitro coculture system that simultaneously cultures osteogenic differentiating hMSCs with endothelial cells (ECs). We utilized the TPS bioreactor as a dynamic coculture environment, which we hypothesize will encourage prevascularization of endothelial cells and early formation of bone tissue and could aid in anastomosis of the graft with the host vasculature after patient implantation. To evaluate the effect of different natural scaffolds for this coculture system, the cells were encapsulated in alginate and/or collagen hydrogel scaffolds. We discovered the necessity of cell-to-cell proximity between the two cell types as well as preference for the natural cell binding capabilities of hydrogels like collagen. We discovered increased osteogenic and angiogenic potential as seen by amplified gene and protein expression of ALP, BMP-2, VEGF, and PECAM. The TPS bioreactor further augmented these expressions, indicating a synergistic effect between coculture and applied shear stress. The development of this dynamic coculture platform for the prevascularization of engineered bone, emphasizing the importance of the construct microenvironments and will advance the clinical use of tissue engineered constructs.
Keywords: mesenchymal stem cells, endothelial cells, hydrogel scaffold, TPS bioreactor, coculture, bone regeneration
Introduction
A major challenge of developing a large bone tissue engineering construct is delivery of nutrients and oxygen to the core once it has been implanted in the patient’s defect site. In vivo, cells are supplied with vital molecules via nearby blood vessels that carry nutrients and waste to and from the cells. However, without a pre-established vascular network developed in vitro prior to implantation, the diffusion of important nutrients is limited to 100–200 µm from the host vasculature, unable to maintain viability of the majority of the construct1,2. Therefore, in order to establish a viable system for in vivo implantation, the complex interaction between the two main cell types for bone tissue engineering applications, human mesenchymal stem cells (hMSCs) and endothelial cells, need to be better understood. This knowledge will allow for the creation of a prevascularized engineered construct prior to insertion into a patient.
The use of in vitro cocultures has been one of the most explored options for this application3–6. A range of coculture methods investigate the interactions between the endothelial cells and osteogenic differentiated mesenchymal stem cells or osteoblast like cells as they are inherently linked during the osteogenic and angiogenic process7–9. The formation of micro-vasculature in some coculture conditions has been demonstrated10–13, however, few have been applied for in high-volume tissue engineering constructs, which require an extensive prevascularized network to remain viable in vivo14.
Here we investigate the important role of coculture parameters that influence the osteogenic and angiogenic potential of hMSCs and endothelial cells, respectively. First, we examine the role of scaffold types on cocultures, specifically, the native content of cell-binding sites that exist in natural polymers like collagen but are less abundant in alginate. The sodium alginate polymeric backbone presents no intrinsic cell-binding domains, but can be used to regulate gel mechanical properties. On the other hand, natural collagen fibrils present specific peptide sequences that can be recognized by cell surface receptors, therefore allowing for cell adhesion and spreading that better recreates many in vivo contexts15,16. The second parameter tested was the influence of shear stress on the coculture system. Dynamic culture conditions can be created in a perfusion bioreactor that mimics in vivo conditions. We have previously demonstrated the benefits of the tubular perfusion system (TPS) bioreactor on osteogenic differentiation of hMSCs due to the applied shear flow and the increased oxygen and nutrient supply to the cultured cells17–21. Although these systems have been utilized to create bone tissue constructs of large sizes14,22–24, the leap from in vitro bench top applications to clinical reality will require the presence of ECs within the construct to allow for the establishment of preexisting vasculature.
To this end, the objective of this study is to determine the importance of specific environmental parameters in HUVEC and hMSCs coculture: 1) scaffold types, 2) cell coculture proximity, and 3) effects of shear stress on hMSC osteogenesis and HUVEC angiogenesis. We hypothesize that MSCs and ECs will prefer scaffolds with natural binding sites, which allow them to spread and interact with one another and that they will flourish in closer culture proximity regardless of the scaffold type, through the secretion of paracrine signals as they would in vivo. Additionally, we predict the enhancement of both angiogenic and osteogenic processes when cultured in the TPS bioreactor, which is able to supply shear stresses similar to those in the bone formation process.
Materials and Methods
Cell culture
Bone marrow derived hMSCs were purchased from RoosterBio, Inc. and cultured in the accompanying high performance media kit from RoosterBio. hMSCs were passaged and given fresh media on Day 5. They were harvested at passage 3 for encapsulation. HUVECs were purchased from Lonza and cultured in endothelial cell growth media (Lonza). HUVECs were given fresh media every 2–3 days and passaged on Day 5. HUVECs were harvested at passage 3 for encapsulation. All cells were cultured at 37°C with 5% CO2.
Alginate bead scaffold fabrication and hMSC encapsulation
Alginate beads were fabricated using 2% alginate solution (Sigma), homogeneously mixed with hMSCs (100,000 hMSCs/bead), and crosslinked in 100mM CaCl2 by drop wise addition of the solution through an 18G needle and syringe. The beads were stirred for 10 minutes to allow for complete crosslinking of the alginate and placed in hMSC growth media until use.
Thin hydrogel fabrication for cell adhesion
2% alginate (Sigma) and 4mg/mL collagen (Corning) hydrogels were fabricated to test hMSC and HUVEC adhesion to the scaffolds. Cylindrical alginate gels were created using molds (20mm in diameter, 2mm in height) and diffused with 100mM CaCl2 for crosslinking and then transferred to 12 well plates. Collagen hydrogels were made directly in the 12-well wells and crosslinked at 37°C for 30 minutes, resulting in cylindrical gels of 22mm in diameter, 2mm in height. Both hydrogels were stored in PBS until cell seeding.
Cell adhesion assay
hMSCs and HUVECs were lifted using trypsin/0.25% EDTA (Life Technologies) and counted. They were seeded onto the hydrogels in a concentrated solution of 100,000 cells/100uL for 1 hour at 37°C. 3 mL of additional media was added after 1 hour and cells were allowed to adhere for 3 more hours. Cells were seeded on TCPS as a positive control for cell attachment using the same amount of cell density and cell-to-media ratio. After 4 total hours of incubation, hydrogels were gently washed 3 times with PBS. To visualize and quantify cell adhesion, a live-dead assay (Invitrogen) was performed following standard protocol. Gels were imaged using a fluorescent microscope (Axiovert 40 CFL with filter set 23; Zeiss) equipped with a digital camera (Diagnostic Instruments 11.2 Color Mosaic). Adhesion was also quantified using a plate reader after 30 min incubation in the live-dead assay solution. Labeling of Calcein-AM by live cells was read at an excitation of 494 nm and emission of 517 nm. Blank hydrogels with no cells were incubated in live-dead solution as a negative control, while confluent cells seeded in a 12-well were used as positive control. Background fluorescence from the gels was subtracted for all hydrogel samples.
Collagen hydrogel formation/encapsulation
All cells were encapsulated in 4mg/mL rat tail derived type I collagen (Corning) and prepared following manufacturer’s instructions. The desired collagen concentration was kept on ice while cells were harvested and centrifuged at 500g for 5 minutes. Collagen was added to the cell pellets and mixed and kept on ice. Small gels were created by pipetting 3.33µL of the collagen/cell mixture onto UV-sterilized parafilm and crosslinking for 7 minutes at 37°C. The beads were washed off the parafilm and collected. We created 6 experimental groups: hMSCs in collagen in static conditions, hMSCs in collagen in dynamic condition, HUVECs in collagen in static conditions, HUVECs in collagen in dynamic conditions, a coculture of hMSCs and HUVECs in collagen in static conditions, and a coculture of hMSCs and HUVECs in collagen in dynamic conditions. All hydrogels contained a total of 100,000 cells/scaffold, utilizing a 1:1 ratio in the cocultured experimental groups.
Static & dynamic culture
Scaffolds in the static groups were cultured in 6 well plates, allowing 20 scaffolds in 5 mL of media per well. Dynamic groups were placed in a TPS bioreactor as previously described, resulting in the same ratio of scaffolds to media per growth chamber17. Each group was loaded into a ¼” by 5” growth chamber and connected to the tubing circuit and media reservoir. The flow was driven by an L/S Multichannel Pump System (Cole Parmer) at a flow rate of 3 mL/min and media was changed every 3 days. All groups were cultured at 37°C with 5% CO2. All coculture groups were cultured in mixture of 1:1 ratio of osteogenic media to endothelial cell growth media. Osteogenic media was prepared by supplementing growth media containing High Glucose Dulbecco’s modified Eagle’s medium with l-Glutamine (Gibco), supplemented with 10% fetal bovine serum (Invitrogen), 1% v/v penicillin/streptomycin (Gibco), and 0.1 mM nonessential amino acids (Invitrogen) with 100 nM dexamethasone (Sigma), 10 mM b-glycerophosphate, and 173 mM ascorbic acid (Sigma).
RNA extraction
Cell samples from each group were isolated from collagen gels by dissolution in 1mg/mL collagenase (Sigma) for 60 min at 37°C and a cell pellet was formed by centrifugation and washed with PBS three times. The RNeasy Plus Mini Kit (Qiagen) was used to isolate total RNA following standard protocols. Total RNA was quantified using a Nanodrop Spectrometer (Thermo Scientific).
Real-time quantitative polymerase chain reaction (RT-qPCR)
Isolated RNA was then reverse transcribed to cDNA using a High Capacity cDNA Archive Kit (Life Technologies). RT-qPCR was performed by combining the cDNA solution with a Universal Master Mix (Life Technologies), along with oligonucleotide primers and Taqman probes for ALP, BMP-2, and OCN (hMSCs and coculture samples) and VEGF and PECAM (HUVEC and coculture samples), and compared to the endogenous gene control glyceraldehyde 3 phosphate dehydrogenase (GAPDH; Life Technologies). The reaction was performed using a 7900HT real-time PCR System (Applied Biosystems) at thermal conditions of 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. The relative gene expression level of each target gene was then normalized to the mean of the GAPDH in each group. Samples were completed in technical triplicates and standard deviations are reported (n = 3).
Immunohistochemistry
At each timepoint, collagen gels were fixed in 4% paraformaldehyde for 2 hours at RT, dehydrated and embedded, and sectioned into 10µm slices. Antigens were retrieved by exposure to steam composed of Tris base and EDTA buffer (pH = 8) containing TWEEN 20 for 15 minutes. Samples were blocked and then stained with the primary antibodies to detect BMP-2 and ALP (Abcam, Cambridge, MA) in hMSCs, PECAM and VEGF in HUVECs, and BMP-2 and PECAM in the coculture. FITC and Cy-5 tagged secondary antibodies were used to visualize the protein, while DAPI counterstained the nucleus. Samples were imaged using a LSM700 confocal microscope (Zeiss).
Cell Viability Assay
Cell viability was assessed using a fluorescent Live/Dead assay (Invitrogen) following standard protocols. Gels were placed in 1.5mL microcentrifuge tubes (Fisher Scientific) and incubated in 1 mM ethidium homodimer and 2 mM calcein AM (Molecular Probes) for 30 min. Fluorescent images were then taken of the entire scaffold using a fluorescence microscope (Axiovert 40 CFL; Zeiss) equipped with a digital camera (11.2 Color Mosaic; Diagnostic Instruments).
Statistical Analysis
Each analysis was performed in triplicate (n=3). Statistical significance was determined by one-way analysis of variance and Tukey’s multiple-comparison test. A confidence interval of 95% (α = 0.05) was used for all analyses. Mean values of triplicates and standard deviation error bars are reported on each figure as well as relevant statistical relationships.
Results
Coculture of hMSCs in alginate and HUVECs in collagen scaffolds
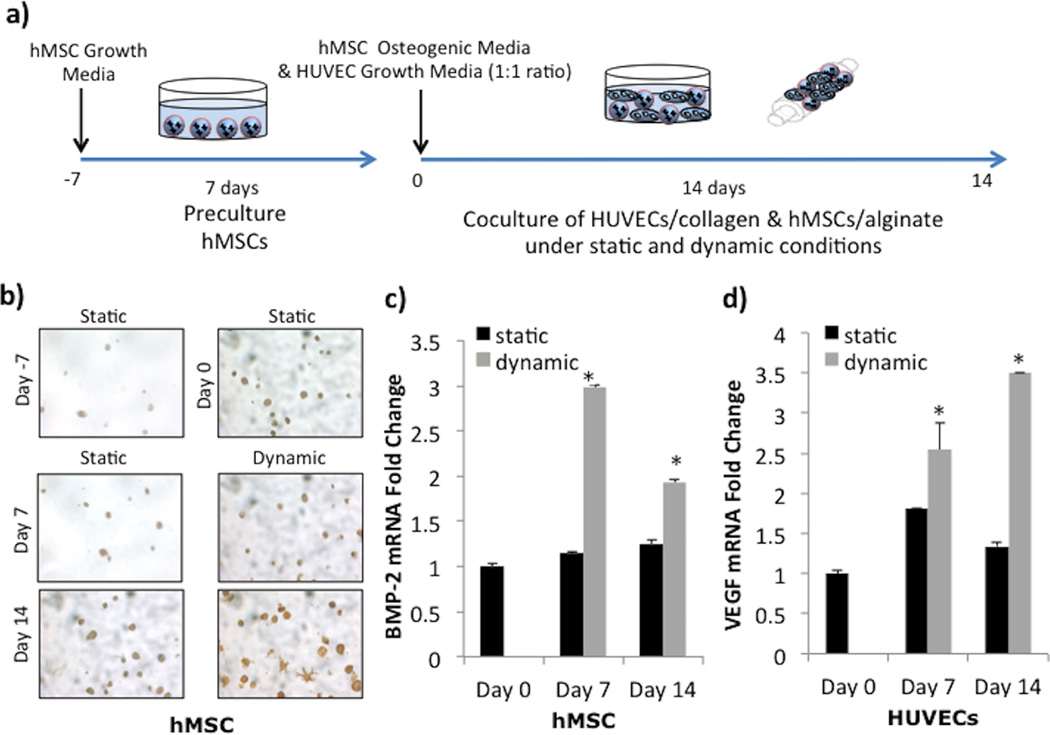
We first examined the effect of culturing distance between hMSCs and HUVECs by culturing them separately in alginate and collagen, respectively, under either static or dynamic conditions (Figure 1a). To allow for cells to acclimate to the 3-dimensional scaffold environment after encapsulation, we cultured the scaffolds under static conditions for 7 days prior to dividing the scaffolds into either static or dynamic culture. BMP-2 immunostaining of hMSCs showed increased presence of protein (brown) after 14 days of coculture, especially in the dynamically cultured group (Figure 1b). The morphological change seen by day 14 in dynamic conditions indicated cell spreading of the hMSCs. Gene expression of mRNA also showed enhanced expression in both cells cultured in the TPS bioreactor. BMP-2 gene expression in dynamically cultured hMSCs increased 3-fold after 7 days and fell to 2-fold after 14 days, compared to day 0 values (Figure 1c, gray). In contrast, static BMP-2 values remained fairly constant over the 14 day period (Figure 1b, black). The impact of dynamic culture was also visible in HUVEC gene expression of VEGF, which linearly increased over 14 days to 3.5-fold change compared to day 0 values (Figure 1d, gray). Statically cultured cells reached only a 1.8-fold increase on day 7 before decreasing back down to basal levels (Figure 1d, black).
Figure 1.
Coculture in Alginate and Collagen Scaffolds. A) Experimental setup depicts a 7 day static preculture of hMSCs encapsulated in alginate scaffolds (labeled Day -7), followed by a 14 day dynamic or static coculture with HUVECs encapsulated in collagen scaffolds (Day 0 - Day 14). B) BMP-2 immunostaining demonstrates increase in BMP-2 production (brown) in hMSCs (dark blue nucleus). C) BMP-2 mRNA expressions in hMSCs significantly increases over 14 days in dynamic culture but stays constant static culture. D) VEGF mRNA expressions in HUVECs significantly increases over 14 days in dynamic culture and shows an increase on day 7 in static before decreasing back close to basal levels. The symbol ‘*’ indicates statistical significance within groups at a timepoint (p<0.05).
Cell adhesion on collagen and alginate
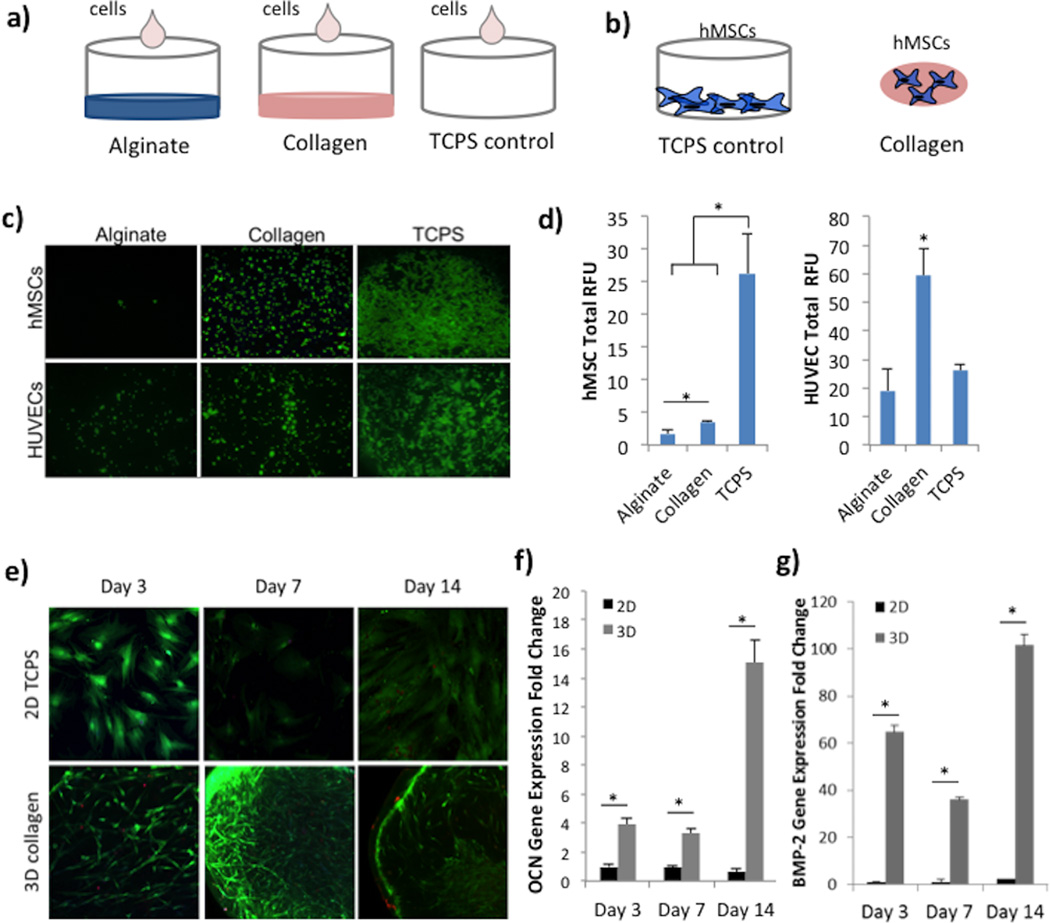
Next, we investigated the importance of binding sites in natural polymers like alginate and collagen on hMSC and HUVEC adhesion and spreading (Figure 2a). The attachment of spreading of both cell types in vitro indicates native behavior observed in vivo. When seeded on top of flat alginate hydrogels discs, hMSCs remained rounded in morphology and very few attached after the 4 hour incubation (Figure 2c, top row). HUVECs showed slightly higher attachment ability in comparison, however, little spreading was observed during this period (Figure 2c, bottom row). On the other hand, greater number of cells remained attached on collagen hydrogels, for both hMSCs and HUVECs, with a distinctly greater number of elongated HUVECs. Cells seeded on TCPS as positive control adhered on the well surface, with clear spreading and elongated morphology visible. It is important to note that HUVECs, while fully spreading out, most adhered around the edge of the well, due to the small and concentrated media volume at seeding. These cell adhesion results were conferred using a microplate reader to determine total fluorescence (in RFU) (Figure 2d). Based on the detected fluorescence, significantly greater number of hMSCs and HUVECs adhered to collagen compared to alginate hydrogel surfaces. In the case of HUVEC, greater fluorescence was detected on collagen compared to TCPS, which can be contributed to the bare well center and a confluent cell ring at the border.
Figure 2.
Cell Adhesion and Osteogenic Differentiation in Collagen Scaffolds. A) Experimental setup for cell adhesion study on alginate and collagen substrates, and TCPS as positive control. B) Experimental setup to investigate the effect of collagen encapsulation on osteogenic differentiation compared to TCPS as a control. C) Fluorescence images of hMSCs or HUVECs seeded on alginate, collagen, or TCPS substrates, taken at 2.5× magnification. D) Quantification of fluorescence signal read via a spectrophotometer at excitation of 494nm and emission of 517nm for both hMSCs and HUVECs. Units are listed as RFU (relative fluorescence units). E) Fluorescence images of hMSCs labeled with live (green) and dead (red) stain on TCPS or encapsulated in 3D collagen scaffolds. F) Gene expression of osteocalcin (OCN) mRNA in hMSCs over 14 days. Production was statistically greater in collagen scaffolds compared to the TCPS control. G) Gene expression of BMP-2 mRNA in hMSCs shows significantly increased expression in 3D collagen compared to TCPS. The symbol ‘*’ indicates statistical significance within groups at a timepoint (p<0.05).
Osteogenic differentiation of hMSCs in collagen scaffolds
In order to validate the analogous behavior of hMSCs in collagen as previously seen in alginate, we assessed their ability to successfully differentiate into osteoblasts while encapsulated in collagen scaffolds through morphological changes and gene expression of late differentiation marker osteocalcin (OCN) and general osteoblast marker bone morphogenic protein-2 (BMP-2). Results were compared to hMSCs seeded in 2D on TCPS. As visible in the fluorescence live-dead staining, hMSCs greatly elongated when encapsulated in collagen compared to 2D TCPS, resulting in greater cell length but smaller cell width (Figure 2e). Additionally, hMSCs expressed significantly greater amounts of OCN and BMP-2 at every timepoint over the 14 day differentiation study (Figure 2f–g).
Effects of dynamic culture on hMSC and HUVEC coculture in collagen scaffolds
Based on literature and our own previously published work, we were able to conclude that collagen scaffolds would allow for differentiation of hMSCs as well as attachment and spreading of HUVECs as a prevascular network. To investigate the simultaneous behavior of both cell types, HUVECs and hMSCs were encapsulated together in collagen scaffolds and cultured for 14 days in static or dynamic conditions to determine effects on osteogenic and angiogenic potential of the cells (Figure 3a). Fluorescence microscopy of the cell-encapsulated hydrogel indicated high viability of all three groups (hMSCs, HUVECs, and hMSCs+HUVECs), with great elongation of hMSCs visible while HUVECs remained more rounded in morphology (Figure 3b). Interestingly, cells formed aggregates when cocultured in collagen, more visibly in dynamic culture compared to static coculture (Figure 3, white arrows).
Figure 3.
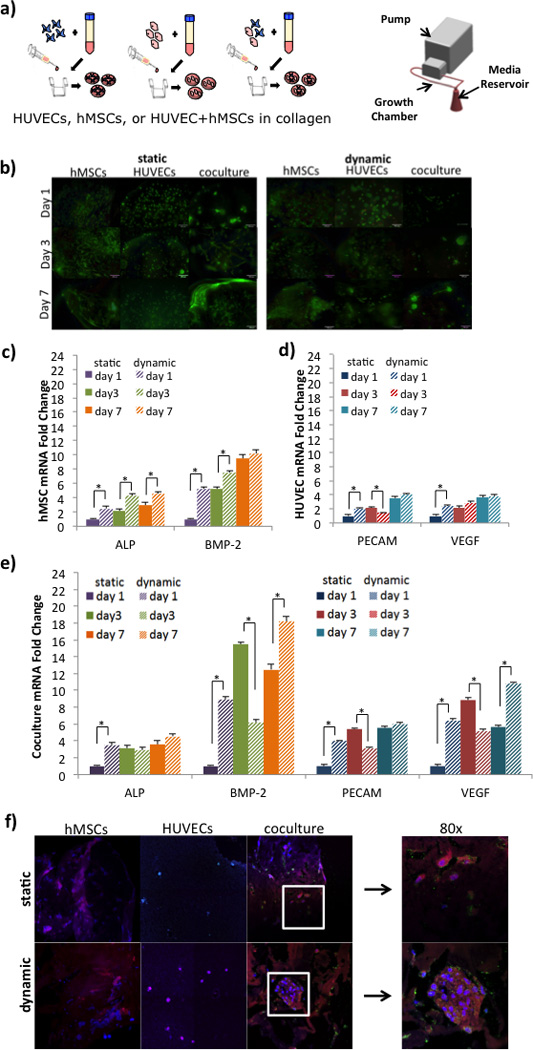
Effect of Dynamic Culture on hMSCs and HUVEC coculture. A) Experimental setup of cell encapsulation groups: hMSCs in collagen, HUVECs in collagen, or hMSCs and HUVECs in collagen. Cell-seeded scaffolds were cultured in static well plates or in the TPS bioreactor under dynamic flow conditions. B) E) Fluorescence viability images of hMSCs labeled with live (green) and dead (red) stain on 3D collagen scaffolds after 1, 3, or 7 days of static or dynamic culture. Scale bar represents 100 µm. C) Gene expression of ALP and BMP-2 in hMSCs monocultured in static (solid bars) or dynamic (striped bars). Overall, dynamic coculture resulted in the highest expression of ALP and BMP-2 expression by day 7. D) Gene expression of PECAM and VEGF in HUVEC monocultured in static (solid bars) or dynamic (striped bars). Overall, dynamic coculture resulted in the highest expression of PECAM and VEGF expression by day 7. E) Gene expression of ALP, BMP-2, PECAM, and VEGF in hMSC and HUVEC cocultured in static (solid bars) or dynamic (striped bars). Overall, the synergistic effect of coculture and dynamic coculture resulted in the highest expression of all four markers by day 7. The symbol ‘*’ indicates statistical significance within groups at a timepoint (p<0.05). F) Immunofluorescence staining of hMSCs, HUVECs, and coculture in static and dynamic. hMSCs were stained for BMP-2 (pink/red), counterstained with DAPI (blue). HUVECs were stained for CD31 (green), VEGF (red), and counterstained with DAPI (blue). Cocultured samples were stained for BMP-2 (pink/red), CD31 (green), and counterstained with DAPI (blue). Images were taken at 40× with an additional 2× zoom (right panel).
We examined gene expression of common osteogenic markers (ALP and BMP-2) in hMSCs and angiogenic markers (PECAM and VEGF) in HUVECs during mono- and cocultures (Figures 3c–e). As seen in Figure 3c, left, ALP mRNA expression in hMSCs cultured alone in collagen increased moderately over 7 days, resulting in a 2.4-fold increase in static conditions and a 4.5-fold increase in dynamic culture. Expression in dynamic cells was statistically greater on days 1 and 7 compared to static cells (purple and orange striped bars vs. purple and orange solid bars). Similarly BMP-2 mRNA expression in hMSC monoculture significantly increased over 7 days, with dynamically cultured cells leading the way until day 7, when statically cultured cells expressed similar levels, 9.5 and 10.2 fold, respectively. Angiogenic marker expressions in monocultured HUVECs in collagen indicated only a conservative increase over the 7 day culture period (Figure 3d). For example, while PECAM expression increased 3.5-fold and 4.0-fold in static and dynamic conditions respectively on day 7, these values were not statistically significant. Similar trends were observed in VEGF mRNA expression in HUVECs encapsulated in collagen.
More interestingly, coculture of hMSCs and HUVECs in collagen scaffolds increased expression of all four markers over 7 days compared to day 1 static expressions, independent of their static or dynamic culture environment (Figure 3e). For example, ALP expression, while staying constant over the culture period, increased 4-fold on day 1 in dynamic culture and day 3 in static culture compared to the day 1 static group, but was not reached until day 7 in hMSC monoculture. During coculture, BMP-2 mRNA saw a similar sharp increase on day 1 in the dynamic group compared to the static groups (8.9-fold increase), which was surpassed by day 3 in the static group (15.5-fold increase), but lost in the dynamic group (6.2-fold increase). However, it impressively recovered by day 7, reaching an 18.2-fold increase in the dynamic group and 12.4-fold increase in the static group. PECAM and VEGF expressions were also greater in coculture conditions compared to the HUVEC monoculture expressions (Figure 3e, right, compared to Figure 3d). Specifically, dynamic culture on day 1 was consistently significant increased in both angiogenic marker expression compared to static conditions on the same day. On day 3, static conditions resulted in statistically greater expression (5.4-fold) compared to dynamic conditions (3.1-fold). By day 7, PECAM expressions increased 5.5- and 6.0-fold over day 1 values in static and dynamic conditions, respectively, albeit they were not statistically different from one another. VEGF mRNA expressions exhibited a similar trend, with cells producing significantly greater amounts compared to static conditions on day 1 and 7, but static culture dominating the expression on day 3.
These mRNA expression results were confirmed using immunofluorescence staining on day 7 (Figure 3f). hMSCs in monoculture were stained with BMP-2 antibody and showed visible staining throughout the cell by day 7 (red with blue nucleus), in both static and dynamic culture. HUVEC monoculture stained more positive for CD-31 (green) on day 7 in static culture than in dynamic culture, but vice versa with VEGF (red) staining. hMSCs and HUVECs cocultured in collagen scaffolds were stained for BMP-2 (red) and CD31 (green) with DAPI labeled nuclei (blue). There were visibly greater amounts of BMP-2 compared to monocultured hMSCs, with the greatest amounts seen in cells cultured in the TPS bioreactor. An 80× zoom of the area (highlighted in white box), displays diffused BMP-2 (red) throughout the cellular space, with more spotty detection of CD31 around the membrane of the cells.
Discussion
In this study, we investigate the role of coculture parameters such as scaffold type and cell-to-cell distance between mesenchymal stem cells and endothelial cells for the application in vascularized bone tissue engineering. Alginate and collagen are natural polymers that have been shown to be biodegradable, non-toxic, and maintain viability of encapsulated cells. Although alginate has many favorable properties for tissue engineering applications, it lacks specific interaction with cells. Binding sites like Arg-Gly-Asp (RGD) sequences are preserved in collagen fibrils, allowing cells to interact and adhere to the hydrogel, which are inherently lacking in alginate. For these reasons, we investigate collagen hydrogel scaffolds as the main coculture cell carrier in our system.
Using alginate hydrogel scaffolds, we have shown successful differentiation of hMSCs into osteoblasts, in both static and dynamic culture conditions. However, in order to progress towards a prevascularized bone construct, we cocultured the two main cell types involved in this process: osteogenic differentiating hMSCs and HUVECs. Type I collagen being the most abundant extracellular protein surrounding endothelial cells, we encapsulated HUVECs in collagen hydrogels. Together with alginate-encapsulated hMSCs, they were cultured in either static or dynamic conditions. The objective behind this coculture system was to provide an environment where cytokines and growth factors could be exchanged between HUVECs and hMSCs, similar to in vivo environments, where cells may be in proximity but not in cell-to-cell contact. After 14 days of coculture, both cell types showed little response towards osteogenesis or angiogenesis when cultured in static conditions. However, dynamic coculture resulted in enhanced BMP-2 gene production in hMSCs and increased VEGF gene production in HUVECs, which indicated progression towards osteogenesis and prevascularization, respectively. More notably was the morphological change of the hMSCs after 14 days of coculture. Cells started to extend outward, which may exhibit behavior of pericytes that support endothelial cells during angiogenesis. The role of MSCs as pericytes has been widely discussed in the field of tissue engineering, with evidence showing that MSCs can transform to take on the role of support cells when cultured in proximity of endothelial cells25,26. This idea would indicate a cytokine or growth factor communication between the cocultured cells, to which the hMSCs can respond and migrate to.
However, the separate and different material scaffold setup (HUVEC/collagen and hMSC/alginate) resulted in little morphological changes in HUVECs towards forming prevascular networks. Therefore, to investigate the effect of close proximity coculture, we encapsulated the cells in the same natural polymer hydrogel scaffold. To determine the optimal scaffold, we tested the cells’ ability to attach, bind, and spread on the two polymer hydrogel surfaces. The natural higher content of RGD-like binding sites on collagen allowed hMSCs and HUVECs to more readily bind to the surface. Both cell types not only adhered but showed early signs of spreading. Although the cells were more confluent and spread on TCPS, with additional time, we believe the cells on collagen could reach similar confluency and adhesion. The greater RFUs detected in hMSCs seeded on collagen than in TCPS can be explained by the seeding and attachment of hMSCs on the outer ring of the TCPS well, rather than forming a homogenous monolayer on the entire surface. This adhesion pattern resulted in lower RFUs detected during the microplate spectrophotometry. With the validation of successful cell attachment and spreading, we moved forward with collagen as our coculture carrier.
We have demonstrated successful hMSC differentiation potential in alginate scaffolds, and wanted to confirm similar outcomes in collagen scaffolds. We attribute significant increases of OCN and BMP-2 expression in hMSCs in collagen compared to 2D TCPS not only do the 3D environment, which mimics the cells’ natural environment, but believe the additional biological cues received from collagen are able to enhance the osteogenic differentiation process.
Next, we proceeded to evaluate the effects of coculture on hMSC osteogenic differentiation and HUVEC angiogenic vascular network formation. Cells were either mono- or cocultured in static or dynamic conditions. Under static conditions, cells were placed into 6 well plates and provided similar amounts of media as in the TPS bioreactor for dynamic culture, ensuring similar cytokine and endogenous growth factor concentrations. Fluorescence microscopy images showed elongation and spreading of hMSC morphology, which was not visible in the HUVEC population (Figure 2b). As others have noted, depending on the encapsulated cell type, hydrogels like collagen will greatly contract and shrink as cells inherently pull on the scaffold during adhesion and spreading. This was evidently in the hMSC and coculture-encapsulating collagen gels, which were half the size as the HUVEC collagen scaffolds by day 3. Interestingly, the aggregates of cells seen in the coculture group (Figure 3f, white boxes) may indicate preferences for the cells to remain in clusters in order to remodel their surrounding extracellular matrix, especially during dynamic culture.
Gene expression results of the study exhibited strong benefits of coculture in dynamic environments for the purpose of osteogenic differentiation in hMSCs and HUVEC prevascularization compared to static and/or monocultures environments. However, it is interesting to remark on the enhanced expression of BMP-2, PECAM, and VEGF mRNA in static coculture on day 3 compared to dynamic coculture on the same day. This trend is not seen in monoculture and is reversed in most genes by day 7. Based on these gene expression trends, we suggest that it may be that hMSCs and HUVECs are able to benefit from static coculture during the first three days as they are acclimating to their environment and release paracrine signals that are vital during the early stages of coculture. This setup could be leveraged to design future studies that may include a static pre-coculture prior a dynamic coculture. The cells would experience a stationary media environment to allow for deposition of matrix and signal exchange, followed by TPS bioreactor culture, where the cells are exposed to dynamic shear stress, which is evident in promoting both osteogenesis and neovascular promotion in later timepoints.
Protein detection using immunofluorescence on day 7 samples displayed greater amounts of staining in samples in all dynamically cultured groups compared to static groups, as was confirmed using gene expression. Additionally, the clustering of cells in the coculture group during dynamic culture was visually significant because this behavior was not seen in static coculture samples. The presence of increased production of proteins, gene expression, and clustering of cells validates the benefits of combinatorial coculture with dynamic shear stress in collagen hydrogel scaffolds.
Conclusion
This series of experiments demonstrated that coculture parameters for hMSCs and HUVECs are extremely important in providing the appropriate environment to push the cells towards osteogenesis and angiogenesis, respectively. Biomaterial properties of collagen, such as its naturally occurring binding sites provide the necessary habitat for the cells to adhere, spread, and perform their distinct functions towards osteogenesis and angiogenesis. While we have shown alginate scaffolds to be beneficial for osteogenic purposes, we conclude that a collagen is ultimately a superior biomaterial scaffold for hMSCs and HUVECs for future simultaneous vascularization of tissue engineered bone constructs. Furthermore, the culture distance between the two cell types has proven to be crucial in enhancing this process. In particular, cell-to-cell contact in the collagen hydrogel scaffold demonstrated the greatest benefit to hMSCs and HUVECs, compared to when they were cocultured in separate scaffolds, such as alginate and collagen. Additionally, while we have previously showed enhancement of osteogenesis in hMSCs under dynamic flow, the TPS bioreactor resulted in further improvements in HUVEC expression of angiogenic markers in both mono- and coculture environments. The interesting trends seen in osteogenic and angiogenic gene expression, mainly the enhanced expression in static conditions on day 3 followed by augmented expression by day 7 in dynamic conditions, support the idea of combining both static and dynamic environments into a coculture regiment for future studies that will further stimulate the prevascularization of engineered bone. With this series of experiments, we have developed and evaluated coculture parameters that, when combined, can provide the fundamental scaffold and cell components for the fabrication of prevascularized bone for future clinical applications.
Acknowledgments
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01 AR061460), as well as by the National Science Foundation (CBET 1264517). Additionally, this work was supported by a National Science Foundation Graduate Fellowship to BNBN.
Conflicts of Interest
J.P.F. is a founder and co-owner of the company 3DBioWorks which focuses on the use of bioreactors for cell proliferation and differentiation.
References
- 1.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng. Part B. Rev. 2009;15(3):353. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiedler T, Belova IV, Murch GE, Poologasundarampillai G, Jones JR, Roether JA, et al. A comparative study of oxygen diffusion in tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2014;25(11):2573. doi: 10.1007/s10856-014-5264-7. [DOI] [PubMed] [Google Scholar]
- 3.Unger RE, Ghanaati S, Orth C, Sartoris A, Barbeck M, Halstenberg S, et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31(27):6959. doi: 10.1016/j.biomaterials.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 4.Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. In: Goncalves R, editor. PLoS One. 12. Vol. 6. 2011. p. e28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma J, van den Beucken JJJP, Yang F, Both SK, Cui F-Z, Pan J, et al. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng. Part C. Methods. 2011;17(3):349. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 6.Dahlin RL, Gershovich JG, Kasper FK, Mikos AG. Flow Perfusion Co-culture of Human Mesenchymal Stem Cells and Endothelial Cells on Biodegradable Polymer Scaffolds. Ann. Biomed. Eng. 2013 doi: 10.1007/s10439-013-0862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melchiorri AJ, Nguyen B-NB, Fisher JP. Mesenchymal stem cells: roles and relationships in vascularization. Tissue Eng. Part B. Rev. 2014;20(3):218. doi: 10.1089/ten.teb.2013.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013;242(8):909. doi: 10.1002/dvdy.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K-I, Park S, Im G-I. Osteogenic differentiation and angiogenesis with cocultured adipose-derived stromal cells and bone marrow stromal cells. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 10.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. U. S. A. 2000;97(16):9191. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29(19):2869. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zigdon-Giladi H, Bick T, Lewinson D, Machtei EE. Co-Transplantation of Endothelial Progenitor Cells and Mesenchymal Stem Cells Promote Neovascularization and Bone Regeneration. Clin. Implant Dent. Relat. Res. 2013;1 doi: 10.1111/cid.12104. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen B-NB, Ko H, Moriarty RA, Etheridge JM, Fisher JP. Dynamic Bioreactor Culture of High Volume Engineered Bone Tissue. Tissue Eng. Part A. 2016;22(3–4):263. doi: 10.1089/ten.tea.2015.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branco da Cunha C, Klumpers DD, Li WA, Koshy ST, Weaver JC, Chaudhuri O, et al. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials. 2014;35(32):8927. doi: 10.1016/j.biomaterials.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeatts AB, Gordon CN, Fisher JP. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng. Part C. Methods. 2011;17(12):1171. doi: 10.1089/ten.tec.2011.0263. [DOI] [PubMed] [Google Scholar]
- 18.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48(2):171. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 19.Yeatts AB, Geibel EM, Fears FF, Fisher JP. Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol. Bioeng. 2012;109(9):2381. doi: 10.1002/bit.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Porta G, Nguyen B-NB, Campardelli R, Reverchon E, Fisher JP. Synergistic effect of sustained release of growth factors and dynamic culture on osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A. 2014 doi: 10.1002/jbm.a.35354. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen B-NB, Ko H, Fisher JP. Tunable osteogenic differentiation of hMPCs in tubular perfusion system bioreactor. Biotechnol. Bioeng. 2016 doi: 10.1002/bit.25929. [DOI] [PubMed] [Google Scholar]
- 22.Gardel LS, Serra LA, Reis RL, Gomes ME. Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng. Part B. Rev. 2014;20(2):126. doi: 10.1089/ten.TEB.2013.0010. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Li M, Liu P, Zhang Y, Lu J, Li J. Tissue-engineered bone constructed in a bioreactor for repairing critical-sized bone defects in sheep. Int. Orthop. 2014 doi: 10.1007/s00264-014-2389-8. [DOI] [PubMed] [Google Scholar]
- 24.Olivier V, Hivart P, Descamps M, Hardouin P. In vitro culture of large bone substitutes in a new bioreactor: importance of the flow direction. Biomed. Mater. 2007;2(3):174. doi: 10.1088/1748-6041/2/3/002. [DOI] [PubMed] [Google Scholar]
- 25.McFadden TM, Duffy GP, Allen AB, Stevens HY, Schwarzmaier SM, Plesnila N, et al. The delayed addition of human MSCs to pre-formed endothelial cell networks results in functional vascularisation of a collagen-GAG scaffold in vivo. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.08.014. null(null) [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of Pericytes Leads to Endothelial Hyperplasia and Abnormal Vascular Morphogenesis. J. Cell Biol. 2001;153(3):543. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]