Abstract
Objective
To determine if a moderate-to-large PDA is responsible for vasopressor-dependent hypotension, occurring at the end of the first postnatal week.
Study design
We performed a retrospective double cohort controlled study of infants delivered at ≤27+6 weeks gestation (n=313). From January 2004 through April 2011, all infants were treated with prophylactic indomethacin (PINDO epoch). From May 2011 through December 2015 no infant was treated with indomethacin until at least 8 postnatal days (Conservative epoch). Echocardiograms were performed on postnatal days 6 or 7. Hypotension was managed by a predefined protocol. The primary outcome was the incidence of dopamine-dependent hypotension, defined as having received at least 6 µg/kg/min dopamine for at least 24 hours during postnatal days 4–7.
Results
As expected, the incidence of moderate-to-large PDA at the end of the first week differed significantly between epochs (PINDO=8%; Conservative=64%). In multivariate analyses, PINDO infants had a significantly lower incidence of vasopressor-dependent hypotension (11%) than Conservative infants (21%)(OR=0.40, 95% CI: 0.20–0.82). PINDO infants also required less Mean Airway Pressure, had a lower Respiratory Severity Score and lower Mode of Ventilation Score than Conservative infants during postnatal days 4–7. The effects of PINDO on both the incidence of vasopressor-dependent hypotension and the need for respiratory support were no longer significant when analyses were adjusted for “presence or absence of a moderate-to-large PDA”.
Conclusion
PINDO decreases vasopressor-dependent hypotension and the need for respiratory support at the end of the first postnatal week. These effects are mediated by closure of the PDA.
Keywords: hypotension, newborn, dopamine, premature birth
Systemic hypotension occurs commonly in extremely preterm infants and is associated with an increased incidence of neonatal mortality and morbidity (intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), neurodevelopmental delay) (1–9). Vasopressors are frequently used to treat this condition despite the absence of clear guidelines to discriminate physiologic from pathologic hypotension (5, 10, 11).
During the immediate postnatal period, the underlying causes of hypotension are multifactorial. They include perinatal asphyxia, hemorrhage/hypovolemia, inflammation/infection, and factors associated with delayed postnatal transition (e.g., myocardial depression, relative adrenal insufficiency, impaired vascular regulation) (12). However, by the end of the first postnatal week, most episodes of hypotension can be attributed to identifiable causes (e.g., bacteremia, necrotizing enterocolitis (NEC), gastrointestinal perforations, surgery, and patent ductus arteriosus (PDA)) or nonspecific causes associated with immaturity and illness (like dysregulated cytokine, vasodilator, and/or cortisol production or release) (13).
Extremely preterm infants frequently develop a moderate-to-large PDA at the end of the first week. When other identifiable causes of hypotension have been ruled out, clinicians often attribute the cause of vasopressor-dependent hypotension to the presence of a PDA (disregarding the possible involvement of any of the nonspecific causes mentioned above). Although it is true that a moderate-to-large PDA can lower systemic blood pressure (14–16) and is associated with the presence of vasopressor-dependent hypotension at the end of the first week (13, 17) no study to date has determined whether the PDA actually is responsible for the vasopressor-dependent hypotension or whether its presence is just a surrogate for nonspecific causes related to immaturity/illness.
Indomethacin, given either prophylactically (within 24 hours of birth) or within the first few days after birth, is effective in achieving ductus closure (18, 19). Although more than 20 RCTs have investigated the effects of early PDA treatment on neonatal morbidities, none have mentioned its effect on the incidence of vasopressor dependent hypotension (18, 19). Therefore we performed the following retrospective double cohort controlled study to examine whether early treatment of the PDA decreases the incidence of vasopressor-dependent hypotension at the end of the first postnatal week.
Prior to May 2011, all infants in our nursery who delivered at ≤27+6 weeks gestation were treated with prophylactic indomethacin (PINDO epoch). After April 30, 2011, prophylactic indomethacin was no longer used and infants were only treated with indomethacin if the PDA persisted beyond 7 days (Conservative epoch; see Methods). In the following study, we hypothesized that infants treated with PINDO would have a lower incidence of vasopressor-dependent hypotension at the end of the first week and that closure of the moderate-to-large PDA would explain this effect.
Methods
This project was approved by the Institutional Review Board of the University of California San Francisco. Infants were included in the study if they were born between January 2004 and December 2015, delivered at ≤27+6 weeks gestation, and were admitted to the intensive care nursery at the University of California San Francisco within 24 hours of birth. Detailed descriptions of our approach to respiratory and hemodynamic support have been previously published (20–22). Two distinct epochs of PDA management existed during this 12-year period. During the first epoch, prior to May 2011, all infants without contraindications (n=284) were treated with a course of prophylactic indomethacin (PINDO) starting within 15 hours of birth. Six potential PINDO doses were given at 24 hours intervals. An echocardiogram was performed before the third PINDO dose and doses 4–6 were given only if there was any evidence (even minimal) of ductus patency on the echocardiogram. An echocardiogram was repeated at the end of the first week. Following the PINDO treatment, infants with a small or closed ductus were examined daily for a change in clinical symptoms indicative of a PDA (systolic murmur, widened pulse pressure, hyperdynamic precordium). If any of these occurred, an echocardiogram was performed within 24 hours. Infants with a persistent moderate-to-large PDA after the first week were followed to determine if or when retreatment or ligation would be necessary.
In May 2011 we made a change to a more conservative treatment approach. During epoch 2 (May 2011 through December 2015, n=127) PINDO was no longer used. PDAs were no longer treated with indomethacin until at least 8 days of age to allow for spontaneous closure (23). During epoch 2, all infants had an echocardiogram on postnatal days 6 or 7.
Our goal was to determine whether a moderate-to-large PDA was responsible for vasopressor-dependent hypotension at the end of the first postnatal week if other identifiable causes of hypotension were excluded. Therefore, infants who died or developed identifiable causes of hypotension (bacteremia, NEC or spontaneous intestinal perforations) during the first 7 days were excluded from our study population (Table I). None of the study infants underwent surgical ligation during the first 7 postnatal days.
Table 1.
Baseline characteristics for the Total and Study Populations
| Characteristics | Total Eligible Population (n=411) |
Study Population (n=313) |
||||
|---|---|---|---|---|---|---|
| Conservative epoch |
Prophylactic epoch |
p-value | Conservative epoch |
Prophylactic epoch |
p-value | |
| (n=127) | (n=284) | (n=98) | (n=215) | |||
| Preeclampsia (%) | 21 | 20 | 0.86 | 21 | 20 | 0.70 |
| Gestational Diabetes (%) | 13 | 6 | 0.01 | 15 | 7 | 0.03 |
| Chorioamnionitis (%) | 18 | 26 | 0.08 | 19 | 23 | 0.50 |
| Antenatal betamethasone * - any (%) | 81 | 73 | 0.09 | 85 | 77 | 0.13 |
| Antenatal betamethasone ≥24 hours ** (%) | 71 | 64 | 0.18 | 73 | 69 | 0.52 |
| Cesarean section (%) | 73 | 67 | 0.23 | 68 | 68 | 0.92 |
| Caucasian race (%) | 47 | 41 | 0.23 | 44 | 40 | 0.52 |
| Male (%) | 41 | 53 | 0.03 | 38 | 53 | 0.02 |
| Gestational age - weeks (mean ± SD) | 25.9 ± 1.2 | 25.9 ±1.2 | 0.60 | 26.1 ± 1.1 | 26.1 ± 1.1 | 0.96 |
| Birth weight -grams (mean ± SD) | 806 ± 206 | 811 ± 206 | 0.81 | 826 ± 205 | 833 ± 203 | 0.78 |
| Small for gestational age (%) | 15 | 16 | 0.75 | 15 | 15 | 0.92 |
| 5 minute Apgar score <6 (%) | 43 | 32 | 0.03 | 40 | 30 | 0.10 |
| Intubation in delivery room (%) | 72 | 83 | 0.01 | 67 | 82 | <0.01 |
| Respiratory distress syndrome (%) | 94 | 93 | 0.68 | 92 | 92 | 0.95 |
| Surfactant (%) | 82 | 95 | <0.0001 | 77 | 94 | <0.0001 |
| Respiratory Severity Score ξ at 24 hours of life (mean ± SD) |
2.4 ±1.9 | 1.8 ±1.7 | <0.0001 | 2.2 ±1.6 | 1.8 ±1.0 | 0.01 |
| Intubated and mechanically ventilated at 24 hours of life (%) |
74 | 65 | 0.09 | 68 | 63 | 0.38 |
| Any dopamine for at least 12 hours during first 24 hours of life (%) |
48 | 39 | 0.07 | 45 | 39 | 0.34 |
| Dopamine ≥6 µg/kg/min for at least 12 hours during first 24 hours of life (%) |
33 | 23 | 0.04 | 28 | 22 | 0.33 |
| Grade III or IV IVH (%) | 20 | 17 | 0.53 | 10 | 12 | 0.71 |
| Fluid intake during 1st 24 hours - ml/kg/day (mean ± SD) |
144 ±43 | 132 ±38 | 0.01 | 141 ±36 | 133 ±38 | 0.08 |
| Patent ductus arteriosus (moderate-to-large) on days 6 or 7 (%) |
66 | 9 | <0.0001 | 64 | 8 | <0.0001 |
| Exclusion Criteria | ||||||
|
Exclusion: Bacteremia before 8 days of life (%) |
6 | 6 | 0.89 | - | - | |
|
Exclusion: Necrotizing Entercolitis φbefore 8 days of life (%) |
6 | 5 | 0.87 | - | - | |
| Exclusion: Death before 8 days of life (%) | 15 | 13 | 0.53 | - | - | |
| Total excluded (%) | 23 | 24 | 0.75 | - | - | |
Antenatal Betamethasone, received betamethasone more than 6 hours prior to delivery
Antenatal Betamethasone ≥24 hours, received betamethasone at least 24 hours prior to delivery
Respiratory Severity Score (RSS), mean airway pressure × fraction of inspired oxygen, measured at 24 hours after birth
Necrotizing enterocolitis, Bell’s classification ≥II (treated medically or surgically) and “spontaneous perforations”
The effects of a moderate-to-large PDA (and of the two different treatment approaches) on other short term and long term neonatal morbidities will be reported separately.
A single neonatologist prospectively evaluated and recorded all of the perinatal/neonatal risk factors during the hospitalization (Table I). Gestational age was determined by the date of last menstrual period and early ultrasounds (before 24 weeks gestation). Small for gestational age was defined as birthweight less than the tenth-percentile for gestational age using the growth curves from Olsen et al (24). All infants were examined with serial bedside cranial ultrasounds initiated within the first week of life. Intraventricular hemorrhage was classified using the four-level grading system (25).
The echocardiographic studies included two dimensional imaging, M-mode, color flow mapping and Doppler interrogation as previously described (26). A moderate-to-large PDA was defined by one or more of the following echocardiographic criteria: Internal ductus diameter ≥ 1.5mm (or PDA:left pulmonary artery ratio ≥0.5); ductus flow velocity ≤2.5m/sec or mean pressure gradient across the ductus ≤8mm; left pulmonary artery diastolic (or mean) flow velocity > 0.2 (or >0.42) m/sec; and/or reversed diastolic flow in the descending aorta (22, 27).
During the time period of our study, a standardized approach was used in our nursery that determined when volume expanders and vasopressors would be initiated, and included the rate at which they would be increased or decreased (see below) (21). An arterial line and transducer were used to measure blood pressure continuously in all infants receiving dopamine infusions or hydrocortisone for blood pressure support.
Hypotension was defined as “mean BP less than the 3rd percentile for postmenstrual age that lasted more than 15 minutes” (28, 29). Operationally, this meant that infants were considered to be hypotensive, and require treatment for their hypotension, if their mean blood pressure was less than [(postmenstrual age in mm Hg) - (3 to 4 mm Hg)].
When infants failed to maintain an adequate BP (defined as “BP greater than the hypotensive range”), no more than 2 fluid boluses could be given initially to correct presumed hypovolemia. If the fluid boluses were unsuccessful in maintaining an adequate BP, dopamine support could be added. Infusion of dopamine was started at a rate of 5 µg/kg/min. The dose could be increased by 2 µg/kg/min every 15 – 30 minutes until an adequate BP was achieved. If a dopamine infusion rate of >15 µg/kg/min failed to maintain an adequate BP, hydrocortisone (starting at 1 mg/kg/day, dosed at 0.25 mg/kg/dose every 6 hours) could be added. When attempting to wean the dopamine infusion, the rate was decreased by 2 µg/kg/min every hour as long as an adequate BP was maintained.
Dopamine infusion rates were recorded hourly for the first 7 days after birth. Our outcome, vasopressor-dependent hypotension at the end of the first week, was defined as having received vasopressors for at least 24 hours during postnatal days 4–7 (non-contiguous; maximum 96 hours) (day of birth = postnatal day 0). Postnatal days 4–7 were chosen because (a) PDAs that respond to PINDO are small or closed by postnatal day 4, (b) most of the factors specifically related to hypotension during the early transitional period are no longer present by postnatal day 4 (12), and (c) indomethacin treatment could be used in the Conservative epoch after postnatal day 7.
The severity of the hypotension was determined by the maximum amount of dopamine that was used for at least 24 hours (any, ≥ 6 or ≥ 10 µg/kg/min). The primary outcome was the incidence of dopamine-dependent hypotension, defined as ≥6 µg/kg/min dopamine for at least 24 hours during postnatal days 4–7. Hydrocortisone administration was counted as 24 hours of vasopressor exposure for each day it was given.
The mode of ventilation (continuous positive nasal airway pressure (CPAP), biphasic nasal ventilation (BiPAP), or intubated mechanical ventilation), mean airway pressure and fraction of inspired oxygen (FiO2) were recorded at noon each day for the first 7 days after birth. The mean airway pressure and FiO2 at the end of the first postnatal week (days 4–7) were calculated by averaging the daily mean airway pressures and FiO2s for days 4–7. For each infant a daily Respiratory Severity Score (RSS = mean airway pressure × FiO2) was calculated and averaged for days 4–7. A weighted Mode of Ventilation Score was created to reflect the duration of different levels of respiratory support during days 4–7 [Mode of Ventilation Score = (3 × number of days intubated) + (2 × number of days of BiPAP) + 1 × (number of days of CPAP)] (range 0–12).
Statistical analyses
STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) was used for all statistical analysis. Chi-Squared tests were used to compare the PINDO and vasopressor-dependent hypotension groups with categorical variables. For continuous variables, Student’s t-tests were used to compare groups for parametric variables and Wilcoxon rank sum tests to compare groups for non-parametric variables. Logistic regression was used to examine the relationship between PINDO and vasopressor-dependent hypotension. Prophylactic indomethacin treatment was assigned by epoch and therefore not confounded by indication. Although sixteen study infants born during the PINDO epoch received their first dose of indomethacin after 24 hours (on days 2 or 3, because of initial oliguria, elevated creatinine, or coagulopathy), all infants born during the PINDO period were analyzed using the intention to treat principle.
Because several differences in baseline characteristics existed between the two epochs that might affect the outcomes (Table I), we created two models: a simple (unadjusted) model (including only indomethacin treatment and outcome) and a model that included all the characteristics that differed significantly (with p-values <0.05) between the epochs (delivery room intubation, surfactant, respiratory severity score at 24 hours after birth, gestational diabetes and male sex [Table I]). Sensitivity analysis was used to compare these models. Potential outcomes estimation using STATA’s margins command was used to estimate a risk difference.
In order to examine whether closing the PDA with PINDO was effective in preventing hypotension among infants who were at highest risk for developing vasopressor dependent hypotension, we used logistic regression to develop a model for predicting which infants would be most likely to develop vasopressor-dependent hypotension (assuming the infants had not previously received indomethacin to close their PDA). Therefore, to develop the model, we only used infants from the Conservative epoch, because they had not previously received indomethacin to close their PDA. Baseline characteristics associated with vasopressor-dependent hypotension (with p values of ≤0.10), were included in the model using forward selection. The final variables included in the model were gestational age, dopamine of ≥6 µg/kg/min for at least 12 hours during the first 24 hours of life, small for gestational age, RSS >2.5 at 24 hours of life, and an interaction term between gestational age and dopamine of ≥6 µg/kg/min for at least 12 hours during the first 24 hours of life. Hosmer-Lemeshow goodness of fit testing was used to assess calibration and the receiver-operating characteristic was used to assess discrimination. The model fit the data well and the area under the receiver-operating characteristic curve was 0.89. We then used the model to predict the probability of vasopressor-dependent hypotension for each of the infants in the Conservative and PINDO groups and used the chi-squared test to compare a subset of infants from the two groups who had a high-predicted probability of vasopressor dependent hypotension (probability ≥0.6).
Linear regression was used to examine the effects of PINDO on respiratory variables (mean airway pressure, FiO2, RSS and Mode of Ventilation Score) on days of life 4–7. The models included all the characteristics that differed between the PINDO and Conservative epochs (see above) and were checked to make sure they satisfied the assumptions of linear regression. Robust standard errors were used to satisfy the constant variance assumption. Linear prediction was used to predict marginal means using STATA’s margins command.
Results
During the study period, 411 infants were born; 98 infants were excluded because of NEC, early onset septicemia or death during the first 7 days (29 in the conservative group; 69 in the PINDO group) (Table I). The two treatment epochs were similar except for the incidences of gestational diabetes, male sex, surfactant administration, intubation in the delivery room and Respiratory Severity Score at 24 hours after birth (Table I). As expected the incidence of moderate-to-large PDA at the end of the first week differed significantly between the two treatment epochs (Table I).
Infants in the PINDO group had a lower incidence of vasopressor dependent hypotension than those in the Conservative group (Tables II–IV and Figures 1 and 2; Table III and Figures 1 and 2 available at www.jpeds.com). As the severity of the vasopressor dependent hypotension increased, the effects of PINDO became more significant (Tables II and IV and Figure 1). For our primary outcome of vasopressor-dependent hypotension (≥ 6 µg/kg/min of dopamine for at least 24 hours between days 4–7) we created 3 logistic regression models to evaluate whether the effects of PINDO on vasopressor-dependent hypotension were mediated through possible confounders that differed between the treatment epochs (Table IV). The odds ratio and confidence interval of the simple unadjusted model (Model 1: OR=0.41, 95% CI: 0.23–0.79) were similar to the odds ratio and confidence interval of the model adjusted for the differences between the epochs listed above (Model 2: OR=0.40, 95% CI: 0.20–0.82) (Table IV). This suggests that the effects of PINDO on vasopressor dependent hypotension were not confounded by any of the differences in baseline characteristics.
Table 2.
Effect of prophylactic indomethacin on vasopressor dependent hypotension
| Incidence of vasopressor use for at least 24 hours during days 4–7 |
Duration of vasopressor use ** | |||||
|---|---|---|---|---|---|---|
| % | Hours Median (10th −90th percentile) |
|||||
| Vasopressor | Conservative Epoch (n=98) |
Prophylactic epoch (n=215) |
p-value | Conservative epoch |
Prophylactic epoch |
p-value |
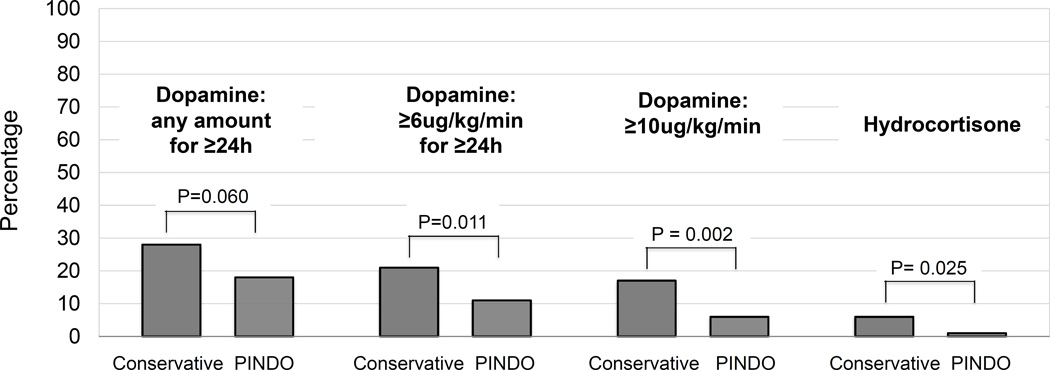
| Dopamine -any | 28 | 18 | 0.060 | 96 (35–96) | 58 (27–96) | 0.002 |
| Dopamine ≥6 µg/kg/min | 21 | 11 | 0.011 | 91 (42–96) | 57 (33–96) | 0.033 |
| Dopamine ≥10 µg/kg/min | 17 | 6 | 0.002 | 80 (37–96) | 62 (28–96) | 0.163 |
| Hydrocortisone * | 6 | 1 | 0.025 | - | - | - |
Incidence of hydrocortisone use when dopamine infusion rate ≥16 µg/kg/min
Duration of vasopressor use among infants who received dopamine at the indicated infusion rate for at least 24 hours during postnatal days 4–7(maximum duration = 96 hours)
Table 4.
Effects of prophylactic indomethacin on vasopressor dependent hypotension at different levels of severity
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
|
Dopamine infusion rate |
Simple Model | Multivariate Model* without PDA variable in model |
Multivariate Model* including PDA variable in the model |
|
| Prophylactic Indomethacin |
Prophylactic Indomethacin |
Prophylactic Indomethacin |
Moderate/largeP DA on day 6–7 |
|
| Dopamine - any | ||||
| Odds Ratio | 0.58 | 0.54 | 1.20 | 3.52 |
| 95% CI | 0.33–1.02 | 0.29–1.01 | 0.51–2.82 | 1.52–8.14 |
| p-value | 0.060 | 0.056 | 0.676 | 0.003 |
|
Dopamine ≥6 µg/kg/min |
||||
| Odds Ratio | 0.41 | 0.40 | 0.89 | 3.38 |
| 95% CI | 0.23–0.79 | 0.20–0.82 | 0.34–2.30 | 1.32–8.60 |
| p-value | 0.007 | 0.011 | 0.806 | 0.011 |
|
Dopamine ≥10 µg/kg/min |
||||
| Odds Ratio | 0.31 | 0.30 | 0.70 | 3.58 |
| 95% CI | 0.14–0.66 | 0.13–0.70 | 0.22–2.26 | 1.10–11.67 |
| p-value | 0.003 | 0.005 | 0.549 | 0.034 |
Multivariate Model adjusted for delivery room intubation, surfactant, respiratory severity score at 24 hours after birth, gestational diabetes and male gender Prophylactic Indomethacin variable = prophylactic group versus conservative group; PDA variable = moderate/large PDA versus small or closed ductus present on postnatal day 6 or 7
Figure 1.
Effect of prophylactic indomethacin on the incidence of vasopressor dependent hypotension (by epoch). Bars represent the incidence of dopamine use (at the indicated infusion rate) for at least 24 hours during days 4–7. Hydrocortisone was only used when the dopamine infusion rate was at least 16 µg/kg/min.
Figure 2.
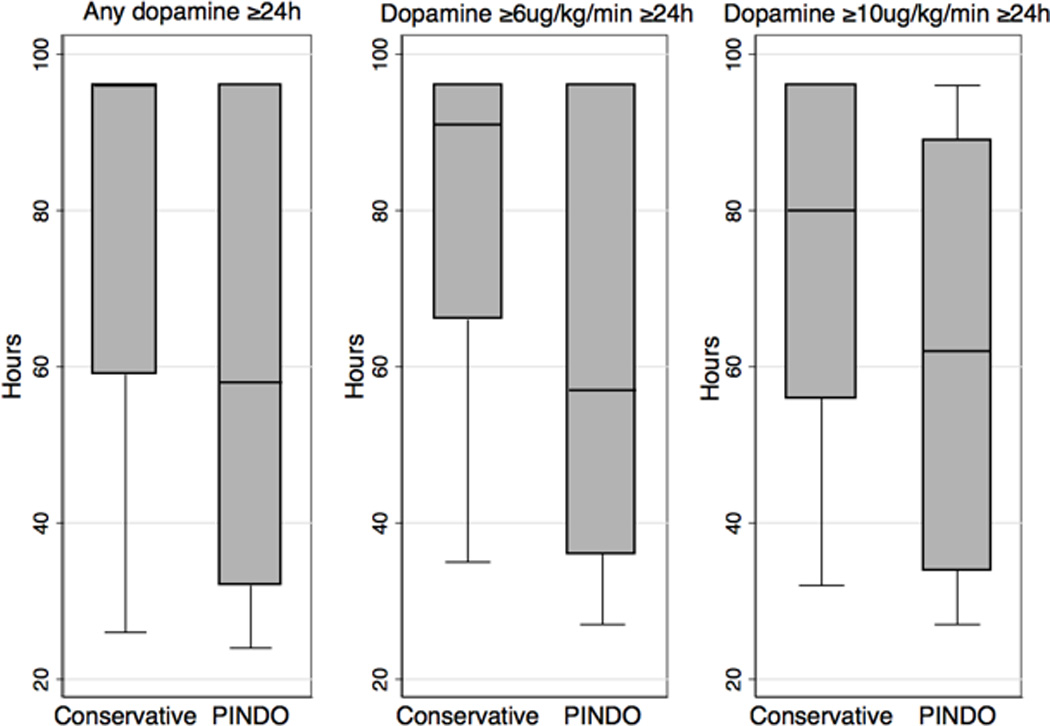
Effect of prophylactic indomethacin on the duration of vasopressor use (by epoch), represented by the duration of vasopressor use among infants who received dopamine at the indicated infusion rate for at least 24 hours during postnatal days 4–7 (maximum duration = 96 hours). The box-and-whisker plots represent the median, first and third quartiles, and minimum and maximum values of the duration of vasopressor use.
Table 3; online.
Effect of prophylactic indomethacin on vasopressor dependent hypotension among infants born at ≤256/7 weeks gestation or ≥26 weeks gestation
| Incidence of vasopressor use for at least 24 hours during days 4–7 | ||||||
|---|---|---|---|---|---|---|
| ≤256/7 weeks gestation (n=130) | ≥26 weeks gestation (n=183) | |||||
| Vasopressor | Conservative epoch (n=41) |
Prophylactic epoch (n=89) |
p-value | Conservative epoch (n=57) |
Prophylactic epoch epoch |
p-value |
| % | % | % | % | |||
| Dopamine -any | 37 | 22 | 0.09 | 21 | 15 | 0.32 |
| Dopamine ≥6 µg/kg/min | 32 | 13 | 0.01 | 14 | 9 | 0.28 |
| Dopamine ≥10 µg/kg/min | 22 | 9 | 0.04 | 14 | 4 | 0.01 |
| Hydrocortisone * | 5 | 2 | 0.65 | 7 | 1 | 0.03 |
Incidence of hydrocortisone use when dopamine infusion rate ≥16 µg/kg/min
During both treatment epochs, infants who had a persistent moderate-to-large PDA had an increased incidence of vasopressor dependent hypotension (Table V). When the variable “presence of a moderate-to-large PDA” was added to the adjusted model, the “presence of a moderate-to-large PDA” continued to be highly associated with the incidence of vasopressor dependent hypotension (Model 3: OR=0.3.38, 95% CI: 1.32–8.60); on the other hand, PINDO treatment no longer was significantly associated with vasopressor dependent hypotension (Model 3: OR=0.89, 95% CI: 0.34–2.30) (Table IV). This suggests that the effect of PINDO on vasopressor-dependent hypotension is mediated through closure of the PDA.
Table 5.
Incidence of vasopressor dependent hypotension among infants with a moderate-to-large PDA during the Conservative and Prophylactic treatment epochs
| Incidence of vasopressor use for at least 24 hours during days 4–7 | ||||||
|---|---|---|---|---|---|---|
| Conservative epoch (n=98) | Prophylactic epoch (n=215) | |||||
| Vasopressor | PDA closed or small (n=35) |
PDA moderate-to- large (n=63) |
p-value | PDA closed or small (n=197) |
PDA moderate-to- large (n=18) |
p-value |
| % | % | % | % | |||
| Dopamine -any | 17 | 33 | 0.09 | 15 | 56 | <0.001 |
| Dopamine ≥6 µg/kg/min | 9 | 29 | 0.02 | 9 | 33 | 0.001 |
| Dopamine ≥10 µg/kg/min | 9 | 22 | 0.09 | 5 | 22 | 0.003 |
| Hydrocortisone * | 3 | 8 | 0.42 | 2 | 0 | 0.990 |
Incidence of hydrocortisone use when dopamine infusion rate ≥16 µg/kg/min
We examined whether closing the PDA with PINDO was effective in preventing hypotension in the subset of infants with a high predicted probability (probability >= 0.6) for developing vasopressor-dependent hypotension (see Methods). Even in this high probability subset, PINDO significantly decreased the incidence of vasopressor-dependent hypotension (incidence of vasopressor-dependent hypotension ≥ 6 µg/kg/min of dopamine for at least 24 hours between days 4–7: PINDO group =44%; conservative group =85% (p = 0.02)).
In addition to its effects on hypotension, we also examined the effects of PINDO on the need for respiratory support at the end of the first postnatal week (days 4–7) (Table VI). Infants in the PINDO group required less Mean Airway Pressure and had a lower Respiratory Severity Score and weighted Mode of Ventilation Score than those in the conservative group. The effects of PINDO were no longer significant when the presence of a moderate-to-large PDA was included in the model (Table VI).
Table 6.
Multivariate models examining the effects of prophylactic indomethacin on respiratory needs at the end of the first postnatal week (days 4–7)
| Respiratory Outcome Variables | Conservative group |
Prophylactic group |
Difference between the groups (95% confidence interval)* |
p-value |
|---|---|---|---|---|
| (mean) | (mean) | |||
|
Multivariate Model* without PDA variable in model |
||||
| Mean airway pressure (cm H2O) | 6.9 | 5.8 | −1.10 (−1.63, −0.51) | <0.001 |
| FiO2 | 0.25 | 0.24 | −0.02 (−0.03, −0.01) | 0.002 |
| Respiratory Severity Score | 1.9 | 1.4 | −0.46 (−0.67, −0.24) | <0.001 |
| Mode of Ventilation Score | 7.8 | 6.6 | −1.19 (−2.06, −0.32) | 0.007 |
|
Multivariate Model* with PDA variable in model |
||||
| Mean airway pressure (cm H2O) | 6.3 | 6.1 | −0.22 (−0.91, 0.46) | 0.522 |
| FiO2 | 0.25 | 0.24 | −0.03 (−0.024, −0.002) | 0.025 |
| Respiratory Severity Score | 1.7 | 1.5 | −0.19 (−0.43, 0.04) | 0.104 |
| Mode of Ventilation Score | 6.9 | 7.0 | −0.04(−1.00, 1.08) | 0.937 |
PDA variable = moderate/large PDA versus small or closed ductus present on day 6 or 7
Multivariate Model adjusted for delivery room intubation, surfactant, respiratory severity score at 24 hours after birth, gestational diabetes and male gender. Linear prediction was used to predict the marginal means using STATA’s margins command.
Discussion
We found that PINDO decreases the incidence of vasopressor-dependent hypotension (and the degree of respiratory support needed) at the end of the first postnatal week when other identifiable causes of hypotension are not involved. The majority of PINDO’s effects can be attributed to its ability to close the moderate-to-large PDA that is present at the end of the first week. From our study, it is not possible to determine whether the PDA-induced hypotension is due to the shunt run-off into the low resistance pulmonary vascular bed (14) or to the increased mean airway pressure needed to overcome the PDA induced changes in lung compliance (30). Even though PINDO decreased the incidence of vasopressor-dependent hypotension, the incidence was not reduced to 0% (Tables II and V and Figure 1). We speculate that nonspecific causes associated with immaturity and/or illness (e.g., dysregulated cytokine, vasodilator, and/or cortisol production or release, delayed maturation of central autonomic or peripheral nervous systems) may be responsible for the vasopressor-dependent hypotension that occurs despite PDA closure (13, 31).
There are several limitations to our study. We used data from a single center; because the definition and treatment thresholds for hypotension differ by center (5, 10, 11), our results may not be generalizable to centers where hypotension is defined and managed differently. The effect size of PINDO on vasopressor-dependent hypotension may be less than our estimate in centers with stricter criteria for treatment of hypotension and greater in centers that treat hypotension liberally. In addition, even though we adjusted our analyses for differences between the epochs, there may have been unmeasured changes in practice that could have affected the rates of hypotension during the study period.
There are also strengths to our study. Prior observational studies that have tried to examine the relationship between the presence of a PDA and neonatal hypotension have been severely limited by the problem of residual confounding (i.e., the presence of a PDA is also a likely surrogate for immaturity). Any study that just compares groups based on whether or not a PDA is present is significantly confounded by the fact that infants in the group with a persistent PDA are also significantly more immature than infants in the group with a closed ductus. In our study, prophylactic indomethacin was used as a surrogate instrument for PDA closure. The planned change in the use of indomethacin allowed us to use a retrospective double cohort controlled study design. With this study design prophylactic indomethacin was not confounded by indication, as it has been in observational retrospective studies. Approximately 92% of infants in our study closed their ductus after prophylactic indomethacin compared with only 36% of infants in the conservative group (Table I). This imperfect correlation between prophylactic indomethacin and PDA closure does produce some misclassification because not all infants who received indomethacin closed their ductus and not all infants in the conservative group had a persistent moderate-to-large PDA. This type of misclassification, however, would bias our estimate towards the null, meaning that the true effect of PINDO is likely greater than what we observed in our study. The single center aspect of the study meant that the same consensus-driven, standardized approaches to respiratory, hemodynamic, fluid, nutrition and PDA evaluation and management were consistent among the infants in each of the study epochs. Although differences other than the use of prophylactic indomethacin administration did exist between the two time periods (Table I), models that included these factors produced similar odds ratios and confidence intervals, suggesting that these factors were not confounders of the relationship between PINDO and vasopressor-dependent hypotension (Table IV).
Over the last decade the use of prophylactic or early PDA treatment has decreased (32). This is primarily due to the results of the prophylactic and early PDA treatment RCTs that failed to show any improvement in long-term morbidities (like BPD and neurodevelopment) (18, 19). Despite the lack of long-term benefits, the PINDO RCTs have demonstrated important reductions in the risks of several short-term morbidities like severe IVH, pulmonary hemorrhage and surgical ligations (18, 19). Our results suggest that PINDO or early PDA treatment has the additional short-term benefit of reducing the incidence of vasopressor-dependent hypotension. Decreasing the incidence of vasopressor-dependent hypotension is important because infants requiring vasopressor support are made nil per os and have more days of intravenous therapies - putting them at increased risk for infection (33). There is also the possibility that vasopressor use may increase the risks of other morbidities (3, 5, 34–36). Neonatologists may wish to consider these additional short-term benefits when they decide whether or not to use PINDO or early PDA treatment in extremely premature infants.
Acknowledgments
We thank Drs Mark Cocalis, Laura Robertson, Michael Brook, Anita Moon-Grady, and Shabnam Peyvandi for their expert help in reading and interpreting the echocardiograms.
Supported by the National Heart, Lung, and Blood Institute (HL109199), the American Academy of Pediatrics Marshall Klaus Neonatal-Perinatal Research Award, and by the Jamie and Bobby Gates Foundation.
Abbreviations
- RCT
randomized controlled trial
- PDA
patent ductus arteriosus
- PINDO
prophylactic indomethacin treatment
- NEC
necrotizing enterocolitis
- BP
systemic blood pressure
- IVH
intraventricular/intracranial hemorrhage
- BPD
bronchopulmonary dysplasia
- CPAP
continuous positive nasal airway pressure
- BiPAP
biphasic nasal ventilation
- STATA
Stata Statistical Software, StataCorp. 2015
- OR
odds ratio
- CI
confidence interval
- RSS
Respiratory Severity Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
The authors declare no conflicts of interest.
References
- 1.Faust K, Hartel C, Preuss M, Rabe H, Roll C, Emeis M, et al. Short-term outcome of very-low-birthweight infants with arterial hypotension in the first 24 h of life. Arch Dis Child Fetal Neonatal Ed. 2015;100:F388–F392. doi: 10.1136/archdischild-2014-306483. [DOI] [PubMed] [Google Scholar]
- 2.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Early blood pressure, antihypotensive therapy and outcomes at 18–22 months’ corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2016;101:F201–F206. doi: 10.1136/archdischild-2015-308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics. 2013;131:e1865–e1873. doi: 10.1542/peds.2012-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham S, Symon AG, Elton RA, Zhu C, McIntosh N. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum Dev. 1999;56:151–165. doi: 10.1016/s0378-3782(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 5.Laughon M, Bose C, Allred E, O’Shea TM, Van Marter LJ, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119:273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara PJ, Stewart L, Shivananda SP, Stephens D, Sehgal A. Patent ductus arteriosus ligation is associated with impaired left ventricular systolic performance in premature infants weighing less than 1000 g. J Thorac Cardiovasc Surg. 2010;140:150–157. doi: 10.1016/j.jtcvs.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr. 1990;117:607–614. doi: 10.1016/s0022-3476(05)80700-0. [DOI] [PubMed] [Google Scholar]
- 8.Murphy DJ, Hope PL, Johnson A. Neonatal risk factors for cerebral palsy in very preterm babies: case-control study. BMJ. 1997;314:404–408. doi: 10.1136/bmj.314.7078.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens SE, Rijken M, Stoelhorst GM, van Zwieten PH, Zwinderman AH, Wit JM, et al. Is hypotension a major risk factor for neurological morbidity at term age in very preterm infants? Early Hum Dev. 2003;75:79–89. doi: 10.1016/j.earlhumdev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol. 2007;27:469–478. doi: 10.1038/sj.jp.7211774. [DOI] [PubMed] [Google Scholar]
- 11.Al-Aweel I, Pursley DM, Rubin LP, Shah B, Weisberger S, Richardson DK. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol. 2001;21:272–278. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- 12.Wu TW, Azhibekov T, Seri I. Transitional Hemodynamics in Preterm Neonates: Clinical Relevance. Pediatr Neonatol. 2016;57:7–18. doi: 10.1016/j.pedneo.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Noori S, Seri I. Pathophysiology of newborn hypotension outside the transitional period. Early Hum Dev. 2005;81:399–404. doi: 10.1016/j.earlhumdev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Clyman RI, Mauray F, Heymann MA, Roman C. Cardiovascular effects of a patent ductus arteriosus in preterm lambs with respiratory distress. J Pediatr. 1987;111:579–587. doi: 10.1016/s0022-3476(87)80126-9. [DOI] [PubMed] [Google Scholar]
- 15.Evans N, Moorcraft J. Effect of patency of the ductus arteriosus on blood pressure in very preterm infants. Arch Dis Child. 1992;67:1169–1173. doi: 10.1136/adc.67.10_spec_no.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans N, Iyer P. Change in blood pressure after treatment of patent ductus arteriosus with indomethacin. Arch Dis Child. 1993;68:584–587. doi: 10.1136/adc.68.5_spec_no.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouissou A, Rakza T, Klosowski S, Tourneux P, Vanderborght M, Storme L. Hypotension in preterm infants with significant patent ductus arteriosus: effects of dopamine. J Pediatr. 2008;153:790–794. doi: 10.1016/j.jpeds.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD000174. CD000174. [DOI] [PubMed] [Google Scholar]
- 19.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003745. CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebowitz MC, Clyman RI. Predicting the Need for Home Oxygen Therapy in Preterm Infants Born Before 28 Weeks’ Gestation. Am J Perinatol. 2016;33:34–39. doi: 10.1055/s-0035-1555122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clyman RI, Wickremasinghe A, Merritt TA, Solomon T, McNamara P, Jain A, et al. Hypotension following patent ductus arteriosus ligation: the role of adrenal hormones. J Pediatr. 2014;164:1449–1455. doi: 10.1016/j.jpeds.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–387. doi: 10.1016/j.jpeds.2010.02.062. 7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–1121. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 24.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 25.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight < 1500 grams. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 26.Keller RL, Tacy TA, Fields S, Ofenstein JP, Aranda JV, Clyman RI. Combined treatment with a non-selective nitric oxide synthase inhibitor (L-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatric Research. 2005;58:1216–1221. doi: 10.1203/01.pdr.0000183659.20335.12. [DOI] [PubMed] [Google Scholar]
- 27.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90:F419–F422. doi: 10.1136/adc.2003.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. J Perinatol. 1995;15:470–479. [PubMed] [Google Scholar]
- 29.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19:103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 30.Szymankiewicz M, Hodgman JE, Siassi B, Gadzinowski J. Mechanics of breathing after surgical ligation of patent ductus arteriosus in newborns with respiratory distress syndrome. Biol Neonate. 2004;85:32–36. doi: 10.1159/000074955. [DOI] [PubMed] [Google Scholar]
- 31.Engle WD. Blood pressure in the very low birth weight neonate. Early Hum Dev. 2001;62:97–130. doi: 10.1016/s0378-3782(01)00124-4. [DOI] [PubMed] [Google Scholar]
- 32.Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA. Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. 2016;175:775–783. doi: 10.1007/s00431-016-2705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS, Canadian Neonatal N. Risk Factors and Outcomes of Late-Onset Bacterial Sepsis in Preterm Neonates Born at < 32 Weeks’ Gestation. Am J Perinatol. 2015;32:675–682. doi: 10.1055/s-0034-1393936. [DOI] [PubMed] [Google Scholar]
- 34.Short BL, Van Meurs K, Evans JR. Summary proceedings from the cardiology group on cardiovascular instability in preterm infants. Pediatrics. 2006;117:S34–S39. doi: 10.1542/peds.2005-0620F. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 36.Dempsey EM, Barrington KJ. Evaluation and treatment of hypotension in the preterm infant. Clin Perinatol. 2009;36:75–85. doi: 10.1016/j.clp.2008.09.003. [DOI] [PubMed] [Google Scholar]